Abstract
Background
We sought to evaluate population-based temporal trends in perioperative management, as well as short- and long-term outcomes associated with the operative management of colorectal liver metastasis (CRLM).
Methods
Using Surveillance, Epidemiology and End Results–Medicare linked data, we identified 2,121 patients with operatively managed CRLM between 1991 and 2006. Clinicopathologic data, trends in operative management, and survival were examined.
Results
Preoperative evaluation included computed tomography (CT; 66%), magnetic resonance imaging (MRI; 5%), and positron emission tomography (PET; 2%) with a temporal increase in the use of all 3 modalities over time (all P < .05). Patients undergoing hepatectomy only (n = 1,267; 60%) decreased over time, whereas the use of ablation alone (n = 668; 32%) and combined resection plus ablation (n = 186; 9%) increased (all P < .05). The use of both preoperative (10% to 16%) and adjuvant chemotherapy (35% to 47%) increased over time (P < .05). There was a marked temporal increase in patient comorbidities (>3 comorbidities: 1991–1995, 3%; 2003–2006, 12%; P < .001); however, perioperative complications (63%) and 30-day mortality (3%) did not change over time (both P > .05); 90-day mortality decreased from 9% to 7% over the study period (P = .007). Overall the 1-, 3-, and 5-year survivals were 74%, 42%, and 28% with no improvement over time (P = .19). On multivariate analysis, synchronous disease (hazard ratio [HR], 1.7) and use of ablation alone (HR, 1.2) were associated independently with a worse survival (both P < .05).
Conclusion
Most patients were evaluated with CT; PET was employed rarely. Although there was a temporal increase in chemotherapy utilization, only one half of patients received perioperative chemotherapy. Mortality associated with hepatic operations was low, but morbidity remained high with no temporal change despite an increased number of patient medical comorbidities.
With approximately 50,000 attributable deaths per year, colorectal cancer (CRC) is the third most common cause of cancer-related death in the United States.1 The liver is the most common site for CRC metastasis (CRLM); 15–25% of patients present with CRLM at the time of diagnosis of their primary cancer2,3 and an additional 15–40% of patients develop metachronous CRLM.2,4 Operative management of CRLM is the only potential for cure and is associated with a 5-year survival ranging from 40 to 55%.5-7 In recent years, advances in surgical care, improvements in preoperative imaging, and operative techniques have emerged, as well as more effective systemic chemotherapy.8-10 The treatment paradigm for CRLM has evolved with expanding indications for hepatic resection and new treatment strategies to compliment resection/ablation and increase the number of patients who are eligible to undergo resection.9,11 Data on temporal trends in perioperative management, utilization of hepatic resection versus thermal ablation, morbidity and mortality, as well as survival after liver-directed surgery for CRLM remain poorly characterized. Most data are derived from case series from large academic institutions,5,12-16 which may provide information that is inappropriate to extrapolate outside of the respective institution.
Population-based data may help to better characterize patterns of care among a more representative cohort of patients with CRLM. Detailed population-based data on patients with CRLM have been scarce owing to the inherent limitations of available datasets.17,18 For example, although the Surveillance, Epidemiology, and End Results (SEER) cancer registry provides patient- and primary tumor-specific data, information about utilization of perioperative services and metastatic disease sites are limited. In contrast, Medicare data are more limited regarding disease-specific information, but have detailed claims-derived data on the provision of services to beneficiaries. Together, the linked SEER–Medicare database allows for a more accurate examination of procedure utilization and outcome.19 The objective of the current study was to evaluate perioperative and operative management, as well as short- and long-term outcomes in a population-based cohort of patients with CRLM derived from the linked SEER–Medicare database with a particular emphasis on identifying any temporal changes over the last 16 years.
METHODS
Data source
This study was a retrospective analysis of prospectively collected data from the linked SEER–Medicare database. The SEER–Medicare database represents the unique linkage of 2 large, population-based sources of data that provide detailed information about Medicare beneficiaries with cancer. SEER data derive from 18 cancer registries, representing approximately 26% of the United States population, and is maintained by the National Cancer Institute.20 SEER data include information on patient demographics, tumor and disease characteristics, course of treatment, use of cancer-directed operative and medical therapy, survival, as well as cause of death for individuals diagnosed with cancer. The linkage of SEER data with Medicare claims is performed by the National Cancer Institute and Center for Medicare and Medicaid Services. For Medicare-eligible individuals, the linked SEER–Medicare database also includes Medicare claims for covered health care services, including hospital, physician, outpatient, home health, and hospice bills, from the individual's time of enrollment until death.21 These linked data are available beginning in 1991, and the SEER–Medicare database has matched successfully 93% of individuals aged ≥65 years at the time of primary cancer diagnosis with their Medicare enrollment file.
Study population
All Medicare-enrolled patients aged ≥65 years diagnosed with incident malignant primary colorectal adenocarcinoma between 1991 and 2006 in a SEER area were evaluated for inclusion. Patients with colorectal adenocarcinoma were identified by the International Classification of Diseases for Oncology topography, behavior, and histology codes.22 Patients were included who had topography codes representative of primary neoplasms located in the cecum, ascending colon, hepatic flexure, transverse colon, splenic flexure, descending colon, sigmoid colon, large intestine, not otherwise specified, rectosigmoid junction, or rectum, as well as a code indicative of malignant behavior. Histology codes (Table I) were selected to identify only patients with adenocarcinoma; other histology codes (eg, carcinoid) were excluded. A gastrointestinal pathologist at the Johns Hopkins Hospital (RAA) reviewed all of the histology codes to determine which codes were relevant to colorectal adenocarcinoma. Identification of patients with primary CRC who had hepatic metastasis was accomplished using an established algorithm23 that employed the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) diagnosis and procedure codes and Current Procedural Terminology (CPT) codes for malignant neoplasm of liver, secondary malignant neoplasm of liver, hepatectomy, and ablation of liver lesion or tissue (Table II). The study cohort included only patients enrolled in both Medicare parts A and B who were not enrolled in a managed care plan during the study period.
Table I.
ICD-O-3 histology codes specific to colorectal adenocarcinoma
| Histology code | Number of patients (%) |
|---|---|
| 8140 | 1,658 (78.2) |
| 8210 | 71 (3.3) |
| 8261 | 64 (3.0) |
| 8263 | 97 (4.6) |
| 8480 | 132 (6.2) |
| 8481 | 82 (3.9) |
| Other (8141, 8144, 8211, 8260, 8262, 8490) | 17 (1.0) |
| Total | 2,121 |
Table II.
CPT/ICD-9-CM codes used in identification of Medicare claims
| CPT codes | ICD-9-CM codes | |
|---|---|---|
| Procedures | ||
| Endoscopy | 43234, 43235, 43239, 43241, 43242, 43245, 43250, 43251, 43256, 43258 | 42.24, 44.14, 45.13, 45.14, 45.16 |
| Cholangiogram | 74320 | 87.52, 87.53, 87.54 |
| Percutaneous transhepatic cholangiography | 47500, 47505, 47510, 74363, 75980 | 51.98, 87.51 |
| MRI abdomen (with and without contrast) | 74181, 74182, 74183, 74185 | 88.97 |
| CT abdomen (with and without contrast) | 74150, 74160, 74170 | 87.41, 87.42, 87.72, 88.01, 88.02 |
| PET | 78811, 78812, 78813, 78814, 78815, 78816 | 88.90, 92.04, 92.18 |
| Portal vein embolization | 37204, 75894 | 39.79 |
| Diagnostic laparoscopy | 49320, 49321, 49329 | 54.21 |
| Hepatectomy | ||
| Biopsy, wedge | 47100 | 50.11, 50.12, 50.19 |
| Partial lobectomy | 47120 | 50.22 |
| Trisegmentectomy | 47122 | |
| Left lobectomy | 47125 | |
| Right lobectomy | 47130 | |
| Lobectomy (either or NOS) | 47125, 47130 | 50.3 |
| Lymphadenectomy | 38747, 38780 | 40.29, 40.50 |
| Hepaticojejunostomy | 47760, 47780, 47785, 47800, 47999 | 51.37 |
| Excision of biliary tree | 47711, 47712 | 51.64, 51.69 |
| Hepatic ablation | ||
| Open | 50.23 | |
| Radiofrequency | 47380 | |
| Cryosurgical | 47381 | |
| Laparoscopic | 50.25 | |
| Radiofrequency | 47370 | |
| Cryosurgical | 47371 | |
| Percutaneous | 50.24 | |
| Radiofrequency | 47382 | |
| Other and unspecified | 50.26, 50.29 | |
| Chemotherapy (intravenous) | 96409, 96411, 96413, 96415, 96416, 96417 | 99.25 |
| Transcatheter arterial chemoembolization | 37204, 75894 | |
| Complications | ||
| Operative reexploration | 49000, 49002 | 54.11, 54.22 |
| Percutaneous drain | 47000, 49021, 49041, 49061, 75989 | 54.91 |
| Accidental laceration | 998.2 | |
| Postoperative hemorrhage | 998.1–998.19 | |
| Post-hemorrhagic anemia | 285.1 | |
| Anesthetic reaction | 995.4 | |
| Wound dehiscence | 998.3, 998.6, 998.83 | |
| Liver abscess | 572.0 | |
| Peritonitis | 567.2 | |
| Gastrointestinal hemorrhage | 578.0, 578.1, 578.9 | |
| Gastrointestinal complications | 997.4 | |
| Biliary fistula | 576.4 | |
| Intestinal fistula | 569.81 | |
| Stomach or duodenal fistula | 537.4 | |
| Postoperative infection | 998.5–998.59 | |
| Paralytic ileus | 560.1 |
NOS, Not otherwise specified.
Outcome and predictor variables
Data on perioperative procedures, treatments, and complications were selected a priori based on clinical relevance and then identified from the Medicare database using both ICD-9-CM diagnosis and procedure codes, as well as CPT codes (Table II). Information on chemotherapy was designated as preoperative (within 6 months prior) and adjuvant (within 3 months after) liver-directed operation. Previous studies have demonstrated the validity of Medicare billing codes to assess a wide range of outcomes.24 Information on age, gender, race, marital status, and geographic region were obtained from the SEER portion of the database. Variables were transformed into categorical and indicator variables where appropriate. The Elix-hauser comorbidity index, a comprehensive set of 30 comorbidity measures, was used to identify and adjust for comorbid conditions.25-29 Comorbid diagnoses related to the patient's admission diagnosis (eg, metastatic cancer), as well as any comorbid condition with a frequency <5, were excluded so that a total of 20 comorbidities remained for analysis. To avoid including patients who underwent wedge biopsy only of CRLM without curative intent, patients with CPT (47100) and ICD-9 (50.11, 50.12, 50.19) codes for wedge biopsy were excluded from the analysis.
Statistical analyses
Mean and median values were used to describe continuous data, with discrete variables displayed as totals and frequencies. Cells with <11 cases per variable cell were relabeled as “<11 (<%)” in compliance with the National Cancer Institute regulation for reporting of SEER–Medicare data. Univariate comparisons were assessed using the 2-sample Student t-test for continuous variables and the Chi-square test for dichotomous and categorical variables. For purposes of analyses, the distribution of the total number of comorbid conditions per patient was divided into quartiles: 0, 1, 2–3, or >3 comorbidities. When assessing temporal trends, the data were separated into quartiles (1991–1995, 1996–1999, 2000–2002, and 2003–2005) based on the year of operation. Trends in ordinal data were evaluated using the linear-by-linear association test.30
Cumulative event rates were calculated using the method of Kaplan and Meier,31 and survival curves were compared using the log-rank test. Overall survival time was calculated from the date of the liver-directed operation for CRLM to the date of last follow-up. A postoperative death was defined as patient survival <30 or 90 days after the liver-directed operation. Univariate and multivariate modeling of survival were performed using Cox proportional hazards models.32 Covariates were included in the multivariate Cox model based on statistical significance in the univariate models (P ≤ .20). The model was validated by checking against a forward stepwise Wald selection model as described by Hosmer and Lemeshow.33,34 The overall fit of the multivariate models were assessed using the likelihood ratio test. Relative risks were expressed as hazard ratios (HR) with a 95% confidence interval (CI). The final model was evaluated for goodness-of-fit using the method proposed by May and Hosmer.33,35 Adherence to the proportional hazards assumption was assessed using Schoenfeld residuals and log–log plots. All reported P-values are 2-tailed. All statistical analyses were performed using SPSS Version 18.0 (SPSS Inc., Chicago, IL).
RESULTS
Patient and primary tumor characteristics
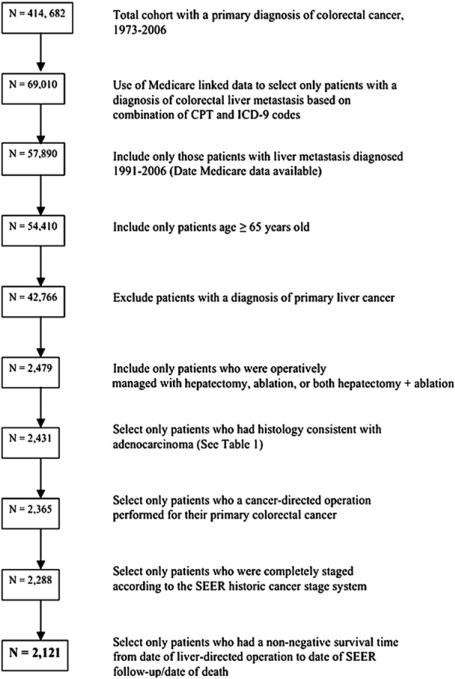
Utilizing the SEER database, 414,682 patients diagnosed with CRC between 1973 and 2006 were identified. After linking to the Medicare data (1991–2006) and selecting patients based on a combination of CPT and ICD-9 codes, while also excluding patients <65 years old and those with primary liver malignancies, 42,766 patients were identified as having a primary CRC with liver metastasis (Fig 1). Of the 42,766 patients with CRLM, 40,645 patients (95.0%) did not undergo operative management of their metastatic liver disease. In turn, 2,121 patients (5.0%) were identified as having undergone operative management of their hepatic metastasis as determined by the established selection algorithm23 and represent the study cohort.
Fig 1.
Flow diagram of patient selection within SEER–Medicare database, 1973–2006.
The demographic and clinical characteristics of the 2,121 patients with operatively managed CRLM are outlined in Table III. The majority of patients were white (n = 1,815, 85.6%), men (n = 1,084; 51.1%), and resided in an urban setting (n = 1,936, 91.3%). The colon was the primary site of CRC in 1,643 patients (77.5%), whereas 478 patients (22.5%) had a primary rectal neoplasm. Approximately one third of patients had locoregional lymph node metastasis associated with the primary colorectal neoplasm (n = 799; 37.7%). The most prevalent histology code (n = 1658; 78.2%) was 8140, “adenocarcinoma not otherwise specified” (Table I).22 At the time of CRLM diagnosis, the median patient age was 73.0 years (standard deviation [SD], 7.0). Presentation of the hepatic metastasis was synchronous in 893 patients (42.1%). Among the 1,228 patients (57.9%) who presented with metachronous disease, the median disease-free interval between diagnosis of the primary CRC neoplasm and the hepatic metastasis was 16.0 months (SD, 29.6). Although most patients did not have any of the 20 Elixhauser medical comorbidities (n = 955; 45.0%), 180 patients (8.5%) had >3 comorbidities. Of note, the number of patients with >3 comorbidities increased over time (1991–1995, 3.4%; 1996–2000, 7.8%; 2001–2002, 9.4%; 2003–2006, 12.0%; P < .001). The most common preoperative comorbidities were hypertension (n = 751; 35.4%), anemia (n = 467; 22.0%), and chronic pulmonary disease (n = 223; 10.5%).
Table III.
Trends in baseline demographic and clinical characteristics of patients with operatively managed CRLM, SEER–Medicare, 1991–2006
| Total | 1991–1995 | 1996–2000 | 2001–2002 | 2003–2006 | |
|---|---|---|---|---|---|
| n | 2,121 | 468 | 574 | 404 | 675 |
| Number of patients (%) | |||||
| Median age at CRC diagnosis (yrs) ± SD | 73.0 ± 7.0 | 72.0 ± 7.0 | 73.0 ± 7.2 | 73.0 ± 7.1 | 72.0 ± 6.7 |
| Median age at CRLM diagnosis (yrs) ± SD | 73.0 ± 6.6 | 74.0 ± 6.6 | 74.0 ± 6.6 | 74.0 ± 6.8 | 73.0 ± 6.5 |
| Women | 1,037 (48.9) | 216 (46.2) | 301 (52.4) | 202 (50.0) | 318 (47.1) |
| White | 1,815 (85.6) | 411 (87.8) | 474 (82.6) | 361 (87.4) | 569 (84.3) |
| Married | 1,304 (61.5) | 292 (62.4) | 337 (58.7) | 253 (62.6) | 422 (62.5) |
| Urban | 1,936 (91.3) | 426 (91.0) | 524 (91.3) | 375 (92.8) | 611 (90.5) |
| Median time from diagnosis of CRC to CRLM (mos) ± SD* | 16.0 ± 29.6 | 16.5 ± 30.2 | 16.0 ± 34.7 | 14.0 ± 29.0 | 16.0 ± 25.4 |
| Location of primary cancer | |||||
| Colon | 1,643 (77.5) | 351 (75.0) | 454 (79.1) | 311 (77.0) | 527 (78.1) |
| Rectum | 478 (22.5) | 117 (25.0) | 120 (20.9) | 93 (23.0) | 148 (21.9) |
| SEER historic stage of CRC primary | |||||
| Localized‡ | 429 (20.2) | 87 (18.5) | 121 (21.1) | 80 (19.8) | 141 (20.9) |
| Regional | 799 (37.7) | 180 (38.5) | 198 (34.5) | 159 (39.4) | 262 (38.8) |
| Distant | 893 (42.1) | 201 (42.9) | 255 (44.4) | 165 (40.8) | 272 (40.3) |
| Elixhauser comorbidities | |||||
| Congestive heart failure | 128 (6.0) | 18 (3.8) | 41 (7.1) | 29 (7.2) | 40 (5.9) |
| Arrhythmias† | 196 (9.2) | 28 (6.0) | 52 (9.1) | 34 (8.4) | 82 (12.1) |
| Valvular heart disease† | 118 (5.6) | <11 (<2.4) | <11 (<1.9) | 29 (7.2) | 54 (8.0) |
| Pulmonary circulatory disease | 11 (0.5) | <11 (<2.4) | <11 (<1.9) | <11 (<2.7) | <11 (<1.6) |
| Peripheral arterial disease† | 125 (5.9) | 23 (4.9) | 23 (4.0) | 17 (4.2) | 62 (9.2) |
| Hypertension† | 751 (35.4) | 104 (22.2) | 200 (34.8) | 173 (42.8) | 274 (40.6) |
| Paralysis | 11 (0.5) | <11 (<2.4) | <11 (<1.9) | <11 (<2.7) | <11 (<1.6) |
| Other neurologic disorder | 34 (1.6) | <11 (<2.4) | <11 (<1.9) | 11 (2.7) | <11 (<1.6) |
| Chronic pulmonary disease† | 223 (10.5) | 24 (5.1) | 54 (9.4) | 56 (13.4) | 89 (13.2) |
| Diabetes complicated | 69 (3.3) | <11 (<2.4) | <11 (<1.9) | 16 (4.0) | 24 (3.6) |
| Thyroid disease† | 162 (7.6) | 21 (4.5) | 38 (6.6) | 44 (10.9) | 59 (8.7) |
| Renal failure | 25 (1.2) | <11 (<2.4) | <11 (<1.9) | <11 (<2.7) | <11 (<1.6) |
| Liver disease† | 14 (0.7) | <11 (<2.4) | <11 (<1.9) | <11 (<2.7) | <11 (<1.6) |
| Peptic ulcer disease† | 40 (1.9) | <11 (<2.4) | 16 (2.8) | <11 (<2.7) | 16 (2.4) |
| Rheumatoid arthritis† | 59 (2.8) | <11 (<2.4) | 15 (2.6) | <11 (<2.7) | 24 (3.6) |
| Coagulopathy† | 32 (1.5) | <11 (<2.4) | 15 (2.6) | <11 (<2.7) | 14 (2.1) |
| Weight loss | 13 (0.6) | <11 (<2.4) | <11 (<1.9) | <11 (<2.7) | <11 (<1.6) |
| Deficiency anemia† | 467 (22.0) | 71 (15.2) | 133 (23.2) | 103 (25.5) | 160 (23.7) |
| Alcohol abuse | <11 (<0.5) | <11 (<2.4) | <11 (<1.9) | <11 (<2.7) | <11 (<1.6) |
| Depression | 40 (1.9) | <11 (<2.4) | <11 (<1.9) | <11 (<2.7) | 19 (2.8) |
| Comorbidities (n) | |||||
| None† | 955 (45.0) | 270 (57.7) | 258 (44.9) | 156 (38.6) | 271 (40.1) |
| 1 | 490 (23.1) | 108 (23.1) | 140 (24.4) | 90 (22.3) | 152 (22.5) |
| 1–2† | 496 (23.4) | 74 (15.8) | 131 (22.8) | 120 (29.7) | 171 (25.3) |
| >3† | 180 (8.5) | 16 (3.4) | 45 (7.8) | 38 (9.4) | 81 (12.0) |
For patients with metachronous disease only (n = 1,228).
Significant at P < .05 by the linear-by-linear association test of trend.
Also includes in situ lesions (less than 1% overall total) to mask totals <11 in compliance with SEER data usage agreement.
CRC, Colorectal cancer; CRLM, colorectal liver metastasis; SD, standard deviation; SEER, Surveillance, Epidemiology, and End-Results Cancer Registry.
Trends in perioperative care: Diagnostic imaging and systemic chemotherapy
Most patients (n = 1505, 71.0%) were evaluated preoperatively by cross-sectional imaging. In the overwhelming majority of patients, computed tomography (CT) was the imaging modality of choice (n = 1,398, 65.9%); magnetic resonance imaging (MRI; n = 107, 5.0%) and positron emission tomography (PET; n = 40, 1.9%) were used in only a minority of patients. The overall utilization of cross-sectional imaging increased over the time periods examined (P < .001). Of note, there was a temporal change in the relative utilization of the different imaging modalities (Table IV). Specifically, there was almost a 50% increase in the use of CT from 52.8% in 1991–1995 to 77.8% in 2003–2006. In contrast, although the use of MRI increased >3-fold (1991–1995, 2.4%; 1996–2000, 3.8%; 2001–2002, 6.2%; 2003–2006, 7.3%; P < .001), MRI was still utilized in <10% of patients with CRLM. Of note, PET was used in only 5.5% of patients, even in the most recent time period examined.
Table IV.
Preoperative staging, operative details, and postoperative outcomes of operatively managed patients with CRLM, SEER–Medicare, 1991–2006
| Total | 1991–1995 | 1996–2000 | 2001–2002 | 2003–2006 | |
|---|---|---|---|---|---|
| n | 2,121 | 468 | 574 | 404 | 675 |
| Preoperative staging within 6 months | |||||
| MRI abdomen* | 107 (5.0) | 11 (2.4) | 22 (3.8) | 25 (6.2) | 49 (7.3) |
| CT abdomen* | 1,398 (65.9) | 247 (52.8) | 330 (57.5) | 296 (73.3) | 525 (77.8) |
| PET* | 40 (1.9) | 0 (0.0) | <11 (<1.9) | <11 (<2.7) | 37 (5.5) |
| Diagnostic laparoscopy* | 54 (2.5) | <11 (<2.4) | <11 (<1.9) | 13 (3.2) | 24 (3.6) |
| Portal vein embolization | <11 (<0.5) | <11 (<2.4) | <11 (<1.9) | <11 (<2.7) | <11 (<1.6) |
| Hepatic resection | |||||
| Hepatectomy alone* | 1,267 (59.7) | 333 (71.2) | 359 (62.5) | 224 (55.4) | 351 (52.0) |
| Partial hepatectomy* | 1,199 (56.5) | 307 (65.6) | 325 (56.6) | 212 (52.5) | 355 (52.6) |
| Hemihepatectomy | 422 (19.9) | 90 (19.2) | 116 (20.2) | 79 (19.6) | 137 (20.3) |
| Extended hepatectomy | 43 (2.0) | 13 (2.8) | 11 (1.9) | <11 (<2.7) | <11 (<1.6) |
| Hepatectomy and ablation* | 186 (8.8) | 29 (6.2) | 38 (6.6) | 36 (8.9) | 83 (12.3) |
| Ablation alone* | 668 (31.5) | 106 (22.6) | 177 (30.8) | 144 (35.6) | 241 (35.7) |
| Systemic therapy | |||||
| Preoperative chemotherapy* | 275 (13.0) | 45 (9.6) | 63 (11.0) | 59 (14.6) | 108 (16.0) |
| Adjuvant chemotherapy*,† | 868 (40.9) | 162 (34.6) | 223 (38.9) | 167 (41.3) | 316 (46.8) |
| Both preoperative and adjuvant chemotherapy* | 151 (7.1) | 26 (5.6) | 29 (5.1) | 31 (7.7) | 65 (9.6) |
| TACE | 13 (0.6) | <11 (<2.4) | <11 (<1.9) | <11 (<2.7) | <11 (<1.6) |
| Morbidity | |||||
| Any | 1,334 (62.9) | 298 (63.7) | 367 (63.9) | 252 (62.4) | 417 (61.8) |
| Reexploration after operation* | 95 (4.5) | 28 (6.0) | 30 (5.2) | 22 (5.4) | 15 (2.2) |
| Postoperative hemorrhage* | 95 (4.5) | 33 (7.1) | 27 (4.7) | 15 (3.7) | 20 (3.0) |
| Postoperative infection | 146 (6.9) | 30 (6.4) | 35 (6.1) | 25 (6.2) | 56 (8.3) |
| Percutaneous drain* | 307 (14.5) | 46 (9.8) | 65 (11.3) | 70 (17.3) | 126 (18.7) |
| Survival from liver-directed therapy | |||||
| Median (mos) | 28.0 | 28.0 | 28.0 | 27.0 | NR |
| 3-Year (%) | 41.9 | 41.7 | 40.6 | 41.2 | 51.7 |
| 30-Day mortality‡ | 59 (2.8) | <11 (<2.4) | 21 (3.7) | <11 (<2.7) | 16 (2.4) |
| 90-Day mortality§ | 210 (9.9) | 43 (9.2) | 73 (12.7) | 46 (11.4) | 48 (7.1) |
Significant at P < .05 by linear-by-linear association test of trend.
Postoperative chemotherapy given within 3 months after liver-directed therapy.
Death within 30 days of liver-directed therapy.
Death within 90 days of liver-directed therapy.
Data are presented as number of patients (%).
NR, Not reached; TACE, transcatheter arterial chemoembolization.
Patients with synchronous disease were less likely to have had a CT or MRI preoperatively as compared with patients who had metachronous CRLM (both P < .05). Patients with a primary rectal neoplasm were more likely to have had a pre-operative CT compared with patients who had a colon lesion (71.8% vs 64.2%; P = .002); there was no difference in use of MRI among patients with rectal versus colon neoplasms (P = .99). PET imaging was utilized more in patients presenting with metachronous disease versus patients with synchronous metastases (2.6% vs 0.9%; P = .007), but there was no difference in use of PET among patients with colon versus rectal neoplasms (P = .57).
Many patients (n = 992, 46.8%) underwent some type of perioperative systemic chemotherapy. Specifically, 275 (13.0%) received preoperative chemotherapy, and 868 patients (40.9%) received adjuvant therapy (Table IV). The use of preoperative chemotherapy increased over the time periods examined (1991–1995, 9.6%; 1996– 2000, 11.0%; 2001–2002, 14.6%; 2003–2006, 16.0%; P < .001; Fig 2, A). The mean duration of preoperative systemic chemotherapy was 2.6 months (median, 1.7; SD, 2.2). Receipt of preoperative chemotherapy was associated with the primary site of the CRC (rectal 15.7% vs colon 12.2%) and the presentation of liver metastasis (synchronous 6.3% vs metachronous 17.8%; both P < .05); however, receipt of preoperative chemotherapy was not associated with the number of preoperative medical comorbidities (P = .37). Of the 275 patients who received preoperative chemotherapy, 151 (54.9%) also received adjuvant chemotherapy. The proportion of patients who received both preoperative and adjuvant systemic therapy increased over time (1991–1995: 5.6% vs 2003–2006: 9.6%; P = .001; Table IV).
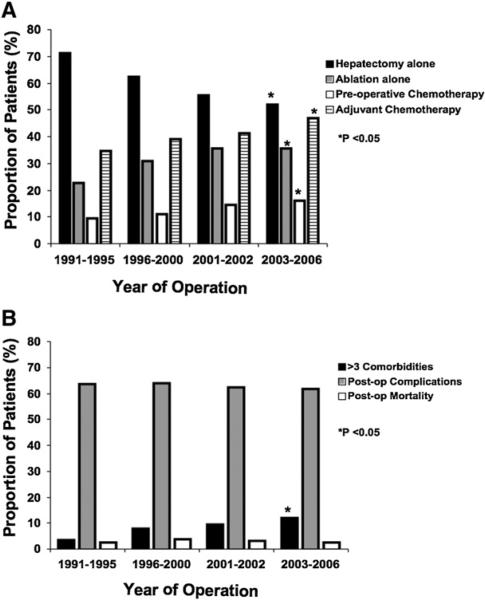
Fig 2.
(A) Temporal trends in liver-directed operations and perioperative medical management for patients with CRLM. (B) Trends in Elixhauser medical comorbidities and perioperative complications and 30-day mortality for patients with CRLM undergoing hepatectomy, ablation, or both.
Overall, 868 patients (40.9%) received adjuvant systemic chemotherapy after treatment of CRLM (preoperative + adjuvant, n = 151; adjuvant only, n = 717). Receipt of adjuvant therapy was associated with the presentation of liver metastasis (synchronous 51.0% vs metachronous 33.6%; P < .001). Receipt of adjuvant systemic therapy was not associated with the primary tumor site of the CRC (ie, rectal vs colon), number of preoperative medical comorbidities or postoperative complications (all P > .05). The mean time from hepatic resection with or without ablation to receipt of adjuvant systemic chemotherapy was 2.4 months (median, 2.5; SD, 0.6).
Trends in liver-directed management and perioperative outcomes
Of the 2,121 patients who underwent liver-directed management for CRLM, 1,267 (59.7%) underwent hepatic resection only, 186 (8.8%) had both resection and ablation, and 668 (31.5%) had ablation only (operative ablations, n = 543 vs percutaneous ablation, n = 125; Table IV). Most patients underwent partial hepatectomy/wedge resection (n = 1,199; 56.5%), whereas a hemihepatectomy was employed in 422 patients (19.9%). Extended hepatic resection was employed rarely (n = 43; 2.0%). Patients with synchronous disease were more likely to undergo a wedge resection (63.3%) than patients with a metachronous presentation (51.6%; P < .001). Among patients who underwent combined resection and ablation (n = 186), the hepatic resection consisted of partial hepatectomy/wedge resections in the vast majority of patients (n = 159; 85.5%). Patients who underwent resection plus ablation were more likely to be men (62.9% vs 37.1%; P = .001), but there were no other differences in race, marital status, rural residence, colon versus rectal site of the primary neoplasm, or Elixhauser comorbidity score compared with those patients who underwent resection alone (all P > .05). In contrast, patients who underwent ablation alone were more likely to have more Elixhauser comorbidities (11.2% vs 7.2%; P = .003) compared with patients managed with hepatic resection alone or resection plus ablation.
There were several shifting trends noted in the liver-directed management of patients treated over the time periods examined (Table IV; Fig 2, A). Specifically, the use of hepatectomy alone decreased over time (1991–1995, 71.2% vs 2003–2006, 52.0%; P < .001), whereas the use of combined resection plus ablation (1991–1995, 6.2% vs 2003–2006, 12.3%; P < .001) and ablation alone increased (1991–1995, 22.6% vs 2003–2006, 35.7%; P < .001). In particular, the use of ablation increased substantially during the last 2 periods (2001–2002, 19.8% vs 2003–2006, 29.6%; P <.001).
The overall proportion of patients who had a complication was 62.9% (n = 1,334) and did not change appreciably over the study period (Table IV; Fig 2, B). The most common complications were need for percutaneous drain (n = 307; 14.5%), postoperative infection (n = 146, 6.9%), and reexploration (n = 95; 4.5%). The risk of complication was associated with having >3 Elixhauser comorbidities (>3 comorbidities, 9.7% vs ≤3 comorbidities, 6.4%; P = .009). The risk of any complications was not associated with other factors, including type of procedure performed or time period examined (both P > .05; Fig 2, B).
There were 59 deaths within 30 days of operation for a perioperative mortality of 2.8%. The 30-day mortality did not change over the study period (P = .84; Fig 2, B). There was no association between perioperative mortality and rural residence, race, >3 Elixhauser comorbidities, or the extent of hepatic resection (all P > .05). In contrast, patients undergoing hepatic resection alone compared with ablation alone had a lesser risk of 30-day mortality (1.2% vs 3.8%, respectively; P = .002). The overall 90-day mortality was 9.9% (n = 210) and decreased from 9.2% to 7.1% from the beginning to the end of the study period (P = .007).
Long-term outcomes
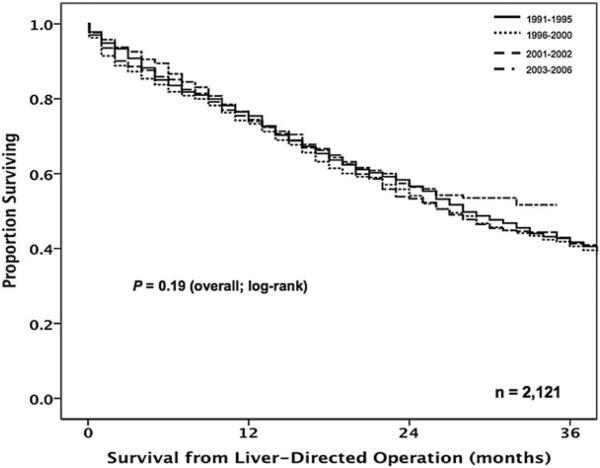
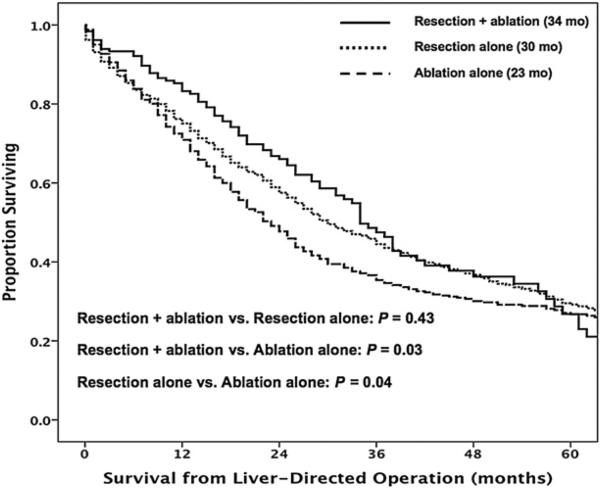
The overall median survival of patients with operatively managed CRLM was 28.0 months (95% CI, 26.0–30. months) with 1-, 3-, and 5-year survivals of 74.5%, 42.0%, and 28.4%, respectively. Of note, no significant change in overall survival was noted over the time periods examined (P = .19; Fig 3). On univariate analyses, however, several clinicopathologic factors were associated with worse survival, including synchronous disease, receipt of both preoperative and adjuvant systemic therapy, and treatment with ablation alone (all P < .05; Table V). Specifically, patients who underwent resection alone had a similar median survival compared with patients treated with resection plus ablation (30.0 vs 34 months, respectively; P = .43). In contrast, patients treated with ablation alone had a lesser median survival (23 months; P = .04; Fig 4). After using multivariate analysis to control for competing risk factors, including age and preoperative comorbidities, synchronous disease (HR, 1.67; 95% CI, 1.49–1.86; P < .001), receipt of both preoperative and adjuvant systemic therapy (HR, 1.62; 95% CI, 1.31–2.01; P < .001), and treatment with ablation alone (HR, 1.18; 95% CI, 1.05–1.33; P = .006) remained associated independently with worse outcome (Table V).
Fig 3.
Overall survival of operatively managed patients from the time of the liver-directed operation, stratified by time period. There was no improvement in survival over time (P = .19). Overall median survival 28 months.
Table V.
Cox regression analyses of variables associated with survival in patients undergoing liver-directed therapy for their colorectal liver metastases
|
Univariate
|
Multivariate
|
|||||
|---|---|---|---|---|---|---|
| Prognostic factor | Hazard ratio | 95% CI | P-value | Hazard ratio | 95% CI | P-value |
| Year of CRLM diagnosis category: 1991–1995 | 0.97 | 0.85–1.09 | .593 | — | — | — |
| Age at diagnosis of CRLM | 1.03 | 1.02–1.04 | <.001 | 1.03 | 1.02–1.04 | <.001 |
| Male gender | 1.06 | 0.95–1.18 | .320 | — | — | — |
| White race | 1.03 | 0.89–1.21 | .676 | — | — | — |
| Unmarried | 1.18 | 1.06–1.32 | .003 | 1.06 | 0.94–1.18 | .356 |
| Rural residence | 1.08 | 0.88–1.32 | .405 | — | — | — |
| Synchronous disease | 1.55 | 1.39–1.73 | <.001 | 1.67 | 1.49–1.86 | <.001 |
| Rectal primary CRC | 0.96 | 0.85–1.10 | .569 | — | — | — |
| Ablation alone | 1.15 | 1.02–1.29 | .022 | 1.18 | 1.05–1.33 | .006 |
| Both pre and postoperative systemic therapy | 1.40 | 1.13–1.72 | .002 | 1.62 | 1.31–2.01 | <.001 |
| Elixhauser index | — | — | — | |||
| Comorbidities | ||||||
| None (referent) | — | — | — | — | — | — |
| 1 | 0.98 | 0.85–1.13 | .780 | 0.93 | 0.81–1.10 | .288 |
| 2–3 | 1.12 | 0.98–1.29 | .102 | 1.02 | 0.89–1.18 | .739 |
| >3 | 1.21 | 0.99–1.49 | .065 | 1.03 | 0.83–1.27 | .806 |
Fig 4.
Overall survival from the time of the liver-directed operation stratified by resection alone, ablation alone, and combined resection plus ablation. Medians months of survival: resection alone (30 months), ablation alone (23 months), and resection plus ablation (34 months).
DISCUSSION
Data on patients undergoing liver-directed therapy for CRLM often come from single institution series5,13,36 or from multicenter series7 that combine data from specialized large hepatobiliary centers. These data, however, are inherently subject to referral and publication bias and may not reflect “true” outcomes and trends occurring on a population-level basis.37 To our knowledge, no previous report has investigated specifically population-based CRLM data to examine the trends in perioperative management, operative procedure utilization, and outcomes. Although several groups have investigated population-based outcomes using data from national cancer registries,17,18,38,39 these data have inherent limitations.40 The SEER dataset offers the ability to assess cancer surgery care in >25% of the US population and, therefore, likely reflects the spectrum of practice patterns found across a wide demographic and institutional range. The SEER data, however, are limited in scope and do not provide robust data concerning perioperative resource and procedure utilization. As such, unlike previous studies that only employed the SEER database,17,18,38 we utilized linked SEER–Medicare data, which can improve the accuracy of analyzing cancer care.19 Furthermore, by using a specific algorithm,23 we were able to identify that population of patients within SEER who had CRC and then identify the subset of patients who underwent liver-directed procedures for their CRLM based on CPT codes from Medicare. Data from the current study are important, because our findings reflect more accurately how CRLM is being managed throughout the United States. In turn, data from the current study can be used to assess how the population-level care of patients with CRLM corresponds to recommendations and outcomes reported from select academic centers.
One interesting finding in the current study was the low proportion of patients with CRLM managed with liver-directed operations. In total, we identified only 2,121 patients (5.0%) as having undergone liver-directed management of their hepatic metastasis. The total sample size of approximately 2,000 patients who underwent liver-directed management of colorectal liver metastasis is similar to other studies in the literature that have used SEER–Medicare data.17,38 Other studies have reported similarly a low utilization of operative management for patients with CRLM (5–7%) when examining population-based data.17,41 The reason for the low utilization of liver-directed therapy for CRLM is likely multifactorial. Given that the study population was derived from Medicare data, all patients in the current study were ≥65 years. The low utilization of liver-directed therapy may in part, therefore, be related to the lesser utilization of liver operations among the elderly population. Several studies, however, have noted that advanced chronologic age cannot be regarded as a medical contraindication for hepatic resection of CRLM.42,43 An additional possible reason for the low use of liver-directed therapy might have been a result of underreporting; however, as others have noted,17 the low utilization of liver-directed therapy is likely not the result of underreporting, because the SEER and Medicare data have a high level of agreement for identifying CRC patients who have not undergone an operation.
The overall utilization of preoperative cross-sectional imaging was 71.0%. The reason for the relative low rate of imaging among study patients was probably multifactorial. The utilization of imaging was time dependent; >90% of patients between 2003 and 2006 had some form of preoperative cross-sectional imaging versus only approximately 55% from 1991 to 1995. Because we limited data collection to within 6 months before liver-directed operation, we may not have captured adequately all data on preoperative imaging that occurred before 6 months. In addition, >40% of patients presented with synchronous disease. Among patients who had synchronous disease, only 59.2% had preoperative imaging compared with 71.7% of patients who presented with metachronous disease (P < .001). We also noted several other interesting trends in the use of cross-sectional imaging over the time periods examined. Specifically, although the use of both CT and MRI increased dramatically over time, MRI was utilized in <10% of CRLM cases. Although some data have suggested that MRI may be preferable sometimes to CT as a diagnostic imaging modality for CRLM,44 CT remains by far the predominant imaging modality of choice for CRLM in the United States. The routine use of PET for patients with CRLM remains controversial. Data from a meta-analysis45 noted that the addition of PET changed the treatment plan in 29% of patients with CRLM. Pawlik et al46 reported that, among patients who underwent a nontherapeutic laparotomy for CRLM, 45% had unsuspected extrahepatic disease. Over the time period examined, the authors noted that nontherapeutic laparotomies decreased from 15% to 5% corresponding with an increased use of PET at their institution.46 In a separate study, Wiering et al47 noted that the additional costs of PET in the diagnostic workup of patients with potentially resectable CRLM were compensated by the decrease in futile laparotomies. Despite National Comprehensive Cancer Network48 guidelines that suggest that PET is indicated in the setting of potentially curable metastatic disease, we noted a very low utilization of PET (~5%) in the population at large.
Clinically important advances in chemotherapy for treating patients with CRLM have been made over the last several decades. Whereas with 5-fluorouracil monotherapy responses were in the range of 20%, now with combined oxaliplatin- or irinotecan-based therapies responses have increased ranging to 40–55%. Although randomized, controlled data on the use of perioperative systemic chemotherapy for resected CRLM are limited,49 there is sound rationale for the use of adjuvant therapy based on robust level 1 data derived from patients with resected stage III CRC.50,51 As such, the predominance of evidence seems to suggest that perioperative chemotherapy has a role in the treatment of patients with resectable colorectal liver metastasis. Despite this, we noted that only about one half of patients received some type of perioperative systemic chemotherapy, with <20% of patients receiving preoperative therapy. The use of chemotherapy did, however, increase over the time periods examined (Fig 2, A). Although older patients with more comorbidities are often less likely to receive chemotherapy,52 we did not find that age or number of comorbidities were associated with receipt of chemotherapy in the current study.
There were also several shifting trends noted in the operative management of patients (Table IV; Fig 2, A). Whereas the use of hepatectomy alone decreased over time, the use of ablation either alone or combined with resection increased significantly. In particular, we noted that the use of ablation combined with wedge resection was utilized in about 1 in 10 patients operated on for CRLM between 2003 and 2006. In contrast, major hepatectomy (hemihepatectomy or extended hepatectomy) was utilized far less frequently. In fact, major hepatectomy accounted for <25% of the operative interventions for CRLM on a population-based level. Rather, most patients with CRLM were managed with a wedge resection or with a combination of wedge resection and ablation.
Advances in operative techniques and perioperative care have minimized mortality associated with operations for CRLM, but postoperative morbidity remains a concern. Most contemporary series from large academic centers report a 30-day mortality of <5%.5,7,37,53,54 In the current study, we noted a 30-day mortality of 2.8%. Although this population-based mortality was comparable with that reported from academic centers, fewer major hepatectomies were performed in the current cohort compared with many academic series---making direct comparisons difficult.5,7,46 Although the perioperative mortality was low, the overall proportion of patients who had a postoperative complication was 62.9%. We noted that the incidence of 30-day mortality and morbidity remained constant over the time periods examined despite a significant increase in the number of patients with comorbidities. We noted also that 90-day mortality decreased from 9% to 7% over the study period (P = .007). These data suggest strongly that, although continued progress needs to be made to decrease morbidity similar to the progress made with perioperative mortality, resection for CRLM in an aging population with more medical comorbidities can be performed safely with low mortality.
The current study had several limitations. The use of large administrative datasets, although providing data with a greater generalizability, suffers from a number of shortcomings. Specifically, despite utilizing a linked dataset such as SEER–Medicare, detailed data on the characteristics of the CRLM were not available. Although SEER captures relatively specific data on characteristics of the primary tumor, detailed data on metastasis are not available. As such, we could not stratify the analyses based on extent of metastatic disease or examine the relative utilization of perioperative or operative procedures based on metastatic disease burden. The trends in management, complications, and procedural utilization are limited by reporting bias of the surgeons and administrative processes involved with billing codes and should, therefore, we believe be viewed in broad terms and not as exact values. In addition, because we utilized a dataset linked to Medicare, only patients aged ≥65 years were included. Whether results from the current study can be extrapolated to younger patient populations requires further study.
In conclusion, we found that the overall utilization of liver-directed operations for CRLM was relatively low (~5%) among Medicare beneficiaries. Although most patients with CRLM were evaluated with a CT before their operation, PET was utilized rarely as part of the staging workup. In addition, only about one half of patients received perioperative chemotherapy in addition to liver-directed operations for CRLM, although there was an increased trend in chemotherapy utilization over time. Mortality associated with liver-directed operations was low, but morbidity remains high with no change over time despite an increase in the number of patients with medical comorbidities. Future studies should seek to better understand the factors that impact the differences and trends in the relative utilization of perioperative and operative interventions aimed at treating patients with CRLM.
Footnotes
Presented at the 6th Annual Academic Surgical Congress, Huntington Beach, CA, February 3, 2011.
REFERENCES
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer Statistics, 2009. CA Cancer J Clin. 2009;59:225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Norstein J, Silen W. Natural history of liver metastases from colorectal carcinoma. J Gastrointest Surg. 1997;1:398–407. doi: 10.1016/s1091-255x(97)80126-6. [DOI] [PubMed] [Google Scholar]
- 3.Blumgart LH, Allison DJ. Resection and embolization in the management of secondary hepatic tumors. World J Surg. 1982;6:32–45. doi: 10.1007/BF01656371. [DOI] [PubMed] [Google Scholar]
- 4.Taylor I, Mullee MA, Campbell MJ. Prognostic index for the development of liver metastases in patients with colorectal cancer. Br J Surg. 1990;77:499–501. doi: 10.1002/bjs.1800770508. [DOI] [PubMed] [Google Scholar]
- 5.Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230:309–18. doi: 10.1097/00000658-199909000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Jong MC, van Vledder MG, Ribero D, Hubert C, Gigot JF, Choti MA, et al. Therapeutic efficacy of combined intraoperative ablation and resection for colorectal liver metastases: an international, multi-institutional analysis. J Gastrointest Surg. 2011;15:336–44. doi: 10.1007/s11605-010-1391-8. [DOI] [PubMed] [Google Scholar]
- 7.de Jong MC, Pulitano C, Ribero D, Strub J, Mentha G, Schulick RD, et al. Rates and patterns of recurrence following curative intent surgery for colorectal liver metastasis: an international multi-institutional analysis of 1669 patients. Ann Surg. 2009;250:440–8. doi: 10.1097/SLA.0b013e3181b4539b. [DOI] [PubMed] [Google Scholar]
- 8.Curley SA, Marra P, Beaty K, Ellis LM, Vauthey JN, Abdalla EK, et al. Early and late complications after radiofrequency ablation of malignant liver tumors in 608 patients. Ann Surg. 2004;239:450–8. doi: 10.1097/01.sla.0000118373.31781.f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pawlik TM, Schulick RD, Choti MA. Expanding criteria for resectability of colorectal liver metastases. Oncologist. 2008;13:51–64. doi: 10.1634/theoncologist.2007-0142. [DOI] [PubMed] [Google Scholar]
- 10.Pawlik TM, Choti MA. Surgical therapy for colorectal metastases to the liver. J Gastrointest Surg. 2007;11:1057–77. doi: 10.1007/s11605-006-0061-3. [DOI] [PubMed] [Google Scholar]
- 11.Berri RN, Abdalla EK. Curable metastatic colorectal cancer: recommended paradigms. Curr Oncol Rep. 2009;11:200–8. doi: 10.1007/s11912-009-0029-z. [DOI] [PubMed] [Google Scholar]
- 12.Abdalla EK, Vauthey JN, Ellis LM, Ellis V, Pollock R, Broglio KR, et al. Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann Surg. 2004;239:818–25. doi: 10.1097/01.sla.0000128305.90650.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choti MA, Sitzmann JV, Tiburi MF, Sumetchotimetha W, Rangsin R, Schulick RD, et al. Trends in long-term survival following liver resection for hepatic colorectal metastases. Ann Surg. 2002;235:759–66. doi: 10.1097/00000658-200206000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fong Y, Cohen AM, Fortner JG, Enker WE, Turnbull AD, Coit DG, et al. Liver resection for colorectal metastases. J Clin Oncol. 1997;15:938–46. doi: 10.1200/JCO.1997.15.3.938. [DOI] [PubMed] [Google Scholar]
- 15.Ambiru S, Miyazaki M, Isono T, Ito H, Nakagawa K, Shimizu H, et al. Hepatic resection for colorectal metastases: analysis of prognostic factors. Dis Colon Rectum. 1999;42:632–9. doi: 10.1007/BF02234142. [DOI] [PubMed] [Google Scholar]
- 16.Aloia TA, Vauthey JN, Loyer EM, Ribero D, Pawlik TM, Wei SH, et al. Solitary colorectal liver metastasis: resection determines outcome. Arch Surg. 2006;141:460–6. doi: 10.1001/archsurg.141.5.460. [DOI] [PubMed] [Google Scholar]
- 17.Cummings LC, Payes JD, Cooper GS. Survival after hepatic resection in metastatic colorectal cancer: a population-based study. Cancer. 2007;109:718–26. doi: 10.1002/cncr.22448. [DOI] [PubMed] [Google Scholar]
- 18.Kopetz S, Chang GJ, Overman MJ, Eng C, Sargent DJ, Larson DW, et al. Improved survival in metastatic colorectal cancer is associated with adoption of hepatic resection and improved chemotherapy. J Clin Oncol. 2009;27:3677–83. doi: 10.1200/JCO.2008.20.5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cooper GS, Virnig B, Klabunde CN, Schussler N, Freeman J, Warren JL. Use of SEER-Medicare data for measuring cancer surgery. Med Care. 2002;40(8 Suppl):IV–43-8. doi: 10.1097/00005650-200208001-00006. [DOI] [PubMed] [Google Scholar]
- 20.National Cancer Institute D, Surveillance Research Program, Cancer Statistics Branch Surveillance, epidemiology, and end results (SEER) program. ( http://www.seer.cancer.gov/) SEER* Stat database: Incidence, SEER 17 regs public-use, Nov 2005 sub (1973–2003 varying), linked to county attributes, total U.S., 1969–2003 counties. Released April 2006, based on the November 2005 submission.
- 21.Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40(8 Suppl):IV–3-18. doi: 10.1097/01.MLR.0000020942.47004.03. [DOI] [PubMed] [Google Scholar]
- 22.Fritz AG. International classification of diseases for oncology: ICD-O. 3rd ed. World Health Organization; Geneva: 2000. [Google Scholar]
- 23.Becker NS, Richardson P, Abraham NS, Anaya DA. Identification of patients with colorectal liver metastases using administrative databases. Abstracts of the Fifth Annual Academic Surgical Congress of the Association for Academic Surgery and Society of University Surgeons. San Antonio, Texas, USA. February 3–5, 2010. J Surg Res. 2010;158:171–424. doi: 10.1016/j.jss.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 24.Potosky AL, Warren JL, Riedel ER, Klabunde CN, Earle CC, Begg CB. Measuring complications of cancer treatment using the SEER-Medicare data. Med Care. 2002;40(8 Suppl):IV–62-8. doi: 10.1097/00005650-200208001-00009. [DOI] [PubMed] [Google Scholar]
- 25.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 26.Baldwin LM, Klabunde CN, Green P, Barlow W, Wright G. In search of the perfect comorbidity measure for use with administrative claims data: does it exist? Med Care. 2006;44:745–53. doi: 10.1097/01.mlr.0000223475.70440.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li B, Evans D, Faris P, Dean S, Quan H. Risk adjustment performance of Charlson and Elixhauser comorbidities in ICD-9 and ICD-10 administrative databases. BMC Health Serv Res. 2008;8:12. doi: 10.1186/1472-6963-8-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Quan H, Parsons GA, Ghali WA. Validity of information on comorbidity derived from ICD-9-CCM administrative data. Med Care. 2002;40:675–85. doi: 10.1097/00005650-200208000-00007. [DOI] [PubMed] [Google Scholar]
- 29.Southern DA, Quan H, Ghali WA. Comparison of the Elix-hauser and Charlson/Deyo methods of comorbidity measurement in administrative data. Med Care. 2004;42:355–60. doi: 10.1097/01.mlr.0000118861.56848.ee. [DOI] [PubMed] [Google Scholar]
- 30.Agresti A. Introduction to categorical data analysis. Wiley; New York: 1996. pp. 231–6. [Google Scholar]
- 31.Kaplan ELMP. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–81. [Google Scholar]
- 32.Cox D. Regression models and life tables. J R Stat Soc B. 1972;34:187–220. [Google Scholar]
- 33.Hosmer DW, Lemeshow S. Applied Survival Analysis: Regression modeling of time to event data. 2nd ed. John Wiley and Sons; New York: 1999. [Google Scholar]
- 34.Hosmer DW, Lemeshow S. Applied logistic regression. 2nd ed. John Wiley and Sons; New York: 2000. [Google Scholar]
- 35.Lemeshow S, Hosmer DW., Jr A review of goodness of fit statistics for use in the development of logistic regression models. Am J Epidemiol. 1982;115:92–106. doi: 10.1093/oxfordjournals.aje.a113284. [DOI] [PubMed] [Google Scholar]
- 36.Adam R, de Haas RJ, Wicherts DA, Aloia TA, Delvart V, Azoulay D, et al. Is hepatic resection justified after chemo-therapy in patients with colorectal liver metastases and lymph node involvement? J Clin Oncol. 2008;26:3672–80. doi: 10.1200/JCO.2007.15.7297. [DOI] [PubMed] [Google Scholar]
- 37.Asiyanbola B, Chang D, Gleisner AL, Nathan H, Choti MA, Schulick RD, et al. Operative mortality after hepatic resection: are literature-based rates broadly applicable? J Gastrointest Surg. 2008;12:842–51. doi: 10.1007/s11605-008-0494-y. [DOI] [PubMed] [Google Scholar]
- 38.Wang X, Hershman DL, Abrams JA, Feingold D, Grann VR, Jacobson JS, et al. Predictors of survival after hepatic resection among patients with colorectal liver metastasis. Br J Cancer. 2007;97:1606–12. doi: 10.1038/sj.bjc.6604093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mayo SC, Shore AD, Nathan H, Edil B, Wolfgang CL, Hirose K, et al. National trends in the management and survival of surgically managed gallbladder adenocarcinoma over 15 years: a population-based analysis. J Gastrointest Surg. 2010;14:1578–91. doi: 10.1007/s11605-010-1335-3. [DOI] [PubMed] [Google Scholar]
- 40.Nathan H, Pawlik TM. Limitations of claims and registry data in surgical oncology research. Ann Surg Oncol. 2008;15:415–23. doi: 10.1245/s10434-007-9658-3. [DOI] [PubMed] [Google Scholar]
- 41.Temple LK, Hsieh L, Wong WD, Saltz L, Schrag D. Use of surgery among elderly patients with stage IV colorectal cancer. J Clin Oncol. 2004;22:3475–84. doi: 10.1200/JCO.2004.10.218. [DOI] [PubMed] [Google Scholar]
- 42.Nagano Y, Nojiri K, Matsuo K, Tanaka K, Togo S, Ike H, et al. The impact of advanced age on hepatic resection of colorectal liver metastases. J Am Coll Surg. 2005;201:511–6. doi: 10.1016/j.jamcollsurg.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 43.Nojiri K, Nagano Y, Tanaka K, Matsuo K, Yamagishi S, Ota M, et al. Validity of hepatic resection of colorectal liver metastases in the elderly (75 years and older). Anticancer Res. 2009;29:583–8. [PubMed] [Google Scholar]
- 44.Niekel MC, Bipat S, Stoker J. Diagnostic imaging of colorectal liver metastases with CT, MR imaging, FDG PET, and/or FDG PET/CT: a meta-analysis of prospective studies including patients who have not previously undergone treatment. Radiology. 2010;257:674–84. doi: 10.1148/radiol.10100729. [DOI] [PubMed] [Google Scholar]
- 45.Huebner RH, Park KC, Shepherd JE, Schwimmer J, Czernin J, Phelps ME, et al. A meta-analysis of the literature for whole-body FDG PET detection of recurrent colorectal cancer. J Nucl Med. 2000;41:1177–89. [PubMed] [Google Scholar]
- 46.Pawlik TM, Assumpcao L, Vossen JA, Buijs M, Gleisner AL, Schulick RD, et al. Trends in nontherapeutic laparotomy rates in patients undergoing surgical therapy for hepatic colorectal metastases. Ann Surg Oncol. 2009;16:371–8. doi: 10.1245/s10434-008-0230-6. [DOI] [PubMed] [Google Scholar]
- 47.Wiering B, Adang EM, van der Sijp JR, Roumen RM, de Jong KP, Comans EF, et al. Added value of positron emission tomography imaging in the surgical treatment of colorectal liver metastases. Nucl Med Commun. 2010;31:938–44. doi: 10.1097/MNM.0b013e32833fa9ba. [DOI] [PubMed] [Google Scholar]
- 48.Podoloff DA, Advani RH, Allred C, Benson AB, 3rd, Brown E, Burstein HJ, et al. NCCN task force report: positron emission tomography (PET)/computed tomography (CT) scanning in cancer. J Natl Compr Canc Netw. 2007;5(Suppl 1):S1–22. [PubMed] [Google Scholar]
- 49.Nordlinger B, Sorbye H, Glimelius B, Poston GJ, Schlag PM, Rougier P, et al. Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomised controlled trial. Lancet. 2008;371:1007–16. doi: 10.1016/S0140-6736(08)60455-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sargent D, Sobrero A, Grothey A, O'Connell MJ, Buyse M, Andre T, et al. Evidence for cure by adjuvant therapy in colon cancer: observations based on individual patient data from 20,898 patients on 18 randomized trials. J Clin Oncol. 2009;27:872–7. doi: 10.1200/JCO.2008.19.5362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Andre T, Boni C, Mounedji-Boudiaf L, Navarro M, Tabernero J, Hickish T, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med. 2004;350:2343–51. doi: 10.1056/NEJMoa032709. [DOI] [PubMed] [Google Scholar]
- 52.Wildes TM, Kallogjeri D, Powers B, Vlahiotis A, Mutch M, Spitznagel EL, et al. The benefit of adjuvant chemotherapy in elderly patients with stage III colorectal cancer is independent of age and comorbidity. J Geriatr Oncol. 2010;1:48–56. doi: 10.1016/j.jgo.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.de Jong MC, Mayo SC, Pulitano C, Lanella S, Ribero D, Strub J, et al. Repeat curative intent liver surgery is safe and effective for recurrent colorectal liver metastasis: results from an international multi-institutional analysis. J Gastrointest Surg. 2009;13:2141–51. doi: 10.1007/s11605-009-1050-0. [DOI] [PubMed] [Google Scholar]
- 54.Vauthey JN, Pawlik TM, Abdalla EK, Arens JF, Nemr RA, Wei SH, et al. Is extended hepatectomy for hepatobiliary malignancy justified? Ann Surg. 2004;239:722–30. doi: 10.1097/01.sla.0000124385.83887.d5. [DOI] [PMC free article] [PubMed] [Google Scholar]