Abstract
The human pathogen Staphylococcus aureus is renowned for the rapid colonization of contaminated wounds, medical implants, and food products. Nevertheless, little is known about the mechanisms that allow S. aureus to colonize the respective wet surfaces. The present studies were therefore aimed at identifying factors used by S. aureus cells to spread over wet surfaces, starting either from planktonic or biofilm-associated states. Through proteomics analyses we pinpoint phenol-soluble modulins (PSMs) as prime facilitators of the spreading process. To dissect the roles of the eight PSMs produced by S. aureus, these peptides were chemically synthesized and tested in spreading assays with different psm mutant strains. The results show that PSMα3 and PSMγ are the strongest facilitators of spreading both for planktonic cells and cells in catheter-associated biofilms. Compared to the six other PSMs of S. aureus, PSMα3 and PSMγ combine strong surfactant activities with a relatively low overall hydropathicity. Importantly, we show that PSM-mediated motility of S. aureus facilitates the rapid colonization of wet surfaces next to catheters and the colonization of fresh meat.
INTRODUCTION
Staphylococcus aureus is an opportunistic human pathogen that can cause a wide range of acute and chronic diseases, which range from superficial skin infections to life-threatening endocarditis and sepsis (1, 2). The ability of this Gram-positive bacterium to cause these infections depends on the production of secreted and cell wall-associated virulence factors. Of increasing concern is the ability of S. aureus to acquire resistance against antibiotics, as underscored by the global spread of methicillin-resistant S. aureus (MRSA) lineages.
Intriguingly, recent proteomics studies have revealed an enormous diversity in the production of virulence factors by different isolates of S. aureus, and only a few of these seem to be invariantly produced (3–5). Among the most commonly identified staphylococcal virulence factors, especially in the community-associated (CA)-MRSA lineages, are the so-called phenol-soluble modulins (PSMs) (6). These PSMs are short, amphipathic, α-helical peptides that have leukocidal activity and biosurfactant properties (7–9). The growth media of S. aureus cultures contain both N-terminally formylated and deformylated PSMs, suggesting that these virulence factors are substrates for the bacterial N-formylmethionine deformylase (9, 10).
To date, eight PSMs have been identified in S. aureus. These include the four PSMα1 to PSMα4 peptides (22 residues each), the PSMβ1 and PSMβ2 peptides (44 residues each), PSMγ (25 residues) and the recently reported PSM-mec (22 residues). The PSMα peptides are encoded by the psmα operon, the PSMβ peptides by the psmβ operon, and PSMγ by the hld gene. Notably, the hld gene is embedded within the regulatory RNAIII molecule that is encoded by the agr locus. The gene for PSM-mec was identified in MRSA strains carrying the staphylococcal cassette chromosome mec (SCCmec) types II or III. The expression of all psm genes is controlled by the Agr system for quorum sensing (8, 11–13). This system modulates gene expression such that cell wall-associated virulence factors (e.g., the immunoglobulin G-binding protein A) are most highly expressed at low cell densities and that secreted virulence factors (e.g., the PSMs) are most highly expressed at high cell densities (14–21).
Notably, PSMs have been implicated in the high virulence of CA-MRSA lineages, which are readily transmitted by direct contact with a carrier (9, 22). The investigated CA-MRSA isolates produce higher amounts of the PSM peptides than the generally less virulent nosocomial MRSA isolates (9, 22). The PSMα peptides have the strongest leukolytic, proinflammatory, and chemotactic activities (9). Consistently, a strain lacking psmα had a decreased ability to cause skin lesions in mice and rabbits (9, 23). In addition to this, Wang et al. (24) have shown that the mortality rates and the levels of the inflammatory cytokine tumor necrosis factor alpha in the blood of mice infected with psmα or psmγ mutant strains were substantially reduced. The PSMβ peptides appear less important for cytolysis and inflammation but, in low concentrations, they seem to promote biofilm formation by Staphylococcus epidermidis. High amounts of the same PSMβ peptides do, however, promote the detachment of staphylococcal cells from biofilms both in vitro and in vivo (24).
Although S. aureus was originally believed to be nonmotile, recent studies have shown that this organism is capable of spreading over wet surfaces (25–27). We have previously shown that a mix of the four PSMα peptides can promote this so-called colony spreading phenotype (27), and we hypothesized that this relates to their strong surfactant properties (9). The Agr system is an important determinant for colony spreading due to its control over the synthesis of PSMs (27). However, Agr is also needed for biofilm formation, which gives it a decisive role in the choice between motile and sessile lifestyles of S. aureus. Furthermore, it was shown that covalently cell wall-anchored proteins, like FnbpA, FnbpB, ClfA, and ClfB, can set a limit to the colony-spreading ability of S. aureus cells (28). To date, very little was known about the roles of individual PSM peptides in colony spreading and whether these are the main factors promoting spreading. Therefore, the present studies were aimed at dissecting the roles of the different PSM peptides in colony spreading. Furthermore, we wanted to test whether N-terminally formylated and deformylated PSMs are equally potent in colony spreading. To achieve these objectives, we constructed multiple psm mutant strains, which were then incubated in the presence or absence of synthetic PSMs. Importantly, our results show that PSMα3 and PSMγ have key roles in colony spreading. Furthermore, our observations link PSMα3 and PSMγ to the spreading of staphylococcal cells from catheter-related biofilms, and they suggest that PSM-mediated spreading plays a major role in the movement of S. aureus over biotic surfaces. Importantly, our findings show that the PSMs that most effectively promote the spreading of S. aureus combine high surfactant activities with low overall hydropathicity.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The bacterial strains and plasmids that were used in this study are listed in Table 1. Escherichia coli strains were grown in Lysogeny broth (LB) at 37°C under vigorous shaking. S. aureus strains were grown in tryptic soy broth (TSB) at 37°C under vigorous shaking, or on tryptic soy agar (TSA) plates. Where necessary, antibiotics were added at the following concentrations: ampicillin, 100 μg/ml (for E. coli); erythromycin, 5 μg/ml (for S. aureus); and chloramphenicol, 10 μg/ml (for S. aureus).
Table 1.
Bacterial strains and plasmids
| Strain or plasmid | Descriptiona | Source or reference |
|---|---|---|
| Strains | ||
| E. coli | ||
| DH5α | λ− ϕ80dlacΖΔΜ15 Δ(lacZYA-argF)U169 recA1 endA hsdR17(rK− mK−) supE44 thi-1 gyrA relA1 | 29 |
| TOP10 | Cloning host for TOPO vector; F− mcrA Δ(mrr-hsdRMS-mcrBC) λ− ϕ80dlacΖΔΜ15 ΔlacX74 recA1 araD139 Δ(ara-leu)7697 galU galK rpsL (Strr) endA1 nupG | Invitrogen Life Technologies |
| S. aureus | ||
| RN4220 | Restriction-deficient derivative of NCTC8325, cured of all known prophages | 30 |
| NCTC8325 | HA-MSSA strain, agr+ ΔrsbU | 31 |
| NCTC8325− | HA-MSSA strain, Δagr ΔrsbU | 27 |
| NCTC8325 Δpsmα | NCTC8325 lacking the psmα genes | This study |
| NCTC8325 Δpsmβ | NCTC8325 lacking the psmβ genes | This study |
| NCTC8325 Δpsmα Δpsmβ | NCTC8325 lacking the psmα and psmβ genes | This study |
| SH1000 | NCTC8325-4 derivative, rsbU+ agr+ | 32 |
| SH1000− | NCTC8325-4 derivative, rsbU+ Δagr | 27 |
| SH1000 Δpsmα | SH1000 lacking the psmα genes | This study |
| SH1000 Δpsmβ | SH1000 lacking the psmβ genes | This study |
| SH1000 Δpsmα Δpsmβ | SH1000 lacking the psmα and psmβ genes | This study |
| HG001 | NCTC8325 derivative, rsbU+ agr+ | 33 |
| HG001− | NCTC8325 derivative, rsbU+ Δagr | 27 |
| HG001 Δpsmα | HG001 lacking the psmα genes | This study |
| HG001 Δpsmβ | HG001 lacking the psmβ genes | This study |
| HG001 Δpsmα Δpsmβ | HG001 lacking the psmα and psmβ genes | This study |
| Newman | ATCC 25904; high-level clumping factor production; σB+ | 34 |
| Newman Δagr | Newman derivative, Δagr::tetM | 35 |
| Newman Δpsmα | Newman lacking the psmα genes | This study |
| Newman Δpsmβ | Newman lacking the psmβ genes | This study |
| Newman Δpsmα Δpsmβ | Newman lacking the psmα and psmβ genes | This study |
| LAC USA300 | CA-MRSA | 9 |
| LAC USA300 Δpsmα | LAC USA300 lacking the psmα genes | 9 |
| Plasmids | ||
| pUC18 | Apr; ColE1, ϕ80dlacΖ; lac promoter | 36 |
| TOPO | pCR-Blunt II-TOPO vector; Kmr | Invitrogen Life Technologies |
| pMAD | E. coli/S. aureus shuttle vector that is temperature sensitive in S. aureus and contains the bgaB gene; Emr Apr | 37 |
| pRIT5H | E. coli/S. aureus shuttle vector; Cmr; spa promoter | 38 |
| pSW4-GFPopt | pSW4 plasmid containing 729-bp AseI/BamHI-cloned gfpopt | 39 |
| GFPopt-pRIT5H | pRIT5H with gfpopt gene; Apr Kmr | This study |
| pAH9 | sarA promoter Pi-RFP | 40 |
CA, community acquired; HA, hospital acquired; MRSA, methicillin-resistant S. aureus. Cmr, chloramphenicol resistance; Apr, ampicillin resistance; Emr, erythromycin resistance; Kmr, kanamycin resistance.
Colony spreading assay.
The colony-spreading assay was performed essentially as described by Kaito et al. (25), but with minor previously described modifications (27). To detect colony spreading of the S. aureus strains SH1000 and Newman along the growth curve, these strains were grown in TSB for 24 h. Samples were collected at hourly intervals for the first 7 h and after 24 h of growth. All samples were immediately tested for colony spreading. Equal amounts of cells from each time point were spotted onto the 0.24% TSA plates. All spreading assays were repeated at least five times.
Mass spectrometric analyses of culture supernatants.
Strains Newman (agr+, Δagr, or Δpsmα), LAC USA300 (agr+ or Δpsmα), NCTC8325 (agr+ or Δagr) and HG001 (agr+ or Δagr) were grown in TSB. At an optical density at 600 nm (OD600) of 2 and 6, 3-ml culture samples were collected, and cells were separated from the growth medium by centrifugation (8,000 × g, 4°C, 10 min). To precipitate the secreted proteins, the medium fractions were incubated at 4°C with 10% trichloroacetic acid overnight. The proteins were pelleted (20,000 × g, 4°C, 20 min), washed with acetone, and subsequently dissolved in 8 M urea. Protein concentrations were determined using the Bio-Rad DC protein assay according to the protocol of the supplier. The samples (2 μg) were reduced with 10 mM dithiothreitol (DTT; Duchefa Biochemie) for 30 min and alkylated with 10 mM iodoacetamide (Sigma-Aldrich) for another 30 min in the dark. Finally, the protein samples were incubated overnight at 37°C with 40 ng of trypsin (Promega). Peptides were purified using ZipTips (4), separated and analyzed by liquid chromatography-tandem mass spectrometry using an Easy-nLCII high-pressure liquid chromatography system (Thermo Fisher Scientific, Waltham, MA) coupled directly to an LTQ Orbi-Trap mass spectrometer (Thermo Fisher Scientific, Waltham, MA). The Easy-nLCII was equipped with a self-packed analytical column (C18-material [Luna 3u C18(2)100A; Phenomenex], 100-μm inner diameter by 200-mm column). Peptide elution was performed by application of a binary gradient of buffer A (0.1% [vol/vol] acetic acid) and B (99.9% [vol/vol] acetonitrile, 0.1% [vol/vol] acetic acid) over a period of 80 min with a flow rate of 300 nl/min. The mass spectrometry (MS) analyses were performed as described by Miller et al. (41). A nonredundant database was constructed that contained all available S. aureus protein sequences (Uni-Prot) that differ in at least one amino acid residue (see Table S2 and FASTA Files S1 and S2 in the supplemental material). This database was subsequently used for the database search (including a concatenated reversed database, 40,098 entries). The database search was performed with Sequest using a fragment ion mass tolerance of 1.00 Da and a parent ion tolerance of 10 ppm. Oxidation of methionine and carbamidomethylation of cysteine were specified in Sequest as variable modifications. Validation of MS/MS-based peptide and protein identifications was performed with Scaffold (version Scaffold 3.3.2; Proteome Software, Inc., Portland, OR). Peptide identifications were accepted if they exceeded specific database search engine thresholds. Sequest identifications required at least deltaCn scores of >0.10 and XCorr scores of greater than 1.9, 2.2, 3.8, and 3.8 for singly, doubly, triply, and quadruply charged peptides. All experiments have been conducted in independent duplicates. Proteins were only accepted as being identified if they were detected in both biological replicates per sample set. With these filter parameters, the false-positive rate was below 1%.
Construction of a GFP expressing vector.
For constitutive expression of GFP in S. aureus the gfpopt gene was amplified from plasmid pSW4-GFPopt using primers GFPoptFR and GFPoptRV (see Table S3 in the supplemental material). The amplified PCR product was then cloned in plasmid pRIT5H using the EcoRI and SalI restriction sites. Expression of GFP was detected using the IVIS Spectrum from Caliper Life Sciences using the specific filter for GFP (excitation, 465 nm; emission, 520 nm).
Construction of PSM mutant strains of S. aureus.
Mutants of S. aureus were constructed using the temperature-sensitive plasmid pMAD (37) and previously described procedures (42). Primers were designed using the genome sequence of S. aureus NCTC8325 (http://www.ncbi.nlm.nih.gov/nuccore/NC007795). To delete the psmα and/or psmβ operons, the primer pairs with the designations F1/R1 and F2/R2 were used for PCR amplification of the respective upstream and downstream regions (each ∼500 bp) (see Table S3 in the supplemental material). The R1 and F2 primers contain a 24-bp linker sequence to fuse the flanking regions by PCR prior to cloning in pMAD. The resulting plasmids were used to transform S. aureus strain RN4220 via electroporation. Next, the plasmids were isolated from the RN4220 strain and used to transform the S. aureus SH1000, HG001, NCTC8325, or Newman strains via electroporation in order to delete their psmα and/or psmβ operons through subsequent plasmid integration and excision steps (43). At the end of the procedure, white colonies were screened for the absence of the psmα and/or psmβ genes by colony PCR using primers F1 and R2.
Complementation of psm mutations by synthetic PSM peptides and determination of surfactant properties.
The PSMα1 to PSMα4, PSMβ1 and PSMβ2, PSMγ, and PSM-mec peptides were synthesized as described previously with a C-terminal four-residue glycyl spacer and an ε-amino biotinyl lysine (27). All peptides were dissolved in dimethyl sulfoxide plus 10 mM DTT to a concentration of 12 mM. The peptides were then diluted 10-fold in phosphate-buffered saline, and 2 μl of each peptide solution was spotted in the center of a soft TSA plate prior to the inoculation of the S. aureus mutant strain to be tested for spreading. The surfactant properties of the PSMs were determined by spotting 2 μl of each peptide solution in the center of a soft TSA plate and by subsequently measuring the diameter of the resulting transparent halo.
Colony spreading of S. aureus from catheter-associated biofilms.
Catheters were positioned on soft agar plates and 2 μl of bacteria grown overnight were spotted next onto these catheters before the plates were incubated overnight at 37°C. Subsequently, the catheters were transferred to fresh soft TSA plates and incubated again overnight at 37°C, which resulted in the formation of catheter-associated biofilms. Catheters with associated biofilms were transferred to fresh soft TSA plates, where 2 μl of PSM peptide solution was spotted prior to the transfer of the catheters. The plates were then incubated overnight at 37°C. Images were recorded with a G:box (Syngene, Leusden, Netherlands).
Colony spreading of S. aureus on pork.
Pieces of pork meat were placed in sterile petri dishes and, approximately in the center, 2 μl of bacteria grown overnight in TSB were spotted. The meat was incubated at 37°C for 48 h, after which images were recorded with a Sony cyber-shot camera.
RESULTS
Secreted factors regulated by agr are responsible for colony spreading.
We have previously shown that the agr locus, which regulates the synthesis of secreted virulence factors, is required for colony spreading of S. aureus. This suggested that secreted virulence factors are of prime importance for colony spreading. Another indication for the role of secreted factors in spreading came from the observation that colonies of S. aureus cells that are capable of spreading were surrounded by a transparent halo (Fig. 1A). This halo was absent from nonspreading agr mutant colonies. Furthermore, when overnight grown agr-proficient bacteria were spotted on soft agar plates, spreading of the cultured cells was visible after a few seconds, suggesting that any secreted factors needed for spreading were already present in the culture. In fact, this area of rapid spreading remained clearly visible on the plates (marked by a box in Fig. 1B), which was due to the emergence of subsequent “waves” of spreading cells from the initial spreading zone. This suggested that the factors needed for spreading were not synthesized continuously. To investigate whether the spreading factors were synthesized growth phase dependently, S. aureus cells were grown to different growth stages and tested for the rapid spreading phenotype. Indeed, the rapid spreading was not observed for cells in the early exponential growth phase (Fig. 2A, time point t3), but it started when the cells reached the late exponential phase (t5) and continued in the stationary phase (t7). Consistent with our previous findings, these time points correspond with the activation of the Agr system (17). To verify that secreted factors promote colony spreading, Δagr cells from different strains were resuspended in filtered supernatants of overnight grown agr+ strains and the cell suspension was spotted on top of soft agar plates. As shown in Fig. 2B, the filtered supernatants were able to promote colony spreading of the Δagr cells. Notably, when the filtered supernatant of an agr+ strain was included within the soft agar, it did not promote the colony spreading of Δagr cells (Fig. 2C, plate 4). Together, these results demonstrate that secreted factors regulated via the Agr system are both needed and sufficient for colony spreading of S. aureus. Importantly, to promote colony spreading, these secreted factors need to be present on the surface of the soft agar on which the bacteria spread.
Fig 1.
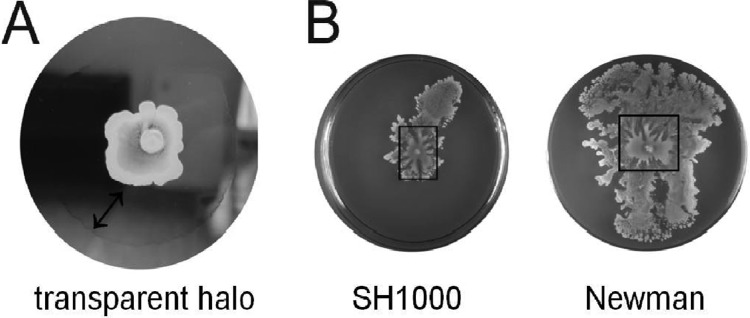
Characteristic features of S. aureus spreading motility. (A) A transparent “halo” is produced by cells of spreading colonies. A similar halo is also generated by surfactants that are spotted on soft agar plates (not shown). (B) Spreading motility involves a rapid phase of spreading by the cells spotted on a plate. Subsequently, waves of cells emerge from the cells in the initial spreading zone. The boxes mark the rapid spreading zones that emerged from the sites of inoculation of strains SH1000 and Newman on soft agar plates.
Fig 2.
Colony spreading depends on the growth phase of inoculated S. aureus cells and on secreted factors produced by agr+ strains. (A) Colony spreading by planktonic cells of S. aureus strains SH1000 or Newman collected from cultures in different growth stages; t3 corresponds to the early exponential growth phase (OD600 = 1.5), t5 to the late exponential phase (OD600 = 6.8), and t7 to early stationary phase (OD600 = 9.0). (B) Filter-sterilized culture medium of agr+ cells of S. aureus strain Newman promotes rapid spreading of Δagr cells of strains SH1000, NCTC8325, and Newman. As shown for Δagr cells of strain SH1000, no spreading is observed when fresh medium is used (left plate labeled “agr−”). (C) Culture supernatants of agr+ cells need to be applied on top of soft agar plates to promote spreading. Fresh soft agar plates were prepared and inoculated as follows: plate 1, regular soft agar with the wild-type strain Newman; plate 2, regular soft agar with the wild-type strain Newman resuspended in 2 μl of filter-sterilized supernatant of the wild-type strain Newman; plate 3, 200 μl of filter-sterilized supernatant of the wild-type strain Newman included within the soft agar prior to inoculation with the wild-type strain Newman; and plate 4, 200 μl of filter-sterilized supernatant of the wild-type strain Newman included in the soft agar prior to inoculation with Δagr cells of strain Newman. (D) Δagr cells carrying plasmid GFPopt-pRIT5H for expression of GFP were coinoculated with agr+ cells carrying plasmid pAH9 for expression of mCherry. GFP fluorescence of the Δagr cells is detectable at the edges of the spreading zone (green color), whereas mCherry fluorescence of the agr+ cells (red color) is detectable in the entire area covered by spreading. All spreading assays were repeated at least five times.
As the spent growth medium of agr+ cells can promote the spreading of Δagr cells, we wondered whether the Δagr cells would also spread if they were grown in the presence of agr+ cells. To test this, an overnight culture of a nonspreading Δagr derivative of S. aureus HG001 expressing the green fluorescent protein (GFP) was mixed with an overnight culture of the authentic S. aureus HG001 strain (agr+) expressing the fluorescent mCherry protein. Next aliquots of the mixed culture were transferred to soft agar plates and incubated overnight. Images were taken using the IVIS spectrum with the specific filters for GFP (excitation, 465 nm; emission, 520 nm) and mCherry (excitation, 570 nm; emission, 620 nm) to monitor the spreading of the two different strains. GFP was only detected at the edges of the colony spreading area, indicating that the Δagr strain was very well able to spread over the soft agar together with the agr+ strain (Fig. 2D). Since the Δagr HG001 strain cannot spread by itself, this observation implies that nonspreading S. aureus cells can passively spread on the soft agar plates with the help of factors that are actively secreted by spreading S. aureus cells.
MS identification of secreted proteins potentially involved in colony spreading.
To investigate which proteinaceous factors are involved in colony spreading, culture supernatants of agr+, Δagr, and Δpsmα variants of the S. aureus strains Newman, LAC USA300, NCTC8325, and HG001 were analyzed using MS. As expected, the MS analyses revealed many strain-dependent differences between the investigated S. aureus strains (see Table S1 in the supplemental material). Importantly, the only proteins that were common in the media of all tested agr+ strains and absent from the media of all tested Δagr strains were PSMα2, PSMα3, PSMα4, PSMβ1, PSMβ2, and the staphylococcal lipase 1. Only the identified PSM peptides have potential surfactant properties that might promote colony spreading, and therefore we focused all our following studies on these peptides.
Dissection of PSM function with synthetic peptides reveals major roles for PSMα3 and PSMγ in colony spreading.
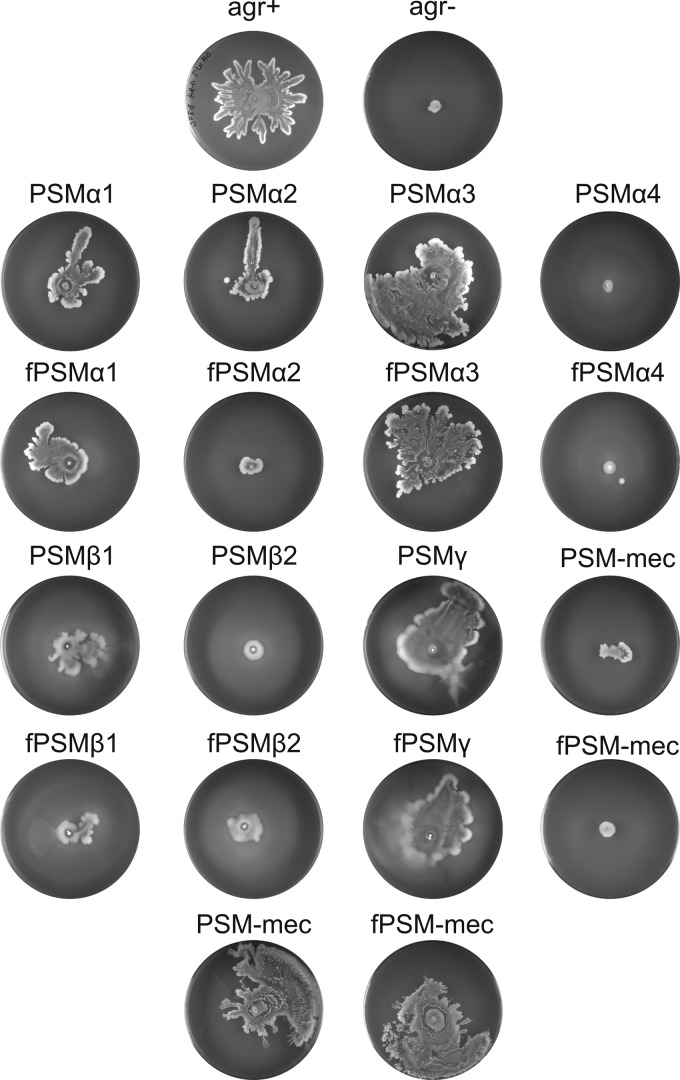
Surfactants lower the surface tension of liquid-to-air interfaces, and due to this activity they facilitate the motility of many different bacterial species (44–46). To determine which of the previously identified PSM peptides might be involved in the spreading of S. aureus, we tested synthetic PSMα1-4, PSMβ1-2, PSMγ, and PSM-mec peptides for their ability to promote colony spreading. To this end, all PSMs were individually spotted at the same concentrations on soft agar plates. This analyses showed that the PSMα3 and PSMγ peptides strongly promoted the colony spreading by otherwise nonspreading Δagr strains (Fig. 3). Other PSMs—such as PSMα1, PSMα2, and PSMβ1—promoted colony spreading to lesser extents, whereas PSMα4, PSMβ2, and PSM-mec did not promote spreading at all (Fig. 3). Based on genetic studies, it was previously proposed that PSM-mec might inhibit colony spreading (47). We therefore tested the synthetic PSM-mec peptide for possible inhibitory effects, but unfortunately, no such effects were detectable in our experimental setting (Fig. 3, plates in bottom row). Furthermore, our studies show that N-terminally formylated PSMs promote spreading equally well as the nonformylated peptides (Fig. 3). It thus seems that both the formylated and nonformylated forms of PSMα3 and PSMγ contribute to the movement of S. aureus cells over wet surfaces. Importantly, both forms of PSMα3 and PSMγ exerted their effect on spreading in a dose-dependent manner (see Fig. S1 in the supplemental material). This underscores the view that these two PSMs actively contribute to colony spreading.
Fig 3.
Particular synthetic PSM peptides facilitate spreading of Δagr cells. To identify factors that facilitate spreading of Δagr cells (labeled “agr−” in the figure), chemically synthesized PSMs from S. aureus (plate rows 2 to 5) were spotted in the center of soft agar plates prior to the inoculation with S. aureus SH1000 Δagr cells. Both N-terminally formylated PSMs (marked with an “f” prefix) and nonformylated peptides were used in the assay. In addition, the PSM-mec peptides were tested for a potentially inhibitory role in spreading by cells of the S. aureus SH1000 agr+ strain (bottom row). All spreading assays were repeated at least five times.
To investigate why different PSMs promote the spreading of S. aureus cells to different extents, we compared their surfactant properties on soft agar plates by spotting 2 μl of each peptide solution in the center of such a plate (without adding bacteria) and by subsequently measuring the diameter of the resulting transparent halo. This revealed that PSMα3, PSMγ, and PSMβ2 have the strongest surfactant properties (Table 2). Furthermore, we assessed other potentially relevant parameters of the investigated PSMs, namely, the grand average of hydropathicity (GRAVY), the hydrophobic moment (μH), the net charge (z), the number of polar residues plus glycine, and the number of nonpolar residues. The results show that the PSMs that most effectively promote the spreading of S. aureus, namely, PSMα3 and PSMγ, combine high surfactant activities with low hydropathicity (Table 2). The other investigated PSM properties seem to be of minor relevance for spreading activity. Notably, while PSMβ2 has relatively strong surfactant properties, it has a high hydropathicity, suggesting that this high hydropathicity may counteract the promotion of spreading. Consistent with this view, PSM-mec combines the lowest surfactant properties with the highest overall hydropathicity (Table 2).
Table 2.
Properties of S. aureus PSM peptidesa
| Parametera | PSMα1 | PSMα2 | PSMα3 | PSMα4 | PSMβ1 | PSMβ2 | PSMγ | PSM-mec |
|---|---|---|---|---|---|---|---|---|
| Surfactant properties (mean length [cm] ± SD) | 1.0 ± 0.4 | 1.9 ± 0.1 | 7.4 ± 0.5 | 0.6 ± 0.1 | 1.4 ± 0.4 | 4.3 ± 2.4 | 6.3 ± 0.4 | 0.6 ± 0.1 |
| Grand avg of hydropathicity (GRAVY) | 0.957 | 0.890 | 0.305 | 1.700 | 0.570 | 0.607 | 0.150 | 1.100 |
| Hydrophobic moment, <μH> | 0.551 | 0.562 | 0.563 | 0.599 | 0.308 | 0.214 | 0.587 | 0.530 |
| Net charge z | 2 | 3 | 2 | 2 | –1 | –1 | –1 | –1 |
| Polar residues + GLY (no./%) | 10/47.62 | 10/47.62 | 10/45.45 | 6/30.00 | 23/52.27 | 22/50.00 | 14/53.85 | 10/45.45 |
| Nonpolar residues (no./%) | 11/52.38 | 11/52.38 | 12/54.55 | 14/70.00 | 21/47.73 | 22/50.00 | 12/46.15 | 12/54.55 |
Surfactant properties were determined by spotting 2 μl of a PSM solution (6 to 1.2 mM) on a soft agar plate and measuring the diameter of the resulting transparent halo. The grand average of hydropathicity (GRAVY) was determined using the ProtParam tool provided by the ExPASy server. The hydrophobic moment (μH), the net charge (z), the amount of polar residues + GLY, and the amount of nonpolar residues were obtained by using Heliquest (http://heliquest.ipmc.cnrs.fr/cgi-bin/ComputParams.py).
Genetic dissection of PSM function reveals additive effects in colony spreading.
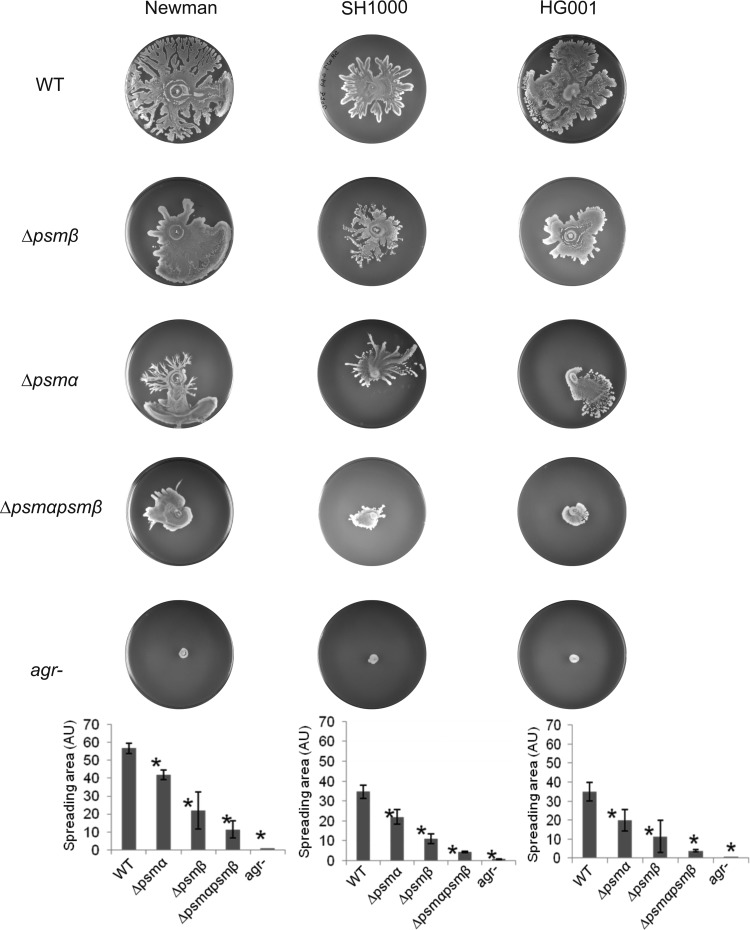
As underscored by our proteomics analyses, agr-deficient strains are completely defective in the synthesis of PSMs. To identify the contributions of the psmα and psmβ operons to spreading, the respective single- and double-mutant strains were constructed. Next, the spreading ability of these mutants was compared to the parental strain and to the equivalent agr mutant, which is fully PSM deficient. To quantify spreading activity, all images of spreading assays were analyzed with ImageJ, and the area covered by the cells was determined. Figure 4 shows that deletion of the psmβ operon had a moderate but reproducible effect on spreading. Deletion of the psmα operon had a more severe effect on spreading and the spreading activity of the psmα psmβ double mutant was even lower (Fig. 4). Notably, the spreading activity of the double mutant was still higher than that of the agr mutant, which suggests that the remaining activity of the double mutant is due to the production of PSMγ. This view is consistent with the above finding that the synthetic PSMγ is sufficient to restore spreading of agr mutant strains (Fig. 3). A comparison of the results obtained for the single and double psmα psmβ mutant variants of strains Newman, SH1000, and HG001 shows that the deletion of the psmα operon has the strongest negative impact on spreading, which is fully consistent with the findings obtained with the synthetic peptides added at equimolar concentrations. Furthermore, our genetic dissection of the function of PSM-encoding loci shows that they contribute additively to colony spreading.
Fig 4.
Additive effects of psmα and psmβ gene deletions on the spreading of S. aureus cells. The psmα and/or psmβ loci of the S. aureus strains Newman, SH1000, or HG001 were deleted, and the effects on colony spreading were compared with the effects of an agr mutation. Subsequently, the spreading areas of the investigated mutant and parental strains were determined by ImageJ, and statistical analyses were performed based on triplicate measurements for each individual strain. The graphs show the areas covered in arbitrary units (AU).
Spreading activity of S. aureus cells from catheter-associated biofilms or on pork meat.
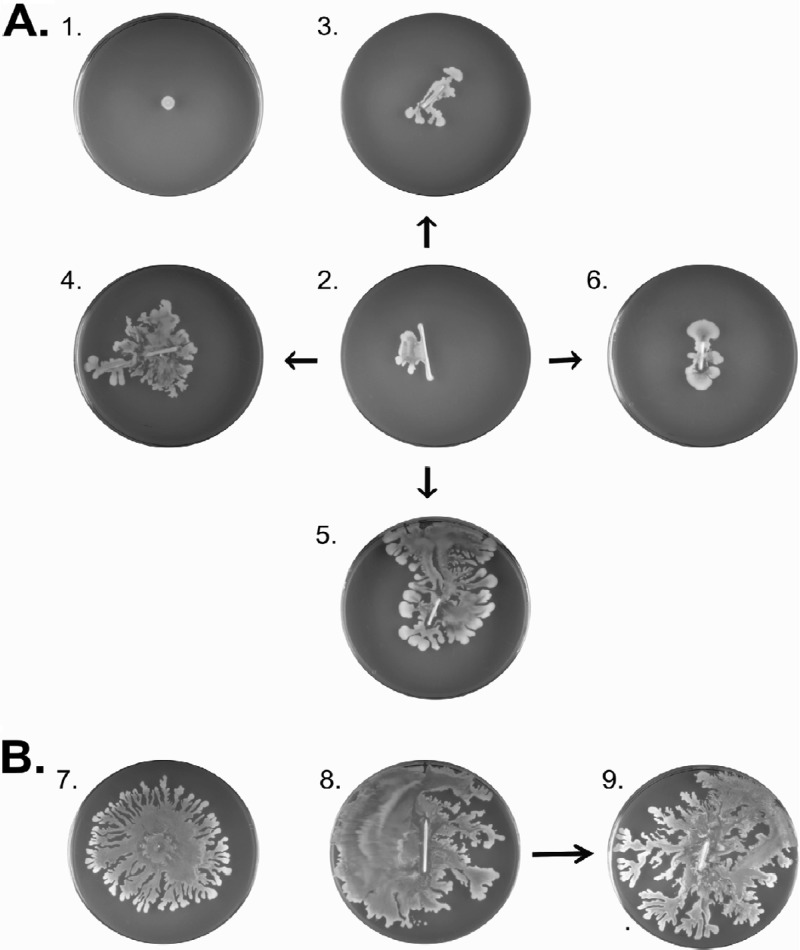
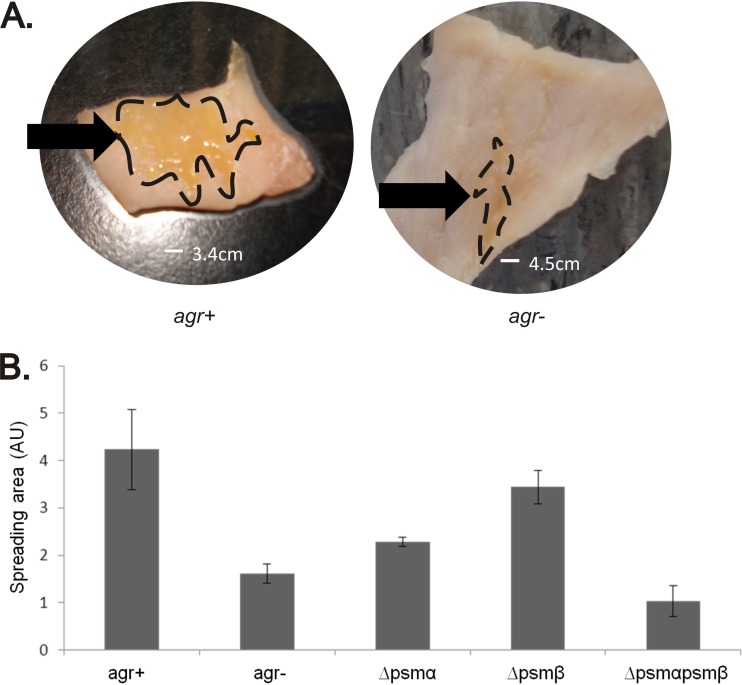
PSMs allow S. aureus to move across wet surfaces. In the assays described above this was demonstrated starting with planktonic cells growing in a broth. However, we wanted to know whether spreading would be detectable also in assays that mimic clinically relevant surfaces and conditions. In one approach, we therefore tested whether S. aureus cells present in a catheter-associated biofilm have the ability to spread. As shown in Fig. 5, both agr+ and Δagr strains were able to form biofilms on catheter material (plates 8 and 2, respectively). Interestingly, when the catheters were transferred to fresh TSA plates, the agr+ strains were well able to detach and spread away from the catheter material (plate 9). This phenomenon was not observed for the tested Δagr strains (plate 3). Importantly, however, when these catheters were transferred to fresh plates on which 2 μl of PSMα3 or PSMγ were spotted beforehand, the Δagr strains were also able to detach and spread away from the catheter (plates 4 and 5, respectively). In contrast, the PSMβ1 did not facilitate the spreading of Δagr cells from the catheter material (plate 6). These findings suggest that the ability of PSMs to promote spreading is important for S. aureus to move away from a biofilm and to colonize the surrounding wet surface. This idea was further tested by studying colony spreading on fresh pork meat. As predicted, agr+ cells were substantially more efficient than Δagr cells in colonizing pieces of pork meat upon 48 h of incubation at 37°C. In the experiment shown in Fig. 6A, the agr+ cells colonized an area that was on average ∼2.5-fold larger than the area colonized by Δagr cells. Importantly, the spreading phenotypes of psm mutant strains on fresh pork meat (Fig. 6B) resembled by-and-large the respective spreading phenotypes on soft agar plates (Fig. 4). While psmβ mutant cells were not significantly inhibited in their spreading on pork, the psmα mutant cells showed a significant spreading defect. Spreading on pork meat was most severely affected by deletion of both the psmα and psmβ genes, even to a slightly higher extent than agr mutant cells. It thus seems that PSM-mediated colony spreading has a general role in the colonization of wet surfaces by S. aureus.
Fig 5.
The spread of S. aureus cells from catheter-associated biofilms is facilitated by particular PSM peptides. (A) To investigate whether the spreading of cells from a catheter-associated biofilm is facilitated by PSMs, biofilms of Δagr cells of S. aureus Newman were grown on ∼1-cm-long strips of catheter material. These strips were then incubated on soft agar plates under differing conditions. Plate 1, 1egative control plate, showing that the used S. aureus Newman Δagr strain is unable to spread; plate 2, biofilms of Δagr cells were grown on catheter strips placed on a soft agar plate as shown with plate 2; plate 3, catheter strip with a biofilm (as on plate 2) transferred to a fresh soft TSA plate without further additions. Some “outgrowth” of the cells is observed but no spreading. Plate 4, catheter strip with a biofilm (as on plate 2) transferred to a soft TSA plate to which the PSMα3 peptide was added prior to the positioning of the catheter strip; plate 5, catheter strip with a biofilm transferred to a plate with the PSMγ peptide (as in plate 4); plate 6, catheter strip with a biofilm transferred to a plate with the PSMβ1 peptide (as in plate 4). (B) Control experiments with agr+ cells of S. aureus Newman. Plate 7, positive control plate showing colony spreading of agr+ cells; plate 8, colony spreading of Newman agr+ cells from a catheter strip; plate 9, catheter strip from plate 8 transferred to a fresh soft TSA plate without added PSMs. All spreading assays were repeated at least five times.
Fig 6.
Spreading of S. aureus on meat. (A) Overnight grown S. aureus SH1000 agr+ or Δagr (labeled “agr−” in the figure) cells were spotted on pork meat, which was subsequently incubated 48 h at 37°C. SH1000 agr+ cells covered a 2.5-fold larger area than SH1000 Δagr cells. The spreading areas are marked with dashed lines. (B) Spreading areas of agr+ and Δagr S. aureus SH1000 cells, and single or double psmα and/or psmβ mutant strains upon spotting onto fresh pork meat. All spreading assays were repeated at least three times.
DISCUSSION
Colony spreading and PSMs.
The present studies have focused attention on the role of secreted factors that are needed for the rapid colony spreading phenotype of S. aureus. The first indication for an important role of secreted factors in colony spreading was the presence of a transparent halo around the expanding colonies. Subsequent analyses showed that culture supernatants of spreaders were sufficient to make nonspreaders move on a wet soft agar surface and that the main common components in the media of spreaders were PSMs. Importantly, culture supernatants of spreaders facilitated the spreading of nonspreaders only when these supernatants were added on top of the soft agar. This is in accordance with the apparent need for high surfactant properties of the PSMs in order to be able to promote spreading. In contrast, when the culture supernatants of spreaders were included within the soft agar, they were unable to promote the spreading of non-spreaders and they even seemed to inhibit the spreading by spreaders (Fig. 2C). With synthetic PSM peptides we subsequently demonstrated conclusively that several PSMs of S. aureus are sufficient to promote colony spreading of otherwise nonspreading strains. This view was confirmed by mutagenesis experiments in which the psmα and/or psmβ operons were deleted. Taken together, our present findings show that PSMα3 and PSMγ are the key players in colony spreading, and that the other PSMα's and PSMβ's have minor roles in spreading. We observed no role for the PSM-mec neither in the promotion nor the inhibition of spreading. The latter observations are intriguing, because it has been reported that the PSM-mec and/or the PSM-mec mRNA can inhibit colony spreading (47). Since the synthetic PSM-mec peptides gave no phenotype, whereas other synthetic PSM peptides were active, it seems most likely that the previously reported effects do not relate to a translated product but rather to a regulatory effect of the psm-mec gene. We were unable to assess this possibility with the strains used in our studies, because they lack type II or type III SCCmec elements that encode PSM-mec. In any case, it is safe to conclude from our studies that addition of the PSM-mec peptide did not interfere with the function of the main spreading-promoting PSMα3 and PSMγ peptides. Indeed, an independent study confirms that deletion of the psm-mec gene does not affect the production of the other PSMs compared to the respective parental strains (11). Lastly, it was previously reported that S. aureus secretes both N-terminally formylated and deformylated PSM peptides. Our present data show that the removal of the N-terminal formyl group has no consequences with respect to the activity of PSMs in colony spreading. This indicates that the surfactant properties of PSM peptides are not substantially influenced by N-terminal formylation or deformylation.
At present, we do not know at what concentrations exactly the different PSMs need to be present locally to promote spreading. In this respect, one has to bear in mind that both the synthetic and the natural PSM peptides investigated in the present studies will diffuse not only over the soft agar surface but also into the agar underneath the surface. In addition, in a regular spreading assay, the S. aureus cells applied to a soft agar plate will initially make use of the PSMs present in the growth medium, which explains the first observed wave of “rapid” spreading. Subsequently, these cells will produce additional PSMs, allowing them to spread further over the plate. Therefore, it is very difficult to make statements about local concentrations of the PSM peptides on plates and how these correlate with the concentrations present in the spent medium. The situation is somewhat different when synthetic PSM peptides are used for a spreading assay, since this essentially represents the first wave of ‘rapid’ spreading. To this end, relatively high amounts of PSM peptides were initially tested. However, as shown by dose response curves with the PSMs that promote effective spreading (see Fig. S1 in the supplemental material), we can mimic effective spreading with concentrations that are only ∼10-fold higher than the concentrations measured in the growth medium (9). We believe that this is a realistic situation, because bacteria that are spreading with the aid of PSMs secreted into the growth medium can employ a mix of several PSMs for spreading. Moreover, the Agr-deficient cells that were used for the spreading assays cannot produce any PSMs by themselves in the course of an assay, while Agr-proficient cells will produce their own PSMs. In this context it is important to note that when the concentrations of PSMs were stepwise increased, higher spreading levels were achieved. This mimics in some way the situation where Agr-proficient cells produce several “waves of PSMs” during the course of a colony spreading experiment.
Is colony spreading by S. aureus clinically relevant?
A key question in the analysis of colony spreading is whether this property is clinically relevant. As a first approach to answer this question, we recently tested the spreading ability of 500 different clinical isolates that are representative for invasive S. aureus infections in Europe. More than 85% of these strains were able to spread (our unpublished observations). While this does not tell us that spreading was important for the actual infections, this finding does show that spreading is a very common feature of strains that have caused invasive infections in humans. Accordingly, it is conceivable that invasive strains make use of their spreading ability to move away from catheters and other implanted devices so that they can efficiently colonize wet surfaces of the human body. Clearly, our experiments with catheter material show that S. aureus cells originating form a biofilm have a similar spreading ability as planktonic cells. In this light it is not surprising that we find the same PSMs (i.e., PSMα3 and PSMγ) to be most effective in both types of assays. These findings therefore suggest that spreading may be a clinically relevant staphylococcal trait.
It has been observed that most clinical S. aureus isolates are agr+, but Δagr isolates are also isolated from patients. This population heterogeneity is likely to be advantageous for S. aureus since agr+ strains are more potent in initiating infections (48), while the Δagr strains are more potent in the establishment of chronic infections through biofilm formation (49). Our finding that nonspreading cells can “hitch-hike” along with the spreaders on soft agar plates would suggest that a similar phenomenon might “help” Δagr cells also in the colonization of wet surfaces, either in the human host or in other habitats. One of these other habitats might be animal meat intended for human consumption. There are many reports on the contamination of meat products with S. aureus, including MRSA (50–52). This is highly unwanted, first, because S. aureus is renowned as a causative agent of food poisoning (53). In addition, the contamination of food products with S. aureus, MRSA in particular, is a high-risk factor for frail and immunocompromised individuals who are more susceptible for staphylococcal infections. As shown by our experiments with pork, agr+ cells can colonize larger surfaces of the investigated meat in shorter periods of time than Δagr cells, reflecting the differences in their spreading ability on soft agar plates. Also, spreading on pork meat was reduced when the psmα genes were deleted, and it was even more reduced when both the psmα and psmβ genes were deleted. Thus, we hypothesize that spreading is an important parameter at least in food spoilage and, consequently, in food poisoning by S. aureus. It will remain a challenge for future studies to verify this hypothesis and to pinpoint any other potentially clinically relevant roles of staphylococcal spreading.
Supplementary Material
ACKNOWLEDGMENTS
We thank Sebastian Grund for expert technical assistance in the proteomics analyses, Despo Ierodiakonou for support in the statistical analyses, Corinna Glasner for support in database assembly, Jolien Seinen and Gijs Bijl for support in spreading analyses, and Alexander Horswill for providing plasmid pAH9.
E.T., E.L.D., D.B., G.B., J.W.B., J.M.V.D., and A.D. were in part supported by CEU projects LSHM-CT-2006-019064 and LSHG-CT-2006-037469, the transnational SysMO initiative through BACELL SysMO projects 1 and 2, and Top Institute Pharma project T4-213. D.B. was supported by the DFG through SFB-TR34.
Footnotes
Published ahead of print 26 November 2012
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.03157-12.
REFERENCES
- 1. Lowy FD. 1998. Staphylococcus aureus infections. N. Engl. J. Med. 339:520–532 [DOI] [PubMed] [Google Scholar]
- 2. Peacock SJ, de Silva I, Lowy FD. 2001. What determines nasal carriage of Staphylococcus aureus? Trends Microbiol. 9:605–610 [DOI] [PubMed] [Google Scholar]
- 3. Ziebandt AK, Kusch H, Degner M, Jaglitz S, Sibbald MJ, Arends JP, Chlebowicz MA, Albrecht D, Pantucek R, Doskar J, Ziebuhr W, Broker BM, Hecker M, van Dijl JM, Engelmann S. 2010. Proteomics uncovers extreme heterogeneity in the Staphylococcus aureus exoproteome due to genomic plasticity and variant gene regulation. Proteomics 10:1634–1644 [DOI] [PubMed] [Google Scholar]
- 4. Dreisbach A, Hempel K, Buist G, Hecker M, Becher D, van Dijl JM. 2010. Profiling the surfaceome of Staphylococcus aureus. Proteomics 10:3082–3096 [DOI] [PubMed] [Google Scholar]
- 5. Dreisbach A, van Dijl JM, Buist G. 2011. The cell surface proteome of Staphylococcus aureus. Proteomics 11:3154–3168 [DOI] [PubMed] [Google Scholar]
- 6. Otto M. 2010. Basis of virulence in community-associated methicillin-resistant Staphylococcus aureus. Annu. Rev. Microbiol. 64:143–162 [DOI] [PubMed] [Google Scholar]
- 7. Queck SY, Jameson-Lee M, Villaruz AE, Bach TH, Khan BA, Sturdevant DE, Ricklefs SM, Li M, Otto M. 2008. RNAIII-independent target gene control by the agr quorum-sensing system: insight into the evolution of virulence regulation in Staphylococcus aureus. Mol. Cell 32:150–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Queck SY, Khan BA, Wang R, Bach TH, Kretschmer D, Chen L, Kreiswirth BN, Peschel A, DeLeo FR, Otto M. 2009. Mobile genetic element-encoded cytolysin connects virulence to methicillin resistance in MRSA. PLoS Pathog. 5:e1000533 doi:10.1371/journal.ppat.1000533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang R, Braughton KR, Kretschmer D, Bach TH, Queck SY, Li M, Kennedy AD, Dorward DW, Klebanoff SJ, Peschel A, DeLeo FR, Otto M. 2007. Identification of novel cytolytic peptides as key virulence determinants for community-associated MRSA. Nat. Med. 13:1510–1514 [DOI] [PubMed] [Google Scholar]
- 10. Somerville GA, Cockayne A, Durr M, Peschel A, Otto M, Musser JM. 2003. Synthesis and deformylation of Staphylococcus aureus delta-toxin are linked to tricarboxylic acid cycle activity. J. Bacteriol. 185:6686–6694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chatterjee SS, Chen L, Joo HS, Cheung GY, Kreiswirth BN, Otto M. 2011. Distribution and regulation of the mobile genetic element-encoded phenol-soluble modulin PSM-mec in methicillin-resistant Staphylococcus aureus. PLoS One 6:e28781 doi:10.1371/journal.pone.0028781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Projan SJ, Brown-Skrobot S, Schlievert PM, Vandenesch F, Novick RP. 1994. Glycerol monolaurate inhibits the production of beta-lactamase, toxic shock toxin-1, and other staphylococcal exoproteins by interfering with signal transduction. J. Bacteriol. 176:4204–4209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sibbald MJ, Ziebandt AK, Engelmann S, Hecker M, de Jong A, Harmsen HJ, Raangs GC, Stokroos I, Arends JP, Dubois JY, van Dijl JM. 2006. Mapping the pathways to staphylococcal pathogenesis by comparative secretomics. Microbiol. Mol. Biol. Rev. 70:755–788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. George EA, Muir TW. 2007. Molecular mechanisms of agr quorum sensing in virulent staphylococci. Chembiochem 8:847–855 [DOI] [PubMed] [Google Scholar]
- 15. Morfeldt E, Janzon L, Arvidson S, Lofdahl S. 1988. Cloning of a chromosomal locus (exp) which regulates the expression of several exoprotein genes in Staphylococcus aureus. Mol. Gen. Genet. 211:435–440 [DOI] [PubMed] [Google Scholar]
- 16. Novick RP, Geisinger E. 2008. Quorum sensing in staphylococci. Annu. Rev. Genet. 42:541–564 [DOI] [PubMed] [Google Scholar]
- 17. Novick RP, Ross HF, Projan SJ, Kornblum J, Kreiswirth B, Moghazeh S. 1993. Synthesis of staphylococcal virulence factors is controlled by a regulatory RNA molecule. EMBO J. 12:3967–3975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Novick RP, Projan SJ, Kornblum J, Ross HF, Ji G, Kreiswirth B, Vandenesch F, Moghazeh S. 1995. The agr P2 operon: an autocatalytic sensory transduction system in Staphylococcus aureus. Mol. Gen. Genet. 248:446–458 [DOI] [PubMed] [Google Scholar]
- 19. Peng HL, Novick RP, Kreiswirth B, Kornblum J, Schlievert P. 1988. Cloning, characterization, and sequencing of an accessory gene regulator (agr) in Staphylococcus aureus. J. Bacteriol. 170:4365–4372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Recsei P, Kreiswirth B, O'Reilly M, Schlievert P, Gruss A, Novick RP. 1986. Regulation of exoprotein gene expression in Staphylococcus aureus by agar. Mol. Gen. Genet. 202:58–61 [DOI] [PubMed] [Google Scholar]
- 21. Ziebandt AK, Becher D, Ohlsen K, Hacker J, Hecker M, Engelmann S. 2004. The influence of agr and sigmaB in growth phase-dependent regulation of virulence factors in Staphylococcus aureus. Proteomics 4:3034–3047 [DOI] [PubMed] [Google Scholar]
- 22. Kobayashi SD, DeLeo FR. 2009. An update on community-associated MRSA virulence. Curr. Opin. Pharmacol. 9:545–551 [DOI] [PubMed] [Google Scholar]
- 23. Li M, Cheung GY, Hu J, Wang D, Joo HS, DeLeo FR, Otto M. 2010. Comparative analysis of virulence and toxin expression of global community-associated methicillin-resistant Staphylococcus aureus strains. J. Infect. Dis. 202:1866–1876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang R, Khan BA, Cheung GY, Bach TH, Jameson-Lee M, Kong KF, Queck SY, Otto M. 2011. Staphylococcus epidermidis surfactant peptides promote biofilm maturation and dissemination of biofilm-associated infection in mice. J. Clin. Invest. 121:238–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kaito C, Sekimizu K. 2007. Colony spreading in Staphylococcus aureus. J. Bacteriol. 189:2553–2557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kaito C, Omae Y, Matsumoto Y, Nagata M, Yamaguchi H, Aoto T, Ito T, Hiramatsu K, Sekimizu K. 2008. A novel gene, fudoh, in the SCCmec region suppresses the colony spreading ability and virulence of Staphylococcus aureus. PLoS One 3:e3921 doi:10.1371/journal.pone.0003921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tsompanidou E, Sibbald MJ, Chlebowicz MA, Dreisbach A, Back JW, van Dijl JM, Buist G, Denham EL. 2011. Requirement of the agr locus for colony spreading of Staphylococcus aureus. J. Bacteriol. 193:1267–1272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tsompanidou E, Denham EL, Sibbald MJ, Yang XM, Seinen J, Friedrich AW, Buist G, van Dijl JM. 2012. The sortase A substrates FnbpA, FnbpB, ClfA, and ClfB antagonize colony spreading of Staphylococcus aureus. PLoS One 7:e44646 doi:10.1371/journal.pone.0044646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hanahan D. 1983. Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 166:557–580 [DOI] [PubMed] [Google Scholar]
- 30. Kreiswirth BN, Lofdahl S, Betley MJ, O'Reilly M, Schlievert PM, Bergdoll MS, Novick RP. 1983. The toxic shock syndrome exotoxin structural gene is not detectably transmitted by a prophage. Nature 305:709–712 [DOI] [PubMed] [Google Scholar]
- 31. Novick R. 1967. Properties of a cryptic high-frequency transducing phage in Staphylococcus aureus. Virology 33:155–166 [DOI] [PubMed] [Google Scholar]
- 32. Horsburgh MJ, Aish JL, White IJ, Shaw L, Lithgow JK, Foster SJ. 2002. sigmaB modulates virulence determinant expression and stress resistance: characterization of a functional rsbU strain derived from Staphylococcus aureus 8325-4. J. Bacteriol. 184:5457–5467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pohl K, Francois P, Stenz L, Schlink F, Geiger T, Herbert S, Goerke C, Schrenzel J, Wolz C. 2009. CodY in Staphylococcus aureus: a regulatory link between metabolism and virulence gene expression. J. Bacteriol. 191:2953–2963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Duthie ES, Lorenz LL. 1952. Staphylococcal coagulase; mode of action and antigenicity. J. Gen. Microbiol. 6:95–107 [DOI] [PubMed] [Google Scholar]
- 35. Wolz C, McDevitt D, Foster TJ, Cheung AL. 1996. Influence of agr on fibrinogen binding in Staphylococcus aureus Newman. Infect. Immun. 64:3142–3147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Norrander J, Kempe T, Messing J. 1983. Construction of improved M13 vectors using oligodeoxynucleotide-directed mutagenesis. Gene 26:101–106 [DOI] [PubMed] [Google Scholar]
- 37. Arnaud M, Chastanet A, Debarbouille M. 2004. New vector for efficient allelic replacement in naturally nontransformable, low-GC-content, Gram-positive bacteria. Appl. Environ. Microbiol. 70:6887–6891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kuroda M, Ito R, Tanaka Y, Yao M, Matoba K, Saito S, Tanaka I, Ohta T. 2008. Staphylococcus aureus surface protein SasG contributes to intercellular autoaggregation of Staphylococcus aureus. Biochem. Biophys. Res. Commun. 377:1102–1106 [DOI] [PubMed] [Google Scholar]
- 39. Sastalla I, Chim K, Cheung GY, Pomerantsev AP, Leppla SH. 2009. Codon-optimized fluorescent proteins designed for expression in low-GC Gram-positive bacteria. Appl. Environ. Microbiol. 75:2099–2110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Boles BR, Horswill AR. 2008. agr-mediated dispersal of Staphylococcus aureus biofilms. PLoS Pathog. 4:e1000052 doi:10.1371/journal.ppat.1000052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Miller M, Dreisbach A, Otto A, Becher D, Bernhardt J, Hecker M, Peppelenbosch MP, van Dijl JM. 2011. Mapping of interactions between human macrophages and Staphylococcus aureus reveals an involvement of MAP kinase signaling in the host defense. J. Proteome Res. 10:4018–4032 [DOI] [PubMed] [Google Scholar]
- 42. Kouwen TR, Trip EN, Denham EL, Sibbald MJ, Dubois JY, van Dijl JM. 2009. The large mechanosensitive channel MscL determines bacterial susceptibility to the bacteriocin sublancin 168. Antimicrob. Agents Chemother. 53:4702–4711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sibbald MJ, Winter T, van der Kooi-Pol MM, Buist G, Tsompanidou E, Bosma T, Schafer T, Ohlsen K, Hecker M, Antelmann H, Engelmann S, van Dijl JM. 2010. Synthetic effects of secG and secY2 mutations on exoproteome biogenesis in Staphylococcus aureus. J. Bacteriol. 192:3788–3800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kearns DB, Losick R. 2003. Swarming motility in undomesticated Bacillus subtilis. Mol. Microbiol. 49:581–590 [DOI] [PubMed] [Google Scholar]
- 45. Kohler T, Curty LK, Barja F, van Delden C, Pechere JC. 2000. Swarming of Pseudomonas aeruginosa is dependent on cell-to-cell signaling and requires flagella and pili. J. Bacteriol. 182:5990–5996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lindum PW, Anthoni U, Christophersen C, Eberl L, Molin S, Givskov M. 1998. N-Acyl-l-homoserine lactone autoinducers control production of an extracellular lipopeptide biosurfactant required for swarming motility of Serratia liquefaciens MG1. J. Bacteriol. 180:6384–6388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kaito C, Saito Y, Nagano G, Ikuo M, Omae Y, Hanada Y, Han X, Kuwahara-Arai K, Hishinuma T, Baba T, Ito T, Hiramatsu K, Sekimizu K. 2011. Transcription and translation products of the cytolysin gene psm-mec on the mobile genetic element SCCmec regulate Staphylococcus aureus virulence. PLoS Pathog. 7:e1001267 doi:10.1371/journal.ppat.1001267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Traber KE, Lee E, Benson S, Corrigan R, Cantera M, Shopsin B, Novick RP. 2008. agr function in clinical Staphylococcus aureus isolates. Microbiology 154:2265–2274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yarwood JM, Schlievert PM. 2003. Quorum sensing in Staphylococcus infections. J. Clin. Invest. 112:1620–1625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. van Loo IH, Diederen BM, Savelkoul PH, Woudenberg JH, Roosendaal R, van Belkum A, Lemmens-den Toom N, Verhulst C, van Keulen PH, Kluytmans JA. 2007. Methicillin-resistant Staphylococcus aureus in meat products, the Netherlands. Emerg. Infect. Dis. 13:1753–1755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. O'Brien AM, Hanson BM, Farina SA, Wu JY, Simmering JE, Wardyn SE, Forshey BM, Kulick ME, Wallinga DB, Smith TC. 2012. MRSA in conventional and alternative retail pork products. PLoS One 7:e30092 doi:10.1371/journal.pone.0030092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kluytmans JA. 2010. Methicillin-resistant Staphylococcus aureus in food products: cause for concern or case for complacency? Clin. Microbiol. Infect. 16:11–15 [DOI] [PubMed] [Google Scholar]
- 53. Le Loir Y, Baron F, Gautier M. 2003. Staphylococcus aureus and food poisoning. Genet. Mol. Res. 2:63–76 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.