Abstract
Staphylococcus aureus and Staphylococcus epidermidis biofilms cause chronic infections due to their ability to form biofilms. The excretions/secretions of Lucilia sericata larvae (maggots) have effective activity for debridement and disruption of bacterial biofilms. In this paper, we demonstrate how chymotrypsin derived from maggot excretions/secretions disrupts protein-dependent bacterial biofilm formation mechanisms.
TEXT
Chronic infections are commonly associated with biofilms formed by staphylococci such as Staphylococcus aureus and Staphylococcus epidermidis (1). Staphylococcal biofilm formation involves a number of steps: first, the attachment of bacteria to a biomaterial surface via cell wall-associated adhesins (2), followed by their accumulation to a multibacterial layer. S. aureus and S. epidermidis use several different intercellular adhesive mechanisms, such as the polysaccharide intercellular adhesin (PIA), also termed polymeric N-acetylglucosamine (PNAG), which is synthesized by the icaADBC locus, to accumulate and form biofilms (3–6); proteinaceous factors independent of icaADBC and PIA have emerged as alternatives and include surface protein G (SasG) (7) and biofilm-associated protein (Bap) (8) in S. aureus and the accumulation-associated protein (Aap; homolog to SasG) (9) and extracellular matrix binding protein (Embp) (10) in S. epidermidis.
Lucilia sericata larvae (maggots) have been applied to chronic wounds for centuries, and sterile maggots have been shown to effectively debride necrotic tissue (11) and disinfect wounds (12) and are also reputed to influence healing (13, 14). Components of maggot secretions that aid debridement, such as metalloproteases, serine-proteases, and aspartyl compounds (15), that have antibacterial activities (16–18), and which may assist healing (19, 20) have been identified. Specifically of interest to this present study is the isolation from excretions/secretions (ES) of a chymotrypsin-like proteinase (15), which, as a recombinant enzyme, effectively degrades wound eschar ex vivo (21, 22). Thus, we studied the potential of this recombinant chymotrypsin (rChymotrypsin) to interfere with staphylococcal biofilms. This was facilitated by the availability of clinically important biofilm-forming S. aureus and S. epidermidis strains that employ either PIA (3, 23) or proteinaceous adhesins such as Aap/SasG (7, 9) for biofilm formation.
The previously described semiquantitative adherence assay using 96-well tissue culture plates (Nunc, United Kingdom) was used to measure attachment and accumulation of S. epidermidis 1457 (icaADBC and PIA positive) (23) and 5179-R1 (Aap positive and icaADBC negative) (9) and S. aureus SA113 (ATCC 35556; icaADBC, PIA, and SasG positive) (3, 7, 24) biofilms on the plastic surface in the presence of 0.1, 1, or 10 μg/ml rChymotrypsin (12, 18, 23). No rChymotrypsin was added to the control wells. rChymotrypsin was prepared and tested as previously described (21, 22) and had a specific activity of 10.1 pmol/min/mg (22). Data were analyzed using a one-way analysis of variance (ANOVA) with Tukey's test and with the level of significance set at a P value of <0.05. All experiments were performed three times, each time in triplicate (n = 9).
The greatest effect of rChymotrypsin on both nascent and preformed biofilms was seen on S. epidermidis 5179-R1, with less of an effect on S. epidermidis 1457 and S. aureus SA113 (Fig. 1). Nascent S. epidermidis 1457 biofilm formation was inhibited by 20 to 33% by 0.1 to 10 μg/ml rChymotrypsin compared to that of the control (Fig. 1a), while a disruption of 11 to 51% was observed on the preformed biofilms (Fig. 1b). The effect of rChymotrypsin was not significant on either the nascent or preformed S. epidermidis 1457 biofilms. In the case of S. epidermidis 5179-R1, a significant decrease of 69 to 72% in nascent biofilm formation was observed (Fig. 1a), while rChymotrypsin disrupted preformed biofilms by 6 to 77%, with a significant difference between 10 μg/ml and the control and 0.1 μg/ml (Fig. 1b). The results for S. aureus SA113 were more variable. A significant decrease of 32 to 61% was seen when nascent biofilms were exposed to 1 and 10 μg/ml rChymotrypsin (Fig. 1a), while no effect was seen with 0.1 μg/ml of rChymotrypsin. On S. aureus SA113 preformed biofilms, an 11 to 51% disruption in biofilm, which was not a significant change from the biofilm formation of the control, was observed (Fig. 1b).
Fig 1.
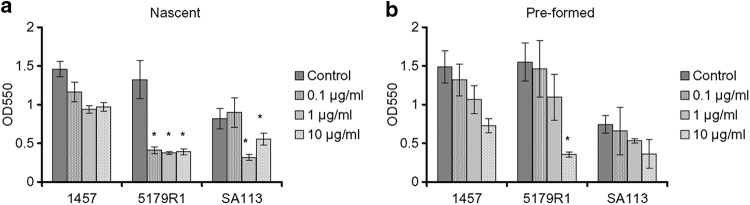
Effect of rChymotrypsin on nascent S. epidermidis 1457 and 5179-R1 and S. aureus SA113 biofilms (a) and preformed S. epidermidis 1457 and 5179-R1 and S. aureus SA113 biofilms (b). Significant effects were seen on nascent S. epidermidis 5179-R1 and S. aureus SA113 biofilm formation (*, P < 0.05), but on the preformed biofilms, rChymotrypsin had a significant effect only on S. epidermidis 5179-R1 (*, P < 0.05). OD550, optical density at 550 nm. Error bars indicate the standard errors of the means.
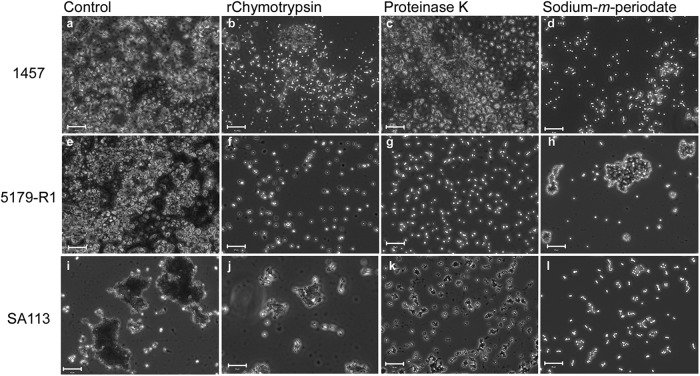
To visualize the effect of rChymotrypsin on the staphylococcal biofilms, light microscopy was used (Fig. 2) (18). rChymotrypsin clearly disrupted S. epidermidis 5179-R1 and S. aureus SA113 biofilms (Fig. 2f and 2j); the effect was not so apparent on S. epidermidis 1457 (Fig. 2b). To support the hypothesis that the cell-cell adhesion disruptions observed were due to the proteolytic activity of rChymotrypsin, the respective biofilms were exposed to sodium meta-periodate and proteinase K, chemicals known to disintegrate the intercellular adhesins PIA/PNAG and Aap/SasG employed by S. epidermidis and S. aureus (3, 7, 9, 23). Results showed similar disruption of cell aggregates to rChymotrypsin (Fig. 2), specifically on S. epidermidis 5179-R1, which is Aap dependent.
Fig 2.
Light-microscopy images showing the effect of rChymotrypsin, proteinase K, and sodium meta-periodate on preformed S. epidermidis 1457 (a to d), S. epidermidis 5179-R1 (e to h), and S. aureus SA113 (i to l) biofilms. (a, e, and i) Untreated bacteria, controls; (b, f, and j) 10 μg/ml rChymotrypsin; (c, g, and k) proteinase K; (d, h, and l) sodium meta-periodate. Bar = 10 μm.
Aap is a cell wall-associated protein comprising an A domain and a repetitive B domain. The intercellular adhesive properties of Aap are located in the N-terminal domain B, which becomes active only after the A domain has been proteolytically cleaved by an endogenous staphylococcal protease or an exogenous host protease (9). Rohde et al. showed that different proteases can either encourage or inhibit Aap-mediated biofilm formation by S. epidermidis 5179-R1 in a dose-dependent manner (9). Thus, rChymotrypsin may affect the proteolytic processing mechanism of Aap in nascent S. epidermidis 5179-R1, which is essential for the activation and mediation of intercellular adhesion and biofilm formation. Alternatively, with preformed biofilms, rChymotrypsin may affect Aap activity by cleaving the Aap peptide bonds as observed with proteinase K (9). The exact influence of rChymotrypsin on Aap is under investigation, as is the reversible nature of its effect. The fact that rChymotrypsin works only on the proteinaceous-adhesin-dependent strains and the fact that different clinical staphylococci, in particular, PIA-dependent S. epidermidis strains and an S. aureus strain, use a polysaccharide and/or proteinaceous biofilm-forming mechanism suggest that chymotrypsin is unlikely to represent a standalone agent. Work is also under way to study the effect of rChymotrypsin on a range of clinical S. epidermidis and S. aureus isolates, including methicillin-resistant S. aureus and other clinically relevant staphylococci.
In conclusion, our study has clearly demonstrated that maggot rChymotrypsin can interfere with bacterial adhesion, adding further to our understanding of the way maggots exert their antibacterial effects. Clearly, protein adhesins are not the only mechanism used by bacteria to adhere to wound tissue, and we believe that maggots attack bacterial adhesins in vivo by secreting a repertoire of bioactive antibiofilm agents, of which chymotrypsin is one key component.
ACKNOWLEDGMENTS
We thank the Technology Strategy Board and MRC DPFS for funding Alan Brown and Gary Telford (Immune Modulation Research Group, School of Pharmacy, University of Nottingham) to produce maggot recombinant chymotrypsin.
Footnotes
Published ahead of print 7 December 2012
REFERENCES
- 1. Costerton JW, Stewart PS, Greenberg EP. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318–1322 [DOI] [PubMed] [Google Scholar]
- 2. Patti JM, Allen BL, McGavin MJ, Hook M. 1994. MSCRAMM-mediated adherence of microorganisms to host tissues. Annu. Rev. Microbiol. 48:585–617 [DOI] [PubMed] [Google Scholar]
- 3. Cramton SE, Gerke C, Schnell NF, Nichols WW, Gotz F. 1999. The intercellular adhesion (ica) locus is present in Staphylococcus aureus and is required for biofilm formation. Infect. Immun. 67:5427–5433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Heilmann C, Schweitzer O, Gerke C, Vanittanakom N, Mack D, Gotz F. 1996. Molecular basis of intercellular adhesion in the biofilm-forming Staphylococcus epidermidis. Mol. Microbiol. 20:1083–1091 [DOI] [PubMed] [Google Scholar]
- 5. Mack D, Fischer W, Krokotsch A, Leopold K, Hartmann R, Egge H, Laufs R. 1996. The intercellular adhesin involved in biofilm accumulation of Staphylococcus epidermidis is a linear β-1,6-linked glucosaminoglycan: purification and structural analysis. J. Bacteriol. 178:175–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Maira-Litran T, Kropec A, Abeygunawardana C, Joyce J, Mark G, III, Goldmann DA, Pier GB. 2002. Immunochemical properties of the staphylococcal poly-N-acetylglucosamine surface polysaccharide. Infect. Immun. 70:4433–4440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Corrigan RM, Rigby D, Handley P, Foster TJ. 2007. The role of Staphylococcus aureus surface protein SasG in adherence and biofilm formation. Microbiology 153:2435–2446 [DOI] [PubMed] [Google Scholar]
- 8. Cucarella C, Solano C, Valle J, Amorena B, Lasa I, Penades JR. 2001. Bap, a Staphylococcus aureus surface protein involved in biofilm formation. J. Bacteriol. 183:2888–2896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rohde H, Burdelski C, Bartscht K, Hussain M, Buck F, Horstkotte MA, Knobloch JK-M, Heilmann C, Herrmann M, Mack D. 2005. Induction of Staphylococcus epidermidis biofilm formation via proteolytic processing of the accumulation-associated protein by staphylococcal and host proteases. Mol. Microbiol. 55:1883–1895 [DOI] [PubMed] [Google Scholar]
- 10. Christner M, Franke GC, Schommer NN, Wendt U, Wegert K, Pehle P, Kroll G, Schulze C, Buck F, Mack D, Aepfelbacher M, Rohde H. 2010. The giant extracellular matrix-binding protein of Staphylococcus epidermidis mediates biofilm accumulation and attachment to fibronectin. Mol. Microbiol. 75:187–207 [DOI] [PubMed] [Google Scholar]
- 11. Golinko MS, Joffe R, Maggi J, Cox D, Chandrasekaran EB, Tomic-Canic RM, Brem H. 2008. Operative debridement of diabetic foot ulcers. J. Am. Coll. Surg. 207:e1–e6 [DOI] [PubMed] [Google Scholar]
- 12. Britland S, Smith A, Finter W, Eagland D, Vowden K, Vowden P, Telford G, Brown A, Pritchard D. 2011. Recombinant Lucilia sericata chymotrypsin in a topical hydrogel formulation degrades human wound eschar ex vivo. Biotechnol. Prog. 27:870–874 [DOI] [PubMed] [Google Scholar]
- 13. Courtenay M, Church JC, Ryan TJ. 2000. Larva therapy in wound management. J. R. Soc. Med. 93:72–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wollina U, Karte K, Herold C, Looks A. 2000. Biosurgery in wound healing—the renaissance of maggot therapy. J. Eur. Acad. Dermatol. Venereol. 14:285–289 [DOI] [PubMed] [Google Scholar]
- 15. Chambers L, Woodrow S, Brown AP, Harris PD, Phillips D, Hall M, Church JCT, Pritchard DI. 2003. Degradation of extracellular matrix components by defined proteinases from the greenbottle larva Lucilia sericata used for the clinical debridement of non-healing wounds. Br. J. Dermatol. 148:14–23 [DOI] [PubMed] [Google Scholar]
- 16. Bexfield A, Bond AE, Roberts EC, Dudley E, Nigam Y, Thomas S, Newton RP, Ratcliffe NA. 2008. The antibacterial activity against MRSA strains and other bacteria of a <500 Da fraction from maggot excretions/secretions of Lucilia sericata (Diptera: Calliphoridae). Microbes Infect. 10:325–333 [DOI] [PubMed] [Google Scholar]
- 17. Bexfield A, Nigam Y, Thomas S, Ratcliffe NA. 2004. Detection and partial characterisation of two antibacterial factors from the excretions/secretions of the medicinal maggot Lucilia sericata and their activity against methicillin-resistant Staphylococcus aureus (MRSA). Microbes Infect. 6:1297–1304 [DOI] [PubMed] [Google Scholar]
- 18. Harris LG, Bexfield A, Nigam Y, Rohde H, Ratcliffe NA, Mack D. 2009. Disruption of Staphylococcus epidermidis biofilms by medicinal maggot Lucilia sericata excretions/secretions. Int. J. Artif. Organs 32:555–564 [DOI] [PubMed] [Google Scholar]
- 19. Bexfield A, Bond AE, Morgan C, Wagstaff J, Newton RP, Ratcliffe NA, Dudley E, Nigam Y. 2010. Amino acid derivatives from Lucilia sericata excretions/secretions may contribute to the beneficial effects of maggot therapy via increased angiogenesis. Br. J. Dermatol. 162:554–562 [DOI] [PubMed] [Google Scholar]
- 20. van der Plas MJA, van der Does AM, Baldry M, Dogterom-Ballering HCM, van Gulpen C, van Dissel JT, Nibbering PH, Jukema GN. 2007. Maggot excretions/secretions inhibit multiple neutrophil pro-inflammatory responses. Microbes Infect. 9:507–514 [DOI] [PubMed] [Google Scholar]
- 21. Telford G, Brown AP, Kind A, English JS, Pritchard DI. 2011. Maggot chymotrypsin I from Lucilia sericata is resistant to endogenous wound protease inhibitors. Br. J. Dermatol. 164:192–196 [DOI] [PubMed] [Google Scholar]
- 22. Telford G, Brown AP, Seabra RA, Horobin AJ, Rich A, English JS, Pritchard DI. 2010. Degradation of eschar from venous leg ulcers using a recombinant chymotrypsin from Lucilia sericata. Br. J. Dermatol. 163:523–531 [DOI] [PubMed] [Google Scholar]
- 23. Mack D, Siemssen N, Laufs R. 1992. Parallel induction by glucose of adherence and a polysaccharide antigen specific for plastic-adherent Staphylococcus epidermidis: evidence for functional relation to intercellular adhesion. Infect. Immun. 60:2048–2057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Iordanescu S, Surdeanu M. 1976. Two restriction and modification systems in Staphylococcus aureus NCTC8325. J. Gen. Microbiol. 96:277–281 [DOI] [PubMed] [Google Scholar]