Abstract
Global warming and decreasing fossil fuel reserves have prompted great interest in the synthesis of advanced biofuels from renewable resources. In an effort to address these concerns, we performed metabolic engineering of the cyanobacterium Synechocystis sp. strain PCC 6803 to develop a strain that can synthesize isobutanol under both autotrophic and mixotrophic conditions. With the expression of two heterologous genes from the Ehrlich pathway, the engineered strain can accumulate 90 mg/liter of isobutanol from 50 mM bicarbonate in a gas-tight shaking flask. The strain does not require any inducer (i.e., isopropyl β-d-1-thiogalactopyranoside [IPTG]) or antibiotics to maintain its isobutanol production. In the presence of glucose, isobutanol synthesis is only moderately promoted (titer = 114 mg/liter). Based on isotopomer analysis, we found that, compared to the wild-type strain, the mutant significantly reduced its glucose utilization and mainly employed autotrophic metabolism for biomass growth and isobutanol production. Since isobutanol is toxic to the cells and may also be degraded photochemically by hydroxyl radicals during the cultivation process, we employed in situ removal of the isobutanol using oleyl alcohol as a solvent trap. This resulted in a final net concentration of 298 mg/liter of isobutanol under mixotrophic culture conditions.
INTRODUCTION
Global energy needs continue to increase rapidly due to industrial and development demands, raising environmental concerns. Much of the worldwide energy consumption comes from the burning of fossil fuels, which produces about 6 gigatons of CO2 annually (1). Increasing CO2 levels may act as a feedback loop to increase the soil emissions of other greenhouse gases, such as methane and nitrous oxide, raising the global temperature (2). For energy security and due to environmental concerns, there is an urgent demand for the development of bioenergy. Bioethanol is the most common biofuel, but it also has low energy density and absorbs moisture. Isobutanol (IB) is a better fuel, because it is less water soluble and has an energy density/octane value close to that of gasoline (3, 4). Among the next-generation biofuels synthesized from pyruvate, IB possesses fewer reaction steps (5 reaction steps from pyruvate to IB) than the synthesis of 1-butanol or biodiesel. IB is less toxic to microbes (5), so it may achieve a higher product titer and yield (6, 7). For example, a maximum titer of 50.8 g/liter of IB can be achieved in an engineered Escherichia coli strain (8).
On the other hand, cyanobacteria not only can convert CO2 into bioproducts, but also can play an important role in environmental bioremediation. The photosynthetic efficiency of cyanobacteria (3 to 9%) is high compared to that of higher plants (≤0.25 to 3%) (9, 10). Furthermore, some species of cyanobacteria are amenable to genetic engineering. Table 1 lists the various biofuels that have been synthesized through the metabolic engineering of cyanobacteria. Autotrophic IB production in cyanobacteria was first demonstrated in Synechococcus sp. strain 7942 (13). Moreover, a model cyanobacterium, Synechocystis sp. strain PCC 6803, is capable of growing under both photoautotrophic and mixotrophic conditions, while the presence of glucose can significantly promote biomass and bioproduct synthesis (20). Therefore, we have engineered a glucose-tolerant Synechocystis 6803 strain with two key genes, kivd and adhA, of the Ehrlich pathway (21) so that the cyanobacterial strain can convert CO2 into IB. Through both metabolic engineering and bioprocess optimization, we have improved our strain's IB production capabilities.
Table 1.
Metabolic engineering of cyanobacterial strains for biofuel production
| Product | Species | Titer or productivity | Overexpressed gene(s) | Promoter(s) | Culture vessel/remarks | No. of culture days | Reference |
|---|---|---|---|---|---|---|---|
| Ethanol | Synechococcus 7942 | 230 mg/liter | pdc, adh | rbcLS | Shake flask | 28 | 11 |
| Ethanol | Synechocystis 6803 | 552 mg/liter | pdc, adh | psbA2 | Photobioreactor | 6 | 12 |
| Isobutyraldehyde | Synechococcus 7942 | 1,100 mg/liter | alsS, ilvC, ilvD, kivd, rbcls | LlacO1, trc, tac | Roux culture bottle with NaHCO3 | 8 | 13 |
| Isobutanol | Synechococcus 7942 | 18 mg/liter | kivd, yqhD | trc | Shake flask with NaHCO3 | 13 | |
| Isobutanol | Synechococcus 7942 | 450 mg/liter | alsS, ilvC, ilvD, kivd, yqhD | LlacO1, trc | Shake flask with NaHCO3 | 6 | 13 |
| Fatty alcohol | Synechocystis 6803 | 200 ± 8 μg/liter | far | rbc | Photobioreactor with 5% CO2 | 18 | 14 |
| Alkanes | Synechocystis 6803 | 162 ± 10 μg/OD unit/liter | accBCDA | rbcl | Shake flask | 14 | |
| Fatty acids | Synechocystis 6803 | 197 ± 14 mg/liter | tesA, accBCDA, fatB1, fatB2, tesA137 | psbA2, cpc, trc | 1% CO2 bubbling | 17 | 15 |
| Hydrogen | Synechococcus 7942 | 2.8 μmol/h/mg Chl-aa | hydEF, hydG, hydA | psbA1, lac | Anaerobic conditions with DCMUb treatment | 16 | |
| 1-Butanol | Synechococcus 7942 | 14.5 mg/liter | hbd, crt, adhE2, ter, atoB | trc, LlacO1 | Dark Roux culture bottle under anoxic conditions | 7 | 17 |
| Fatty alcohol | Synechocystis 6803 | 20 ± 2 μg/liter/OD | far, aas | rbc, psbA2 | Shake flask | 18 | |
| 1-Butanol | Synechococcus 7942 | 30 mg/liter | ter, nphT7, bldh, yqhD, phaJ, phaB | trc, LlacO1 | Shake flask | 18 | 19 |
Chl-a, chlorophyll a.
DCMU, 3-(3,4-dichlorophenyl)-1,1-dimethylurea.
MATERIALS AND METHODS
Chemicals and reagents.
Restriction enzymes, T4 DNA ligase, DNase, and a Revertaid first-strand cDNA synthesis kit were purchased from Fermentas or New England BioLabs. Oligonucleotides were purchased from Integrated DNA Technologies. Toluene, IB, α-ketoisovaleric acid, phenol, and chloroform were purchased from Sigma-Aldrich (St. Louis, MO). KlenTaq-LA (22) was purchased from DNA Polymerase Technology (St. Louis, MO). TRI Reagent was purchased from Ambion. 13C-labeled glucose was purchased from Cambridge Isotope Laboratories, Cambridge, MA.
Culture medium and growth conditions.
A glucose-tolerant wild-type (WT) strain of Synechocystis 6803 and the recombinant strain AV03 were grown at 30°C in liquid blue-green medium (BG-11 medium) or solid BG-11 medium at a light intensity of 50 μmol of photons m−2 s−1 in ambient air. Kanamycin at a concentration of 20 μg/ml was added to the BG 11 medium when required. Growth of the cells was monitored by measuring the optical density at 730 nm (OD730) of the cultures on an Agilent Cary 60 UV-vis spectrophotometer. Cultures for the synthesis of IB were grown in 10 ml medium (initial OD730, 0.4) in 50-ml shake flasks for 4 days. The mid-log-phase cultures were then closed with rubber caps to prevent the loss of IB during incubation, and the cultures were supplemented with 50 mM NaHCO3 as an inorganic carbon source. Mixotrophic cultures of Synechocystis 6803 were started in BG-11 medium containing a known amount of glucose (0.5%) as an organic carbon source. E. coli strain DH10B was the host for all plasmids constructed in this study. E. coli cells were grown in Falcon tubes containing Luria-Bertani (LB) medium at 37°C with continuous shaking. Ampicillin (100 μg/ml) or kanamycin (50 μg/ml) was added to the LB medium when required for the propagation of plasmids in E. coli.
Plasmid construction and transformation of Synechocystis 6803.
The vector pTAC-KA, containing an ampicillin resistance cassette (Ampr) and two genes (kivd and adhA from Lactococcus lactis), was constructed as described previously (40). The pTAC-KA vector was modified using the following steps to convert it into a Synechocystis 6803 vector. To clone the flanking regions of a potential neutral site into pTAC-KA, a Synechocystis 6803 vector, pSL2035, containing both the flanking regions and the kanamycin resistance cassette (Kmr), was used as a template. pSL2035 is a Synechocystis 6803 vector designed to integrate any foreign DNA into the genome of Synechocystis 6803 by replacing the psbA1 gene and its promoter. psbA1 is a member of the psbA gene family and has been found to be silent under most conditions (23, 24). pSL2035 was constructed by cloning the flanking regions for the psbA1 gene and the Kmr into pUC118. The 5′ flanking region from pSL2035 was PCR amplified, along with the Kmr, by the respective primers (AMV14F and AMV15R) (Table 2) and cloned into the PciI and Bsu36I site of pTAC-KA, resulting in the vector pTKA2. The 3′ flanking region was PCR amplified from pSL2035 by the respective primers (AMV16F and AMV17R) and inserted into the AhdI site of pTKA2, disrupting the native Ampr and thus creating the vector pTKA3.
Table 2.
Sequences of primers used in this study
| Primer name | Sequence (5′→3′) |
|---|---|
| AMV14F | GCGCACATGTCGGAACAGGACCAAGCCTTGAT |
| AMV15R | GCGC CCTGAGGCCTTTACCATGACCTGCAGGG |
| AMV16F | GCGCGACGGGGAGTCAATTGTGCCATTGCCATAACTGCTTTCG |
| AMV17R | GCGCGACTCCCCGTCTTTGACTATCCTTTTTAGGATGGGGCA |
| ps1_up_fwda | TACCGGAACAGGACCAAGCCTT |
| AMV01 | GCGCCATATGTATACAGTAGGAGATTACCTATTAGAC |
| AMV12 | GCAGCAGCAACATCAACTGGTAAG |
| AdhA-TMs | TCAACTAGTGGTACCAGGAGATATAATATGAAAGCAGCAGTAGTAAGAC |
| adhA_RTr | GACAATTCCAATTCCTTCATGACCAAG |
| Rnpbr | CGGTATTTTTCTGTGGCACTGTCC |
| Rnpbf | CAGCGGCCTATGGCTCTAATC |
| AV03_6F | GAATCCGTAATCATGGTCATAGCTG |
| AV03_4R | GCCAAAGCTAATTATTTCATGTCCTGT |
| AV03_1R | TGTCGGGGCGCAGCCATGA |
| AV03_2F | AGAGGATCCTTCTGAAATGAGCTG |
| AV03_3F | CAGAGCCTAATCTTAAAGAATTCGTGG |
| AV03_4F | GGGTAAACTATTTGCTGAACAAAATAAATC |
| AV03_2R | CCGCTTCTGCGTTCTGATTTAATC |
| AV03_5F | GTTGATCGGCGCGAGATTTAATCG |
| AV03_7F | CCGTTGAAATTGACCGAGTACTTTCT |
| AV03_8F | CAGTCGAAAGAGAAATTCATGGACC |
| AV03_5R | CGCTACGGCGTTTCACTTCTG |
Transformation was performed by using a double homologous-recombination system, and the genes were integrated into the target site of the Synechocystis genomic DNA. Specifically, 2 ml of Synechocystis 6803 cells from a mid-log-phase (1 × 108 to 3 × 108 cells ml−1) culture was centrifuged at 10,000 × g for 2 min. The pellet was suspended in fresh BG-11 medium (200 μl) to a final cell density of 1 × 109 to 3 × 109 cells ml−1. Plasmid DNA was added to this dense Synechocystis 6803 cell culture to a final DNA concentration of 5 to 10 μg/ml (25). The mixture was then incubated under normal light conditions (50 microeinsteins m−2 s−1) overnight. The culture was then spread onto a BG-11 agar plate containing 20 μg/ml of kanamycin. Recombinant colonies usually appear between 7 and 10 days postinoculation. Colonies were propagated on a fresh BG-11 plate containing kanamycin, and a colony PCR was performed to verify successful integration of the insert into the genomic DNA of the recombinant. The positive colonies were propagated continuously onto BG-11 plates containing kanamycin to get high segregation of the insert in the recombinant (26). To verify the integrity of the promoter and gene sequences, the heterologous DNA integrated into the genome of the mutant AV03 was PCR amplified and sent for sequencing with the respective primers (AV03_6F, AV03_4R, AV03_1R, AV03_2F, AV03_3F, AV03_4F, AV03_2R, AV03_5F, AV03_7F, AV03_8F, and AV03_5R).
Reverse transcription-PCR (RT-PCR).
Total RNA isolation of Synechocystis 6803 was performed using a TRI Reagent (Ambion) by following the manufacturer's protocol with modifications. One milliliter of RNAwiz (the TRI reagent for the isolation of total RNA) was prewarmed to 70°C and pipetted directly into the frozen cells. The mixture was immediately vortexed and incubated for 10 min at 70°C in a heater block. Chloroform (0.2 ml) was added to the mixture and mixed vigorously, followed by incubation at room temperature for 10 min. The aqueous and organic phases were separated by centrifugation at 10,000 × g at 4°C. The RNA containing the aqueous phase was transferred into an Eppendorf tube, to which equal volumes of phenol and chloroform were added. The mixture was mixed vigorously, followed by centrifugation to separate the aqueous and organic phases. The aqueous phase was removed to a clean tube, to which 0.5 ml of diethyl pyrocarbonate (DEPC)-treated water was added. RNA in the solution was precipitated by the addition of room temperature isopropanol and centrifuged at 10,000 × g at 4°C to pellet the RNA. The RNA was washed with ethanol and resuspended in a fresh 50 μl of DEPC-treated water. The quantity and quality of the isolated RNA were determined using a Nanodrop ND-1000 (Thermo Scientific). The RNA was incubated at room temperature with DNase to degrade any genomic DNA present in the RNA sample. Synthesis of cDNA was performed by utilizing a reverse transcriptase enzyme from Fermentas, along with deoxynucleoside triphosphates (dNTPs) and random primers in a reaction buffer. The mixture was incubated at 42°C for 60 min. The synthesized cDNA was used as a template for PCR to detect the expression of the mRNA of interest. The oligonucleotides used for the RT-PCR were as follows: for gene kivd, AMV01 and AMV12; for gene adhA, AdhA-TMs and adhA_RTr; and for gene rnpB (control), Rnpbr and Rnpbf.
Isobutanol quantification assay.
IB synthesized in the culture was quantified using a gas chromatograph (GC) (Hewlett Packard model 7890A [Agilent Technologies] equipped with a DB5-MS column [J&W Scientific]) and a mass spectrometer (MS) (5975C; Agilent Technologies). IB extraction was done using a modified procedure (27). Samples of the cyanobacterial culture (400 μl) were collected and centrifuged at 10,000 × g for 5 min. IB was extracted from the supernatant by vortexing for 1 min with 400 μl of toluene, and methanol was used as the internal standard. A 1-μl sample of the organic layer was injected into the GC with helium as the carrier gas. The GC oven was held at 70°C for 2 min and then raised to 200°C with a temperature ramp of 30°C min−1, and the postrun was set at 300°C for 6 min. The range of the MS scan mode was set between m/z of 20 and 200. The concentration of IB present in the culture was determined based on a calibration curve prepared with known concentrations of IB ranging from 25 mg/liter to 400 mg/liter.
13C experiment to detect the carbon contribution of glucose.
The 13C abundance in some important metabolites was measured for both the wild type and the mutant strain AV03 to estimate the carbon contributions of both glucose (fully labeled by 13C) and nonlabeled bicarbonate for biomass and IB synthesis. Mixotrophic cultures of both the wild-type Synechocystis 6803 and the mutant AV03 were grown in BG-11 medium (with 50 mM nonlabeled NaHCO3), which contained 0.5% glucose (U-13C; Cambridge Isotope Laboratories). Cultures were collected on days 3, 6, and 9, and proteinogenic amino acids were hydrolyzed and then derivatized with TBDMS [N-(tert-butyldimethylsilyl)-N-methyl-trifluoroacetamide; Sigma-Aldrich]. The derivatized amino acids were analyzed for their mass isotopomer abundance by GC-MS, as described previously (28, 29). The m/z ion [M-57]+, which corresponds to the entire amino acid, was used to calculate the 13C abundance in amino acids [m0 m1…. mn]. The fraction of carbon (FA) derived from fully labeled glucose for each amino acid was estimated based on the following equation:
| (1) |
where i is the number of labeled carbons, mi is the mass fraction for different isotopomers of the corresponding amino acid, and n represents the total number of carbons in the corresponding amino acid. The m/z of [M-15]+ was used only for leucine and isoleucine, since their [M-57]+ overlaps with other mass peaks (30). IB extraction was performed for samples obtained from the above-mentioned cultures and was analyzed using the GC-MS. The fraction of carbon derived from glucose for isobutanol (FIB) was estimated based on the isobutanol MS peak abundances:
| (2) |
where Ai is the abundance of the mass-to-charge ratio peaks for the various isobutanol isotopomers (i.e., A0 to A4 for an m/z of 74 to 78).
RESULTS
Construction of an isobutanol-producing Synechocystis 6803 strain.
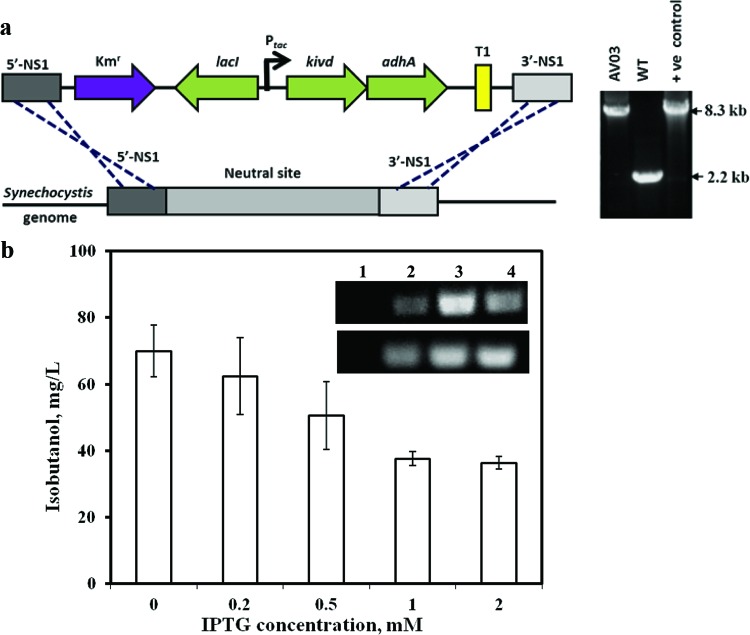
IB synthesis in Synechocystis 6803 requires the expression of two heterologous genes of the Ehrlich pathway. The enzymes 2-keto-acid decarboxylase and alcohol dehydrogenase can convert 2-keto acids into alcohols. In this work, we constructed a plasmid, pTKA3, containing the genes kivd and adhA from L. lactis under the control of an isopropyl β-d-1-thiogalactopyranoside (IPTG)-inducible promoter, Ptac. The plasmid was designed to integrate the genes into a neutral site in the genome of Synechocystis 6803, along with a kanamycin resistance cassette (Fig. 1a, left). The wild-type strain of Synechocystis 6803 was transformed with pTKA3, resulting in the recombinant strain AV03. The integration of the inserted genes into the genome was verified by a colony PCR after several rounds of segregation (Fig. 1a, right).
Fig 1.
(a) Schematic representation showing the integration of the genes kivd and adhA into the genome of Synechocystis 6803. Colony PCR was performed to verify the integration of the insert into the genomic DNA of the mutant (AV03). The vector pTKA3 was used as a template for the positive control, and wild-type cells were used as a negative control. Colony PCR of AV03 showed the presence of a band (8.3 kb) the same size as that of the positive (+ve) control and the absence of the negative-control (WT) band. (b) IB synthesized by engineered Synechocystis 6803 under different IPTG concentrations (n = 3). (Inset) Results of an RT-PCR performed to detect the expression of the heterologous genes kivd (top; 500 bp from kivd) and adhA (bottom; 200 bp from adhA). Lane 1, wild-type 6803; lane 2, AV03 with 0 mM IPTG; lane 3, AV03 with 0.5 mM IPTG; lane 4, AV03 with 1 mM IPTG. The error bars indicate standard deviations.
To identify the optimal IPTG concentration required for IB synthesis, the AV03 strain was grown under different concentrations of IPTG. IB analysis of the cultures indicated that IB was highly synthesized even without the addition of IPTG (Fig. 1b). To verify if this observation was an artifact of any mutations that might have occurred in lacI or the promoter, the foreign DNA integrated into the chromosome of AV03 was sequenced. Sequencing results for lacI and the promoter Ptac in the genome of AV03 revealed that the nucleotide sequence was completely intact. There have been reports of leaky expression with IPTG-inducible promoters (31). Also, Fig. 1b indicates that as the concentration of IPTG rose higher than 1 mM, IB synthesis was reduced. The OD730 values of the different cultures indicated that the addition of IPTG did not apparently interfere with the growth rate of the culture. RT-PCR showed the expression levels of the genes kivd and adhA under different IPTG concentrations. The result of the RT-PCR experiment (Fig. 1b, inset) indicated that the levels of kivd and adhA mRNA synthesized in the mutant were higher with IPTG than without. Hence, lower expression of the two genes is sufficient for IB synthesis, possibly because the Ehrlich pathway may not be the rate-limiting step for IB production.
Isobutanol synthesis under autotrophic and mixotrophic growth.
Under autotrophic conditions, Synechocystis 6803 utilizes light as an energy source (ATP and NADPH) for the conversion of CO2 into biomass and IB. Figure 2A compares the autotrophic growth of the mutant and the wild strain. Under autotrophic conditions, we found that the growth rate of the mutant AV03 remained unaltered compared to the wild-type strain. IB accumulation in the mutant was tested under autotrophic conditions (Fig. 2B), and the strain was found to synthesize a maximum of 90 mg/liter of IB (the only extracellular product detected by GC-MS) in a 6-day culture. In a sealed shaking flask, NaHCO3 in the medium (50 mM) was consumed by AV03 within 6 days, and then both the biomass and IB started declining.
Fig 2.
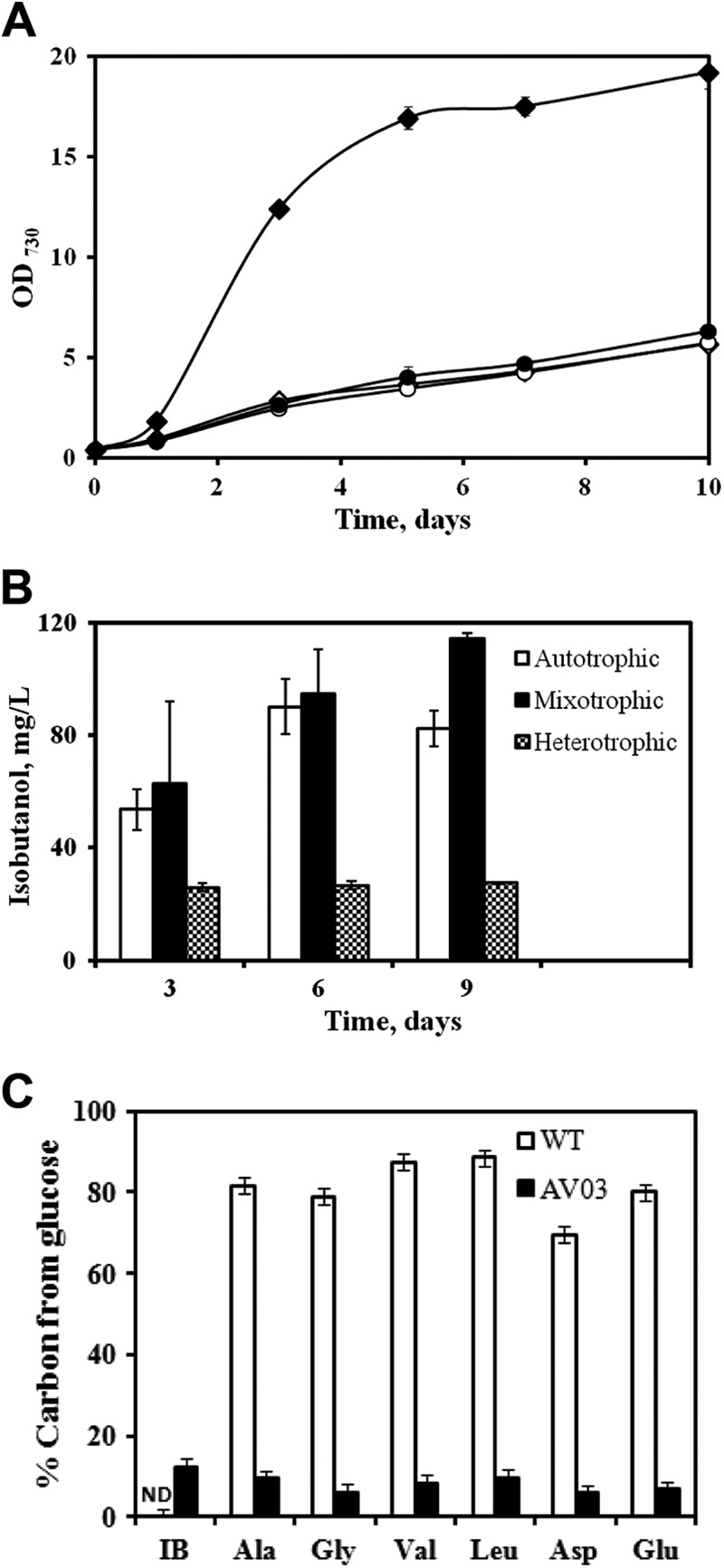
(A) Growth curves of Synechocystis 6803 WT and AV03 (n = 3; shake flask cultures). ♢, WT under autotrophic conditions; ◆, WT under mixotrophic conditions; ○, AV03 under autotrophic conditions; and ●, AV03 under mixotrophic conditions (note that the growth curve of AV03 under mixotrophic conditions overlaps the autotrophic growth curves of AV03 and the WT). (B) IB synthesized in AV03 under autotrophic conditions (only HCO3), heterotrophic conditions (only glucose), and mixotrophic conditions (both HCO3 and glucose) (n = 3; shake flask cultures with closed caps). (C) Percent carbon contribution of glucose for synthesizing amino acids and isobutanol in the WT and the mutant strain (AV03) as measured on day 9 (shake flask cultures with closed caps). Isotopomer analysis (TBDMS-based method) of proteinogenic amino acids confirmed the low [13C]glucose utilization by the mutant. The error bars in panels A and B indicate standard deviations, while those in panel C represent the 2% technical error of the instrument.
The wild-type strain of Synechocystis 6803 grows about 5 times faster under mixotrophic conditions than under autotrophic conditions (Fig. 2A). However, our mutant, AV03, did not exhibit an increased growth rate under mixotrophic conditions. To measure the glucose utilization by the wild type and the mutant AV03, we fed cells with 0.5% fully labeled glucose and nonlabeled bicarbonate. Isotopomer analysis of 13C abundance in cell metabolites (Fig. 2c) showed that the wild type synthesized 70 to 90% of its amino acids using carbon from glucose, whereas the mutant produced biomass using only 5 to 10% carbon from glucose, and 12% of the carbon of IB was labeled (i.e., derived from glucose). These results indicated that the mutant tended to limit glucose metabolism for IB production. The AV03 strain was found to synthesize a maximum of 114 mg/liter of IB mixotrophically after 9 days (Fig. 2b), whereas cells with only glucose (heterotrophic, without bicarbonate or CO2) synthesized a maximum of 27 mg/liter of IB. This result suggests that the Synechocystis 6803 mutant is unable to take significant advantage of its glucose metabolism to have a high rate of IB production.
In situ alcohol-concentrating system using a solvent trap.
IB is toxic to cells, and our study revealed that IB inhibited Synechocystis 6803 growth at external concentrations of only 2 g/liter (Fig. 3). Moreover, our control experiments indicated the loss of IB after 9 days of continuous incubation. IB can be slowly degraded by photochemically produced hydroxyl radicals in aerobic cyanobacterial cultures (32–34). Therefore, a system with continuous removal of the synthesized alcohol products would be beneficial (35, 36). The use of an in situ alcohol removal system utilizing oleyl alcohol (37, 38) as a solvent trap for increasing the IB titer has been demonstrated. In previous studies, gas stripping was one efficient method for IB recovery, but it requires an expensive cooling system due to very low concentrations of IB from photobioreactors. Here, inside each cultivation flask, we placed a small glass vial containing 0.5 or 1 ml oleyl alcohol solvent, so that oleyl alcohol was not mixed with the culture solution (Fig. 4). Volatile IB in the headspace can be trapped in the solvent vial because of the high solubility of IB in oleyl alcohol. This method will effectively trap the IB, while the solvent will not directly interfere with light and cell culture conditions.
Fig 3.
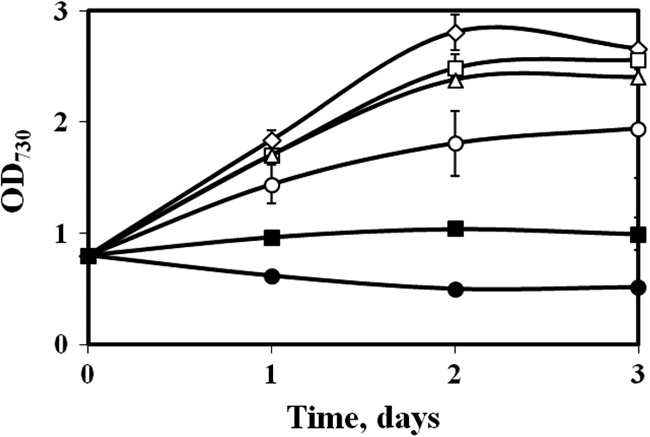
Toxic effects of IB on the growth of Synechocystis 6803. IB was added to final concentrations (g/liter; n = 2) of 0 (♢), 0.2 (□), 0.5 (△), 1 (○), 2 (■), and 5 (●) to a Synechocystis 6803 culture with an initial OD730 of ∼0.8. The error bars indicate standard deviations.
Fig 4.
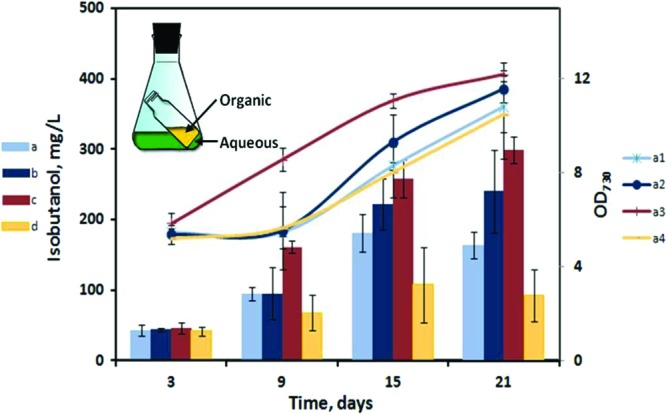
Net concentration of IB synthesized (bars) and biomass growth (curves) by the AV03 culture under different conditions (n = 3). a, IB with 0.5 ml oleyl alcohol (autotrophic); b, IB with 1 ml oleyl alcohol (autotrophic); c, IB with 0.5 ml oleyl alcohol and glucose (mixotrophic); d, IB with no oleyl alcohol (autotrophic, negative control); a1, OD730 with 0.5 ml oleyl alcohol (autotrophic); a2, OD730 with 1 ml oleyl alcohol (autotrophic); a3, OD730 with 0.5 ml oleyl alcohol and glucose (mixotrophic); a4, OD730 with no oleyl alcohol (autotrophic, negative control). (Inset) Schematic representation of the in situ IB removal system used to increase the production of IB. The error bars indicate standard deviations.
To test the effect of oleyl alcohol on IB productivity, we did a 3-week time course study (Fig. 4) by adding 50 mM NaHCO3 intermittently (every 4 days). During the cultivation, the pH of the cultures was also adjusted to between 8 and 9 before the addition of excess bicarbonate. Every 3 days, the oleyl alcohol in the vials was taken out for IB measurement and then replaced with fresh oleyl alcohol. The mixotrophic cultures with an alcohol trap (0.5 ml) reached the highest net IB concentration of 298 mg/liter. The autotrophic cultures with 0.5 ml and 1 ml oleyl alcohol had maximum net IB titers of 180 mg/liter and 240 mg/liter, respectively, whereas the autotrophic cultures without the oleyl alcohol trap were able to achieve a maximum of only 108 mg/liter of IB. IB levels in the organic phase reached concentrations of up to 500 mg/liter with only 3 days of trapping.
DISCUSSION
IB is a promising biofuel for the replacement of gasoline. So far, E. coli has remained the most successful microbial host for IB production. In this study, we focused our efforts on a cyanobacterial species, Synechocystis 6803, which can grow on both CO2 and glucose. The mixotrophic cultivation may offer industrial flexibility and economic benefits because the gas-liquid mass transfer of CO2 is often a rate-limiting step in efficient photobioreactor operations. Attempts to create a stable strain of Synechococcus 7942 that can transport and utilize glucose have been barely successful (39). The glucose-tolerant strain Synechocystis 6803, unlike other cyanobacterial strains, can perform both autotrophic and mixotrophic metabolism. In our work, we found that the wild-type strain Synechocystis 6803 under mixotrophic conditions grew at a rate 5 times higher than under autotrophic conditions. Moreover, the engineered Synechocystis 6803 strain accumulated 90 mg/liter of IB, whereas the Synechococcus 7942 strain expressing the same two enzymes (keto-acid decarboxylase and alcohol dehydrogenase) accumulated a maximum of only 18 mg/liter (13). Switching the conditions from autotrophic to mixotrophic for the mutant AV03 increased the maximum IB titer to 114 mg/liter. Interestingly, the mutant tended to grow autotrophically and had minimal glucose utilization compared to the wild-type strain (Fig. 2C).
IB can be inhibitory to cell physiologies. Moreover, our experiments also observed IB degradation (by hydroxyl radicals) during the incubation process. Therefore, efforts to come up with efficient product recovery are important to improve IB productivity in cyanobacterial culture. This work employed an in situ IB removal system by growing cultures in shake flasks with vials containing oleyl alcohol. Mixotrophic growth of AV03, along with in situ IB removal, synthesized a maximum of 298 mg/liter IB, which is lower than the highest IB titer (450 mg/liter) reported in a Synechococcus 7942 mutant expressing 3 more genes of the keto acid pathway. On the other hand, our strain design has two apparent advantages for industrial applications. First, our strain does not require any antibiotics to maintain its IB production because the two heterologous genes in the mutant show good stability under normal cultivation conditions. Second, the strain does not need any inducer (IPTG) for IB production, which can significantly reduce industrial costs.
Overexpressing the keto acid pathway can increase the IB titer in Synechococcus 7942 (13). Furthermore, optimizing the CO2 and light conditions of the cyanobacterial strain can also increase the final titer and productivity. Liu et al. (15) have reported a doubling time of 7.4 h for Synechocystis 6803 by growing the cells under light at 140 μmol of photons m−2 s−1 and by bubbling 1% CO2-enriched air. Therefore, our strain can serve as a springboard for future development of higher-performance Synechocystis 6803 strains with increased IB titers and productivity.
In summary, IB synthesis under autotrophic conditions in a cyanobacterium, Synechocystis 6803, was demonstrated by the expression of two heterologous genes. It was further demonstrated that mixotrophic cultures of the mutant can significantly increase IB synthesis with minimal glucose consumption. The mechanism behind the reduced glucose-utilizing metabolism of AV03 compared to the wild-type strain remains unclear. A possible explanation is that the cells tend to avoid the intracellular metabolic imbalance or IB intermediate inhibition by downregulating glucose uptake. Using oleyl alcohol as a simple solvent trap, IB production can be improved by 2 to 3 times. Therefore, in situ IB recovery may reduce the product loss and separation cost. We have also demonstrated that a simple expression of the Ehrlich pathway with bioprocess modification can synthesize IB without other major waste products while still achieving levels of IB comparable to those of an extensively genetically modified Synechococcus 7942 strain (Table 1) (13).
ACKNOWLEDGMENTS
We thank Abhay Singh and Anindita Bandyopadhyay for their technical guidance. We also thank Amelia Nguyen and Amelia Chen for their assistance with data collection for a few experiments and Seema Mukhi Dahlheimer from the Engineering Communication Center of Washington University in St. Louis, MO, for her close reading of the manuscript.
This research was funded by an NSF Career Grant to Y.J.T. (MCB0954016); a grant to H.B.P. from the Office of Science (BER), U.S. Department of Energy; and a grant to H.B.P. from the Consortium for Clean Coal Utilization at Washington University.
Y.J.T. initiated this study. H.B.P., Y.J.T., and A.M.V. designed the experiments. A.M.V. and Y.X. performed the experiments. A.M.V. wrote the manuscript. All authors revised and approved the final manuscript.
Footnotes
Published ahead of print 26 November 2012
REFERENCES
- 1. Rittmann BE. 2008. Opportunities for renewable bioenergy using microorganisms. Biotechnol. Bioeng. 100:203–212 [DOI] [PubMed] [Google Scholar]
- 2. van Groenigen KJ, Osenberg CW, Hungate BA. 2011. Increased soil emissions of potent greenhouse gases under increased atmospheric CO2. Nature 475:214–216 [DOI] [PubMed] [Google Scholar]
- 3. Keasling JD, Chou H. 2008. Metabolic engineering delivers next-generation biofuels. Nat. Biotechnol. 26:298–299 [DOI] [PubMed] [Google Scholar]
- 4. Lamsen EN, Atsumi S. 2012. Recent progress in synthetic biology for microbial production of C3–C10 alcohols. Front. Microbiol. 3:196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Atsumi S, Hanai T, Liao JC. 2008. Non-fermentative pathways for synthesis of branched-chain higher alcohols as biofuels. Nature 451:86–89 [DOI] [PubMed] [Google Scholar]
- 6. Colletti P, Goyal Y, Varman A, Feng X, Wu B, Tang Y. 2011. Evaluating factors that influence microbial synthesis yields by linear regression with numerical and ordinal variables. Biotechnol. Bioeng. 108:893–901 [DOI] [PubMed] [Google Scholar]
- 7. Varman A, Xiao Y, Leonard E, Tang Y. 2011. Statistics-based model for prediction of chemical biosynthesis yield from Saccharomyces cerevisiae. Microb. Cell Fact. 10:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Baez A, Cho K-M, Liao JC. 2011. High-flux isobutanol production using engineered Escherichia coli: a bioreactor study with in situ product removal. Appl. Microbiol. Biotechnol. 90:1681–1690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ducat DC, Way JC, Silver PA. 2011. Engineering cyanobacteria to generate high-value products. Trends Biotechnol. 29:95–103 [DOI] [PubMed] [Google Scholar]
- 10. Zhou J, Li Y. 2010. Engineering cyanobacteria for fuels and chemicals production. Protein Cell 1:207–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Deng MD, Coleman JR. 1999. Ethanol synthesis by genetic engineering in cyanobacteria. Appl. Environ. Microbiol. 65:523–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dexter J, Fu P. 2009. Metabolic engineering of cyanobacteria for ethanol production. Energy Environ. Sci. 2:857–864 [Google Scholar]
- 13. Atsumi S, Higashide W, Liao JC. 2009. Direct photosynthetic recycling of carbon dioxide to isobutyraldehyde. Nat. Biotechnol. 27:1177–1180 [DOI] [PubMed] [Google Scholar]
- 14. Tan X, Yao L, Gao Q, Wang W, Qi F, Lu X. 2011. Photosynthesis driven conversion of carbon dioxide to fatty alcohols and hydrocarbons in cyanobacteria. Metab. Eng. 13:169–176 [DOI] [PubMed] [Google Scholar]
- 15. Liu XY, Sheng J, Curtiss R. 2011. Fatty acid production in genetically modified cyanobacteria. Proc. Natl. Acad. Sci. U. S. A. 108:6899–6904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ducat DC, Sachdeva G, Silver PA. 2011. Rewiring hydrogenase-dependent redox circuits in cyanobacteria. Proc. Natl. Acad. Sci. U. S. A. 108:3941–3946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lan EI, Liao JC. 2011. Metabolic engineering of cyanobacteria for 1-butanol production from carbon dioxide. Metab. Eng. 13:353–363 [DOI] [PubMed] [Google Scholar]
- 18. Gao Q, Wang W, Zhao H, Lu X. 2012. Effects of fatty acid activation on photosynthetic production of fatty acid-based biofuels in Synechocystis sp. PCC6803. Biotechnol. Biofuels 5:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lan EI, Liao JC. 2012. ATP drives direct photosynthetic production of 1-butanol in cyanobacteria. Proc. Natl. Acad. Sci. U. S. A. 109:6018–6023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Okamoto S, Ikeuchi M, Ohmori M. 1999. Experimental analysis of recently transposed insertion sequences in the cyanobacterium Synechocystis sp. PCC 6803. DNA Res. 6:265–273 [DOI] [PubMed] [Google Scholar]
- 21. Sentheshanmuganathan S, Elsden SR. 1958. The mechanism of the formation of tyrosol by Saccharomyces cerevisiae. Biochem. J. 69:210–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Barnes WM. 1994. PCR amplification of up to 35 kb DNA with high fidelity and high yield from bacteriophage templates. Proc. Natl. Acad. Sci. U. S. A. 91:2216–2220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mohamed A, Eriksson J, Osiewacz HD, Jansson C. 1993. Differential expression of the psbA genes in the cyanobacterium Synechocystis 6803. Mol. Gen. Genet. 238:161–168 [DOI] [PubMed] [Google Scholar]
- 24. Mohamed A, Jansson C. 1989. Influence of light on accumulation of photosynthesis-specific transcripts in the cyanobacterium Synechocystis 6803. Plant Mol. Biol. 13:693–700 [DOI] [PubMed] [Google Scholar]
- 25. Zang XN, Liu B, Liu SM, Arunakumara K, Zhang XC. 2007. Optimum conditions for transformation of Synechocystis sp. PCC 6803. J. Microbiol. 45:241–245 [PubMed] [Google Scholar]
- 26. Vermaas W. 1996. Molecular genetics of the cyanobacterium Synechocystis sp. PCC 6803: principles and possible biotechnology applications. J. Appl. Phycol. 8:263–273 [Google Scholar]
- 27. Bond-Watts BB, Bellerose RJ, Chang MC. 2011. Enzyme mechanism as a kinetic control element for designing synthetic biofuel pathways. Nat. Chem. Biol. 7:222–227 [DOI] [PubMed] [Google Scholar]
- 28. Feng X, Bandyopadhyay A, Berla B, Page L, Wu B, Pakrasi HB, Tang YJ. 2010. Mixotrophic and photoheterotrophic metabolism in Cyanothece sp. ATCC 51142 under continuous light. Microbiology 156:2566–2574 [DOI] [PubMed] [Google Scholar]
- 29. You L, Page L, Feng X, Berla B, Pakrasi HB, Tang YJ. 2012. Metabolic pathway confirmation and discovery through 13C-labeling of proteinogenic amino acids. J. Vis. Exp. 59:e3583 doi:10.3791/3583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wahl SA, Dauner M, Wiechert W. 2004. New tools for mass isotopomer data evaluation in 13C flux analysis: mass isotope correction, data consistency checking, and precursor relationships. Biotechnol. Bioeng. 85:259–268 [DOI] [PubMed] [Google Scholar]
- 31. Huang H-H, Camsund D, Lindblad P, Heidorn T. 2010. Design and characterization of molecular tools for a synthetic biology approach towards developing cyanobacterial biotechnology. Nucleic Acids Res. 38:2577–2593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cameron JC, Pakrasi HB. 2011. Glutathione facilitates antibiotic resistance and photosystem I stability during exposure to gentamicin in cyanobacteria. Appl. Environ. Microbiol. 77:3547–3550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Grosjean D. 1997. Atmospheric chemistry of alcohols. J. Braz. Chem. Soc. 8:433–442 [Google Scholar]
- 34. Tichy M, Vermaas W. 1999. In vivo role of catalase-peroxidase in Synechocystis sp. strain PCC 6803. J. Bacteriol. 181:1875–1882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kraemer K, Harwardt A, Bronneberg R, Marquardt W. 2010. Separation of butanol from acetone-butanol-ethanol fermentation by a hybrid extraction-distillation process. Computers Chem. Eng. 35:949–963 [Google Scholar]
- 36. Shi ZP, Zhang CY, Chen JX, Mao ZG. 2005. Performance evaluation of acetone-butanol continuous flash extractive fermentation process. Bioprocess Biosyst. Eng. 27:175–183 [DOI] [PubMed] [Google Scholar]
- 37. Ezeji T, Milne C, Price ND, Blaschek HP. 2010. Achievements and perspectives to overcome the poor solvent resistance in acetone- and butanol-producing microorganisms. Appl. Microbiol. Biotechnol. 85:1697–1712 [DOI] [PubMed] [Google Scholar]
- 38. Green EM. 2011. Fermentative production of butanol: the industrial perspective. Curr. Opin. Biotechnol. 22:337–343 [DOI] [PubMed] [Google Scholar]
- 39. Zhang CC, Jeanjean R, Joset F. 1998. Obligate phototrophy in cyanobacteria: more than a lack of sugar transport. FEMS Microbiol. Lett. 161:285–292 [DOI] [PubMed] [Google Scholar]
- 40. Xiao Y, Feng X, Varman AM, He L, Yu H, Tang YJ. 2012. Kinetic modeling and isotopic investigation of isobutanol fermentation by two engineered Escherichia coli strains. Ind. Eng. Chem. Res. 51:15855–15863 [Google Scholar]