Abstract
Rapid and efficient enzymatic degradation of plant biomass into fermentable sugars is a major challenge for the sustainable production of biochemicals and biofuels. Enzymes that are more thermostable (up to 70°C) use shorter reaction times for the complete saccharification of plant polysaccharides compared to hydrolytic enzymes of mesophilic fungi such as Trichoderma and Aspergillus species. The genus Myceliophthora contains four thermophilic fungi producing industrially relevant thermostable enzymes. Within this genus, isolates belonging to M. heterothallica were recently separated from the well-described species M. thermophila. We evaluate here the potential of M. heterothallica isolates to produce efficient enzyme mixtures for biomass degradation. Compared to the other thermophilic Myceliophthora species, isolates belonging to M. heterothallica and M. thermophila grew faster on pretreated spruce, wheat straw, and giant reed. According to their protein profiles and in vitro assays after growth on wheat straw, (hemi-)cellulolytic activities differed strongly between M. thermophila and M. heterothallica isolates. Compared to M. thermophila, M. heterothallica isolates were better in releasing sugars from mildly pretreated wheat straw (with 5% HCl) with a high content of xylan. The high levels of residual xylobiose revealed that enzyme mixtures of Myceliophthora species lack sufficient β-xylosidase activity. Sexual crossing of two M. heterothallica showed that progenies had a large genetic and physiological diversity. In the future, this will allow further improvement of the plant biomass-degrading enzyme mixtures of M. heterothallica.
INTRODUCTION
Replacing petrochemical-based fuels and chemicals with truly sustainable alternatives requires biological conversion of agricultural waste plant material to fermentable sugars. The enzyme mixtures for the degradation of biomass-derived polysaccharides (e.g., cellulose, hemicellulose, and pectin) are most commonly produced by fungal strains belonging to the genera Trichoderma and Aspergillus (1). However, these mixtures are not sufficient for economically viable production of low-value products such as biofuels. A major hurdle of the enzyme mixtures from these mesophilic ascomycetes is that they are most effective at temperatures around 50°C (2–4). Higher thermostability of enzymes allows saccharification of biomass polysaccharides at elevated temperatures. Consequently, reaction times will shorten drastically, mass transfer will increase, and substrate viscosity will be reduced (5, 6). Another issue is the initial treatment by physical and/or chemical means (e.g., high temperature or acid or base treatment). This pretreatment should preferably be as mild as possible, since it involves undesirable chemicals and/or a high-energy input in the process (7). Unfortunately, current enzyme mixtures are incapable of releasing efficiently all available monomeric sugars from mildly pretreated biomass.
These issues can be solved by searching for other plant-biomass degrading fungi that produce enzymes with a higher efficiency and thermostability. The division of Ascomycetes contains several thermophilic fungi that produce hydrolytic enzymes with stability up to 70°C (2, 4, 8, 9). Several of these thermostable enzymes have been characterized (e.g., lipases, amylases, laccases, and phytases [10]). The value of thermophilic fungal enzymes has also been studied for plant-biomass degradation, in particular cellulases and xylanases (11–15). Myceliophthora is a genus consisting of mesophilic and thermophilic fungi. Their taxonomy has only recently been elucidated by phylogenetic analysis (Fig. 1) (16). The genus now includes 10 species, of which three originally belonged to the genus Corynascus (e.g., Corynascus thermophilus was renamed to Myceliophthora fergusii). Furthermore, based on phylogeny and the ability to cross sexually, the isolates originally belonging to M. thermophila have been divided into two species, M. thermophila and M. heterothallica. Four species, M. thermophila, M. heterothallica, M. hinnulea, and M. fergusii, have been described as thermophilic based on their optimal growth at 45°C and were suggested as producers of industrially interesting enzymes with a stability up to 70°C (2, 14). M. thermophila has been extensively described as a producer of thermostable enzymes, such as amylase (17), keratinase (18), laccase (19–21), cellulase (22–24), and aldonolactonase (25), and its genome has been completely sequenced (6). Myceliophthora species can also hydrolyze and grow efficiently on plant substrates (14, 26).
Fig 1.
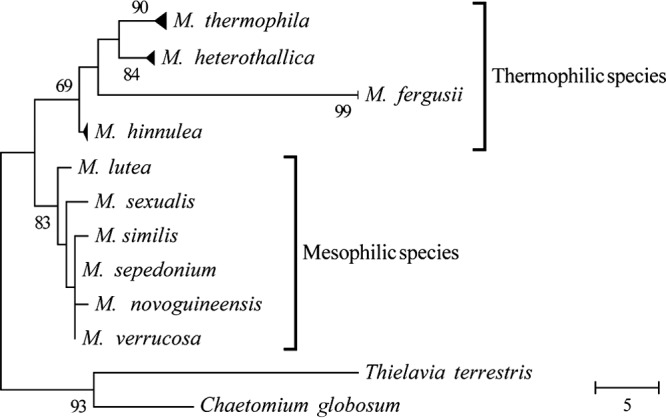
Parsimonious consensus tree of ITS1 region of Myceliophthora species (based on reference 16). The numbers next to the branches indicate the percentage of replicate trees, in which the associated taxa clustered together in the bootstrap test (1,000 replicates).
In the present study, isolates of M. heterothallica were analyzed for their ability to grow on and degrade mildly pretreated plant biomass. The physiology of this species is largely unknown, even though its thermophilic character is very interesting for applications involving thermostable enzymes. In contrast to the better studied and closely related species M. thermophila, M. heterothallica has the ability to cross sexually, which is particularly useful for strain improvement. M. heterothallica isolates were compared to other thermophilic Myceliophthora isolates for their growth on five industrial biomasses with different contents of xylan. The enzyme mixtures of M. heterothallica isolates with the most promising growth profiles were compared to the ones of M. thermophila and mesophilic enzyme producers Aspergillus niger and Trichoderma reesei. Furthermore, sexual crossing of M. heterothallica isolates was assessed as a strategy to improve enzyme mixtures for plant biomass degradation.
MATERIALS AND METHODS
Strains and growth conditions.
All Myceliophthora isolates examined in the present study are listed in Table 1. Aspergillus niger NW249 and Trichoderma reesei QM9414 were used for the comparison between M. heterothallica and mesophilic fungi. Growth profiling on solid medium was performed on minimal medium (27) containing 1.5% agar and 3% pretreated plant biomass. All strains were initially grown on malt extract agar (MEA) (31). A small agar plug containing mycelium (1 mm in diameter) was transferred from the edge of a vigorously growing 1-day-old colony to the center of the petri dishes with the different media. The cultures were incubated in the dark at 40°C. The growth test was conducted twice for each strain. Growth profiling on 32 different carbon sources is explained in the supplemental material.
Table 1.
Thermophilic Myceliophthora strains examined in this study
| Species | Isolate | Source | Mating type |
|---|---|---|---|
| M. thermophila | CBS 117.65 | Dry pasture soil, United Kingdom | |
| CBS 173.70 | Wheat straw compost, United Kingdom | ||
| CBS 669.85 | Unknown source; mutant of CBS 866.85 | ||
| CBS 866.85 | Unknown source | ||
| ATCC 42464 | Unknown source | ||
| M. heterothallica | CBS 131.65 | Birch chips, Sweden | − |
| CBS 202.75 | Garden soil, Germany | + | |
| CBS 203.75 | Soil, Indiana, USA | − | |
| CBS 375.69 | Wood pulp, New Brunswick, Canada | − | |
| CBS 663.74 | Soil under a baobab (Adansonia digitata), Senegal | + | |
| M. hinnulea | CBS 539.82 | Soil from cultivated garden, New Zealand | |
| CBS 540.82 | Soil under Monterey Pine (P. radiata), New Zealand | ||
| CBS 541.82 | Sun-exposed garden soil, New Zealand | ||
| CBS 542.82 | Sun-exposed garden soil, New Zealand | ||
| CBS 544.82 | Soil, New Zealand | ||
| CBS 597.83 | Cultivated soil, Japan | ||
| M. fergusii | CBS 405.69 | Mushroom compost, Pennsylvania, USA | + |
| CBS 406.69 | Mushroom compost, Pennsylvania, USA | − | |
| M. heterothallica | FP 711.01 | Progeny of CBS 203.75 × CBS 663.74 | |
| FP 711.02 | Progeny of CBS 203.75 × CBS 663.74 | ||
| FP 711.03 | Progeny of CBS 203.75 × CBS 663.74 | ||
| FP 711.04 | Progeny of CBS 203.75 × CBS 663.74 | ||
| FP 711.05 | Progeny of CBS 203.75 × CBS 663.74 | ||
| FP 711.06 | Progeny of CBS 203.75 × CBS 663.74 | ||
| FP 711.07 | Progeny of CBS 203.75 × CBS 663.74 | ||
| FP 711.08 | Progeny of CBS 203.75 × CBS 663.74 | ||
| FP 711.09 | Progeny of CBS 203.75 × CBS 663.74 | ||
| FP 711.10 | Progeny of CBS 203.75 × CBS 663.74 | ||
| FP 711.11 | Progeny of CBS 203.75 × CBS 663.74 | ||
| FP 711.12 | Progeny of CBS 203.75 × CBS 663.74 | ||
| FP 711.13 | Progeny of CBS 203.75 × CBS 663.74 | ||
| FP 711.14 | Progeny of CBS 203.75 × CBS 663.74 |
Growth experiments in liquid media were performed in 250-ml shake flasks containing 50 ml of minimal medium and 1.5 g of pretreated plant biomass. All strains were initially grown on MEA. Three agar plugs containing mycelium (5 mm in diameter) were transferred from the edge of a vigorously growing 1-day-old colony to the shake flasks with the different media. The cultures were incubated at 200 rpm and 45°C.
Materials.
The following three sources of plant biomass were used for growth and saccharification experiments: spruce (Sekab E-Technology, Sweden), giant reed (Arundo donax; Chemtex, Italy), and wheat straw (WS; GreenSugar, Germany). Batches of spruce, giant reed, and WS were exposed to 20% hydrochloric acid. Another batch of spruce was pretreated with sulfur dioxide and 6 to 8 min steam at 195°C, and giant reed was pretreated by exposure to steam for 3 min at 200°C. Their different pretreatments, and their glucan and xylan content of the dry matter are given in Table 2. To remove the easily accessible sugars, the pretreated plant biomasses were dissolved in demineralized water, autoclaved, and filtered (using filter papers, quality 4; Whatman) before using them.
Table 2.
Pretreated biomasses of spruce, giant cane, and wheat straw and their glucan and xylan content
| Type of plant biomass | Pretreatment | Content (% [wt/wt]) |
Company | |
|---|---|---|---|---|
| Glucan | Xylan | |||
| Spruce | 1 day with 20% HCl | 55 | 0.0 | GreenSugar |
| Spruce | 15 min with SO2 and 6 to 8 min at 195°C | 48 | 0.5 | Sekab |
| Giant reed | 1 day with 20% HCl | 54 | 9 | GreenSugar |
| Wheat straw | 1 day with 20% HCl | 47 | 12 | GreenSugar |
| Giant reed | 3 min with 200°C | 35 | 13 | Chemtex |
| Wheat straw | 1 day with 5% HCl | 36 | 21 | GreenSugar |
Construction of a phylogenetic tree.
The construction of a parsimonious consensus tree was performed according to van den Brink et al. (16).
Protein analysis.
Protein concentrations were determined using a modified Lowry protein assay reagent kit adapted for 96-well microtiter plates (no. 23240; Pierce). The extracellular proteins within the culture filtrates were separated on an SDS-PAGE and stained with a Coomassie stain using GelCode blue stain reagent (no. 24590; Pierce).
Enzyme assays.
Specific enzyme activities in the culture filtrates were measured using p-nitrophenol-linked substrates (4-nitrophenyl α-d-galactopyranoside, 4-nitrophenyl β-d-galactopyranoside, 4-nitrophenyl β-d-xylopyranoside, 4-nitrophenyl α-l-arabinofuranoside, 4-nitrophenyl β-d-cellobioside, 4-nitrophenyl β-d-glucopyranoside; Sigma-Aldrich, Germany). The assays contained a total volume of 100 μl using 10 to 40 μl of the culture filtrates, 10 μl of 0.01% p-nitrophenol linked substrates, and 25 mM sodium acetate (pH 5.0). Samples were incubated in microtiter plates for 60 min at 45°C. Reactions were stopped by addition of 100 μl of 0.25 M Na2CO3. Absorbance was measured at 405 nm in a microtiter plate reader (FLUOstar Optima; BMG LabTech). The activities were calculated using a standard curve ranging from 0 to 80 nmol of p-nitrophenol per assay volume.
Cellulase, xyloglucanase, and xylanase activities were determined against carboxymethyl cellulose (Sigma-Aldrich), tamarind xyloglucan (Megazyme, Ireland), and beechwood xylan (Sigma-Aldrich). The assays contained a total volume of 200 μl using 10 to 50 μl of culture filtrates and 150 μl of 1% substrate in 50 mM sodium acetate (pH 5.0). The samples were incubated in microtiter plates for 30 to 120 min at 50 and 70°C. Subsequently, 100 μl of supernatant was mixed with 150 μl of 3,5-dinitrosalicylic acid (DNS) solution (28). After an incubation of 25 min at 95°C, absorbance was measured at 540 nm in a microtiter plate reader (FLUOstar Optima). The activities were calculated using a standard curve ranging from 0 to 2 g of glucose liter−1.
Saccharification of pretreated plant biomasses.
Three large agar plugs of mycelium (5 mm in diameter) from the edge of a vigorously growing 1-day-old colony were used to inoculate a 250-ml shake flask with 50 ml of minimal medium (27) and 1.5 g of pretreated plant biomass. Before harvesting the culture filtrate, culture flasks were incubated for 3 days at 200 rpm and 45°C. Then, 4 ml of culture filtrate were mixed with 16 ml of medium consisting of 50 mM sodium acetate buffer (pH 5.0) and 0.6 g of pretreated plant biomass. The hydrolysates were sampled each day for 4 days, boiled for 10 min, and filtered for saccharide analysis.
The analysis of saccharides was performed using a Dionex ICS-3000 HPLC system equipped with a Dionex CarboPac PA-10 (2-mm inner diameter [ID] by 250 mm) column in combination with a CarboPac PA guard column (1 mm [ID] by 25 mm) and a Dionex ED1 PAD detector (Dionex Co., Sunnyvale, CA). An isocratic step was performed at a flow rate of 0.25 ml min−1 with 16 mM NaOH for 15 min, followed by a gradient of 16 to 100 mM NaOH for 5 min. Then, a gradient of sodium acetate was performed in 0.1 M NaOH: 0 to 200 mM in 20 min and 200 to 1,000 mM in 5 min. Each elution was followed by a washing step of 5 min of 1,000 mM sodium acetate in 0.1 M NaOH and a re-equilibration step of 15 min of 16 mM NaOH. Appropriate dilutions (25 μl) were injected on the column by means of an autosampler. Standards (arabinose, galactose, glucose, xylose, cellobiose, and xylobiose) were included at concentrations of 0.5 to 4 μg ml−1 in order to quantify those saccharides.
Mating.
A small agar plug containing mycelium (1 mm in diameter) from the edge of a vigorously growing 1-day-old colony on MEA medium was transferred to petri dishes with MEA medium, followed by incubation in the dark at 37°C (16, 29, 30). After 3 weeks, ascomata were taken out of the agar and ascospores were separated and diluted in ACES buffer (31). The ascospores were cultured on minimal medium containing 1.5% agar and 3% “5% HCl” pretreated wheat straw.
The genotype of parents M. heterothallica CBS203.75 and CBS663.74 and their 14 progenies was determined using amplified fragment length polymorphism (AFLP) fingerprint analysis, as described previously by Boekhout et al. (32).
RESULTS
M. heterothallica and M. thermophila grow well on plant substrates with high xylan content.
The potential of M. heterothallica to degrade plant biomass was evaluated by growth profiling on plant biomass. Five M. heterothallica isolates were compared to six M. hinnulea, two M. fergusii, and five M. thermophila isolates for their ability to grow on pretreated plant biomass (Fig. 2). The five tested substrates, all pretreated differently, contained a high amount of cellulose (>35% [wt/wt]), but varied strongly in their amount of xylan (Table 2). The pretreated spruce did not contain any xylan, while giant reed and wheat straw contain significant amounts of xylan (>8% [wt/wt]). In general Myceliophthora isolates grew better with an increasing content of xylan in the media. Compared to isolates of the other three species, M. hinnulea isolates grew slowly on the plant biomass (Fig. 2). Only M. hinnulea isolate CBS 542.82 showed densely grown colonies after an incubation of 11 days. The two isolates of M. fergusii showed dense colonies after 4 days on medium with giant reed and wheat straw. Still, the densest colonies after 2 days of incubation were observed for the 10 isolates of the genetically related species, M. thermophila and M. heterothallica. Using visual inspection, ATCC 42464 grew better among the M. thermophila isolates on xylan-rich substrates, whereas CBS 866.85 and CBS 669.85 grew better on cellulose-rich and xylan-poor spruce. M. heterothallica CBS 663.74, CBS 202.75, and CBS 375.69 grew better than the two other isolates of this species.
Fig 2.
Growth of thermophilic isolates of Myceliophthora on five industrial substrates at 40°C. Spruce, wheat straw, and giant reed (Arundo donax) were pretreated with 20% HCl or with a steam treatment (indicated between brackets). The pictures of M. thermophila and M. heterothallica were taken after 2 days, M. fergusii after 4 days, and M. hinnulea after 11 days.
The isolates of M. thermophila and M. heterothallica were grown on 35 different media with monomeric, dimeric and polymeric sugars, and crude substrates (see the File S1A in the supplemental material). Differences in the growth of M. thermophila isolates were mostly visible on media with monomeric and dimeric sugars. Compared to the other isolates, M. heterothallica CBS 131.65, CBS 375.69, CBS 202.75, and CBS 663.74 grew better on most monomeric and dimeric sugars. However, M. thermophila ATCC 42464, CBS 866.85, CBS 173.70, and CBS 117.65 grew better on medium with lactose. The differences were less pronounced for growth on polymers and crude substrates. Except for medium with α-cellulose, all M. thermophila and M. heterothallica isolates grew well on polymeric and crude substrates.
M. thermophila and M. heterothallica produce different enzyme mixtures.
Good growth of M. thermophila and M. heterothallica on plant biomass indicated that these fungi produce enzyme mixtures, which efficiently degrade complex polysaccharides. To test the enzyme mixtures produced, the fastest-growing M. heterothallica isolates on plant substrates, CBS 202.75 and CBS 663.74 were compared to M. thermophila ATCC 42464 and the mesophilic enzyme producers Aspergillus niger and Trichoderma reesei. The five strains were grown in liquid medium containing 3% WS as a substrate. The Myceliophthora strains produced most protein after 4 days of growth at 45°C, whereas most proteins of A. niger and T. reesei were produced after 5 days of growth at 30°C. The enzyme mixtures were evaluated by measuring (hemi-)cellulolytic activities against cellulose, xyloglucan, and xylan substrates at 50 and 70°C. Measuring at 50°C is most optimal for mesophilic enzymes, while 70°C is the optimum temperature for Myceliophthora strains (File S1B in the supplemental material shows the activities at different temperatures). The activities between the five strains were all in the same range (Fig. 3). However, the activities at 70°C of A. niger and T. reesei were similar or lower compared to 50°C, whereas the activities of Myceliophthora strains were much higher at 70°C. The cellulase, xyloglucanase, and xylanase activities differed strongly between A. niger and T. reesei. T. reesei had higher cellulase and xyloglucanase activity, whereas A. niger had higher xylanase activity. The (hemi-)cellulolytic activities of M. heterothallica were between the activities of A. niger and T. reesei. In general, the activities of M. heterothallica isolates were slightly lower compared to M. thermophila after growth on WS pretreated with 20% HCl.
Fig 3.
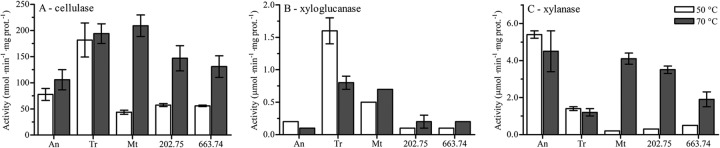
Cellulase, xyloglucanase, and xylanase activities of Aspergillus niger (An), Trichoderma reesei (Tr), Myceliophthora thermophila ATCC 42464 (Mt), and Myceliophthora heterothallica CBS 202.75 and CBS 663.74 after growth on wheat straw pretreated with 20% HCl. The activities are measured by the amount of reduced sugar released and are given in nmol or μmol reduced sugar per min per mg of total protein. The averages and standard deviations represent two independent cultivations and at least four technical replicates.
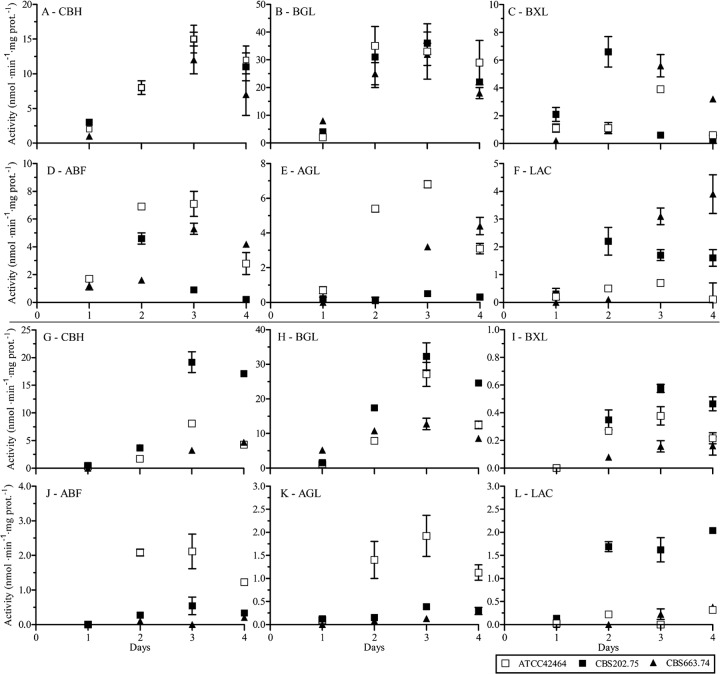
To analyze the enzyme mixtures in more detail, M. heterothallica CBS 202.75 and CBS 663.74 were compared to M. thermophila by growing them on liquid medium containing two different WS substrates. One substrate was pretreated with 20% HCl and contained 12% xylan, while the other was exposed to 5% HCl and contained 21% xylan. Between both WS substrates tested, no differences were found in the protein profiles of the isolates. However, between the three isolates there was a large difference in the protein patterns (see File S1C in the supplemental material). M. thermophila ATCC 42464 grew the fastest and produced the broadest protein set. The two M. heterothallica isolates were very different in their protein profiles. M. heterothallica CBS 663.74 grew the slowest and produced its highest amount of proteins after 3 days growth (see File S1C in the supplemental material). The enzyme activities of six main (hemi-)cellulolytic activities were also very diverse during 4 days of culture on both WS substrates. The two cellulolytic activities, for enzymes cellobiohydrolase (CBH) and β-glucosidase (BGL), showed a similar pattern and activity levels for all three isolates on WS incubated with 20% HCl (20% HCl WS) (Fig. 4A and B). On 5% HCl WS, the CBH and BGL activities of CBS 202.75 were highest, while CBS 663.74 displayed the lowest activities (Fig. 4G and H). The hemicellulolytic activities for β-xylosidase (BXL), α-arabinofuranosidase (ABF), α-galactosidase (AGL), and β-galactosidase (LAC) showed diverse patterns for the isolates (Fig. 4C to F and I to L). All hemicellulolytic activities of M. heterothallica CBS 663.74 increased most after 3 days of culture, which correlated with the extracellular protein profiles. BXL activities were highest for M. heterothallica CBS 202.75 on 20% HCl WS and low for all three isolates on 5% HCl WS. This does not correlate with the xylan content of WS. On both WS substrates, the ABF and AGL activities were highest for M. thermophila ATCC 42464, whereas the LAC activities were higher for the M. heterothallica isolates.
Fig 4.
Enzyme activities of M. thermophila ATCC 42464 and M. heterothallica CBS 202.75 and CBS 663.74 during 4 days growth on wheat straw pretreated with 20% HCl (A to F) or 5% HCl (G to L). The enzymatic activities are measured against p-nitrophenol (PNP)-linked substrates and given in nmol of PNP per min per mg of total protein. The averages and standard deviations represent two independent cultivations and six technical replicates.
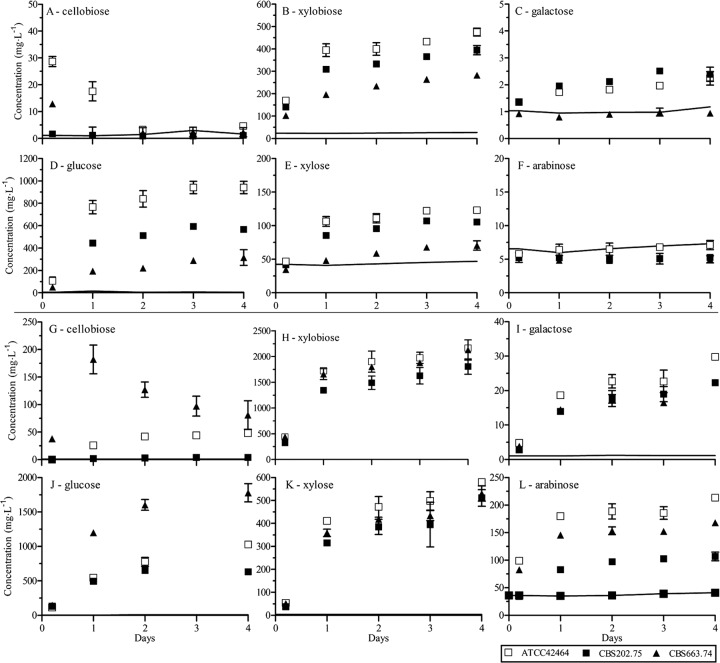
Based on the protein profiles and enzyme activities described above, the saccharification of 3% WS solutions was performed using filtrates of the 3-day-old culture. The mono- and disaccharides in the solution were identified by high-pressure liquid chromatography over 4 days of incubation (Fig. 5). Most sugars were mainly released during the first day of incubation, although glucose, xylobiose, xylose, and galactose were still increasing at the end of the incubation (Fig. 5B to E and H to J). In contrast, cellobiose increased strongly during the initial stages of saccharification, followed by a gradual decrease. In particular, the culture filtrate of CBS 663.74 released a high amount of cellobiose initially from the 5% HCl WS (Fig. 5G). This likely reflected the inability of the BGLs to cope with the high level of cellobiose release. The glucose levels of 20% HCl and 5% HCl WS were similar for enzyme mixtures of CBS 202.75 and ATCC 42464. The glucose release by enzyme mixtures of CBS 663.74 was much better in the 5% HCl WS. The higher hemicellulose fraction in the 5% HCl WS resulted in a higher amount of released xylobiose, xylose, galactose, and arabinose. Xylobiose and xylose release was at least four times higher in 5% HCl WS. Galactose and arabinose release was absent or negligible in the 20% HCl WS, while in 5% HCl WS ATCC 42464 released 30 and 213 g liter−1, respectively. That the concentrations of both sugars were highest using ATCC 42464 enzymes reflected the higher activities of ABF and AGL in the ATCC 42464 enzyme mixtures. Interestingly, this saccharification experiment showed high concentrations of xylobiose in all Myceliophthora samples, which indicates a problem in xylobiose to xylose conversion.
Fig 5.
Saccharification of wheat straw pretreated with 20% HCl (A to F) or 5% HCl (G to L) during 4 days at 45°C using an enzyme mixture of M. thermophila ATCC 42464 and M. heterothallica CBS 202.75 and CBS 663.74. The sugar concentrations are given in mg per liter of filtrate. The black lines are representing the sugar concentrations in wheat straw without an enzyme mixture. The averages and standard deviations represent two independent cultivations and six technical replicates.
M. heterothallica isolates produce genetically diverse progeny.
Isolates with opposite mating type, CBS 663.74 and CBS 203.75, were used to evaluate the strategy of sexual crosses for improving enzyme mixtures produced by M. heterothallica. CBS 663.74, isolated from soil under a baobab tree in Senegal, was shown to be genetically different from CBS 203.75, isolated from soil in Indiana, USA (16). CBS 663.74 and CBS 203.75 produced ascomata in the agar media at their contact zone after 3 weeks. The dark brown ascospores were released from the ascomata and plated on media containing 5% HCl WS. Fourteen colonies with rapid growth were transferred to new plates with 5% HCl WS before further analysis.
The genetic diversity of the 14 selected progenies and their parents was investigated by AFLP. The banding patterns of AFLP were clustered in five main groups (Fig. 6). The pattern of progeny FP 711.01 was very similar to CBS 663.74, while the patterns of FP 711.10 and FP 711.13 were very similar to CBS 203.75. Progenies FP 711.04, FP 711.05, FP 711.06, FP 711.07, FP 711.08, and FP 711.14 were similar to the pattern of CBS 663.74 but had also some bands fitting to the pattern of CBS 202.75. The two patterns of FP 711.09 and FP 711.11 showed a limited amount of bands, whereas the patterns of FP 711.02 and FP 711.03 showed a mix between bands of CBS 203.75 and CBS 663.74.
Fig 6.

Hierarchical clustering (UPGMA) of AFLP banding patterns of Myceliophthora progenies and their parents M. heterothallica CBS 203.75 and CBS 663.74. Similarity of the banding patterns is given in percentage.
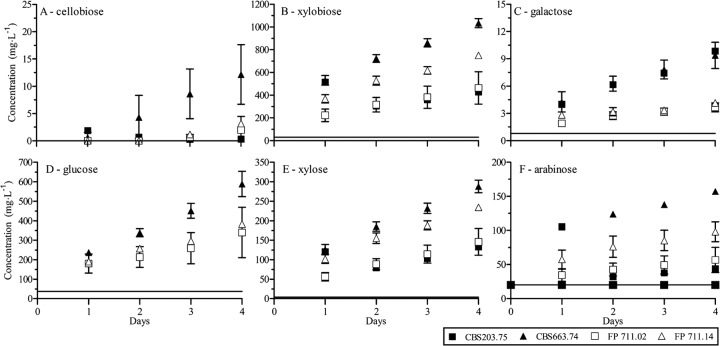
Compared to their parents, most progeny had a similar growth rate and enzyme activities after growth on wheat straw. However, after 3 days of growth on 5% HCl WS, progenies FP 711.02 and FP 711.14 showed higher CBH activities than their parents (data not shown). FP 711.02 and FP 711.14 were therefore chosen for a saccharification experiment with their parents. Similar to the previous section, the filtrates of 3-day-old cultures were used for the saccharification of a 3% WS solution at 45°C. To have a more gradual increase of the sugar concentrations during the 4 days, less protein was loaded compared to the saccharification experiments of the previous section (Fig. 7). The glucose and galactose concentrations were lower after incubation with FP 711.02 and FP 711.14 filtrates than the parental strains. Xylobiose, xylose, and arabinose concentrations of CBS 203.75 and FP 711.02 were similar to each other, and CBS 663.74 and FP 711.14 followed a comparable trend. This similarity in trend correlated with the clustering of the AFLP pattern of FP 711.14 and CBS 663.74. Nevertheless, CBS 663.74 released more mono- and disaccharides than CBS 203.75 and the two progenies.
Fig 7.
Saccharification of mildly pretreated wheat straw (5% HCl) with an enzyme mixture of M. heterothallica CBS 203.75 and CBS 663.74 and their two progeny “FP 711.02” and “FP 711.14” during 4 days at 45°C. The sugar concentrations are given in mg per liter of filtrate. The black lines are representing the sugar concentrations in wheat straw without an enzyme mixture. The averages and standard deviations represent two independent cultures.
DISCUSSION
This study showed that M. heterothallica and M. thermophila rapidly grew on industrial media at temperatures above 40°C. Most of their strains formed dense colonies on media containing xylose, mannose, beechwood xylan, birchwood xylan, and, in particular, industrial substrates with a high content of xylans (e.g., giant reed and wheat straw). Only M. thermophila isolates CBS 866.85 and CBS 669.85 grew faster on cellulose-rich and xylan-poor spruce, which could be expected as they were selected for their ability to produce cellulases (9). The fast growth of M. thermophila and M. heterothallica on plant biomass indicated that these fungi produce enzyme mixtures that efficiently degrade complex polysaccharides. M. thermophila has already been established as a producer of efficient plant-degrading enzyme mixtures (6, 26). However, the characteristics of enzyme mixtures produced by M. heterothallica isolates were unknown. The hydrolytic enzymes of M. heterothallica had a similar thermostability compared to M. fergusii and M. thermophila with optimal activities around 67 to 70°C (see File S1B in the supplemental material) (14). The (hemi-)cellulolytic activities of M. heterothallica are comparable to those of A. niger and T. reesei, even though T. reesei is the most commonly used industrial fungus for cellulose production and A. niger is renowned for its xylanase production (33, 34). We also showed here that the enzymes of M. heterothallica were able to efficiently release fermentable sugars from plant biomass. Compared to M. thermophila, enzyme mixtures of M. heterothallica released more sugars from milder pretreated wheat straw with higher xylan content. The experiments also showed that M. heterothallica isolates were very different in their physiology, which supported their genetic diversity, as previously shown using AFLP patterns (16).
A remarkable observation in the saccharification experiments was the high concentrations of xylobiose compared to the other released sugars. It seems that Myceliophthora isolates were limited in converting xylobiose to xylose, although the enzyme mixtures showed activity against p-nitrophenyl-β-d-xylopyranoside and putative β-xylosidases have been identified (6, 35). The β-xylosidases of Myceliophthora species could be cell-bound, have high transglycosylation activity, or have strong product inhibition (36–38). Since activity has been measured in the culture filtrate and xylotriose was absent during the saccharification, the conversion of xylobiose to xylose was likely prevented by-product inhibition of the β-xylosidases. This strong competitive type of inhibition by xylose has already been described for other fungal β-xylosidases (38–40). The introduction of a heterologous β-xylosidase with a lower inhibitor affinity can improve the release of fermentable xylose residues (41). This will be feasible since heterologous expression has already been achieved with the closely related M. thermophila (26).
The main advantage of M. heterothallica isolates compared to M. thermophila is their natural ability to cross sexually, which is rare among industrially used fungi. Although a larger experiment is needed, the crossings in the pilot test showed the potential to create progeny with changed physiological characteristics. In conclusion, this study showed that M. heterothallica has a high potential to become an enzyme producer for the efficient degradation of mildly pretreated plant biomass.
Supplementary Material
ACKNOWLEDGMENTS
This study has been supported by the EC 7th Framework Program (NEMO, project grant agreement 222699). J.V.D.B. was supported by a grant from The Netherlands Organization for Scientific Research (NWO) via the China-Netherlands Joint Scientific Thematic Research Programme (jstp.10.005) to R.P.D.V.
Sekab E-Technology, Chemtex, and GreenSugar are gratefully acknowledged for the provision of the pretreated plant biomasses.
Footnotes
Published ahead of print 14 December 2012
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.02865-12.
REFERENCES
- 1. van den Brink J, de Vries RP. 2011. Fungal enzyme sets for plant polysaccharide degradation. Appl. Microbiol. Biotechnol. 91:1477–1492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rosgaard L, Pedersen S, Cherry JR, Harris P, Meyer AS. 2006. Efficiency of new fungal cellulase systems in boosting enzymatic degradation of barley straw lignocellulose. Biotechnol. Prog. 22:493–498 [DOI] [PubMed] [Google Scholar]
- 3. Tengborg C, Galbe M, Zacchi G. 2001. Influence of enzyme loading and physical parameters on the enzymatic hydrolysis of steam-pretreated softwood. Biotechnol. Prog. 17:110–117 [DOI] [PubMed] [Google Scholar]
- 4. Viikari L, Alapuranen M, Puranen T, Vehmaanpera J, Siika-Aho M. 2007. Thermostable enzymes in lignocellulose hydrolysis. Adv. Biochem. Eng. Biotechnol. 108:121–145 [DOI] [PubMed] [Google Scholar]
- 5. Doig AR. 1974. Stability of enzymes from thermophilic microorganisms, p 17–21 In Pye EK, Wingard LB. (ed), Enzyme engineering, vol 2 Plenum Press, Inc, New York, NY [Google Scholar]
- 6. Berka RM, Grigoriev IV, Otillar R, Salamov A, Grimwood J, Reid I, Ishmael N, John T, Darmond C, Moisan MC, Henrissat B, Coutinho PM, Lombard V, Natvig DO, Lindquist E, Schmutz J, Lucas S, Harris P, Powlowski J, Bellemare A, Taylor D, Butler G, de Vries RP, Allijn IE, van den Brink J, Ushinsky S, Storms R, Powell AJ, Paulsen IT, Elbourne LD, Baker SE, Magnuson J, Laboissiere S, Clutterbuck AJ, Martinez D, Wogulis M, de Leon AL, Rey MW, Tsang A. 2011. Comparative genomic analysis of the thermophilic biomass-degrading fungi Myceliophthora thermophila and Thielavia terrestris. Nat. Biotechnol. 29:922–927 [DOI] [PubMed] [Google Scholar]
- 7. da Costa Sousa L, Chundawat SP, Balan V, Dale BE. 2009. “Cradle-to-grave” assessment of existing lignocellulose pretreatment technologies. Curr. Opin. Biotechnol. 20:339–347 [DOI] [PubMed] [Google Scholar]
- 8. Morgenstern I, Powlowski J, Ishmael N, Darmond C, Marqueteau S, Moisan M-C, Quenneville G, Tsang A. 2012. A molecular phylogeny of thermophilic fungi. Fungal Biol. 116:489–502 [DOI] [PubMed] [Google Scholar]
- 9. Komura I, Awao T, Yamada K. August 1978. Thermostable cellulase and a method for producing the same. U.S. patent 4,106,989
- 10. Maheshwari R, Bharadwaj G, Bhat MK. 2000. Thermophilic fungi: their physiology and enzymes. Microbiol. Mol. Biol. Rev. 64:461–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Puchart V, Biely P. 2008. Simultaneous production of endo-β-1,4-xylanase and branched xylooligosaccharides by Thermomyces lanuginosus. J. Biotechnol. 137:34–43 [DOI] [PubMed] [Google Scholar]
- 12. Maalej I, Belhaj I, Masmoudi NF, Belghith H. 2009. Highly thermostable xylanase of the thermophilic fungus Talaromyces thermophilus: purification and characterization. Appl. Biochem. Biotechnol. 158:200–212 [DOI] [PubMed] [Google Scholar]
- 13. Singh S, Reddy P, Haarhoff J, Biely P, Janse B, Pillay B, Pillay D, Prior BA. 2000. Relatedness of Thermomyces lanuginosus strains producing a thermostable xylanase. J. Biotechnol. 81:119–128 [DOI] [PubMed] [Google Scholar]
- 14. Maijala P, Kango N, Szijarto N, Viikari L. 2012. Characterization of hemicellulases from thermophilic fungi. Antonie Van Leeuwenhoek 101:905–917 [DOI] [PubMed] [Google Scholar]
- 15. Li DC, Li AN, Papageorgiou AC. 2011. Cellulases from thermophilic fungi: recent insights and biotechnological potential. Enzyme Res. 2011:308730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. van den Brink J, Samson RA, Hagen F, Boekhout T, de Vries RP. 2012. Phylogeny of the industrial relevant, thermophilic genera Myceliophthora and Corynascus. Fungal Diversity 52:10 [Google Scholar]
- 17. Sadhukhan R, Roy SK, Raha SK, Manna S, Chakrabarty SL. 1992. Induction and regulation of alpha-amylase synthesis in a cellulolytic thermophilic fungus Myceliophthora thermophila D14 (ATCC 48104). Indian J. Exp. Biol. 30:482–486 [PubMed] [Google Scholar]
- 18. Liang JD, Han YF, Zhang JW, Du W, Liang ZQ, Li ZZ. 2011. Optimal culture conditions for keratinase production by a novel thermophilic Myceliophthora thermophila strain GZUIFR-H49-1. J. Appl. Microbiol. 110:9. [DOI] [PubMed] [Google Scholar]
- 19. Berka RM, Schneider P, Golightly EJ, Brown SH, Madden M, Brown KM, Halkier T, Mondorf K, Xu F. 1997. Characterization of the gene encoding an extracellular laccase of Myceliophthora thermophila and analysis of the recombinant enzyme expressed in Aspergillus oryzae. Appl. Environ. Microbiol. 63:3151–3157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bulter T, Alcalde M, Sieber V, Meinhold P, Schlachtbauer C, Arnold FH. 2003. Functional expression of a fungal laccase in Saccharomyces cerevisiae by directed evolution. Appl. Environ. Microbiol. 69:987–995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Babot ED, Rico A, Rencoret J, Kalum L, Lund H, Romero J, del Rio JC, Martinez AT, Gutierrez A. 2011. Towards industrially feasible delignification and pitch removal by treating paper pulp with Myceliophthora thermophila laccase and a phenolic mediator. Bioresour. Technol. 102:6717–6722 [DOI] [PubMed] [Google Scholar]
- 22. Badhan AK, Chadha BS, Kaur J, Saini HS, Bhat MK. 2007. Production of multiple xylanolytic and cellulolytic enzymes by thermophilic fungus Myceliophthora sp. IMI 387099. Bioresour. Technol. 98:504–510 [DOI] [PubMed] [Google Scholar]
- 23. Bhat KM, Maheshwari R. 1987. Sporotrichum thermophile growth, cellulose degradation, and cellulase activity. Appl. Environ. Microbiol. 53:2175–2182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Roy SK, Dey SK, Raha SK, Chakrabarty SL. 1990. Purification and properties of an extracellular endoglucanase from Myceliophthora thermophila D-14 (ATCC 48104). J. Gen. Microbiol. 136:1967–1971 [DOI] [PubMed] [Google Scholar]
- 25. Beeson WTt., Iavarone AT, Hausmann CD, Cate JH, Marletta MA. 2010. Extracellular aldonolactonase from Myceliophthora thermophila. Appl. Environ. Microbiol. 77:650–656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Visser H, Joosten V, Punt PJ, Gusakov AV, Olson PT, Joosten R, Bartels J, Visser J, Sinitsyn AP, Emalfarb MA, Verdoes JC, Wery J. 2011. Development of a mature fungal technology and production platform for industrial enzymes based on a Myceliophthora thermophila isolate, previously known as Chrysosporium lucknowense C1. Ind. Biotechnol. 7:214–223 [Google Scholar]
- 27. de Vries RP, Burgers K, van de Vondervoort PJ, Frisvad JC, Samson RA, Visser J. 2004. A new black Aspergillus species, A. vadensis, is a promising host for homologous and heterologous protein production. Appl. Environ. Microbiol. 70:3954–3959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Miller GL. 1956. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 31:3 [Google Scholar]
- 29. von Klopotek A. 1974. Revision of thermophilic Sporotrichum species: Chrysosporium thermophilum (Apinis) comb. nov. and Chrysosporium fergusii spec. nov. equal status conidialis of Corynascus thermophilus Fergus and (Sinden) comb. nov. Arch. Microbiol. 98:365–369 [DOI] [PubMed] [Google Scholar]
- 30. Von Klopotek A. 1976. Thielavia heterothallica spec. nov., die perfekte Form von Chrysosporium thermophilum. Arch. Microbiol. 107:223. [DOI] [PubMed] [Google Scholar]
- 31. Samson RA, Houbraken J, Thrane U, Frisvad JC, Andersen B. 2010. Food and indoor fungi, vol 2 CBS Fungal Biodiversity Centre, Utrecht, Netherlands [Google Scholar]
- 32. Boekhout T, Theelen B, Diaz M, Fell JW, Hop WC, Abeln EC, Dromer F, Meyer W. 2001. Hybrid genotypes in the pathogenic yeast Cryptococcus neoformans. Microbiology 147:891–907 [DOI] [PubMed] [Google Scholar]
- 33. Montenecourt BS, Eveleigh DE. 1977. Preparation of mutants of Trichoderma reesei with enhanced cellulase production. Appl. Environ. Microbiol. 34:777–782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. de Vries RP, Visser J. 2001. Aspergillus enzymes involved in degradation of plant cell wall polysaccharides. Microbiol. Mol. Biol. Rev. 65:497–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hinz SW, Pouvreau L, Joosten R, Bartels J, Jonathan MC, Wery J, Schols AM. 2009. Hemicellulase production in Chrysosporium lucknowense C1. J. Cereal Sci. 50:6 [Google Scholar]
- 36. Katapodis P, Nerinckx W, Claeyssens M, Christakopoulos P. 2006. Purification and characterization of a thermostable intracellular beta-xylosidase from the thermophilic fungus Sporotrichum thermophile. Proc. Biochem. 41:7 [Google Scholar]
- 37. Bohlin C, Praestgaard E, Baumann MJ, Borch K, Praestgaard J, Monrad RN, Westh P. 2013. A comparative study of hydrolysis and transglycosylation activities of fungal β-glucosidases. Appl. Microbiol. Biotechnol. 97:159–169 [DOI] [PubMed] [Google Scholar]
- 38. Selig MJ, Knoshaug EP, Decker SR, Baker JO, Himmel ME, Adney WS. 2008. Heterologous expression of Aspergillus niger β-d-xylosidase (XlnD): characterization on lignocellulosic substrates. Appl. Biochem. Biotechnol. 146:57–68 [DOI] [PubMed] [Google Scholar]
- 39. van Peij NN, Brinkmann J, Vrsanska M, Visser J, de Graaff LH. 1997. β-Xylosidase activity, encoded by xlnD, is essential for complete hydrolysis of xylan by Aspergillus niger but not for induction of the xylanolytic enzyme spectrum. Eur. J. Biochem. 245:164–173 [DOI] [PubMed] [Google Scholar]
- 40. Semenova MV, Drachevskaya MI, Sinitsyna OA, Gusakov AV, Sinitsyn AP. 2009. Isolation and properties of extracellular β-xylosidases from fungi Aspergillus japonicus and Trichoderma reesei. Biochem. Biokhim. 74:1002–1008 [DOI] [PubMed] [Google Scholar]
- 41. Jordan DB, Wagschal K, Fan Z, Yuan L, Braker JD, Heng C. 2011. Engineering lower inhibitor affinities in β-d-xylosidase of Selenomonas ruminantium by site-directed mutagenesis of Trp145. J. Ind. Microbiol. Biotechnol. 38:1821–1835 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.