Abstract
A study of oropharyngeal candidiasis (OPC) in Indian human immunodeficiency virus (HIV)/AIDS patients was conducted over a period of 15 months. This study revealed that 75% of the HIV/AIDS patients had OPC. MIC testing revealed that 5% of the Candida isolates were fluconazole resistant. A correlation between CD4+-T-cell counts and development of OPC in HIV/AIDS patients was also observed. Molecular typing of C. albicans isolates showed that all were genetically unrelated.
Oropharyngeal candidiasis (OPC) was at the top of the list of opportunistic infections in Indian human immunodeficiency virus (HIV)/AIDS patients before the era of highly active antiretroviral therapy (1, 20, 27, 33). Although the incidences of opportunistic infection have been reduced around the globe by highly active antiretroviral therapy, the situation remains the same in most developing countries, including India, where patients can hardly afford this treatment. World Health Organization predictions have put India as one of the biggest repositories of HIV/AIDS patients in the coming decades (38, 39). In order to get an insight into the present scenario of OPC in an Indian hospital, in the present prospective study, an attempt was made to characterize the Candida species isolated from OPC over a period of 15 months in HIV/AIDS patients, mainly to know (i) the spectrum of isolates and (ii) its in vitro pattern of susceptibility to fluconazole (FLC), a common antifungal used for prophylaxis and treatment of OPC. In addition, the genetic relatedness of the isolates was also investigated to get some idea about the primary source of infection.
All of the isolates included in this study were collected from 125 randomly selected HIV/AIDS patients. Whereas 100 patients showed clinical signs of OPC, 25 patients reported discomfort or pain during chewing. All of them were attending the clinic as outpatients of the All India Institute of Medical Sciences, New Delhi, India, from December 1999 to March 2001. These patients were attending the clinic for the first time and had no history of prior treatment with antifungal and antiretroviral drugs. Samples were collected with two sterile cotton swabs. One swab was used to detect the presence of any yeast by Gram staining, and the other was used to test growth on Sabouraud dextrose agar slants containing 2 μg of gentamicin per ml and 0.5% cycloheximide. In the case of positive growth, yeast identification was done by conventional methods. The method used was the germ tube production test, which was done by inoculation of a single colony into 0.5 ml of horse serum, followed by incubation at 37°C for 2 h. Morphology testing for the presence of chlamydospores, pseudohyphae, true mycelium, and blastospore arrangement was done on corn meal agar. An enzymatic triphenyl tetrazolium chloride reduction test was performed in which each Candida species grows with a distinct texture and color. For further characterization, each isolate was subjected to carbohydrate assimilation and fermentation tests (2, 3). To differentiate Candida albicans from C. dubliniensis, all C. albicans isolates (confirmed by conventional methods) were subjected to growth at 45°C and a Tween 80 opacity test (13, 31). Out of 100 samples, 75 were found to be positive for Candida species infection by direct microscopy and culture. This value (75% positive) is lower than that reported (90%) in western populations (5, 10, 23, 35), and one can speculate that the Candida carriage rate in the Indian population is probably lower. Species identification revealed that C. albicans (86%) was the most predominant species; as reported earlier (20), a small percentage of other species has also been identified, i.e., C. parapsilosis (8%) and C. glabrata and C. krusei (3%). The most interesting fact is that C. tropicalis and C. dubliniensis were not isolated. This is a surprising observation in view of reports from developed countries, where non-C. albicans candidiasis is a major problem in HIV/AIDS patients (9, 11, 12, 18, 19). Antifungal drug prophylaxis is rarely practiced in India, and this may be the reason for this observation. Currently, the most widely used drug for treating candidiasis is FLC (16, 17, 24, 28, 36). In vitro FLC susceptibility testing of all of the isolates was done by microdilution susceptibility assay (22). More than 93% of the Candida isolates were found to be susceptible, with MICs of ≤8 μg/ml; 2% were found to be dose-dependently susceptible, with MICs of 16 to 32 μg/ml; and only 5% (two isolates of C. albicans and one each of C. glabrata and C. krusei) were found to be resistant, with MICs of ≥64 μg/ml. The resistant strains (5%) were from patients who never took FLC. These resistant isolates can be described as primarily or intrinsically resistant strains. It is not clear why FLC resistance was low in Candida species isolated from these patients. A couple of years ago, it could have been explained on the basis of the nonaffordability of expensive drugs like FLC, which was not in use as a routine prophylactic antifungal drug. However, over the years the situation has changed. Now, increasing numbers of patients are getting treatment by FLC. Factors contributing to FLC resistance are numerous and include the degree of immunosuppression of the patient, the degree of prior exposure to FLC, the contribution of other chemotherapeutic drugs, and the intrinsic resistance of Candida species (8, 37). However, this is only one-time testing; the study should be continued with repeated collection of isolates from the same patients at different time intervals.
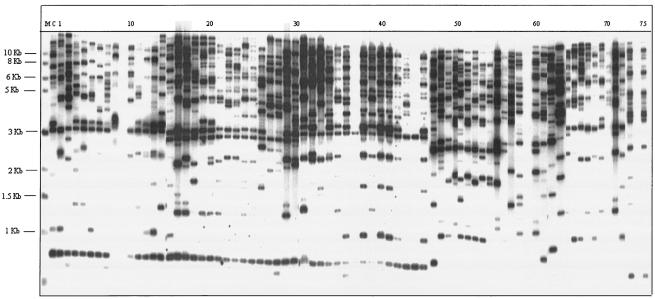
There has been a rapid growth in interest in assessing the genetic relatedness of isolates of the same species in recent years, especially for epidemiology (32). However, no serious attempt has been made so far to detect the source of primary infection and genetic relatedness of the strains isolated in Indian hospitals. To assess the genetic relatedness of C. albicans isolates obtained from HIV/AIDS patients with OPC, we used Southern blot hybridization with the moderately repetitive, C. albicans-specific DNA fragment CARE-2 (14, 15, 29, 32). To accomplish this, we hybridized the EcoRI-digested genomic DNAs of all of the Candida isolates obtained from OPC-positive patients with the labeled probe CARE-2 (7, 30). The CARE-2 hybridization patterns of these isolates were found to be relatively complex (Fig. 1). However, it was apparent from the hybridization patterns that each fingerprinted isolate was different. When these patterns were analyzed quantitatively with the Dendron software package (version 3.0; Solltech, Iowa City, Iowa) as described by Pujol et al. (26), it was further confirmed that all of the C. albicans isolates were different and genetically unrelated (data not shown). The total unrelatedness of the isolates indicates different sources of infection. Since we do not have any knowledge about the commensal status of the outpatients surveyed, it is not possible to speculate about the primary source of infection. The possibility of a commensal organism becoming a pathogen, however, cannot be ruled out totally.
FIG. 1.
DNA fingerprinting patterns of all of the Candida strains obtained from HIV/AIDS patients with OPC. The numbers at the top are isolate numbers. Lanes: M, molecular size marker (1-kb ladder; New England Biolabs); C, control C. albicans strain ATCC 10261; 8, 9, 11, 12, 37, 44, 56, 59, 70, and 74, non-C. albicans Candida strains (no hybridization or nontypical hybridization with the CARE-2 probe); all others, C. albicans isolates (a total of 65 isolates).
The relationship between CD4+-T-cell counts and the onset of OPC in HIV/AIDS patients has been observed in several western reports (4-6, 17, 21, 25, 34). However, such observations are lacking in Indian hospitals. In order to correlate CD4+-T-cell counts and OPC in a selected number of patients (n = 100), CD4+-T-cell counts were determined with a Coulter Manual CD4+-T-cell count kit (Coulter Corporation, Miami, Fla.). Of the 100 patients tested, 75 had clinically observable OPC (confirmed by microbiological methods) and 25 patients did not have any clinical lesions. On the basis of the severity of the OPC clinical lesions versus CD4+-T-cell counts, the patients included in this study could be classified into three broad categories. In the first category, the range of CD4+-T-cell counts was 42 to 100 cells/μl, which was observed among 20 patients with severe OPC lesions (a very thick, curdy membrane spreading over the tongue and oropharynx). The second category had CD4+-T-cell counts ranging from 125 to 200 cells/μl, which were observed among 39 patients having moderate OPC lesions (a membrane on the tongue and stomatitis). The third category had CD4+-T-cell counts ranging from 201 to 245 cells/μl, which were observed among 16 patients with mild OPC lesions (no membrane but stomatitis or gingivitis). In 25 patients having CD4+-T-cell counts in the range of 325 to 626 cells/μl (mean, 424 cells/μl), no such lesions were detected. In India, antiretroviral therapy is prescribed on the basis of CD4+-T-cell counts. CD4+-T-cell counting is expensive, and the necessary equipment is available in only a few selected institutions. This study suggests that the severity of OPC detected clinically can provide a rough estimate of CD4+-T-cell counts, depending on which antiretroviral therapy can be instituted if needed. Finally, this study is probably the first ever survey of OPC in HIV/AIDS patients in India in which comprehensive laboratory data were generated; this will help not only in patient management but also in future clinical research.
Acknowledgments
We specially thank the reviewers for valuable comments on improving the manuscript. The CARE-2 probe was a kind gift from B. A. Lasker. FLC was a kind gift from Pfizer Ltd., Sandwich, Kent, United Kingdom. We thank Sudhakar Jha for valuable help.
The work presented in this paper has been supported in part by grants to R.P., U.B., and G.M. from the Indian Council of Medical Research (5/3/3/17/2000-ECD-I) and to R.P. from the Department of Biotechnology, New Delhi, India (BT/RP1110/MED/09/186/98); the Volkswagen Foundation, Hannover, Germany (1/76 798); and the European Commission, Brussels, Belgium (QLK-CT-2001-02377). Ali Abdul Lattif acknowledges the fellowship from the Indian Council for Cultural Relations, New Delhi, India.
REFERENCES
- 1.Aggarwal, O. P., A. K. Sharma, and A. Inddrayan (ed.). 1997. HIV/AIDS research in India, p. 713-720. Ministry of Health and Family Welfare, Government of India, New Delhi.
- 2.Anonymous. 1999. Guidelines for preparing standard operating procedure for laboratory diagnosis of HIV-opportunistic infection, p. 49-69. W. H. O. Collaboration Centre for Training on HIV/AIDS. Clinical management and counseling. Ministry of Public Health, Bangkok, Thailand.
- 3.Anonymous. 1999. Laboratory diagnosis of HIV-opportunistic infections, p. 93-105. W. H. O. Collaboration Centre for Training on HIV/AIDS. Clinical management and counseling. Ministry of Public Health, Bangkok, Thailand.
- 4.Brambilla, A. M., A Castagna., B. Nocita, H. Hasson, E. Boeri, F. Veglia, and A. Lazzarin. 2001. Relation between CD4 cell counts and HIV RNA levels at onset of opportunistic infections. J. Acquir. Immun. Defic. Syndr. 27:44-48. [DOI] [PubMed] [Google Scholar]
- 5.Fidel, P. L., Jr. 2002. Immunity to Candida. Oral Dis. 8:69-75. [DOI] [PubMed] [Google Scholar]
- 6.Fidel, P. L., Jr. 2002. Distinct protective host defenses against oral and vaginal candidiasis. Med. Mycol. 40:359-375. [PubMed] [Google Scholar]
- 7.Franz, R., S. L. Kelly, D. C. Lamb, D. E. Kelly, M. Ruhnke, and J. Morschhäuser. 1998. Multiple molecular mechanisms contribute to a stepwise development of fluconazole resistance in clinical Candida albicans strains. Antimicrob. Agents Chemother. 42:3065-3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harry, J. B., J. L. Song, C. N. Lyons, and T. C. White. 2002. Transcription of genes associated with azole resistance in Candida albicans. Med. Mycol. 40:73-81. [DOI] [PubMed] [Google Scholar]
- 9.Hoegl, L., E. Thoma-Geber, M. Rocken, and H. C. Korting. 1998. Persistent oral candidosis by non-albicans Candida strains including Candida glabrata in a human immunodeficiency virus-infected patient observed over a period of 6 years. Mycoses 41:335-338. [DOI] [PubMed] [Google Scholar]
- 10.Holmstrup, P., and L. P. Samaranayake. 1990. Acute and AIDS-related oral candidosis, p. 133-550. In L. P. Samaranayake and T. W. MacFarlane (ed.), Oral candidosis. Wright, London, United Kingdom.
- 11.Jabra-Rizk, M. A., A. A. M. A. Baqui, J. I. Kelley, W. A. Falkler, Jr., W. G. Merz, and T. F. Meiller. 1999. Identification of Candida dubliniensis in a prospective study of patients in the United States. J. Clin. Microbiol. 37:321-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jabra-Rizk, M. A., S. M. S. Ferreira, M. Sabet, W. A. Falkler, W. G. Merz, and T. F. Meiller. 2001. Recovery of Candida dubliniensis and other yeasts from human immunodeficiency virus-associated periodontal lesions. J. Clin. Microbiol. 39:4520-4522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jabra-Rizk, M. A., W. A. Falkler, Jr., W. G. Merz, A. A. M. A. Baqui, J. I. Kelley, and T. F. Meiller. 2000. Retrospective identification and characterization of Candida dubliniensis isolates among Candida albicans clinical laboratory isolates from human immunodeficiency virus (HIV)-infected and non-HIV-infected individuals. J. Clin. Microbiol. 38:2423-2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lasker, B. A., L. S. Page, T. J. Lott, and G. S. Kobayashi. 1992. Isolation, characterization, and sequencing of Candida albicans repetitive element 2. Gene 116:51-57. [DOI] [PubMed] [Google Scholar]
- 15.Lischewski, A., D. Harmsen, K. Wilms, G. Baier, U. Gunzer, M. Wilhelm, A. Schwinn, and J. Hacker. 1999. Molecular epidemiology of Candida albicans isolates from AIDS and cancer patients using a novel standardized CARE-2 DNA fingerprinting technique. Mycosis 42:371-383. [DOI] [PubMed] [Google Scholar]
- 16.Maenza, J. R., W. G. Merz, M. J Romagnoli, J. C. Keruly, R. D. Moore, and J. E. Gallant. 1997. Infection due to fluconazole-resistant Candida in patients with AIDS: prevalence and microbiology. Clin. Infect. Dis. 24:28-34. [DOI] [PubMed] [Google Scholar]
- 17.Makarova, N. U., V. V. Pokrowisky, A. V. Kravchnko, L. V. Serebrovskaya, M. J. James, M. M. McNeil, B. A. Lasker, D. W. Warnock, and E. Reiss. 2003. Persistence of oropharyngeal Candida albicans strain with reduced susceptibilities to fluconazole among human immunodeficiency virus-seropositive children and adults in a long-term care facility. J. Clin. Microbiol. 41:1833-1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martinez, M., J. L. López-Ribot, W. R. Kirkpatrick, B. J. Coco, S. P. Bachmann, and T. F. Patterson. 2002. Replacement of Candida albicans with C. dubliniensis in human immunodeficiency virus-infected patients with oropharyngeal candidiasis treated with fluconazole. J. Clin. Microbiol. 40:3135-3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meiller, T. F., M. A. Jabra-Rizk, A. A. M. A. Baqui, J. I. Kelley, V. I. Meeks, W. G. Merz, and W. A. Falker, Jr. 1999. Oral Candida dubliniensis as a clinically important species in HIV-seropositive patients in the United States. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 88:573-580. [DOI] [PubMed] [Google Scholar]
- 20.Mirdha, B. R., U. Banerjee, S. Sethi, J. C. Samnatray, and A. N. Malaviya. 1993. Spectrum of opportunistic fungal infections and parasitic infections in hospitalised AIDS patients. CARC Calling 6:9-10. [Google Scholar]
- 21.Myers, T. A., J. E. Leigh, A. R. Arribas, S. Hager, R. Clark, E. Lilly, and P. L. Fidel, Jr. 2003. Immunohistochemical evaluation of T cells in oral lesions from human immunodeficiency virus-positive persons with oropharyngeal candidiasis. Infect. Immun. 71:956-963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.National Committee for Clinical Laboratory Standards. 1997. Reference method for broth dilution antifungal susceptibility testing of yeast. Approved standard M27-A. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 23.Odds, F. C. 1988. Candida and candidiasis: a review and bibliography, 2nd ed. Bailliere Tindall, Toronto, Ontario, Canada.
- 24.Perea, S., J. L. López-Ribot, W. R. Kirkpatrick, R. K. McAtee, R. A. Santillan, M. Martínez, D. Calabrese, D. Sanglard, and T. F. Patterson. 2001. Prevalence of molecular mechanisms of resistance to azole antifungal agents in Candida albicans strains displaying high-level fluconazole resistance isolated from human immunodeficiency virus-infected patients. Antimicrob. Agents Chemother. 45:2676-2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pereiro, M., Jr., A. Losada, and J. Toribio. 1997. Adherence of Candida albicans strain isolated from AIDS patients. Comparison with pathogenic yeast isolated from patients without HIV infection. Br. J. Dermatol. 137:76-80. [PubMed] [Google Scholar]
- 26.Pujol, C., S. Joly, S. R. Lockhart, S. Noel, M. Tibayrenc, and D. R. Soll. 1997. Parity among the randomly amplified polymorphic DNA method, multilocus enzymes electrophoresis, and Southern blot hybridization with the moderately repetitive DNA probe Ca3 for fingerprinting Candida albicans. J. Clin. Microbiol. 35:2348-2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramkrishna, B., U. M. Tendolkar, A. Varaiya, R. Dhura, and A. Gogate. 2000. Fluconazole sensitivity of oral isolates of Candida species from HIV positive patients. Indian J. Med. Microbiol. 18:30-32. [Google Scholar]
- 28.Rex, J. H., T. J. Walsh, J. D. Sobel, S. G. Filler, P. G. Pappas, W. E. Dismukes, and J. E. Edwards. 2000. Practice guidelines for the treatment of candidiasis. Clin. Infect. Dis. 30:662-678. [DOI] [PubMed] [Google Scholar]
- 29.Ruhnke, M., I. G. Worner, A. Lischewski, A. Neubauer, A. Etsinmuller, M. Trautmann, and J. Morschhauser. 1999. Genotypic relatedness of yeast isolates from women infected with human immunodeficiency virus and their children. Mycosis 42:385-394. [DOI] [PubMed] [Google Scholar]
- 30.Schmid, J., E. Voss, and D. R. Soll. 1990. Computer-assisted methods for assessing strain relatedness in Candida albicans by fingerprinting with the moderately repetitive sequence Ca3. J. Clin. Microbiol. 28:1236-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Slifkin, M. 2000. Tween 80 opacity test responses of various Candida species. J. Clin. Microbiol. 38:4626-4628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Soll, D. R. 2000. The ins and outs of DNA fingerprinting the infectious fungi. Clin. Microbiol. Rev. 13:332-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Solomon, S., and A. K. Ganesh. 2002. HIV in India. Top. HIV Med. 10:19-24. [Google Scholar]
- 34.Varagas, K. G., and J. Sophie. 2002. Carriage frequency, intensity of carriage, and strains of oral yeast species vary in the progression to oral candidiasis in human immunodeficiency virus-positive individuals. J. Clin. Microbiol. 40:341-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vazquez, J. 2000. Therapeutic options for the management of esophageal candidiasis in HIV/AIDS patients. HIV Clin. Trials 1:47-59. [DOI] [PubMed] [Google Scholar]
- 36.Vazquez, J. 2003. Invasive esophageal candidiasis: current and developing treatment options. Drugs 63:971-989. [DOI] [PubMed] [Google Scholar]
- 37.White, T. C., M. A. Pfaller, M. G. Rinaldi, J. Smith, and S. W. Redding. 1997. Stable azole drug resistance associated with a substrain of Candida albicans from an HIV-infected patient. Oral Dis. 3(Suppl. 1):S102-S109. [DOI] [PubMed] [Google Scholar]
- 38.World Health Organization. 3 December 1999, posting date. Global AIDS surveillance. WHO Wkly. Epidemiol. Rec. 74:409-420. [Online.] www.who.int/wer/en/. [Google Scholar]
- 39.World Health Organization. 3 December 2003, posting date. HIV/AIDS in Asia and the Pacific region 2001. In HIV/AIDS in Asia and the Pacific region 2001. [Online.] World Health Organization, Geneva, Switzerland. http://w3.whosea.org/hivaids/index.htm.