Abstract
We report the recovery of Escherichia coli or Klebsiella pneumoniae containing the extended-spectrum β-lactamase gene blaCTX-M from 24 of 1,495 (1.6%) swine fecal samples in 8 of 50 (16%) finishing barns located in 5 U.S. states. We did not detect an association between antimicrobial use and recovery of blaCTX-M.
TEXT
We previously reported the blaCTX-M gene in fecal Escherichia coli from cattle in Ohio (1), described their distribution among dairy herds in Ohio (2), and reported the recovery of Salmonella harboring blaCTX-M from livestock diagnostic submissions (3). blaCTX-M has been recovered from livestock pathogens and commensal flora in Europe and Asia (4–7) and from sporadic human cases of salmonellosis in the United States (8–10). However, the epidemiology of blaCTX-M in U.S. finishing swine populations has not been described. Our objectives were to estimate the frequency and distribution of Salmonella and coliform species harboring blaCTX-M from finishing swine barns. We also characterized the genetic diversity of the isolates and investigated the association between antimicrobial use and the recovery of enteric bacteria harboring blaCTX-M.
We obtained fecal samples from swine finishing barns in Ohio (n = 20), Kansas (n = 10), Illinois (n = 10), Michigan (n = 8), and Minnesota (n = 2) during June through October in 2011. Approximately 30 individual fecal samples were obtained from throughout each barn for a total of 1,495 samples. The barns sampled housed a mean of 1,236 pigs (standard deviation [SD] = 510) in 35 pens (SD = 20.1) that had been in the barns for 98 days (SD = 33.1). The fecal samples were screened for extended-spectrum cephalosporin-resistant enteric bacteria using selective media as previously described (2).
We recovered coliform bacteria harboring blaCTX-M from 24 (1.6%) of the 1,495 fecal samples, representing 8 (16%) of the 50 finishing barns (Table 1). Of these 24 isolates, 22 were E. coli recovered from 5 barns in Ohio (n = 10) and 1 barn in Michigan (n = 12), and the remaining 2 isolates were Klebsiella pneumoniae recovered from 2 barns in Illinois. No blaCTX-M-harboring coliforms were recovered from barns in Kansas or Minnesota. E. coli bacteria carrying blaCMY-2 were recovered from 1,174 (79%) fecal samples representing all 50 barns, with proportions ranging from 20% to 100% of samples within individual barns. Salmonella bacteria were recovered from 109 (7.3%) fecal samples from 25 (50%) barns, but no Salmonella isolates carried blaCTX-M.
Table 1.
Recovery of the blaCTX-M and blaCMY-2 genes from fecal samples collected from swine finishing barns located in five statesa
| State | Barns |
Fecal samples |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n |
blaCTX-M recovered |
blaCMY-2 recovered |
n |
blaCTX-M recovered |
blaCMY-2 recovered |
|||||
| n | Proportion | n | Proportion | n | Proportion | n | Proportion | |||
| All five states | 50 | 8 | 0.16 | 50 | 1 | 1,495 | 24 | 0.016 | 1,174 | 0.785 |
| Illinois | 10 | 2 | 0.2 | 10 | 1 | 300 | 2 | 0.007 | 291 | 0.97 |
| Kansas | 10 | 0 | 0 | 10 | 1 | 298 | 0 | 0 | 192 | 0.644 |
| Michigan | 8 | 1 | 0.125 | 8 | 1 | 240 | 12 | 0.05 | 178 | 0.742 |
| Minnesota | 2 | 0 | 0 | 2 | 1 | 60 | 0 | 0 | 59 | 0.983 |
| Ohio | 20 | 5 | 0.25 | 20 | 1 | 597 | 10 | 0.017 | 454 | 0.76 |
Recovery of the extended-spectrum cephalosporin resistance genes blaCTX-M and blaCMY-2 from 1,495 fecal samples collected from 50 swine finishing barns located in 5 U.S. states.
The use of antimicrobial agents was common with only 3 (6%) barns reporting no antimicrobial use (Table 2). Pigs in 44 (88%) barns received antimicrobial agents in feed, most commonly tetracyclines (25 barns), lincosamides (13 barns), or macrolides (11 barns) with some pigs (14 barns) receiving 2 or 3 different antimicrobial classes in feed. Antimicrobials were also delivered in water in 7 (14%) barns, and individual sick pigs received antimicrobial therapy in 37 (74%) barns during the finishing period. Exposure to ceftiofur, an extended-spectrum cephalosporin ostensibly favoring selection of organisms with blaCTX-M, was common with 41 (82%) barns reporting its use prior to finishing in either farrowing or nursery barns. During finishing, individual sick pigs in 24 (48%) barns received ceftiofur therapy with a median reported ceftiofur treatment rate of 1.2% of pigs, ranging from treatment of a single individual to 8.7% of the barn population receiving ceftiofur.
Table 2.
Reported antimicrobial use among 50 swine finishing barns located in 5 U.S. states screened for the presence of CTX-M cephalosporinase-producing enteric bacteriac
| Antimicrobial use | All barns (n = 50) |
CTX-M (+) barnsa (n = 8) |
CTX-M (−) barnsa (n = 42) |
|||
|---|---|---|---|---|---|---|
| n | Proportion | n | Proportion | n | Proportion | |
| Ceftiofurb | 43 | 0.86 | 7 | 0.88 | 36 | 0.86 |
| Therapy | 24 | 0.48 | 6 | 0.75 | 18 | 0.43 |
| Prefinishing | 41 | 0.82 | 7 | 0.88 | 34 | 0.81 |
| Antimicrobials in feed | 43 | 0.86 | 7 | 0.88 | 37 | 0.88 |
| Bacitracin | 2 | 0.04 | 0 | 0 | 2 | 0.05 |
| Carbadox | 7 | 0.14 | 3 | 0.38 | 4 | 0.10 |
| Lincomycin | 13 | 0.26 | 2 | 0.25 | 0 | 0.26 |
| Macrolide | 11 | 0.22 | 0 | 0 | 0 | 0.26 |
| Tetracycline | 25 | 0.5 | 5 | 0.63 | 20 | 0.48 |
| Tiamulin | 9 | 0.18 | 3 | 0.38 | 6 | 0.14 |
| Virginiamycin | 1 | 0.02 | 0 | 0 | 1 | 0.02 |
| Antimicrobials in water | 7 | 0.14 | 1 | 0.13 | 6 | 0.14 |
| Gentamicin | 1 | 0.02 | 1 | 0.13 | 0 | 0 |
| Lincomycin | 1 | 0.02 | 0 | 0 | 1 | 0.02 |
| Penicillin | 3 | 0.06 | 0 | 0 | 3 | 0.07 |
| Sulfamethazine | 1 | 0.02 | 0 | 0 | 1 | 0.02 |
| Tetracycline | 3 | 0.06 | 0 | 0 | 3 | 0.07 |
| Tiamulin | 2 | 0.04 | 0 | 0 | 2 | 0.05 |
| Parenteral antimicrobialsd | 37 | 0.74 | 8 | 1 | 29 | 0.69 |
| Florfenicol | 1 | 0.02 | 0 | 0 | 1 | 0.02 |
| Fluoroquinolone | 3 | 0.06 | 0 | 0 | 3 | 0.07 |
| Lincomycin | 15 | 0.3 | 4 | 0.50 | 11 | 0.26 |
| Penicillin | 17 | 0.34 | 5 | 0.63 | 12 | 0.29 |
| Tetracycline | 7 | 0.14 | 1 | 0.13 | 6 | 0.14 |
| Tylosin | 5 | 0.1 | 0 | 0 | 5 | 0.12 |
CTX-M (+) barns, barns in which CTX-M cephalosporinase-producing enteric bacteria were found; CTX-M (−) barns, barns in which CTX-M cephalosporinase-producing enteric bacteria were not found.
Ceftiofur therapy indicates that one or more individual sick pigs in the finishing barn received ceftiofur therapy. Ceftiofur prefinishing indicates that one or more pigs in the barn had received therapeutic or prophylactic ceftiofur treatment in the farrowing or nursery facility prior to entering the finishing barn.
Four rows are shown in boldface type because they present summary values for the categories of antimicrobial use.
Parenteral antimicrobials indicate treatment of one or more individual pigs in a barn with the antimicrobial drug.
E. coli bacteria with blaCTX-M were recovered from 12 (40%) of the 30 fecal samples from a single finishing barn in Michigan representing a single pulsotype. Plasmid analysis indicated that each isolate carried an approximately 40-kb IncN sequence type 1 (ST1) plasmid bearing blaCTX-M-1.
Ten E. coli isolates with blaCTX-M were recovered from 5 Ohio finishing barns belonging to the same company. Of these, 2 isolates from the same barn represented a single clonal strain carrying blaCTX-M-15 on IncF plasmids. The remaining 8 isolates from 4 barns represented a single pulsotype, each carrying blaCTX-M-1 on an IncF plasmid. All 10 isolates from Ohio expressed fluoroquinolone resistance (Fig. 1) conferred by gyrA and parC mutations (11), despite the absence of fluoroquinolone use in these operations.
Fig 1.
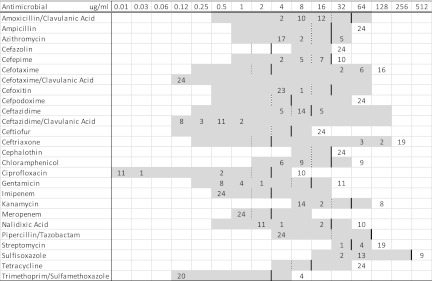
MICs of 26 antimicrobial agents for 22 Escherichia coli and 2 Klebsiella pneumoniae isolates containing blaCTX-M recovered from fecal samples from finishing swine in Ohio, Michigan, and Illinois. The MICs (in micrograms per milliliter) are shown at the top of the figure, and the numbers of isolates are shown in the body of the figure. The broken vertical lines represent susceptible breakpoints, and the solid vertical lines represent resistant breakpoints where available. The range of concentrations of each antimicrobial agent included in this study is indicated by the gray blocks.
We recovered two blaCTX-M-1 K. pneumoniae isolates of the same pulsotype from two finishing barns belonging to the same company in Illinois. Their plasmids, which harbored blaCTX-M-1, could not be typed using our incompatibility group typing procedure (2, 3).
We observed no association between reported antimicrobial use, including therapeutic ceftiofur use, and the recovery of E. coli harboring blaCTX-M using a multivariable logistic regression model (P > 0.05) with a backward selection procedure and generalized estimating equations to account for expected clustering within barns and companies. Similar results have been reported for dairy cattle where ceftiofur treatment rates were not associated with the probability of recovery of fecal E. coli with blaCTX-M (2). Our data suggest that pigs are commonly exposed to ceftiofur either prior to or during the finishing phase of production. Ceftiofur treatment of individual pigs has been associated with a higher recovery of fecal coliform bacteria harboring blaCTX-M (12), but similar associations between ceftiofur use and the recovery of blaCTX-M in livestock populations have not been reported.
Pulsed-field gel electrophoresis (PFGE) analysis of these blaCTX-M-positive isolates indicated that clonal dissemination of a single strain occurred within barns and among barns of the same company. We previously reported that strain homogeneity was common among E. coli isolates carrying blaCTX-M within dairy herds (2). U.S. finishing swine are housed in population-dense environments conducive to the sharing of flora. Our results suggest that clonal expansion of resistant organisms and sharing of enteric flora are the primary dissemination mechanisms of blaCTX-M in U.S. finishing barns.
We found that the blaCTX-M gene was carried by plasmids classified as incompatibility groups N and F based on the standardized classification system utilizing plasmid replication control and portioning functions (13). The ST1 IncN plasmids we recovered from the barn in Michigan have been previously reported to be epidemic in humans, livestock, and food in Europe (14). In addition, ST1 IncN plasmids carrying blaCTX-M-1 have been recovered in the United States from fecal E. coli from dairy cattle (2) and from Salmonella diagnostic submissions from livestock (3). The mechanism for the pandemic dissemination of this unique combination of plasmid and resistance gene is not understood but could result from widespread antimicrobial selection pressure together with frequent movement of animals, people, and food products.
In addition to blaCTX-M, we also recovered E. coli with blaCMY-2 from 100% of the barns and from 79% of the individual fecal samples. Similar recovery rates have been reported in U.S. dairy cattle populations and appear to be increasing (2, 15, 16). We also recovered two Salmonella isolates from two barns belonging to the same company in Ohio representing a single clonal strain that carried blaCMY-2 on IncI1 plasmids, which suggests that enteric bacteria can serve as a reservoir of extended-spectrum cephalosporin resistance genes for pathogens. These resistant pathogens can contaminate fresh retail pork products (17) and pose a risk to public health.
ACKNOWLEDGMENT
Funding for this project was provided by The National Pork Board.
Footnotes
Published ahead of print 30 November 2012
REFERENCES
- 1. Wittum TE, Mollenkopf DF, Daniels JB, Parkinson AE, Mathews JL, Fry PR, Abley MJ, Gebreyes WA. 2010. CTX-M-type extended-spectrum β-lactamases present in Escherichia coli from the feces of cattle in Ohio, United States. Foodborne Pathog. Dis. 7:1575–1579 [DOI] [PubMed] [Google Scholar]
- 2. Mollenkopf DF, Weeman MF, Daniels JB, Abley MJ, Mathews JL, Gebreyes WA, Wittum TE. 2012. Variable within- and between-herd diversity of CTX-M cephalosporinase-bearing Escherichia coli isolates from dairy cattle. Appl. Environ. Microbiol. 78:4552–4560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wittum TE, Mollenkopf DF, Erdman MM. 2012. Detection of Salmonella enterica isolates producing CTX-M cephalosporinase in U.S. livestock populations. Appl. Environ. Microbiol. 78:7487–7491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Horton R, Randall L, Snary E, Cockrem H, Lotz S, Wearing H, Duncan D, Rabie A, McLaren I, Watson E. 2011. Faecal carriage and shedding density of CTX-M ESBL Escherichia coli in cattle, chickens, and pigs: implications for environmental contamination and food production. Appl. Environ. Microbiol. 77:3715–3719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kameyama M, Chuma T, Yokoi T, Yabata J, Tominaga K, Miyasako D, Iwata H, Okamoto K. 2012. Emergence of Salmonella enterica serovar infantis harboring IncI1 plasmid with blaCTX-M-14 in a broiler farm in Japan. J. Vet. Med. Sci. 74:1213–1216 [DOI] [PubMed] [Google Scholar]
- 6. Moodley A, Guardabassi L. 2009. Transmission of IncN plasmids carrying blaCTX-M-1 between commensal Escherichia coli in pigs and farm workers. Antimicrob. Agents Chemother. 53:1709–1711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tamang MD, Nam HM, Kim TS, Jang GC, Jung SC, Lim SK. 2011. Emergence of extended-spectrum β-lactamase (CTX-M-15 and CTX-M-14)-producing nontyphoid Salmonella with reduced susceptibility to ciprofloxacin among food animals and humans in Korea. J. Clin. Microbiol. 49:2671–2675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sjölund M, Yam J, Schwenk J, Joyce K, Medalla F, Barzilay E, Whichard JM. 2008. Human Salmonella infection yielding CTX-M β-lactamase, United States. Emerg. Infect. Dis. 14:1957–1959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sjölund-Karlsson M, Howie R, Krueger A, Rickert R, Pecic G, Lupoli K, Folster JP, Whichard JM. 2011. CTX-M–producing non-Typhi Salmonella spp. isolated from humans, United States. Emerg. Infect. Dis. 17:97–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sjölund-Karlsson M, Rickert R, Matar C, Pecic G, Howie RL, Joyce K, Medalla F, Barzilay EJ, Whichard JM. 2010. Salmonella isolates with decreased susceptibility to extended-spectrum cephalosporins in the United States. Foodborne Pathog. Dis. 7:1503–1509 [DOI] [PubMed] [Google Scholar]
- 11. Sáenz Y, Zarazaga M, Briñas L, Ruiz-Larrea F, Torres C. 2003. Mutations in gyrA and parC genes in nalidixic acid-resistant Escherichia coli strains from food products, humans and animals. J. Antimicrob. Chemother. 51:1001–1005 [DOI] [PubMed] [Google Scholar]
- 12. Cavaco LM, Abatih E, Aarestrup FM, Guardabassi L. 2008. Selection and persistence of CTX-M-producing Escherichia coli in the intestinal flora of pigs treated with amoxicillin, ceftiofur, or cefquinome. Antimicrob. Agents Chemother. 52:3612–3616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Carattoli A, Bertini A, Villa L, Falbo V, Hopkins KL, Threlfall EJ. 2005. Identification of plasmids by PCR-based replicon typing. J. Microbiol. Methods 63:219–228 [DOI] [PubMed] [Google Scholar]
- 14. Naseer U, Sundsfjord A. 2011. The CTX-M conundrum: dissemination of plasmids and Escherichia coli clones. Microb. Drug Resist. 83:83–97 [DOI] [PubMed] [Google Scholar]
- 15. Heider LC, Funk JA, Hoet AE, Meiring RW, Gebreyes WA, Wittum TE. 2009. Identification of Escherichia coli and Salmonella enterica organisms with reduced susceptibility to ceftriaxone from fecal samples of cows in dairy herds. Am. J. Vet. Res. 70:389–393 [DOI] [PubMed] [Google Scholar]
- 16. Tragesser LA, Wittum TE, Funk JA, Winokur PL, Rajala-Schultz PJ. 2006. Association between ceftiofur use and isolation of Escherichia coli with reduced susceptibility to ceftriaxone from fecal samples of dairy cows. Am. J. Vet. Res. 67:1696–1700 [DOI] [PubMed] [Google Scholar]
- 17. Mollenkopf DF, Kleinhenz KE, Funk JA, Gebreyes WA, Wittum TE. 2011. Salmonella enterica and Escherichia coli harboring blaCMY in retail beef and pork products. Foodborne Pathog. Dis. 8:333–336 [DOI] [PubMed] [Google Scholar]


