Abstract
A high prevalence of Bartonella infection is found in many natural systems; however, the transmission dynamics leading to observations of these infections is not fully understood. The capability of Xenopsylla ramesis fleas to serve as competent vectors of Bartonella sp. OE 1-1 (a strain closely related to the zoonotic Bartonella elizabethae) to Meriones crassus jirds was investigated. Naïve X. ramesis fleas were placed for 72 h on naïve jirds or jirds that were either experimentally or naturally infected with Bartonella sp. strain OE 1-1, after which they were placed on naïve jirds. Postfeeding, 69 to 100% of the fleas collected from each Bartonella-positive jird contained Bartonella DNA, and all naïve jirds became positive for Bartonella sp. OE 1-1 after infestation with the infected fleas. In addition, maternal transmission of Bartonella sp. OE 1-1 in jirds was tested by mating 5 Bartonella-positive and 5 naïve female jirds with 10 naïve male jirds in the absence of fleas. Fifteen offspring were delivered by each group. Cultures of blood drawn from all offspring on days 35 and 47 postdelivery were found to be negative for Bartonella. A single spleen sample from the offspring of a Bartonella-positive mother was found molecularly positive for Bartonella sp. OE 1-1. This study demonstrates that X. ramesis fleas are competent vectors of Bartonella sp. OE 1-1 to M. crassus jirds and indicates that maternal transmission is probably not the major transmission route from female jirds to their offspring. We suggest that the dynamics of Bartonella sp. OE 1-1 in the M. crassus jird population in nature is mostly dependent on its vectors.
INTRODUCTION
Bartonella species are fastidious Gram-negative bacteria capable of causing persistent bacteremia in humans and other mammals (1). They are transmitted from one host to another by blood-sucking arthropods (2). In recent years, increasing numbers of Bartonella species and genotypes have been detected in a wide number of hosts and vectors, mainly rodents and their fleas. Only a few were confirmed experimentally as competent vectors, and sound information on the transmission routes of Bartonella spp. is lacking (3).
Several Bartonella spp. of rodent origin have been associated with human illness. These include Bartonella elizabethae, Bartonella grahamii, Bartonella vinsonii subsp. arupensis, Bartonella washoensis, Bartonella tamiae (4), and Bartonella tribocorum (5). Investigation of the transmission routes by competence studies and elucidation of the dispersal dynamics of Bartonella among animals and their vectors are crucial for understanding how these organisms are being maintained in nature and how they cause illness. A blood-sucking arthropod has to be capable of acquiring, maintaining, and transmitting a specific microbial agent to a specific host in order to be considered a competent vector (6). Its ability to transmit an infectious agent to a specific host depends on two factors: it must allow the pathogen to survive in its body and be transmitted as a viable organism to a new host. Proven and putative vectors of Bartonella species were reviewed recently (7).
Bartonella dynamics in nature is substantially influenced by the variety and number of competent vectors in a specific region. In previous studies, Bartonella sp. strain OE 1-1, a strain closely related to the zoonotic Bartonella elizabethae, was detected in blood collected from Meriones crassus jirds and Gerbillus nanus gerbils from Israel (8) and Gerbillus pyramidum gerbils from Egypt (9). In another study, we detected a high prevalence of this Bartonella strain in several flea species collected from several rodent species (10), suggesting that it is a common Bartonella strain in rodents and fleas in this region. In the latter study, 6 distinct Bartonella genotypes were identified in X. ramesis fleas, necessitating further investigation of the capability of X. ramesis to serve as a competent vector for these genotypes (10).
Several mammalian hosts have been shown to contribute to the bacterium's dispersal by maternal (vertical) transmission. A previous study reported that Bartonella spp. designated as groups A and D are transmitted vertically from wild-captured female cotton rats (Sigmodon hispidus) and white-footed mice (Peromyscus leucopus) to their offspring (11). Another study reported similar findings in BALB/c mice infected with Bartonella birtlesii (12). Conversely, no maternal transmission of Bartonella grahamii and Bartonella taylorii was reported in female bank voles (3). The aim of the current study was to expand our knowledge on the dispersal dynamics of Bartonella in the wild rodent population by investigating whether X. ramesis is a competent vector of Bartonella sp. OE 1-1 to M. crassus jirds and to elucidate whether this Bartonella sp. can be transmitted maternally from the mother jird to its offspring.
MATERIALS AND METHODS
Bartonella sp. OE 1-1.
The Bartonella sp. OE 1-1 isolate used in this study was obtained from a naturally infected Sundevall's jird from Israel (8); the isolate was cultured on chocolate agar plates in a 5% CO2 incubator (model no. 3111; Thermo Forma, Ohio) at 37°C. Bacterial colonies used were passaged twice before inoculation (first-passage colonies were harvested after 20 days and then reinoculated and harvested again 14 days later). The colonies were added into a solution containing 80% Luria-Bertani medium (Difco Microbiology, Kansas) and 20% glycerol and were kept frozen at −80°C until thawed and used for inoculation. The bacterial concentration of each inoculation dose was determined by a series of 10-fold dilutions. The isolate used was confirmed to be identical to Bartonella sp. OE 1-1 by PCR and high-resolution melt (HRM) real-time quantitative PCR. The Bartonella sp. OE 1-1 citrate synthase (gltA) gene sequence is in GenBank (under accession number HM771297).
Inoculation of jirds with Bartonella sp. OE 1-1.
Ten male and 18 naïve female M. crassus jirds (Sundevall's jirds), captured in the Negev Desert in Israel, were used in this study (see Table S1 in the supplemental material). They were screened for Bartonella infection by 2 consecutive blood cultures, 14 days apart, and by PCR targeting the gltA and the RNA polymerase B (rpoB) genes as described previously (8), and they were confirmed to be free of Bartonella spp. Six of the female jirds (MB9 to MB14) were subcutaneously inoculated with 107 CFU of Bartonella sp. OE 1-1 diluted in 1.0 ml phosphate-buffered saline (PBS). Six other naïve jirds (MN1 to MN6) were used as negative controls and injected subcutaneously with 1.0 ml PBS. Another 6 naïve female jirds (MN15 to MN20) were used for succeeding stages of the study. Two additional female jirds (MP7 and MP8; captured in the Negev desert) naturally infected with Bartonella sp. OE 1-1, as confirmed by blood cultures and molecular screening of the colonies, were also included in the study. This study was carried out in strict accordance with the recommendations in the National Institutes of Health's Guide for the Care and Use of Laboratory Animals (13) and approved by The Israel Nature and National Parks Protection Authority (approval number 2011/38279) and Hebrew University's Animal Care and Use Committee (approval number MD-10-12720-3).
Fleas.
Bartonella-free X. ramesis fleas, maintained as described previously (14, 15), were used in this study. Briefly, 3 Bartonella-free Sundevall's jirds were placed in individual cages containing a steel nest box with a screen floor and a pan with a mixture of autoclaved sand and dried bovine blood that was examined and verified to be Bartonella-free using PCR, as described below. The jirds were infested with 10 to 15 newly emerged fleas. Every 2 weeks, all substrate and bedding material was collected from the cage and transferred into an incubator (model FOC225E; Velp Scientifica srl, Milano, Italy), where flea emergence and development took place at 25°C and 75% relative humidity.
Acquisition of Bartonella by fleas and transmission to naïve jirds.
Forty newly emerged female and 20 newly emerged male laboratory-reared naïve X. ramesis fleas were placed on each of the 6 experimentally infected female jirds (MB9 to MB14) when they were confirmed to be bacteremic (day 15 postinoculation), as well as on the 2 naturally infected M. crassus jirds (MP7 and MP8) and the 6 naïve female jirds (MN1 to MN6) (Table 1). After 72 h, the fleas were collected manually and separately from each jird and its cage substrate and transferred into a disinfected Erlenmeyer flask for overnight incubation (10 to 12 h at 25°C and 75% relative humidity). The following morning, each flea pool collected from 5 experimentally infected jirds (MB9 to MB13) and from the 2 naturally infected jirds (MP7 and MP8) was placed on a single naïve jird (MN1 to MN6 and MN15) for another 72 h. The flea pool from the sixth experimentally infected jird (MB14) was screened for Bartonella using PCR as described below. Flea pools collected from 3 Bartonella-naïve jirds (MN1 to MN3) were placed separately on another 3 Bartonella-naïve jirds (MN16 to MN18) for 72 h. Fleas collected from 3 other Bartonella-naïve jirds (MN4 to MN6) were screened for Bartonella using PCR (Table 1).
Table 1.
Infection of fleas and jirds with Bartonella
| No. of fleas applied |
Jird to which fleas were applied (day 15)a,b | No. of fleas collected on day 18 |
Jird to which fleas were transferred (day 19)c | No. of female fleas collected (day 22) | No. (%) of Bartonella-positive female fleas | No. of male fleas collected (day 22) | No. (%) of Bartonella-positive male fleas | ||
|---|---|---|---|---|---|---|---|---|---|
| Female | Male | Female | Male | ||||||
| 40 | 20 | MN1 | 37 | 13 | MN16 | 29 | 0 (0) | 11 | 0 (0) |
| 40 | 20 | MN2 | 36 | 20 | MN18 | 33 | 0 (0) | 18 | 0 (0) |
| 40 | 20 | MN3 | 40 | 19 | MN17 | 38 | 0 (0) | 17 | 0 (0) |
| 40 | 20 | MN4 | 33 | 17 | —e | 28d | 0 (0)d | 13d | 0 (0)d |
| 40 | 20 | MN5 | 36 | 20 | — | 30d | 0 (0)d | 18d | 0 (0)d |
| 40 | 20 | MN6 | 39 | 12 | — | 33d | 0 (0)d | 9d | 0 (0)d |
| 40 | 20 | MP7 | 30 | 12 | MN3 | 24 | 21 (88) | 8 | 7 (88) |
| 40 | 20 | MP8 | 33 | 15 | MN1 | 28 | 21 (75) | 13 | 11 (85) |
| 40 | 20 | MB9 | 37 | 20 | MN4 | 37 | 36 (97) | 19 | 16 (84) |
| 40 | 20 | MB10 | 37 | 19 | MN6 | 35 | 29 (83) | 17 | 16 (94) |
| 40 | 20 | MB11 | 36 | 20 | MN5 | 28 | 28 (100) | 17 | 13 (76) |
| 40 | 20 | MB12 | 29 | 20 | MN2 | 23 | 20 (87) | 14 | 12 (86) |
| 40 | 20 | MB13 | 34 | 19 | MN15 | 33 | 28 (85) | 16 | 11 (69) |
| 40 | 20 | MB14 | 29 | 13 | — | 22d | 19 (86)d | 12d | 9 (75)d |
MN1 to MN6 represent Bartonella-negative jirds, MP7 and MP8 represent jirds naturally infected with Bartonella, and MB9 to MB14 represent jirds experimentally infected with Bartonella.
On day 15, fleas were placed on jirds and collected after 72 h.
On day 19, fleas that had been previously placed on naïve or Bartonella-positive jirds were transferred to naïve jirds.
Fleas that were subjected to Bartonella PCR without being transferred to naïve jirds for a second infestation period.
—, fleas were not transferred to new jirds but were subjected to PCR.
Maternal transmission of Bartonella in female jirds.
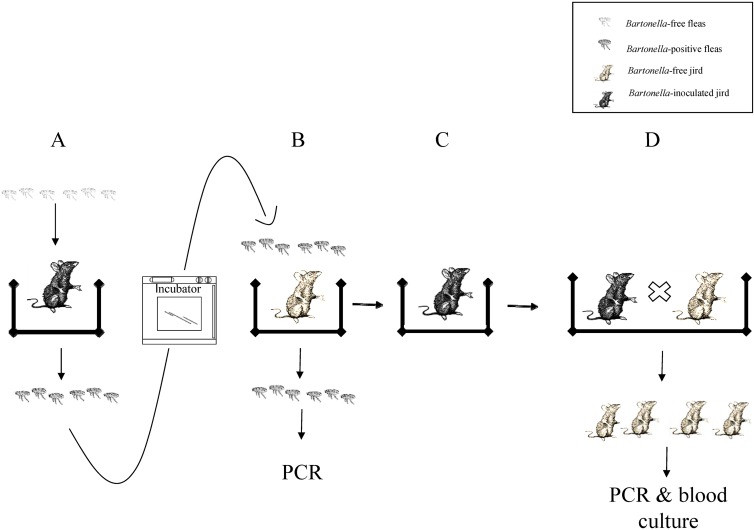
Following the vector competence experiment, 5 culture-confirmed Bartonella-positive female jirds (group 1: MB9 to MB13) and 5 Bartonella-negative female jirds (group 2: MN16 to MN20) were mated with 10 Bartonella-negative male jirds. Each jird couple was kept in a separate cage and free of fleas. All jirds were thoroughly examined and confirmed to be free of fleas. In addition, one drop of fipronil (Frontline; Merial, Georgia) was applied to the skin of each jird between the scapulae to prevent flea infestation. After copulation, the males were removed from the cages, and the pregnant females were kept separately until delivery took place. Postdelivery, the females remained with their offspring for 4 weeks. Thereafter, the offspring were separated into individual cages and maintained for an additional 2 months. The jirds in this study were housed under laboratory conditions as described previously (14, 16). In brief, each jird was maintained separately in a plastic cage (60 by 50 by 40 cm) and offered millet seed and fresh alfalfa (Medicago sp.) ad libitum. They were inspected daily for observable clinical abnormalities. Three months after delivery, all offspring were euthanized. Tissue samples from the lung, liver, kidney, spleen, and brain from each offspring were collected during necropsy (Fig. 1).
Fig 1.
Schematic description of the basic experimental design (shown for one jird). (A) Forty female and 20 male naïve fleas were placed on each of 6 jirds experimentally inoculated with Bartonella and on 2 naturally infected jirds on day 15 for 72 h. (B) Fleas collected from Bartonella-inoculated jirds were placed on Bartonella-free jirds for another 72 h. (C) Bartonella-free jirds became bacteremic after infestation with the fleas. (D) Bartonella-inoculated female jirds were mated with Bartonella-free male jirds and produced offspring.
Bartonella isolation and DNA extraction.
During the course of the 245-day study, blood was periodically collected (into EDTA tubes) from the orbital sinus of each jird (15 blood collections per jird) under isoflurane general anesthesia (see Table S1 in the supplemental material). On days 35 and 47 postdelivery, blood was collected from all jird offspring of the 2 groups. Two hundred microliters of blood was cultured in an incubator on a chocolate agar plate for up to 11 weeks in 5% CO2 at 37°C. Bartonella colonies were diagnosed morphologically as small creamy-white colonies. For each positive plate, the number of colonies per plate was counted. The numbers of colonies counted were rounded to the nearest multiple of 10, and when more than 1,000 colonies were counted, they were reported as 1,000 (see Table S1).
DNA was extracted from Bartonella colonies using a DNA extraction kit (Illustra tissue minispin kit; GE Healthcare, Little Chalfont, United Kingdom) according to the manufacturer's instructions and confirmed for Bartonella sp. OE 1-1 using PCR and sequencing as described below.
Fleas were collected 3 days after infestation of the naïve jirds and were kept in 70% ethyl alcohol until further analyzed. Each single flea was sliced into minute pieces by a separate new sterile scalpel blade. DNA was extracted separately from each single flea using the DNA extraction kit described above.
Following necropsy of the jird offspring, DNA was extracted from 50 mg of each tested tissue by the DNA extraction kit described above and screened molecularly as described below.
PCR analysis and sequencing.
Molecular screening for Bartonella in the fleas and cultured colonies was carried out using conventional PCR with Bhcs.781p and Bhcs.1137n primers targeting the 313-bp fragment of the gltA gene (17, 18). The PCR procedure was performed in a 50-μl reaction volume containing 2 μl of DNA, 20 pmol of each primer, 21 μl of double-distilled water (DDW), and 25 μl of PCR-Ready high specificity (Syntezza, Jerusalem, Israel). For tissue samples, HRM real-time PCR analysis was performed using primers 600f and 800r targeting a 194-bp fragment of the rpoB gene (19). DNA extracted from a cultured Bartonella Tel Aviv Rr. strain (GenBank accession number FJ577651) was used as a positive control, and two samples containing all the ingredients of the reaction mixture except DNA were used as negative controls for all PCR procedures. Randomly chosen Bartonella-positive samples of blood cultures and fleas (2 to 3 fleas, minimum) from each flea group were selected for further sequence analysis. Amplicons were purified using the ExoSAP-IT PCR purification kit (Affymetrix, Santa Clara, CA). DNA sequencing was carried out utilizing the BigDye Terminator cycle sequencing chemistry (Applied Biosystems, Foster City, CA), the ABI 3700 DNA analyzer (Applied Biosystems), and the accompanying data collection and sequence analysis software. Further analysis was performed using the Sequencher software, version 4.8 for Mac (Gene Codes Corporation, Ann Arbor, MI). The obtained sequences were analyzed initially by BLAST through the NCBI's Mega-BLAST algorithm and further aligned with other Bartonella sequences using ClustalW 1.83 (MegAlign; DNAStar Lasergene software).
RESULTS
Inoculation of jirds with Bartonella sp. OE 1-1.
All 6 inoculated jirds (MB9 to MB14) became infected with Bartonella sp. OE 1-1 as confirmed by blood cultures and molecular screening of the colonies. Bacteremia lasted for 100 to 161 days. The mean bacteremia levels of the 6 jirds increased rapidly through day 18. A slight decrease occurred thereafter, followed by another increase peaking on day 78. Then, the mean bacteremia levels started decreasing until day 185, when all jirds became Bartonella negative (Fig. 2A). In this jirds group, the bacteremia levels ranged from 30 to more than 1,000 colonies per plate (see Table S1 in the supplemental material). The 6 Bartonella-naïve jirds (MN1 to MN6) that were injected with PBS and served as controls for the first stage of the study remained negative at this stage.
Fig 2.
Mean bacteremia levels of flea-infected, subcutaneously inoculated, and naturally infected jirds. (A) Mean bacteremia levels (in numbers of CFU) in jirds throughout the study (245 days). Shown is a normalized graph comparing the mean bacteremia levels between jirds infested with Bartonella-infected fleas (n = 7) and jirds subcutaneously inoculated with Bartonella sp. OE 1-1 (n = 6). Vertical lines represent standard errors. (B) Bacteremia in jirds naturally infected with Bartonella sp. OE 1-1 (n = 2).
Acquisition of Bartonella by fleas.
During flea collection at the different stages of the study, not all applied fleas could be collected (Table 1), as some were probably eaten by the hosting jird. Sixty-nine to 100% of the individual fleas, collected from each Bartonella-positive flea-infected jird (MP7 and MP8 and MB9 to MB14) (Table 1), contained Bartonella sp. OE 1-1 DNA. The infection rate of female fleas (88%) was slightly higher than that of the male fleas (82%) but did not differ significantly (Mann Whitney test, P = 0.2265) (Table 1). The fleas which were collected from one Bartonella-positive jird (MB14) and were not placed on a naïve jird showed the same trend, with mean infection rates of 86% for female fleas and 75% for male fleas. All fleas that infested Bartonella-negative jirds remained negative during the entire experiment. All obtained sequences of amplified Bartonella DNA from blood cultures and fleas were identical and identified as Bartonella sp. OE 1-1.
Transmission of Bartonella to naïve jirds.
All 5 naïve jirds (MN2, MN4 to MN6, and MN15) that were infested for 72 h with the fleas that had been previously placed on the Bartonella-inoculated jirds (MB9 to MB14) became bacteremic (see Table S1 in the supplemental material). The 2 naïve jirds (MN1 and MN3) that were infested with the fleas that had been previously placed on the 2 jirds that were naturally infected with Bartonella (MP7 and MP8) became bacteremic as well. All 3 naïve jirds (MN16 to MN18) that were infested with fleas that had been previously placed on naïve jirds (MN1 to MN3) remained negative.
The kinetics of bacteremia of the jirds that were infested with Bartonella-infected fleas was similar to that of the jirds that were experimentally inoculated by subcutaneous injection of Bartonella (Fig. 2A). The flea-infected jirds showed slower increases in bacteremia levels from day 19 until day 37 (18 days postapplication of the fleas). Then, the levels of bacteremia were relatively stable until day 108 (89 days postapplication of fleas), after which they started to decline until day 169 (150 days postapplication of fleas), when most jirds (except MN2) became Bartonella negative (see Table S1 in the supplemental material). In this group, the bacteremia levels ranged from 20 to more than 1,000 colonies per plate (see Table S1).
The two naturally infected jirds (MP7 and MP8) were bacteremic for at least 212 and 168 days, respectively (see Table S1). Distinctly different bacteremia patterns were observed in these two jirds (Fig. 2B). Their bacteremia levels ranged from 20 to more than 1,000 colonies per plate (see Table S1).
Transmission of Bartonella from female jirds to their offspring.
Following pregnancy, the Bartonella-positive female jirds delivered 15 live offspring (MB9 delivered 4 males; MB10, 5 males and 1 female; and MB12, 3 males and 2 females). The Bartonella-negative female jirds delivered 11 live offspring (MN17, 2 males and 2 females; MN18, 1 female; and MN20, 4 males and 2 females). In this group, the morning following the delivery, an additional 4 dead pups were found in one female jird's (MN19) cage (2 males and 2 partially bitten offspring that were severely damaged, so their sex could not be determined). The male-to-female ratio was higher in the Bartonella-positive female jird group (12:3) than in the Bartonella-negative female jird group (8:5); however, the difference was not found to be significant (Fisher's exact test, P = 0.218). When the data were reanalyzed including the 2 bitten offspring with unknown genders as possibly 2 males, 2 females, or 1 female and 1 male, the difference was still found to be nonsignificant (Fisher's exact test, P = 0.68, 0.245, and 0.427, respectively). Cultures from blood drawn on days 35 and 47 postpartum from all of the offspring were negative for Bartonella. All offspring tissues that were molecularly screened for Bartonella DNA were found to be negative except for one spleen sample from a male offspring of a Bartonella-positive jird (MB10).
DISCUSSION
In this study, the transmission dynamics of Bartonella sp. OE 1-1 in Sundevall's Jirds (M. crassus) was investigated. Xenopsylla ramesis fleas were found to be competent vectors of Bartonella sp. OE 1-1 to M. crassus jirds. A period of 72 h was sufficient to acquire Bartonella from positive bacteremic jirds, and the same period was sufficient for transmission of the bacteria to naïve jirds. The high percentage (69 to 100%) of fleas that acquired Bartonella sp. OE 1-1 suggests the great efficiency of this vector in acquiring this Bartonella species. Male and female fleas did not differ in their capability to acquire Bartonella, as we noted in our previous study (10), indicating that both flea sexes are capable of acquiring Bartonella efficiently. Our results are similar to those of a previous study where B. grahamii and B. taylorii were detected in 21 out of 28 bank voles after Bartonella-positive fleas were added to their arena (3). In the same study, all 10 flea pools examined were Bartonella positive, and 7 of 10 randomly collected individual fleas were also positive.
In our study, all naïve jirds became infected following the application of Bartonella-infected fleas. The higher rodent infection rate in our study was probably due to the application of more fleas per jird and the separation of each jird and its fleas in a single cage rather than using one common arena. To the best of our knowledge, no information regarding the minimal number of fleas required for the transmission of Bartonella is present in the biological-medical literature. In our study, the number of Bartonella-positive fleas applied to naïve jirds varied from 1 jird to another. It can be noted that out of 42 fleas placed on jird MN3, 32 were collected after 72 h, and 28 of them were found to be Bartonella positive, indicating that 28 to 38 fleas (the latter number includes the 10 fleas that were probably eaten) were sufficient to transmit this Bartonella species (Table 1). It should be noted that the number of fleas on a rodent host in nature varies, depending on the rodent species, the flea species, the habitat type, and the season (20). Fleas in nature are known to spend considerably more time on a host than is required to obtain a blood meal, but they also spend a significant amount of time off-host in their burrows (21). It was reported previously that wild M. crassus jirds from the Negev Desert (our study area) were infested with a mean of 12.56 fleas (usually mixed flea species, dominated by X. ramesis) per jird (20).
By continuously culturing and counting the numbers of Bartonella CFU in the blood of the experimentally infected jirds, we noticed that the jirds in this study were persistently infected for periods of 100 to 161 days. Moreover, bacteremia levels increased rapidly in the first 18 days after subcutaneous inoculation of Bartonella or infestation with infected fleas, remained relatively high for an additional 90 to 100 days, and decreased thereafter until the disappearance of infection (Fig. 2A and B). Bartonella CFU numbers in jirds varied within the same jird and between jirds, ranging from 20 to more than 1,000 CFU per 200 μl of blood during the peak of bacteremia. A previous study reported that Bartonella coopersplainensis-like (strain Rn1691yn)-infected Swiss Webster mice produced bacteremia at levels as high as 105 bacteria/ml of blood that lasted from 3 to 8 weeks (22). In our study, a longer duration of bacteremia was recorded (up to 26 weeks), suggesting a better evolutionary adaptation of Bartonella sp. OE 1-1 to M. crassus jirds than that of the rat-associated B. coopersplainensis-like strain to Swiss Webster mice.
The kinetics of Bartonella sp. OE 1-1 bacteremia in the subcutaneously injected jirds resembled that of the flea-infested jirds. Pronounced fluctuations in the CFU counts could be noticed, suggesting a possible cyclic bacteremia in naturally infected jirds. Cyclic bacteremias in laboratory and wild-captured Bartonella-infected rodents have been described (12, 23). In an experimental study, bacteremia onset was shown to occur several days after inoculation by a synchronous wave of bacterial invasion into mature erythrocytes, followed by intracellular bacterial replication until a stagnant number was reached and sustained for the remaining life span of the infected erythrocyte. The initial wave of erythrocyte infection was followed by reinfection waves occurring at intervals of several days. It was proposed as a bacterial persistence strategy, adapted to a nonhemolytic intracellular colonization of erythrocytes, that permits blood-sucking arthropods to efficiently transmit the bacteria (1).
The occurrence of maternal transmission of Bartonella from the mother jird to its offspring was also investigated in this study. Blood cultures of the jird offspring were all found to be negative for Bartonella. However, Bartonella sp. OE 1-1 DNA was detected in 1 spleen sample from a pup of a Bartonella-positive female. Our results indicate that maternal transmission from a female jird to its offspring can occur, although whether the route is transplacental, via the milk, via saliva, or by bites cannot be concluded from the current study and have to be further investigated. It seems that even if maternal transmission of this Bartonella strain exists in jirds, it is not a major route of transmission, and as such, a noneffective route would be in disagreement with the high prevalence of this Bartonella strain in the Negev jirds (10). The results of the current study suggest that fleas probably have a major role in maintaining Bartonella sp. OE 1-1 among M. crassus jirds in nature. No evidence of either horizontal or maternal transmission was found in bank voles inoculated with B. taylorii maintained in an arthropod-free environment (3). An absence of maternal transmission was also reported in cats experimentally infected with B. henselae (24, 25). On the other hand, Bartonella could be isolated from neonates and embryos of naturally infected wild-captured North American cotton rats (Sigmodon hispidus) and white-footed mice (Peromyscus leucopus) (11). In another study, transplacental transmission of Bartonella was demonstrated when 76% of the fetal resorptions in BALB/c mice were found to be culture positive for B. birtlesii, although none of the viable offspring was found to be bacteremic (12). The contradicting results on maternal transmission of Bartonella in rodents in several studies conducted to date might suggest different transmission patterns of different Bartonella spp. in different rodent species.
The same number of offspring was delivered by both Bartonella-infected and Bartonella-free female jirds. However, when the sex ratios of the offspring were compared, more male newborn jirds were observed in the Bartonella-positive group than in the Bartonella-negative group (12 males and 3 females in the former versus 8 males and 5 females in the latter). Although the differences were not significant, they might suggest a possible effect of Bartonella infection on M. crassus newborn jirds, such as a higher mortality rate for female embryos. It has been reported that B. birtlesii infection in BALB/c mice affected the outcome of pregnancy, causing resorption, fetal death, and fetal weight loss (12). In order to test the possible occurrence of this effect, a larger group of female jirds would have to be included and compared in future studies.
Bartonella sp. OE 1-1 and B. elizabethae show 10 bp differences between their 313-bp gltA gene fragments, suggesting that they are closely related and supporting the inclusion of the former in the B. elizabethae species complex, as previously suggested (26). As B. elizabethae is a confirmed zoonotic species (27), the zoonotic potential of Bartonella sp. OE 1-1 has to be further investigated. During this study, no observable clinical abnormalities were noticed in any of the infected jirds. Future studies, including gross pathology and histopathology, are required to assess whether this Bartonella strain is virulent for jirds and other rodents.
In conclusion, this study demonstrates that X. ramesis fleas are competent vectors of Bartonella sp. OE 1-1 (a strain closely related to the zoonotic B. elizabethae) to M. crassus jirds. Maternal transmission of Bartonella sp. OE 1-1 from female jirds to their offspring seems to be feasible but is not a major transmission route. The current results suggest that X. ramesis fleas are probably playing an essential role in maintaining this Bartonella strain in the jird population in nature.
Supplementary Material
ACKNOWLEDGMENTS
This research was supported by the Israel Science Foundation (grant no. 30/11, to Shimon Harrus).
This is publication no. 785 of the Mitrani Department of Desert Ecology.
Footnotes
Published ahead of print 14 December 2012
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.03011-12.
REFERENCES
- 1. Schulein R, Seubert A, Gille C, Lanz C, Hansmann Y, Piemont Y, Dehio C. 2001. Invasion and persistent intracellular colonization of erythrocytes. A unique parasitic strategy of the emerging pathogen Bartonella. J. Exp. Med. 193:1077–1086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Harms A, Dehio C. 2012. Intruders below the radar: molecular pathogenesis of Bartonella spp. Clin. Microbiol. Rev. 25:42–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bown KJ, Bennet M, Begon M. 2004. Flea-borne Bartonella grahamii and Bartonella taylorii in bank voles. Emerg. Infect. Dis. 10:684–687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kaiser PO, Riess T, O'Rourke F, Linke D, Kempf VA. 2011. Bartonella spp.: throwing light on uncommon human infections. Int. J. Med. Microbiol. 301:7–15 [DOI] [PubMed] [Google Scholar]
- 5. Kosoy M, Bai Y, Sheff K, Morway C, Baggett H, Maloney SA, Boonmar S, Bhengsri S, Dowell SF, Sitdhirasdr A, Lerdthusnee K, Richardson J, Peruski LF. 2010. Identification of Bartonella infections in febrile human patients from Thailand and their potential animal reservoirs. Am. J. Trop. Med. Hyg. 82:1140–1145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lane RS. 1994. Competence of ticks as vectors of microbial agents with an emphasis on Borrelia burgdorferi, p 4–61 In Sonenshine DE, Mather TN. (ed), Ecological dynamics of tick-borne zoonoses. Oxford University Press, New York, NY [Google Scholar]
- 7. Tsai YL, Chang CC, Chuang ST, Chomel BB. 2011. Bartonella species and their ectoparasites: selective host adaptation or strain selection between the vector and the mammalian host? Comp. Immunol. Microbiol. Infect. Dis. 34:299–314 [DOI] [PubMed] [Google Scholar]
- 8. Morick D, Krasnov BR, Khokhlova IS, Gottlieb Y, Harrus S. 2011. Investigation of Bartonella acquisition and transmission in Xenopsylla ramesis fleas (Siphonaptera: Pulicidae). Mol. Ecol. 20:2864–2870 [DOI] [PubMed] [Google Scholar]
- 9. Inoue K, Maruyama S, Kabeya H, Hagiya K, Izumi Y, Une Y, Yoshikawa Y. 2009. Exotic small mammals as potential reservoirs of zoonotic Bartonella spp. Emerg. Infect. Dis. 15:526–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Morick D, Krasnov BR, Khokhlova IS, Shenbrot GI, Kosoy MY, Harrus S. 2010. Bartonella genotypes in fleas (Insecta: Siphonaptera) collected from rodents in the Negev Desert, Israel. Appl. Environ. Microbiol. 76:6864–6869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kosoy MY, Regnery RL, Kosaya OI, Jones DC, Marston EL, Childs JE. 1998. Isolation of Bartonella spp. from embryos and neonates of naturally infected rodents. J. Wildl. Dis. 34:305–309 [DOI] [PubMed] [Google Scholar]
- 12. Boulouis HJ, Barrat F, Bermond D, Bernex F, Thibault D, Heller R, Fontaine JJ, Piemont Y, Chomel BB. 2001. Kinetics of Bartonella birtlesii infection in experimentally infected mice and pathogenic effect on reproductive functions. Infect. Immun. 69:5313–5317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. National Research Council 1996. Guide for the care and use of laboratory animals. National Academies Press, Washington, DC [Google Scholar]
- 14. Krasnov BR, Khokhlova IS, Fielden LJ, Burdelova NV. 2001. Effect of air temperature and humidity on the survival of pre-imaginal stages of two flea species (Siphonaptera: Pulicidae). J. Med. Entomol. 38:629–637 [DOI] [PubMed] [Google Scholar]
- 15. Krasnov BR, Khokhlova IS, Fielden LJ, Burdelova NV. 2001. Development rates of two Xenopsylla flea species in relation to air temperature and humidity. Med. Vet. Entomol. 15:249–258 [DOI] [PubMed] [Google Scholar]
- 16. Krasnov BR, Khokhlova IS, Fielden LF, Burdelova NV. 2002. The effect of substrate on survival and development of two species of desert fleas (Siphonaptera: Pulicidae). Parasite 9:135–142 [DOI] [PubMed] [Google Scholar]
- 17. Norman AF, Regnery R, Jameson P, Greene C, Krause DC. 1995. Differentiation of Bartonella-like isolates at the species level by PCR-restriction fragment length polymorphism in the citrate synthase gene. J. Clin. Microbiol. 33:1797–1803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Relman DA, Loutit JS, Schmidt TM, Falkow S, Tompkins LS. 1990. The agent of bacillary angiomatosis. An approach to the identification of uncultured pathogens. N. Engl. J. Med. 323:1573–1580 [DOI] [PubMed] [Google Scholar]
- 19. Morick D, Baneth G, Avidor B, Kosoy MY, Mumcuoglu KY, Mintz D, Eyal O, Goethe R, Mietze A, Shpigel N, Harrus S. 2009. Detection of Bartonella spp. in wild rodents in Israel using HRM real-time PCR. Vet. Microbiol. 139:293–297 [DOI] [PubMed] [Google Scholar]
- 20. Krasnov BR, Shenbrot GI, Medvedev SG, Vatschenok VS, Khokhlova IS. 1997. Host-habitat relations as an important determinant of spatial distribution of flea assemblages (Siphonaptera) on rodents in the Negev Desert. Parasitology 114:159–173 [DOI] [PubMed] [Google Scholar]
- 21. Krasnov BR, Khokhlova IS, Shenbrot GI. 2004. Sampling fleas: the reliability of host infestation data. Med. Vet. Entomol. 18:232–240 [DOI] [PubMed] [Google Scholar]
- 22. Colton L, Zeidner N, Kosoy MY. 2011. Experimental infection of Swiss Webster mice with four rat Bartonella strains: host specificity, bacteremia kinetics, dose dependent response, and histopathology. Comp. Immunol. Microbiol. Infect. Dis. 34:465–473 [DOI] [PubMed] [Google Scholar]
- 23. Kosoy M, Mandel E, Green D, Marston E, Jones D, Childs J. 2004. Prospective studies of Bartonella of rodents. II. Diverse infections in a single rodent community. Vector Borne Zoonotic Dis. 4:296–305 [DOI] [PubMed] [Google Scholar]
- 24. Guptill L, Slater LN, Wu CC, Lin TL, Glickman LT, Welch DF, Tobolski J, HogenEsch H. 1998. Evidence of reproductive failure and lack of perinatal transmission of Bartonella henselae in experimentally infected cats. Vet. Immunol. Immunopathol. 65:177–189 [DOI] [PubMed] [Google Scholar]
- 25. Abbott RC, Chomel BB, Kasten RW, Floyd-Hawkins KA, Kikuchi Y, Koehler JE, Pedersen NC. 1997. Experimental and natural infection with Bartonella henselae in domestic cats. Comp. Immunol. Microbiol. Infect. Dis. 20:41–51 [DOI] [PubMed] [Google Scholar]
- 26. Kosoy M, Hayman DT, Chan KS. 2012. Bartonella bacteria in nature: where does population variability end and a species start? Infect. Genet. Evol. 12:894–904 [DOI] [PubMed] [Google Scholar]
- 27. Daly JS, Worthington MG, Brenner DJ, Moss CW, Hollis DG, Weyant RS, Steigerwalt AG, Weaver RE, Daneshvar MI, O'Connor SP. 1993. Rochalimaea elizabethae sp. nov. isolated from a patient with endocarditis. J. Clin. Microbiol. 31:872–881 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.