Abstract
Staphylococcus aureus is a major pathogen that is responsible for mastitis in dairy herds. S. aureus mastitis is difficult to treat and prone to recurrence despite antibiotic treatment. The ability of S. aureus to invade bovine mammary epithelial cells (bMEC) is evoked to explain this chronicity. One sustainable alternative to treat or prevent mastitis is the use of lactic acid bacteria (LAB) as mammary probiotics. In this study, we tested the ability of Lactobacillus casei strains to prevent invasion of bMEC by two S. aureus bovine strains, RF122 and Newbould305, which reproducibly induce acute and moderate mastitis, respectively. L. casei strains affected adhesion and/or internalization of S. aureus in a strain-dependent manner. Interestingly, L. casei CIRM-BIA 667 reduced S. aureus Newbould305 and RF122 internalization by 60 to 80%, and this inhibition was confirmed for two other L. casei strains, including one isolated from bovine teat canal. The protective effect occurred without affecting bMEC morphology and viability. Once internalized, the fate of S. aureus was not affected by L. casei. It should be noted that L. casei was internalized at a low rate but survived in bMEC cells with a better efficiency than that of S. aureus RF122. Inhibition of S. aureus adhesion was maintained with heat-killed L. casei, whereas contact between live L. casei and S. aureus or bMEC was required to prevent S. aureus internalization. This first study of the antagonism of LAB toward S. aureus in a mammary context opens avenues for the development of novel control strategies against this major pathogen.
INTRODUCTION
Staphylococcus aureus is an opportunistic pathogen with a broad host range, and it is a leading cause of chronic and acute infections in humans and domesticated animals worldwide (1–4). Among these infections mastitis is a major disease, affecting dairy herds and resulting in huge economic losses all along the milk production chain (5–7). In milk production, Staphylococcus species are the main contagious pathogens responsible for clinical and subclinical mastitis in lactating cows (5). S. aureus generally causes more acute infections than other staphylococcal species, which can be linked to its ability to colonize the host tissue and thus cause persisting and relapsing infections (8).
To date, intramammary administration of antibiotics is the most common method to treat bovine mastitis (9, 10). However, antibiotic treatments have a low cure rate during lactation for many mastitis pathogens and especially for S. aureus, frequently resulting in chronic and recurrent infections. The mechanism of persistence of S. aureus in its host is still not fully understood. One confirmed mechanism used to evade host defenses is internalization into host cells. It is now well established that S. aureus can adhere to and internalize into mammary gland epithelial cells (8). A variety of surface-exposed (protein A and fibrinogen- and fibronectin-binding proteins) and secreted (enterotoxins, hemolysins, and coagulase) virulence factors allow it to colonize, invade, and multiply in host tissues (8, 11–14).
Many strategies have been proposed to counteract the infectious cycle of S. aureus within the mammary gland. Critical steps, like adhesion and invasion of the host cells, can be targeted by innovative strategies that take account of the increasing social demand for a sustainable agriculture with reduced inputs such as antibiotics. In recent years, the concept of biological control has emerged as one interesting sustainable alternative to fight against pathogens. The range of applications of probiotic bacteria thus has broadened, and they are now considered a possibility for alternative treatments against mastitis (15, 16). The inhibitory activities of lactic acid bacteria (LAB) with a GRAS (generally recognized as safe) status against pathogens have been under scrutiny to address the problem of pathogen colonization in different ecosystems. Lactobacilli are known to have a protective effect against some infections. This ability has been related to the adhesion properties of epithelial cells, which inhibit pathogen adhesion by specific competition or by steric hindrance, as well as to growth inhibition of pathogens by the secretion of bacteriocins, H2O2, or other antimicrobial compounds, and to competition for nutrients and modulation of the host immune response (17). Such properties are harnessed in the development of vaginal probiotics used to prevent urogenital infections (18). Recently, the use of a bacteriocin-producing Lactococcus lactis strain was reported to be as efficient as a conventional antibiotic therapy to treat staphylococcal mastitis (19, 20). Encouraging results were also obtained with a Lactobacillus perolens strain which was able to inhibit several mastitis-causing pathogens in vitro, to coaggregate with all of them, and to adhere to bovine teat canal epithelial cells without affecting udder aspect or the appearance of milk (21). These alternative insights into intramammary infections provide new leads in the fight against mastitis.
In this work, we evaluated the ability of Lactobacillus casei to counteract S. aureus adhesion to and internalization into bovine mammary epithelial cells (bMEC). L. casei CIRM-BIA 667 was selected for this study on the basis of (i) its probiotic effects in the intestinal ecosystem (22, 23) and (ii) its inhibitory activity against staphylococcal biofilm formation (personal observation). The main results were further confirmed with two additional strains, BL23 and CIRM-BIA 1542, a strain isolated from bovine teat canal.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
Two bovine strains of Staphylococcus aureus were used in this study: S. aureus RF122 (renamed ET-3 in Herron-Olson [24]) and S. aureus Newbould 305 (here referred to as NB305). These strains are well characterized and reproducibly induce severe or mild mastitis in experimental infections (24, 25). The Lactobacillus casei strain, CIRM-BIA 667 (here referred to as 667 and also known as CNRZ 313 and ATCC 393), was used to assess inhibitory capabilities against staphylococcal infection in vitro. It is the type strain for L. casei. Two additional L. casei strains were included: L. casei BL23, known for its probiotic properties (26, 27), and L. casei CIRM-BIA 1542 (here referred to as 1542), isolated from bovine teat canal (this study).
S. aureus strain RF122 carrying the plasmid pCtuf-gfp (28) was constructed in this study to allow constitutive expression of green fluorescent protein (GFP) in this strain and direct visualization of S. aureus adhered to or internalized into MAC-T cells by confocal microscopy (see below). Subcultures prior to invasion assays were performed overnight as follows. For S. aureus strains, culture was carried out in brain heart infusion medium (BHI; pH 7.4; BD, Le Pont de Claix, France) at 37°C under agitation (180 rpm), and L. casei was cultured in Man Rogosa Sharpe medium (MRS; pH 6.8; BD, Le Pont de Claix, France) at 30°C without shaking. Subcultures were washed once with phosphate-buffered saline (PBS) and suspended at different concentrations in Dulbecco's modified Eagle's medium (DMEM; pH 7.4; D. Dutscher, Brumath, France).
Bacterial concentrations in subcultures were estimated by spectrophotometric measurements at 600 nm with a VWR V-1200 spectrophotometer. They were further confirmed by determination of the bacterial population using a micromethod as previously described (29). The S. aureus population (in CFU/ml) was determined on mannitol salt agar (MSA; D. Dutscher, Brumath, France) after 24 h of incubation at 37°C. The L. casei population was determined on MRS (pH 5.4) and incubated anaerobically for 48 h at 37°C in an anaerobic jar.
Mammary epithelial cells and culture conditions.
The established bovine mammary epithelial cell (MAC-T) line (30) (Nexia Biotechnologies, Quebec, Canada) has been widely used for invasion assays (8) and thus was retained for this study. MAC-T cells were cultured in T75 cell culture flasks using MAC-T medium: DMEM containing 10% heat-inactivated fetal calf serum (FCS), 100 U/ml penicillin, 10 mg/ml streptomycin, and 5 μg/ml insulin (D. Dutscher). Cells were incubated at 37°C in a humidified incubator with 5% CO2. They were cultured to a confluent monolayer, treated with 0.05% trypsin (Gibco-BRL, Grand Island, NY), and suspended in fresh MAC-T medium at a concentration of 2 × 105 cells/ml. For adhesion and internalization assays, cells were then seeded in 12-well plates (2 × 105 cells/well) and incubated overnight at 37°C in 5% CO2 to obtain a confluent monolayer.
Adhesion assays.
Adhesion assays were adapted from Almeida et al. (8) and modified as follows. Confluent monolayers of MAC-T cells (2.5 × 105 cells/well) were washed twice with PBS and incubated at 37°C in 5% CO2 with 1 ml of S. aureus suspension in DMEM at 2.5 × 106, 1 × 107, or 2.5 × 107 CFU/ml to achieve a multiplicity of infection (MOI; ratio of S. aureus organisms to cells) of 10:1, 40:1, or 100:1, respectively. Adhesion assays with L. casei were performed by adding 1 ml of L. casei at 1 × 108 CFU/ml or 5 × 108 CFU/ml to achieve a ratio of interaction (ROI; ratio of L. casei organisms to cells) of 400:1 or 2,000:1. S. aureus and L. casei adhesion was measured 1 h postinfection.
For adhesion inhibition assays, cells were primarily incubated with L. casei at an ROI of 200:1, 400:1, or 2,000:1 for 2 h at 37°C with 5% CO2 and washed twice with PBS prior to infection with S. aureus for 1 h. When specified, the L. casei suspension was separated from the cell monolayer using a 0.4-μm cell culture insert filter (Millicell; Millipore Corporation, Switzerland). After incubation steps, MAC-T monolayers were washed four times with PBS and treated with 0.05% trypsin for 10 min at 37°C. Cells were centrifuged for 5 min at 800 × g and lysed using 100 μl of 0.01% Triton in sterile water. The population of S. aureus that adhered (CFU/ml) was determined using a micromethod as described above.
The adhesion assay of S. aureus alone was used as a reference. Adhesion rates were then defined as the adhered S. aureus population in the presence of L. casei relative to the adhered S. aureus population in the reference experiment.
For some experiments, heat-killed L. casei 667 cells were prepared by incubating the L. casei suspension in DMEM at 95°C for 15 min prior to addition to bMEC. Supernatant samples were prepared from a 2-h culture on DMEM of L. casei 667 inoculated at 5 × 108 CFU/ml, and the pH was adjusted to 7.4.
Internalization assays.
Internalization assays were adapted from Almeida et al. (8) and modified as follows. Confluent monolayers of MAC-T cells (2.5 × 105 cells/well) were washed twice with PBS and incubated at 37°C in 5% CO2 with 1 ml of S. aureus and/or L. casei suspension in DMEM at an MOI of 10:1, 40:1, or 100:1 for S. aureus and an ROI of 200:1, 400:1, or 2,000:1 for L. casei. S. aureus and L. casei internalizations were measured 2 h postinfection. For internalization inhibition assays, L. casei and S. aureus were simultaneously added to the cells for 2 h. When specified, L. casei was separated from the cell monolayer and S. aureus using a 0.4-μm cell culture insert filter (Millicell; Millipore Corporation, Switzerland). S. aureus internalization was measured 2 h postinfection following an additional 2-h incubation step with DMEM supplemented with gentamicin (100 μg/ml). This step resulted in the killing of extracellular bacteria and allowed the numeration of the internalized bacterial population only. Subsequently, MAC-T monolayers were washed four times with PBS, treated with trypsin, centrifuged for 5 min at 800 × g, and lysed in 0.01% Triton. S. aureus and L. casei populations were determined as described above.
The internalization assay of S. aureus alone was used as a reference. Internalization rates were then defined as the internalized S. aureus population in the presence of L. casei relative to the internalized S. aureus population in the reference experiment. Heat-killed L. casei 667 and supernatant of L. casei 667 cells were prepared as described above.
Intracellular survival assays.
Internalization assays were performed as described above with S. aureus RF122 at an MOI of 10:1, 40:1, or 100:1 and in the absence or presence of L. casei 667 (ROI of 2,000:1). The internalized S. aureus population measured after these 2 h of infection was used as the starting point for intracellular survival assay. Cells were further incubated in DMEM containing gentamicin (25 μg/ml) at 37°C in 5% CO2, and the remaining internalized S. aureus population was measured 24, 48, and 72 h postinfection. DMEM-gentamicin medium was changed every 24 h.
Cell counting and cell viability assays.
Cell density and viability were determined using a hemocytometer by the trypan blue exclusion method 2, 24, 48, and 72 h postinfection.
MTT cell viability assays.
Cell viability was measured during the intracellular survival assay (see above) at 2, 24, 48, and 72 h postinfection using methylthiazolyldiphenyltetrazolium bromide (MTT) as previously described (31). Briefly, following incubation with DMEM containing 25 μg/ml gentamicin, cells were washed four times and incubated in 0.5 mg/ml MTT in PBS for 4 h at 37°C in 5% CO2. The medium was removed, and isopropanol was added for 30 min with shaking at 350 rpm. Absorbance was read at 570 nm with a background at 690 nm. Uninfected cells were used as a negative control (100% viability), and cells treated with 0.01% Triton served as a positive control of mortality (0% viability). Relative viability was expressed with regard to uninfected cells.
Analysis of cellular morphology during internalization assay by confocal microscopy.
MAC-T cells were cultured in 8-well Labtek chamber slides (NalgeNunc International, Naperville, IL). A total of 5 × 104 cells were seeded in each well and incubated overnight at 37°C with 5% CO2. Internalization assays were performed as described above, including a 2-h step with gentamicin (100 μg/ml) to kill extracellular bacteria, with an MOI of 100:1 for S. aureus RF122 and an ROI of 2,000:1 for L. casei 667. Following the internalization assay, cells were washed four times with PBS, fixed for 30 min in PBS containing 4% paraformaldehyde, and permeabilized with PBS containing 0.1% saponin for 10 min. Staining was performed in darkness at room temperature. Cells were stained using the fluorescent nucleic acid stain SYTO 9 from the LIVE/DEAD BacLight stain kit (Molecular Probes Inc., Leiden, The Netherlands) for 30 min, which allowed staining of both bacteria and MAC-T cells. Alternatively, the S. aureus strain RF122 carrying the plasmid pCtuf-gfp was used and combined with the staining of cell actin cytoskeleton with phalloidin (Interchim, Montluçon, France) at 1 U/ml in PBS containing 1% bovine serum albumin for 30 min. This allowed visualization of S. aureus (green) and cytoskeleton (red). Images were acquired using the confocal Nikon C1Si microscope (Nikon, Tokyo, Japan) with an excitation wavelength of 488 (for SYTO 9 and GFP) and of 543 nm (for phalloidin) and using a lens with ×100 magnification. Emission of fluorescence was monitored at 515 nm (± 15 nm) for SYTO 9 and GFP and 590 nm for phalloidin. Image analysis was performed with Image J (National Institutes of Health, Bethesda, MD; http://imagej.nih.gov/ij/).
Statistical analysis.
Each experiment was done in triplicate (biological repeats). Statistical analysis was performed with R software (R Development Core Team, 2007). The differences among the groups were assessed using Student's t test with Bonferroni's correction considering a P value lower than 0.05.
RESULTS
Adhesion and internalization capacities of S. aureus and L. casei.
We tested in vitro the adhesion and internalization abilities of two S. aureus bovine strains, namely, RF122 and NB305, which induce severe and mild bovine mastitis, respectively. Interestingly, we found that the adhesion and internalization capacities of NB305 were higher than those of RF122. Adhered and internalized S. aureus populations were 5- and 40-fold larger, respectively, for strain NB305 than for RF122 at an MOI of 100:1 (Fig. 1). As a comparison, adhesion and internalization capacities of L. casei were also assessed. The three L. casei strains used exhibited poor adhesion capacities compared to S. aureus, as illustrated in Fig. 1. Indeed, despite a higher bacterium/cell ratio (2,000:1 and 100:1 for L. casei and S. aureus, respectively), the adhered and internalized populations were smaller for L. casei than for S. aureus.
Fig 1.
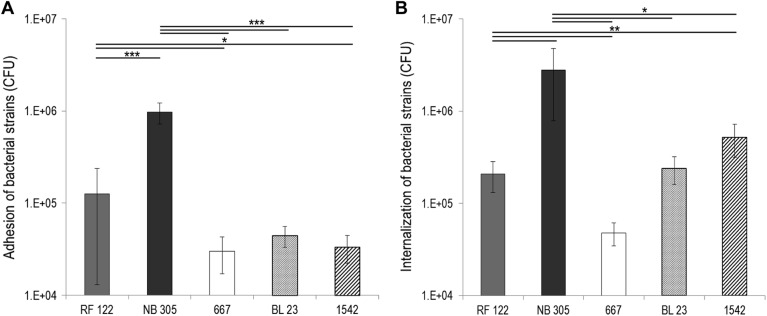
Adhesion to and internalization into bMEC of S. aureus strains RF122 and NB305 and L. casei strains CIRM-BIA 667, BL23, and CIRM-BIA 1542. S. aureus (MOI of 100:1) and L. casei (ROI of 2,000:1) populations adhered to (A) and internalized into (B) bMEC were determined after 1 and 2 h of interaction, respectively. Data are presented as mean populations per well (i.e., corresponding to 2.5 × 105 bMEC) ± standard deviations. Each experiment was done in triplicate, and differences between groups were compared using Student's t test. *, P < 0.05; ***, P < 0.0005.
L. casei 667 reduced the adhesion of S. aureus RF122.
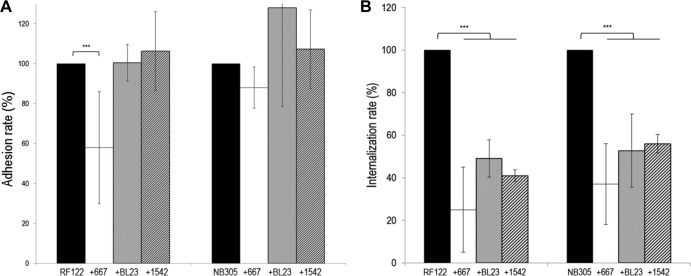
Several conditions of adhesion were tested to evaluate the capacities of L. casei to prevent adhesion of S. aureus RF122 and NB305 to bMEC: preincubation of bMEC with L. casei prior to infection by S. aureus or coinfection of both species. Three MOIs were tested for S. aureus in combination with three ROIs for L. casei 667. Conditions leading to L. casei-mediated inhibition were preincubation of MAC-T cells for 2 h with L. casei at an ROI of 200:1, 400:1, or 2,000:1, followed by the addition of S. aureus at an MOI of 100:1. Under these conditions, a significant reduction of the adhesion rate was observed for S. aureus RF122, down to ∼60% of the adhesion observed with S. aureus RF122 alone (Fig. 2). It should be noted that during this experiment and subsequent assays of adhesion and internalization, the density of the MAC-T cell monolayer was conserved, as confirmed by direct microscopic observation and cell counting. Thus, the lower adhered population of S. aureus RF122 to MAC-T cells did not result from a smaller amount of attached MAC-T cells in wells when incubated with L. casei. Under the same experimental conditions, L. casei 667 did not significantly affect the adhesion rate of S. aureus NB305 (Fig. 2). Similarly, no significant inhibition of S. aureus RF122 or NB305 was observed with the two additional L. casei strains tested, BL23 and 1542 (see Fig. 4A). This result indicates that the inhibitory efficacy of L. casei depends on both the S. aureus and L. casei strains used.
Fig 2.
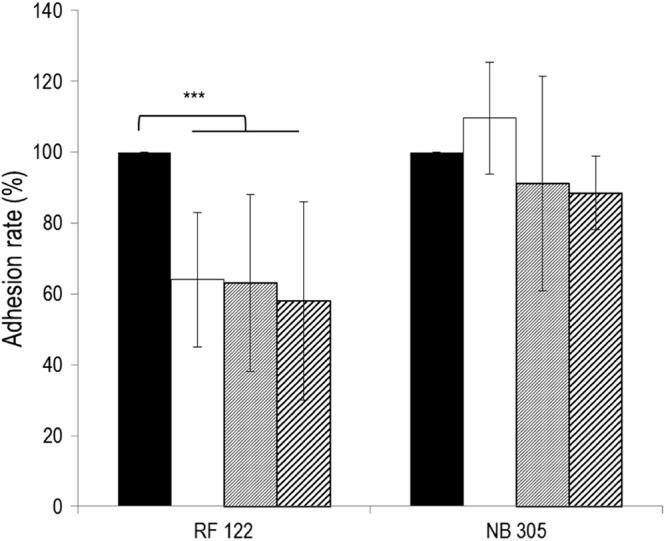
Inhibition of S. aureus RF122 and NB305 adhesion to bMEC by L. casei CIRM-BIA 667. Shown are adhesion rates of S. aureus strains after 1 h of interaction with bMEC and following 2 h of preincubation of cells with L. casei at an ROI of 200:1 (white bars), 400:1 (gray bars), and 2,000:1 (hatched bars). S. aureus was used at an MOI of 100:1. An adhesion assay of S. aureus alone was used as a reference (black bars). Adhesion rates were then defined as the adhered S. aureus population in the presence of L. casei relative to the adhered S. aureus population in the reference experiment. Data are presented as means ± standard deviations. Each experiment was done in triplicate, and differences between groups were compared using Student's t test with Bonferroni's correction. ***, P < 0.0005.
Fig 4.
Inhibition of adhesion and internalization of S. aureus RF122 and NB305 by L. casei strains. (A) Rates of adhesion of S. aureus RF122 and NB305 strains to bMEC following preincubation of cells with L. casei CIRM-BIA 667 (white bars), BL23 (gray bars), and CIRM-BIA 1542 (hatched bars) at an ROI of 2,000:1. (B) Rates of internalization of S. aureus RF122 and NB305 into bMEC in the presence of L. casei CIRM-BIA 667 (white bars) and BL23 (gray bars) at an ROI of 2,000:1 and CIRM-BIA 1542 (hatched bars) at an ROI of 400:1. Adhesion and internalization assays were performed with an S. aureus MOI of 100:1. Adhesion/internalization assays of S. aureus alone were used as a reference (black bars). Adhesion/internalization rates were then defined as the adhered/internalized S. aureus population in the presence of L. casei relative to the adhered/internalized S. aureus population in the reference experiment. Data are presented as means ± standard deviations. Each experiment was done in triplicate, and differences between groups were compared using Student's t test with Bonferroni's correction. ***, P < 0.0005.
L. casei reduced internalization of S. aureus RF122 and NB305.
Beyond L. casei's ability to impair S. aureus adhesion, we tested its inhibitory potential against S. aureus RF122 and NB305 internalization into MAC-T cells. As mentioned for adhesion assays, several conditions were tested. Conditions resulting in L. casei-mediated inhibition were the coincubation of L. casei at an ROI of 2,000:1 with S. aureus at an MOI of 10:1, 40:1, or 100:1. Under these conditions, coinfection of S. aureus RF122 or NB305 with L. casei led to a significant decrease of their internalization rates by 61 to 75% (Fig. 3). This result was further confirmed with the two additional L. casei strains (Fig. 4B). Of note, due to the strong acidification of the medium by mixed culture of L. casei 1542 and S. aureus, the ROI was only 400:1 in mixed cultures for this L. casei strain, compared to an ROI of 2,000:1 for strains 667 and BL23. Despite this lower ROI, L. casei 1542 efficiently inhibited S. aureus internalization. It should be observed here that the total number of viable staphylococci was unaffected by the presence of L. casei in adhesion or internalization assays. S. aureus was indeed able to grow in DMEM during incubation with bMEC, and the population reached after 1 or 2 h of infection was similar with or without preincubation or coinfection with L. casei (see Fig. S1 in the supplemental material). Interestingly, inhibition of internalization was reciprocal. Hence, the rate of L. casei 667 internalization was reduced by 58 and 50% in coinfection experiments with S. aureus RF122 and NB305, respectively.
Fig 3.
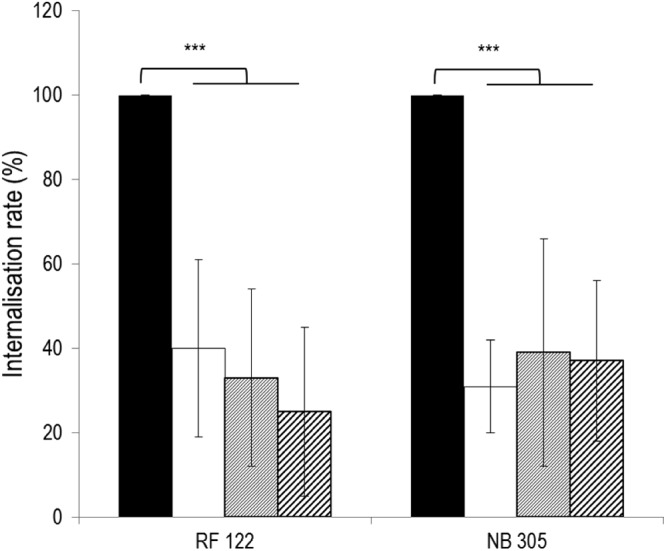
Inhibition of S. aureus RF122 and NB305 internalization into bMEC by L. casei CIRM-BIA 667. Shown are internalization rates of S. aureus strains after 2 h of interaction with bMEC with coincubation with L. casei at an ROI of 2,000:1. S. aureus strains were used at an MOI of 10:1 (white bars), 40:1 (gray bars), or 100:1 (hatched bars). The internalization assay of S. aureus alone was used as a reference (black bars). Internalization rates were then defined as the internalized S. aureus population in the presence of L. casei relative to the internalized S. aureus population in the reference experiment. Data are presented as means ± standard deviations. Each experiment was done in triplicate, and differences between groups were compared using Student's t test with Bonferroni's correction. ***, P < 0.0005.
L. casei 667 did not alter intracellular survival of S. aureus RF122.
To investigate the fate of internalized bacteria, intracellular survival of S. aureus RF122 was monitored 24, 48, and 72 h postinfection. A rapid decrease of the S. aureus internalized population was observed with only 6 and 0.26% of the initial internalized S. aureus population after 24 and 48 h of infection (Fig. 5). The S. aureus internalized population was lower in the presence of L. casei but a similar decrease of the S. aureus internalized population was observed, as illustrated by half-lives of internalized S. aureus into MAC-T of 6.1 and 5.1 h with and without L. casei, respectively (P = 0.12). This result was confirmed with S. aureus MOIs of 40:1 and 10:1 (data not shown). Intracellular survival of L. casei was greater than that of S. aureus RF122, as illustrated by a lower rate of decrease of the L. casei population (P = 0.002) (Fig. 5).
Fig 5.
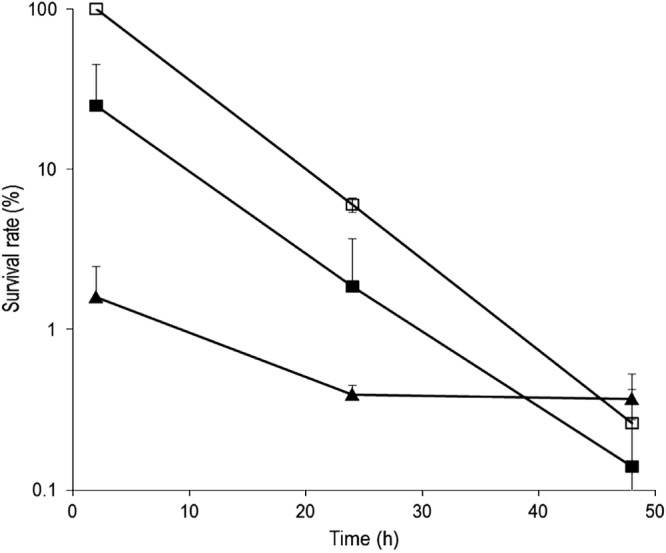
Survival rate of S. aureus RF122 and L. casei CIRM-BIA 667 within bMEC. bMEC were incubated for 2 h with S. aureus RF122 (MOI, 100:1) with or without L. casei CIRM-BIA 667 (ROI, 2,000:1) in DMEM. Following a 2-h incubation step with gentamicin (100 μg/ml) to kill extracellular bacteria, cells were further incubated with gentamicin (25 μg/ml) for 24 and 48 h. The initial internalized S. aureus population measured after the 2 h of infection by S. aureus alone was used as the reference. The remaining internalized population of S. aureus alone (□) or in coinfection with L. casei (■) and L. casei (▲) then were measured and expressed relative to an S. aureus reference population. Data are presented as the mean survival rate ± standard deviations. Each experiment was done in triplicate, and differences between half-lives were compared using Student's t test.
L. casei 667 treatment did not affect MAC-T cell viability.
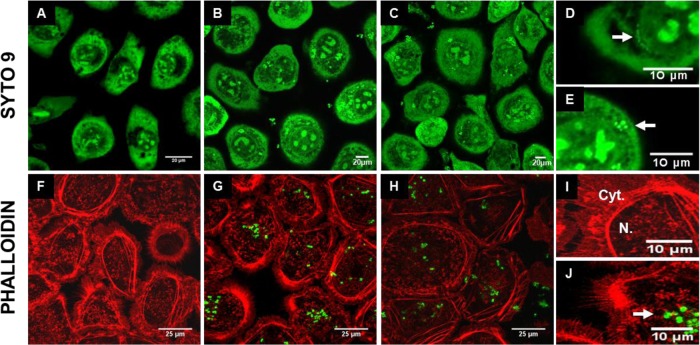
We showed that L. casei was able to inhibit S. aureus invasion into MAC-T cells without affecting the cell monolayer density. Additional studies were done to investigate the effect of L. casei 667 on cell viability and morphology. Cell viability was estimated by trypan blue exclusion and indicated that viability was above 99% for all of the bacterial concentrations tested (data not shown). In agreement, cell viability assessed by MTT assays revealed the same decrease of viability for infected and uninfected cells, i.e., a drop in cell viability of 20 to 25% from 48 h onwards (see Fig. S2 in the supplemental material). Finally, direct observation of bMEC by confocal microscopy during internalization assays confirmed that the general cell architecture was similar in untreated control cells and cells treated with L. casei or S. aureus RF122 either alone or in combination (Fig. 6).
Fig 6.
Fluorescent confocal microscopy of mammary epithelial cells during bacterial infections. SYTO 9 (A to E) and phalloidin (F to J) stainings were used to observe bMEC structure following internalization assays with S. aureus RF122 (carrying plasmid pCtuf-gfp in the case of phalloidin staining) at an MOI of 100:1. MAC-T cells were either untreated (control; A, F, and I) or treated with S. aureus alone (B, E, G, and J), L. casei CIRM-BIA 667 alone at an ROI of 2,000:1 (D), or S. aureus and L. casei in cocultures (C and H). A lens with a ×100 magnification was used, and panels D to E and I to J are electronically zoomed. Arrows indicate internalized L. casei (D) or internalized S. aureus (E and J). Cyt., cytoplasm; N., nucleus.
Inhibition of internalization required live L. casei, whereas adhesion did not.
To further characterize the inhibition observed with L. casei, additional adhesion and internalization experiments were carried out by replacing L. casei with either (i) DMEM artificially acidified to pH 6.8 with lactic acid (corresponding to the pH reached after 2 h of incubation of MAC-T cells with L. casei at an ROI of 2,000:1), (ii) supernatant of L. casei grown in DMEM, or (iii) heat-killed L. casei. Neither DMEM containing lactic acid nor L. casei supernatant affected S. aureus RF122 adhesion or internalization rates (Fig. 7). Heat-killed L. casei was still able to inhibit S. aureus RF122 adhesion, whereas the inhibitory effect of L. casei on S. aureus internalization occurred only in the presence of live L. casei (Fig. 7). Surprisingly, heat-killed L. casei even seemed to favor S. aureus internalization, although the difference was not statistically significant (P = 0.13).
Fig 7.
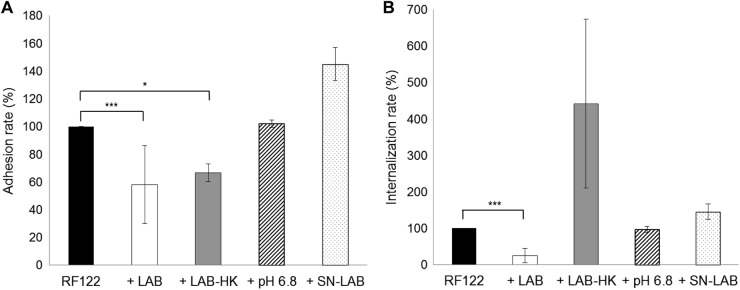
Adhesion and internalization rates of S. aureus RF122 with different treatments. (A) Adhesion rates of S. aureus RF122 to bMEC at an MOI of 100:1, either alone or with preincubation of cells with L. casei CIRM-BIA 667 at an ROI of 2,000:1 (+LAB), heat-killed L. casei CIRM-BIA 667 at an ROI of 2,000:1 (+LAB-HK), DMEM acidified to pH 6.8 with lactic acid (+pH 6.8), or L. casei CIRM-BIA 667 supernatant (+SN-LAB). (B) Internalization rates of S. aureus RF122 into bMEC at an MOI of 100:1, either alone or with coincubation with L. casei CIRM-BIA 667 at an ROI of 2,000:1 (+LAB), heat-killed L. casei CIRM-BIA 667 at an ROI of 2,000:1 (+LAB-HK), DMEM acidified to pH 6.8 with lactic acid (+pH 6.8), or L. casei CIRM-BIA 667 supernatant (+SN-LAB). Adhesion/internalization assays of S. aureus alone were used as references. Adhesion/internalization rates were then defined as the adhered/internalized S. aureus population in the presence of L. casei, lactic acid, or supernatant relative to the adhered/internalized S. aureus population in the reference experiment. Data are presented as means ± standard deviations. Each experiment was done in triplicate, and differences between groups were compared using Student's t test with Bonferroni's correction. *, P < 0.05; ***, P < 0.0005.
Inhibition of adhesion and internalization required contact with L. casei.
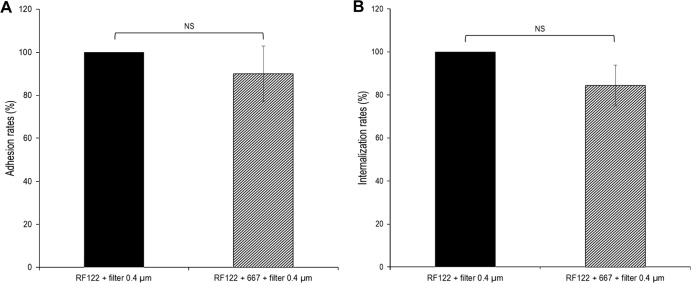
To further characterize the mechanism of inhibition, additional adhesion and internalization experiments were carried out using cell culture insert filters in order to separate L. casei from bMEC and S. aureus. L. casei-mediated inhibition of adhesion was released when L. casei was separated from bMEC during the preincubation step (Fig. 8A). Likewise, L. casei-mediated inhibition of internalization was not retained using cell culture insert filters, indicating that the contact of live L. casei with bMEC and/or S. aureus was required (Fig. 8B).
Fig 8.
L. casei inhibition of S. aureus adhesion and internalization requires contact with bMEC and/or S. aureus. Adhesion and internalization assays were performed, as previously described (see the legends to Fig. 2 and 3), using S. aureus RF122 at an MOI of 100:1 and L. casei CIRM-BIA 667 at an ROI of 2,000:1, except that L. casei was separated from S. aureus and bMEC using a cell culture insert. Adhesion/internalization assays of S. aureus alone with the cell culture insert were used as references. Adhesion/internalization rates were then defined as the adhered/internalized S. aureus population in the presence of L. casei relative to the adhered/internalized S. aureus population in the reference experiment. Data are presented as means ± standard deviations. Each experiment was done in triplicate, and differences between groups were compared using Student's t test. NS, not significant.
DISCUSSION
In this work, we established the ability of L. casei to reduce adhesion to and/or internalization into MAC-T cells of two bovine S. aureus strains. The ability of L. casei to affect adhesion was strain dependent. L. casei 667 was the only L. casei strain able to inhibit adhesion of S. aureus RF122 by 40%. This was not confirmed on the highly adherent S. aureus strain NB305, indicating that L. casei 667 inhibition of S. aureus adhesion to bMEC was restricted to the S. aureus strain with low adhesion capacity (i.e., RF122). The poor capacity of L. casei to inhibit S. aureus adhesion is probably related to its low adhesion capacity compared to that of S. aureus. Rates of adhesion to MAC-T cells were 4- to 30-fold lower for L. casei than for S. aureus, although the bacterium/bMEC ratio was 20-fold higher. Such low-adherence properties of L. casei 667 to epithelial cells had been previously reported (22). More striking in this work was the ability of L. casei to impair S. aureus internalization. As of this time, very few studies have investigated the ability of probiotic lactic acid bacteria to modulate internalization of pathogens within host cells (32). One interesting outcome was the inhibition of internalization of the two S. aureus strains tested by the three L. casei strains tested, whereas inhibition of S. aureus adhesion to bMEC was limited to one S. aureus/L. casei couple. Following internalization, the S. aureus bacterial population decreased. This was in agreement with a study by Martinez-Pulgarin et al., who reported that after a short period of intracellular replication (2 h), internalized S. aureus concentration in MAC-T cells gradually decreased over time (33). In fact, the fate of internalized S. aureus was similar with or without L. casei, suggesting that L. casei did not affect S. aureus physiology once internalized.
During these internalization assays, we also established the capacity of L. casei to internalize, and this was strongly strain dependent. Interestingly, L. casei 1542, isolated from the teat canal, internalized more efficiently into bMEC cells. This might reflect an adaptation of some L. casei strains to the bovine host, as previously shown for S. aureus ruminant isolates (34–36). The internalization capacities of the three L. casei strains were lower than those of S. aureus NB305 and, to a lesser extent, S. aureus RF122. Contrary to the adhesion capacity of LAB, which is well documented, only a few studies report the internalization of lactic acid bacteria (37). A fibronectin-binding protein has been identified in the genomes of Lactobacillus species, suggesting a capacity to adhere and to be internalized (38–41). Interestingly, the survival rate of L. casei in MAC-T cells was better than that of S. aureus. This improved survival may be due to a better resistance to acid and oxidative stresses (42).
L. casei inhibition of S. aureus adhesion and internalization required specific conditions. Two features were common to adhesion and internalization inhibition: (i) in all cases, posttreatment of S. aureus-adhered or -internalized bMEC with L. casei did not alter S. aureus adhesion or internalization rates (data not shown), implying that the use of L. casei was indicated for prevention rather than for treatment of S. aureus mastitis; and (ii) in all cases, contact with L. casei cells was required, indicating that inhibition did not rely on diffusible compounds. However, other features differed between adhesion and internalization inhibition. Inhibition of S. aureus RF122 adhesion required preincubation with L. casei, whereas inhibition of internalization occurred only when S. aureus was coincubated with L. casei. In addition, live L. casei was required to inhibit internalization, whereas heat-killed L. casei still was able to affect S. aureus adhesion. This suggests that the mechanisms of adhesion and internalization inhibition involve two distinct processes.
The results of adhesion inhibition are in agreement with a competitive exclusion mechanism. Preincubation of MAC-T cells with live or heat-killed L. casei allowed saturation of adhesion sites prior to inoculation by S. aureus. This is consistent with other studies where a similar decrease in the S. aureus adhesion rate (approximately 40%) was observed by competition with viable or heat-killed Lactobacillus strains (43–46).
L. casei inhibition of S. aureus internalization probably involves one or more means acting alone or in combination: (i) modulation of bMEC physiology or integrity induced by contact; (ii) direct effect on S. aureus, including coaggregation, as observed for vaginal lactobacilli (47, 48), although preliminary experiments indicated that L. casei 667 exhibited poor aggregative abilities (data not shown); (iii) inhibition of S. aureus virulence expression, including major virulence regulators, as previously reported (48–51); and (iv) competition for attachment sites involved in internalization. In agreement with a competition mechanism, the L. casei internalization rate was also affected by S. aureus. To our knowledge, this is the first time such reciprocal competition for internalization has been demonstrated.
In conclusion, the basic requirements for a strain to be used as a probiotic against epithelial cell infection are that it must be able to adhere to the host epithelium, have no cytotoxic effect on host cells, and show antagonistic activity toward pathogenic bacteria (16, 17, 52). In this study, we showed that L. casei meets all of these criteria. L. casei was able to adhere to and even internalize into MAC-T cells and to prevent S. aureus internalization and, to a lesser extent, adhesion without modifying cell viability and morphology. The use of LAB to prevent S. aureus invasion into bMEC leads to interesting perspectives on new topical strategies to improve the efficiency of mastitis treatment and to reduce the chronicity of S. aureus infection.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to Marie-Noëlle Madec for her help and technical assistance during this work. We thank Gwenaël Jan for his critical reading of the manuscript. L. casei CIRM-BIA 667 was kindly provided by the Centre International de Ressources Microbiennes—Bactéries d'Intérêt Alimentaire, INRA, Rennes, France.
Damien Bouchard is the recipient of a Ph.D. fellowship from the French Ministry of Research. This work was financially supported by the French National Research Agency (ANR) project NABAB (ANR-08-ALIA-11).
Footnotes
Published ahead of print 26 November 2012
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.03323-12.
REFERENCES
- 1. Akers RM, Nickerson SC. 2011. Mastitis and its impact on structure and function in the ruminant mammary gland. J. Mammary Gland Biol. Neoplasia 16:275–289 [DOI] [PubMed] [Google Scholar]
- 2. Archer GL. 1998. Staphylococcus aureus: a well-armed pathogen. Clin. Infect. Dis. 26:1179–1181 [DOI] [PubMed] [Google Scholar]
- 3. Delgado S, Garcia P, Fernandez L, Jimenez E, Rodriguez-Banos M, Del CR, Rodriguez JM. 2011. Characterization of Staphylococcus aureus strains involved in human and bovine mastitis. FEMS Immunol. Med. Microbiol. 62:225–235 [DOI] [PubMed] [Google Scholar]
- 4. Zadoks RN, Middleton JR, McDougall S, Katholm J, Schukken YH. 2011. Molecular epidemiology of mastitis pathogens of dairy cattle and comparative relevance to humans. J. Mammary Gland Biol. Neoplasia 16:357–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Contreras GA, Rodriguez JM. 2011. Mastitis: comparative etiology and epidemiology. J. Mammary Gland Biol. Neoplasia 16:339–356 [DOI] [PubMed] [Google Scholar]
- 6. Le Maréchal C, Thiéry R, Vautor E, Le Loir Y. 2011. Mastitis impact on technological properties of milk and quality of milk products—a review. Dairy Sci. Technol. 91:247–282 [Google Scholar]
- 7. Steeneveld W, van Barkema WTHW, Hogeveen H. 2011. Cow-specific treatment of clinical mastitis: an economic approach. J. Dairy Sci. 94:174–188 [DOI] [PubMed] [Google Scholar]
- 8. Almeida RA, Matthews KR, Cifrian E, Guidry AJ, Oliver SP. 1996. Staphylococcus aureus invasion of bovine mammary epithelial cells. J. Dairy Sci. 79:1021–1026 [DOI] [PubMed] [Google Scholar]
- 9. Barlow J. 2011. Mastitis therapy and antimicrobial susceptibility: a multispecies review with a focus on antibiotic treatment of mastitis in dairy cattle. J. Mammary Gland Biol. Neoplasia 16:383–407 [DOI] [PubMed] [Google Scholar]
- 10. Nickerson SC. 2009. Control of heifer mastitis: antimicrobial treatment—an overview. Vet. Microbiol. 134:128–135 [DOI] [PubMed] [Google Scholar]
- 11. Heilmann C. 2011. Adhesion mechanisms of staphylococci. Adv. Exp. Med. Biol. 715:105–123 [DOI] [PubMed] [Google Scholar]
- 12. Le Marechal C, Seyffert N, Jardin J, Hernandez D, Jan G, Rault L, Azevedo V, Francois P, Schrenzel J, van de Even GMS, Berkova N, Thiery R, Fitzgerald JR, Vautor E, Le LY. 2011. Molecular basis of virulence in Staphylococcus aureus mastitis. PLoS One 6:e27354 doi:10.1371/journal.pone.0027354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Qazi SN, Harrison SE, Self T, Williams P, Hill PJ. 2004. Real-time monitoring of intracellular Staphylococcus aureus replication. J. Bacteriol. 186:1065–1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sinha B, Fraunholz M. 2010. Staphylococcus aureus host cell invasion and post-invasion events. Int. J. Med. Microbiol. 300:170–175 [DOI] [PubMed] [Google Scholar]
- 15. Espeche MC, Pellegrino M, Frola I, Larriestra A, Bogni C, Nader-Macias ME. 2012. Lactic acid bacteria from raw milk as potentially beneficial strains to prevent bovine mastitis. Anaerobe 18:103–109 [DOI] [PubMed] [Google Scholar]
- 16. Reid G. 2006. Probiotics to prevent the need for, and augment the use of, antibiotics. Can. J. Infect. Dis. Med. Microbiol. 17:291–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Reid G, Burton J. 2002. Use of Lactobacillus to prevent infection by pathogenic bacteria. Microbes Infect. 4:319–324 [DOI] [PubMed] [Google Scholar]
- 18. Reid G. 2012. Probiotic and prebiotic applications for vaginal health. J. AOAC Int. 95:31–34 [DOI] [PubMed] [Google Scholar]
- 19. Beecher C, Daly M, Berry DP, Klostermann K, Flynn J, Meaney W, Hill C, McCarthy TV, Ross RP, Giblin L. 2009. Administration of a live culture of Lactococcus lactis DPC 3147 into the bovine mammary gland stimulates the local host immune response, particularly IL-1β and IL-8 gene expression. J. Dairy Res. 76:340–348 [DOI] [PubMed] [Google Scholar]
- 20. Klostermann K, Crispie F, Flynn J, Ross RP, Hill C, Meaney W. 2008. Intramammary infusion of a live culture of Lactococcus lactis for treatment of bovine mastitis: comparison with antibiotic treatment in field trials. J. Dairy Res. 75:365–373 [DOI] [PubMed] [Google Scholar]
- 21. Frola ID, Pellegrino MS, Espeche MC, Giraudo JA, Nader-Macias ME, Bogni CI. 2011. Effects of intramammary inoculation of Lactobacillus perolens CRL1724 in lactating cows' udders. J. Dairy Res. 14:1–9 [DOI] [PubMed] [Google Scholar]
- 22. Hsueh HY, Yueh PY, Yu B, Zhao X, Liu JR. 2010. Expression of Lactobacillus reuteri Pg4 collagen-binding protein gene in Lactobacillus casei ATCC 393 increases its adhesion ability to caco-2 cells. J. Agric. Food Chem. 58:12181–12191 [DOI] [PubMed] [Google Scholar]
- 23. Lazar V, Miyazaki Y, Hanawa T, Chifiriuc MC, Ditu LM, Marutescu L, Bleotu C, Kamiya S. 2009. The influence of some probiotic supernatants on the growth and virulence features expression of several selected enteroaggregative E. coli clinical strains. Roum. Arch. Microbiol. Immunol. 68:207–214 [PubMed] [Google Scholar]
- 24. Herron-Olson L, Fitzgerald JR, Musser JM, Kapur V. 2007. Molecular correlates of host specialization in Staphylococcus aureus. PLoS One 2:e1120 doi:10.1371/journal.pone.0001120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Newbould FH. 1974. Antibiotic treatment of experimental Staphylococcus aureus infections of the bovine mammary gland. Can. J. Comp. Med. 38:411–416 [PMC free article] [PubMed] [Google Scholar]
- 26. Foligne B, Nutten S, Grangette C, Dennin V, Goudercourt D, Poiret S, Dewulf J, Brassart D, Mercenier A, Pot B. 2007. Correlation between in vitro and in vivo immunomodulatory properties of lactic acid bacteria. World J. Gastroenterol. 13:236–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rochat T, Bermudez-Humaran L, Gratadoux JJ, Fourage C, Hoebler C, Corthier G, Langella P. 2007. Anti-inflammatory effects of Lactobacillus casei BL23 producing or not a manganese-dependant catalase on DSS-induced colitis in mice. Microb. Cell Fact. 6:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Biswas R, Voggu L, Simon UK, Hentschel P, Thumm G, Gotz F. 2006. Activity of the major staphylococcal autolysin Atl. FEMS Microbiol. Lett. 259:260–268 [DOI] [PubMed] [Google Scholar]
- 29. Baron F, Cochet MF, Ablain W, Grosset N, Madec MN, Gonnet F, Jan S, Gautier M. 2006. Rapid and cost-effective method for micro-organism enumeration based on miniaturization of the conventional plate-counting technique. Dairy Sci. Technol. 3:251–257 [Google Scholar]
- 30. Huynh HT, Robitaille G, Turner JD. 1991. Establishment of bovine mammary epithelial cells (MAC-T): an in vitro model for bovine lactation. Exp. Cell Res. 197:191–199 [DOI] [PubMed] [Google Scholar]
- 31. Maudsdotter L, Jonsson H, Roos S, Jonsson AB. 2011. Lactobacilli reduce cell cytotoxicity caused by Streptococcus pyogenes by producing lactic acid that degrades the toxic component lipoteichoic acid. Antimicrob. Agents Chemother. 55:1622–1628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Campana R, Federici S, Ciandrini E, Baffone W. 2012. Antagonistic activity of Lactobacillus acidophilus ATCC 4356 on the growth and adhesion/invasion characteristics of human Campylobacter jejuni. Curr. Microbiol. 64:371–378 [DOI] [PubMed] [Google Scholar]
- 33. Martinez-Pulgarin S, Dominguez-Bernal G, Orden JA, de la Fuente R. 2009. Simultaneous lack of catalase and beta-toxin in Staphylococcus aureus leads to increased intracellular survival in macrophages and epithelial cells and to attenuated virulence in murine and ovine models. Microbiology 155:1505–1515 [DOI] [PubMed] [Google Scholar]
- 34. Ben Zakour NL, Sturdevant DE, Even S, Guinane CM, Barbey C, Alves PD, Cochet MF, Gautier M, Otto M, Fitzgerald JR, Le Loir Y. 2008. Genome-wide analysis of ruminant Staphylococcus aureus reveals diversification of the core genome. J. Bacteriol. 190:6302–6317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Alves PD, McCulloch JA, Even S, Le MC, Thierry A, Grosset N, Azevedo V, Rosa CA, Vautor E, Le Loir Y. 2009. Molecular characterisation of Staphylococcus aureus strains isolated from small and large ruminants reveals a host rather than tissue specificity. Vet. Microbiol. 137:190–195 [DOI] [PubMed] [Google Scholar]
- 36. Guinane CM, Ben Zakour NL, Tormo-Mas MA, Weinert LA, Lowder BV, Cartwright RA, Smyth DS, Smyth CJ, Lindsay JA, Gould KA, Witney A, Hinds J, Bollback JP, Rambaut A, Penades JR, Fitzgerald JR. 2010. Evolutionary genomics of Staphylococcus aureus reveals insights into the origin and molecular basis of ruminant host adaptation. Genome Biol. Evol. 2:454–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Guimaraes VD, Innocentin S, Lefevre F, Azevedo V, Wal JM, Langella P, Chatel JM. 2006. Use of native lactococci as vehicles for delivery of DNA into mammalian epithelial cells. Appl. Environ. Microbiol. 72:7091–7097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Castaldo C, Vastano V, Siciliano RA, Candela M, Vici M, Muscariello L, Marasco R, Sacco M. 2009. Surface displaced alfa-enolase of Lactobacillus plantarum is a fibronectin binding protein. Microb. Cell Fact. 8:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hynonen U, Westerlund-Wikstrom B, Palva A, Korhonen TK. 2002. Identification by flagellum display of an epithelial cell- and fibronectin-binding function in the SlpA surface protein of Lactobacillus brevis. J. Bacteriol. 184:3360–3367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lorca G, Torino MI, Font D, Ljungh VAA. 2002. Lactobacilli express cell surface proteins which mediate binding of immobilized collagen and fibronectin. FEMS Microbiol. Lett. 206:31–37 [DOI] [PubMed] [Google Scholar]
- 41. Munoz-Provencio D, Perez-Martinez G, Monedero V. 2010. Characterization of a fibronectin-binding protein from Lactobacillus casei BL23. J. Appl. Microbiol. 108:1050–1059 [DOI] [PubMed] [Google Scholar]
- 42. De Angelis M, Gobbetti M. 2004. Environmental stress responses in Lactobacillus: a review. Proteomics 4:106–122 [DOI] [PubMed] [Google Scholar]
- 43. Boris S, Suarez JE, Vazquez F, Barbes C. 1998. Adherence of human vaginal lactobacilli to vaginal epithelial cells and interaction with uropathogens. Infect. Immun. 66:1985–1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chan RC, Reid G, Irvin RT, Bruce AW, Costerton JW. 1985. Competitive exclusion of uropathogens from human uroepithelial cells by Lactobacillus whole cells and cell wall fragments. Infect. Immun. 47:84–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ren D, Li C, Qin Y, Yin R, Li X, Tian M, Du S, Guo H, Liu C, Zhu N, Sun D, Li Y, Jin N. 2012. Inhibition of Staphylococcus aureus adherence to Caco-2 cells by lactobacilli and cell surface properties that influence attachment. Anaerobe 18:508–515 [DOI] [PubMed] [Google Scholar]
- 46. Vesterlund S, Karp M, Salminen S, Ouwehand AC. 2006. Staphylococcus aureus adheres to human intestinal mucus but can be displaced by certain lactic acid bacteria. Microbiology 152:1819–1826 [DOI] [PubMed] [Google Scholar]
- 47. Kmet V, Lucchini F. 1997. Aggregation-promoting factor in human vaginal Lactobacillus strains. FEMS Immunol. Med. Microbiol. 19:111–114 [DOI] [PubMed] [Google Scholar]
- 48. Younes JA, van der Mei HC, van den Heuvel E, Busscher HJ, Reid G. 2012. Adhesion forces and coaggregation between vaginal staphylococci and lactobacilli. PLoS One 7:e36917 doi:10.1371/journal.pone.0036917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Cretenet M, Laroute V, Ulve V, Jeanson S, Nouaille S, Even S, Piot M, Girbal L, Le Loir Y, Loubiere P, Lortal S, Cocaign-Bousquet M. 2011. Dynamic analysis of the Lactococcus lactis transcriptome in cheeses made from milk concentrated by ultrafiltration reveals multiple strategies of adaptation to stresses. Appl. Environ. Microbiol. 77:247–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Even S, Charlier C, Nouaille S, Ben Zakour NL, Cretenet M, Cousin FJ, Gautier M, Cocaign-Bousquet M, Loubiere P, Le Loir Y. 2009. Staphylococcus aureus virulence expression is impaired by Lactococcus lactis in mixed cultures. Appl. Environ. Microbiol. 75:4459–4472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Li J, Wang W, Xu SX, Magarvey NA, McCormick JK. 2011. Lactobacillus reuteri-produced cyclic dipeptides quench agr-mediated expression of toxic shock syndrome toxin-1 in staphylococci. Proc. Natl. Acad. Sci. U. S. A. 108:3360–3365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Reid G. 1999. The scientific basis for probiotic strains of Lactobacillus. Appl. Environ. Microbiol. 65:3763–3766 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.