Abstract
For exhaustive detection of diarrheagenic Escherichia coli, we previously developed a colony-hybridization method using hydrophobic grid-membrane filters in combination with multiplex real-time PCR. To assess the role of domestic animals as the source of atypical enteropathogenic E. coli (aEPEC), a total of 679 samples (333 from foods, fecal samples from 227 domestic animals, and 119 from healthy people) were examined. Combining 48 strains previously isolated from patients and carriers, 159 aEPEC strains were classified by phylogroup, virulence profile, and intimin typing. Phylogroup B1 was significantly more prevalent among aEPEC from patients (50%) and bovine samples (79%) than from healthy carriers (16%) and swine strains (23%), respectively. Intimin type β1 was predominant in phylogroup B1; B1-β1 strains comprised 26% of bovine strains and 25% of patient strains. The virulence profile groups Ia and Ib were also observed more frequently among bovine strains than among porcine strains. Similarly, virulence group Ia was detected more frequently among patient strains than strains of healthy carriers. A total of 85 strains belonged to virulence group I, and 63 of these strains (74%) belonged to phylogroup B1. The present study suggests that the etiologically important aEPEC in diarrheal patients could be distinguished from aEPEC strains indigenous to humans based on type, such as B1, Ia, and β1/γ1, which are shared with bovine strains, while the aEPEC strains in healthy humans are different, and some of these were also present in porcine samples.
INTRODUCTION
Enteropathogenic Escherichia coli (EPEC), one of the six diarrheagenic E. coli (DEC) pathotypes, is a major cause of diarrheal diseases among young children in developing countries (1). A characteristic phenotype of EPEC is the ability to produce attaching and effacing (A/E) lesions (2). The genes responsible for A/E lesion formation are located in a chromosomal pathogenicity island, known as the locus of enterocyte effacement (LEE). The LEE carries a set of genes, including the intimin gene (eae), which plays a crucial role in the A/E phenotype (3).
EPEC can be further classified into typical EPEC (tEPEC) and atypical EPEC (aEPEC), depending on the presence or absence of the plasmid E. coli adherence factor (EAF). EAF has an important operon for bundle-forming pilus (BFP), a type IV fimbrial adhesin (4), which contributes to the phenotype of localized adherence (LA) to HEp-2 cell monolayers. While tEPEC, so-called class I EPEC (5), is a well-recognized pathogen in developing countries (6), aEPEC organisms have been reported to be more prevalent in both developing and developed countries (7). Animals can be reservoirs of aEPEC, whereas the only reservoir of tEPEC is generally considered to be humans (8).
Thus, EPEC is a well-recognized DEC; however, neither the origin nor the etiological role of human aEPEC has been clarified to date (9, 10). Our previous study did not show any significant differences between the isolation rates of EPEC among healthy individuals or among diarrheal patients (11), although EPEC was significantly prevalent among patients aged 1 to 3 years when study populations were stratified by age (12). Clinical microbiologists and food microbiologists often find it difficult to assess the significance of EPEC isolates, particularly when the organisms are isolated from sporadic patients and foods. Therefore, it is helpful for inspectors to understand the properties associated specifically with EPEC isolated from diarrheal patients.
Intimin, an outer membrane protein encoded by eae, is assigned to 17 genetic variants (α1, α2, β1, ξR/β2B, δ/κ/β2O, γ1, θ/γ2, ε1, νR/ε2, ζ, η, ι1, μR/ι2, λ, μB, νB, and ξB) (13). Torres et al. found that the heterogeneous C-terminal (3′) end of intimin is responsible for receptor binding, and different intimin variants may be responsible for different host and tissue cell tropisms (14).
In this study, we examined whether intimin typing, phylogenetic grouping (15), and virulence profile (16) are able to distinguish between aEPEC isolated from diarrheic patients and the organisms from foods or fecal samples of cattle, swine, and healthy carriers. A total of 679 foods and fecal specimens from domestic animals and healthy carriers were examined for EPEC using our multiplex real-time PCR method (17). By combined use with our newly developed hydrophobic grid membrane filter-colony hybridization (HGMF-CH) method (18), 111 EPEC strains were isolated. To accumulate precise information on the properties of EPEC, 48 EPEC strains isolated from humans in our previous studies (11, 19) were also examined.
MATERIALS AND METHODS
Specimens.
A total of 333 food samples of various types (fishes, fruits, meats, shellfish, vegetables, and ready-to-eat foods) were obtained from local retail markets and the Osaka Municipal Central Wholesale Market. A total of 227 domestic animal fecal samples (109 samples of bovine feces and 118 samples of swine feces) and 119 fecal samples from healthy carriers were collected from the Osaka Municipal Meat Inspection Centre and the Osaka City Institute of Public Health and Environmental Sciences, respectively.
Isolation and identification of EPEC from foods and fecal specimens.
Food samples were cultured using brain heart infusion broth (BHI; Oxoid, Basingstoke, United Kingdom) for 3 h at 37°C and double-strength tryptone phosphate (TP) broth for 20 h at 44°C, as reported previously (17). Fecal samples were cultured in BHI for 20 h at 42°C for bacterial enrichment. Enrichment culture broths were screened for the 10 target enterovirulence genes (eae, stx1, stx2, elt, est for human heat-stable toxin [STh], est for porcine heat-stable toxin [STp], virB, aggR, astA, and afaB) using our multiplex real-time PCR method (17), and eae-positive enrichment broths were processed for EPEC isolation using our recently developed HGMF-CH method (18). One-milliliter aliquots of the enrichment broths were pipetted onto an Iso-Grid HGMF (QA Lifesciences Inc., San Diego, CA) placed on the HGMF Spreadfilter (Filtaflex Ltd., Almonte, Canada), and the suspension was then filtered through the HGMF using an HGMF Spreadfilter. Filters were then placed on MacConkey agar (Becton, Dickinson, Sparks, MD) or tryptic soy agar (TSA; Nissui, Tokyo, Japan) plates and cultured overnight at 37°C; filtered bacteria formed colonies in an HGMF array. Colonies were replicated from the incubated HGMF (master HGMF) to fresh HGMFs using a microbial colony replicator (Filtaflex Ltd.). Filters were then cultured on TSA at 37°C to produce replicated filters for HGMF-CH. The cultured HGMFs were placed on Whatman 3MM filter paper (GE Healthcare, Tokyo, Japan) soaked with pretreatment solution (5 mmol liter−1 sodium phosphate buffer [pH 6.0], 100 mmol liter−1 sodium bicarbonate, and 0.0066% polyethyleneimine) and incubated at room temperature for 30 min. After blotting and air drying for 10 min, HGMFs were transferred to fresh Whatman 3MM filter paper soaked with lysis solution (150 mmol liter−1 NaOH in 70% ethanol) (3 ml/HGMF), followed by heating in a microwave oven for 30 s at the highest setting. Heat-treated HGMFs were then gently shaken (100 strokes min−1) in 20 ml 2× SSC (1×SSC is 0.15 M NaCl plus 0.015 M sodium citrate) supplemented with 0.01% proteinase K (Wako Pure Chemical Industries, Ltd., Osaka, Japan) and 0.1% sodium dodecyl sulfate (SDS) for at least 1 h in a 37°C water bath. HGMFs then were washed for 5 min in 2× SSC containing 0.1% SDS followed by 5 min in 2× SSC solution (50 ml/HGMF), and bacterial debris was then gently removed with a Kimwipe tissue (Nippon Paper Crecia Co., Ltd., Tokyo, Japan). HGMFs were placed on blotting paper, air dried for 30 min, and then exposed to 120 mJ of UV light to cross-link bacterial DNA to the filter surface. The HGMFs with cross-linked DNA were placed in 6 ml digoxigenin (DIG) Easy Hyb (Roche Diagnostics) in a hybridization bag (Roche Diagnostics) and were incubated in a water bath for 1 h with gentle shaking (30 strokes min−1) at 39°C to reduce nonspecific hybridization. The DIG Easy Hyb used for prehybridization was discarded and replaced with fresh DIG Easy Hyb (6 ml/HGMF) containing 10 μl of denatured DIG probe solution for eae. HGMFs were incubated for 24 h at 39°C with gentle shaking (30 strokes min−1), similar to the prehybridization step. After 24 h of hybridization, immunological detection of DIG-labeled probes was carried out using the DIG wash and block buffer set, anti-DIG antibody solution, and detection buffer supplemented with 5-bromo-4-chloro-3-indolylphosphate (BCIP; 375 μg ml−1) and nitroblue tetrazolium (NBT; 188 μg ml−1) (Roche Diagnostics). Individual grid cells of HGMFs turned purple when the target gene was present on the square. Each isolate was examined for the presence of other enterovirulence genes in addition to eae by conventional PCR in order to exclude other DECs, particularly Shiga toxin-producing E. coli (STEC) strains carrying the eae gene. Only the strains possessing eae with or without astA were identified as EPEC and were subjected to further study.
Strains.
Twenty EPEC strains were recovered from food samples; 43 strains each were isolated from bovine feces and swine feces, and five strains were isolated from healthy carriers. In addition, 32 EPEC strains from fecal samples of healthy carriers and 16 from fecal samples of diarrheal patients were investigated in this study (11, 19). A total of 159 EPEC strains were used to compare the subtypes of eae, phylogenetic group, and virulence profile. DH5α was used as a nondiarrheagenic control.
Serotyping of EPEC strains.
EPEC strains were serogrouped with 50 specific O antisera designed for pathogenic Escherichia coli (Denka Seiken Co. Ltd., Tokyo, Japan) in accordance with the manufacturer's protocol.
Virulence profiling.
Virulence profiles were based on the scheme of Afset et al. (16). PCR was employed for detection of 12 virulence genes or markers, including OI-122 genes (efa1 [lifA], set [ent], nleB, and nleE) and genes in other locations (lpfA, ehxA, ureD, paa, yjaA, ibeA, b1121, and astA), which were found to be significantly associated with diarrhea (16). In this scheme, aEPEC strains were classified into two main virulence groups based on the presence of these genes: group I strains were defined by the presence of OI-122 genes and/or lpfA genes as well as the absence of the yjaA gene, while group II strains were classified by the presence of the yjaA gene and the absence of OI-122 and lpfA genes. Group I strains were further divided into subgroups Ia and Ib depending on whether they contained the gene with the strongest association with diarrhea, efa1 (lifA). The 14 pairs of primers (including three variants of lpfA) and the PCR conditions used in this study are listed in Table 1 (15–17, 20–24).
Table 1.
Primers and PCR conditions used for identification of virulence profiles of EPEC strains in this study
| Gene | Primer sequence |
PCR conditions |
Reference or source | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Temp (°C) and time for each PCR step |
No. of cycles | Product length (bp) | ||||||||
| Forward | Reverse | Initial denaturation | Denaturation | Annealing | Extension | Final extension | ||||
| efa1 (lifA) | AAGGTGTTACAGAGATTA | TGAGGCGGCAGGATAGTT | 94 (5 min) | 94 (1 min) | 51 (1 min) | 72 (1 min) | 72 (10 min) | 30 | 266 | 20 |
| set (ent) | TTCCTGGGTTGCTTTTAGCTCT | CATGTCCATTTTGAAGGGCCTG | 94 (2 min) | 94 (1 min) | 60 (30 s) | 72 (40 s) | 72 (10 min) | 30 | 171 | This study |
| nleB | GGTGTGCTGGTAGATGGA | CAGGGTATGATTCTTGTTTATG | 94 (2 min) | 94 (30 s) | 53 (30 s) | 72 (40 s) | 72 (10 min) | 30 | 175 | 16 |
| nleE | CTAATACTCAGGGCGTGTCC | ACCGTCTGGCTTTCTCGTTA | 94 (2 min) | 94 (30 s) | 53 (30 s) | 72 (40 s) | 72 (10 min) | 30 | 192 | 16 |
| lpfAO113 | ATGAAGCGTAATATTATAG | TTATTTCTTATATTCGAC | 94 (2 min) | 94 (1 min) | 50 (50 s) | 72 (1 min) | 72 (5 min) | 30 | 573 | 16 |
| lpfA1 | CTGCGCATTGCCGTAAC | ATTTACAGGCGAGATCGTG | 94 (5 min) | 94 (1 min) | 52 (1 min) | 72 (1.5 min) | 72 (10 min) | 30 | 412 | 16 |
| lpfAR141 | GAAAAAGGTTCTGTTTG | GTTGTAAGTCAGGTTGA | 94 (5 min) | 94 (1 min) | 42 (1 min) | 72 (1 min) | 72 (10 min) | 30 | 520 | 16 |
| ehxA | GCATCATCAAGCGTACGTTCC | AATGAGCCAAGCTGGTTAAGCT | 94 (5 min) | 94 (1 min) | 63 (1 min) | 72 (1 min) | 72 (10 min) | 30 | 534 | 21 |
| ureD | CGTCATCATGTCGGTCTGCTCA | GCGTGGCTCCGGCGTAGTTTT | 94 (5 min) | 94 (1 min) | 63 (1 min) | 72 (40 s) | 72 (10 min) | 30 | 569 | 22 |
| paa | ATGAGGAACATAATGGCAGG | TCTGGTCAGGTCGTCAATAC | 94 (5 min) | 94 (1 min) | 55 (1 min) | 72 (1 min) | 72 (10 min) | 30 | 360 | 16 |
| yjaA | TGAAGTGTCAGGAGACGCTG | ATGGAGAATGCGTTCCTCAAC | 94 (4 min) | 94 (5 s) | 59 (10 s) | 72 (5 min) | 30 | 211 | 15 | |
| ibeA | TGGAACCCGCTCGTAATATAC | CTGCCTGTTCAAGCATTGCA | 94 (5 min) | 94 (1 min) | 57 (45 s) | 72 (1 min) | 72 (10 min) | 30 | 342 | 23 |
| b1121 | CGCCTGGGCTGCGACGTTTAT | GCCCTGCCCAGAGTGGCGATA | 94 (2 min) | 94 (30 s) | 60 (30 s) | 72 (40 s) | 72 (5 min) | 30 | 176 | This study |
| astA | CCATCAACACAGTATATCCGA | GGTCGCGAGTGACGGCTTTGT | 94 (2 min) | 94 (30 s) | 53 (30 s) | 72 (40 s) | 72 (10 min) | 30 | 111 | 17 |
| bfpA | GGTCTGTCTTTGATTGAATC | TTTACATGCAGTTGCCGCTT | 94 (2 min) | 94 (1 min) | 55 (1 min) | 72 (1 min) | 72 (8 min) | 30 | 485 | 24 |
| perA | AACAAGAGGAGAATTTAGCG | CTTGTGTAATAGAATAAACGC | 94 (2 min) | 94 (1 min) | 56 (1 min) | 72 (1 min) | 72 (8 min) | 30 | 770 | 24 |
Phylogenetic group determination.
EPEC strains were classified into four major phylogenetic groups (A, B1, B2, and D) as proposed by Clermont et al. (15) according to the presence or absence in the PCR using chuA, yjaA, and DNA fragment TspE4.C2. Briefly, the primer pairs for chuA (5′-GACGAACCAACGGTCAGGAT-3′ and 5′-TGCCGCCAGTACCAAAGACA-3′), yjaA (5′-TGAAGTGTCAGGAGACGCTG-3′ and 5′-ATGGAGAATGCGTTCCTCAAC-3′), and TspE4C2.1 (5′-GAGTAATGTCGGGGCATTCA-3′ and 5′-CGCGCCAACAAAGTATTACG-3′) were added to the standard PCR mixture, and PCR was performed under the following conditions: denaturation for 4 min at 94°C, 30 cycles of 5 s at 94°C and 10 s at 59°C, and a final extension step of 5 min at 72°C. Strains that reacted with the chuA primers were assigned to group B2 or D based on the positive or negative reaction, respectively, with yjaA primers. Similarly, the chuA-negative strains were classified into group B1 or A based on the positive or negative reaction, respectively, of the PCR for TspE4.C2.
Subtyping of eae genes.
In accordance with a report by Blanco et al. (13), eae genotypes (α1, α2, β1, ξR/β2B, δ/κ/β2O, γ1, θ/γ2, ε1, νR/ε2, ζ, η, ι1, μR/ι2, λ, μB, νB, and ξB) were identified using 17 pairs of intimin type-specific PCR primers complementary to the heterogeneous 3′ end of the genes.
HEp-2 cell adherence assay.
HEp-2 cells that had been grown in Dulbecco's modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS; Gibco) were plated onto coverslips (diameter, 13 mm) in 24-well microtiter plates in the absence of antibiotics, and then they were incubated at 37°C for 2 days in the presence of 5% CO2 to form monolayers of HEp-2 cells. Bacterial strains were grown statically overnight at 37°C in 1% buffered peptone water (Oxoid). After washing the monolayers once with 1× Dulbecco's phosphate-buffered saline (PBS), 0.5 ml of basal Eagle's medium containing d-mannose (1%, wt/vol) without antibiotics or sera was added to each well. Overnight bacterial culture (20 μl) was inoculated into each well, and plates were incubated at 37°C in the presence of 5% CO2 for 3 h. Monolayers were washed three times with 1× PBS, and 0.5 ml of medium was added to each well. After a further 3-h incubation period, monolayers were washed thoroughly three times with 1× PBS, fixed with absolute methanol, and stained with 10% (vol/vol) Giemsa, as described previously (25).
Statistics.
The differences between the EPEC strains isolated from different sources were analyzed by performing a chi-squared test with Yates' continuity correction or Fisher's exact probability test. The chi-squared statistic for an M × N contingency table was used to compare the overall distribution of phylogenetic groupings between the EPEC strains isolated from healthy carriers and those from patients and of adherence between EPEC isolates from domestic animals and patients.
RESULTS
Serotyping.
Forty-eight (30%) of the 159 EPEC strains belonged to 22 O serogroups, and the 111 (70%) strains for which serotypes could not be determined with the set of commercially available antisera were designated UT (untypeable) (Table 2). More than half (53%) of the O-typeable strains were of five serogroups: O26 (four strains), O74 (nine strains), O103 (four strains), O153 (five strains), and O157:H7 (three strains).
Table 2.
Distribution of phylogenetic groups and O antigen serotype among 159 EPEC strains used in this studya
| Source | No. (%) and serotype of strains in group: |
Subtotal | |||
|---|---|---|---|---|---|
| A | B1 | B2 | D | ||
| Foods | 7 (35.0); O18, O115, UT (5)b | 7 (35.0); O103 (2), UT (5) | 3 (15.0); O20, UT (2) | 3 (15.0); O8, UT (2) | 20 |
| Cattle | 6 (14.0); O15, UT (5) | 34 (79.1)***; O26, O74, O153 (3), UT (29) | 0 | 3 (7.0); O157 (2), O169 | 43 |
| Swine | 23 (53.5)***; O8, O26 (3), O74 (2), O115, UT (16) | 10 (23.3); O103, UT (9) | 8 (18.6)*; O74 (3), UT (5) | 2 (4.7); O145, UT | 43 |
| Healthy carriers | 7 (18.9); O27, O74, O145, UT (4) | 6 (16.2); O74, O103, O168, UT (3) | 20 (54.1)*; O15, O74, O127a, O128, O166, UT (15) | 4 (10.8); O55, O124, O167, UT | 37 |
| Patients | 1 (6.3); UT | 8 (50.0)*; O119 (2), O153 (2), UT (4) | 3 (18.8); O63, UT (2) | 4 (25.0); O55, O157, UT (2) | 16 |
| Total | 44 (27.7); O8, O15, O18, O26 (3), O27, O74 (3), O115 (2), O145, UT (31) | 65 (40.9); O26, O74 (2), O103 (4), O119 (2), O153 (5), O168, UT (50) | 34 (21.4); O15, O20, O63, O74 (4), O127a, O128, O166, UT (24) | 16 (10.1); O8, O55 (2), O124, O145, O157 (3), O167, O169, UT (6) | 159 |
P values were determined by chi-squared tests with Yates continuity correction or Fisher's exact probability test; * and *** indicate significantly greater among cattle vs. swine, and among healthy carriers vs. patients at P < 0.05 and 0.001, respectively.
Numbers in parentheses after serotype designation indicate the number of strains. UT, could not be serotyped with commercial antisera.
Detection of bfpA and perA by PCR.
Several EPEC strains reportedly react with the bfpA probe but lack a true pEAF (26); production or nonproduction of BFP should be the best distinguishing characteristic for tEPEC and aEPEC strains (8). Six strains, two from foods, three from feces of domestic animals, and one from healthy carriers, showed positive reactions with PCR primers for bfpA (Table 1); however, none of these strains responded to PCR for the gene perA, another virulence marker of tEPEC (27), and these strains did not adhere to HEp-2 cells in a 3-h assay, in contrast to the characteristic adhesion of tEPEC. Consequently, all of these strains were included as aEPEC for further analysis as suggested by Hernandes et al. (28).
Phylogenetic distribution.
Triplex PCR indicated that the 159 EPEC strains examined in this study were distributed in all four phylogenetic groups (Table 2 and Fig. 1). Statistically significant differences were recognized in the overall distribution of phylogenetic groups between healthy carriers and patients (P = 0.013 by the chi-squared M × N method). Phylogroup B1 was more prevalent among patients (50%; P = 0.01) than among healthy carriers. Group B1 was also significantly more predominant among bovine EPEC strains (79%) than among swine strains (23%; P < 0.001), healthy carriers (16%; P < 0.001), and foods (15%; P < 0.001).
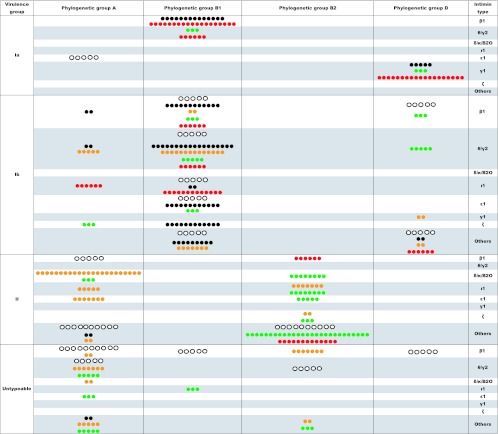
Fig 1.
Distribution of aEPEC strains from different sources. This graph shows the percentage of strains from each source distributed to each zone by specific property (virulence group, phylogenetic group, and intimin type). White dots are for the strains isolated from foods; black, yellow, green, and red are from cattle, swine, healthy carriers, and patients, respectively. One circle represents 1%.
In contrast, phylogroup A was more predominant among the swine strains (54%) than among bovine strains (14%; P < 0.001), patients (6%; P < 0.001), and healthy carriers (19%; P = 0.0015). B2 strains were most prevalent among healthy humans (54%), followed by patients (19%; P < 0.05), foods (15%; P < 0.01), and animals (9%; P < 0.001). Phylogroup B2 was significantly more common among swine strains (19%; P < 0.01) than bovine EPEC strains (0%).
Typing of eae genes.
By subtyping of intimin (eae) genes, strains isolated from cattle, swine, foods, healthy carriers, and patients were assigned into 8, 9, 7, 14, and 7 groups, respectively (Table 3). EPEC strains isolated from human feces showed more intimin types than the strains isolated from foods or feces of domestic animals. Intimins α2 (one strain from healthy carriers and one from patients), η (two strains from healthy carriers), and μB (one strain from healthy carriers) were detected in only five human-derived EPEC strains, which belonged to phylogroup B2, with the exception of one intimin α2-phylogroup D (α2-D) strain from healthy carriers. Intimins μR/ι2, λ, νB, and ξB were not observed in this study. Twenty-four (15.1%) EPEC strains did not produce amplicons with the typing primers used in this study and were designated UT.
Table 3.
Diversity of intimin subtypes and phylogenetic groups among EPEC strains from different sourcesa
| Intimin | No. (%) and phylogenetic groups of strains from: |
|||||
|---|---|---|---|---|---|---|
| Foods | Cattle | Swine | Healthy carriers | Patients | Subtotal | |
| α1 | 1 (5.0); B2 | 0 | 1 (2.3); B2 | 3 (8.1); B2 (3) | 0 | 5 (3.1); B2 (5) |
| α2 | 0 | 0 | 0 | 1 (2.7); D | 1 (6.3); B2 | 2 (1.3); B2, D |
| β1 | 8 (40.0); A (3)b, B1 (3), D (2) | 12 (27.9)*; A, B1 (11) | 5 (11.6); A, B1, B2 (3) | 2 (5.4); B1, D | 5 (31.3)*; B1 (4), B2 | 32 (20.1); A (5), B1 (20), B2 (4), D (3) |
| ξR/β2B | 1 (5.0); B2 | 0 | 0 | 4 (10.8); B2 (4) | 1 (6.3); B2 | 6 (3.8); B2 (6) |
| δ/κ/B2O | 0 | 0*** | 11 (25.6); A(11) | 4 (10.8); A, B2 (3) | 0 | 15 (9.4); A (12), B2 (3) |
| γ1 | 0 | 2 (4.7); D (2) | 1 (2.3); D | 2 (5.4); A,D | 3 (18.8); D (3) | 8 (5.0); A, D (7) |
| θ/γ2 | 3 (15.0); A, B1, B2 | 9 (20.9); A, B1 (8) | 11 (25.6); A (5), B1 (6) | 7 (18.9); A (2), B1 (3), D (2) | 2 (12.5); B1 (2) | 32 (20.1); A (9), B1 (20), B2, D (2) |
| ε1 | 2 (10.0); A, B1 | 5 (11.6); B1 (5) | 3 (7.0); A (3) | 4 (10.8); A, B1, B2 (2) | 0 | 14 (8.8); A (5), B1 (7), B2 (2) |
| νR/ε2 | 0 | 6 (14.0); B1 (6) | 0 | 1 (2.7); B1 | 0 | 7 (4.4); B1 (7) |
| ζ | 0 | 5 (11.6); B1 (5) | 1 (2.3); B2 | 2 (5.4); A, B2 | 0 | 8 (5.0); A, B1 (5), B2 (2) |
| η | 0 | 0 | 0 | 2 (5.4); B2 (2) | 0 | 2 (1.3); B2 (2) |
| ι1 | 1 (5.0); B1 | 3 (7.0); A (2), B1 | 3 (7.0); B2 (3) | 5 (13.5); A, B1, B2 (3) | 3 (18.8); A, B1 (2) | 15 (9.4); A (4), B1 (5), B2 (6) |
| μR/ι2 | 0 | 0 | 0 | 0 | 0 | 0 |
| λ | 0 | 0 | 0 | 0 | 0 | 0 |
| μB | 0 | 0 | 0 | 1 (2.7); B2 | 0 | 1 (0.6); B2 |
| νB | 0 | 0 | 0 | 0 | 0 | 0 |
| ξB | 0 | 0 | 0 | 0 | 0 | 0 |
| UTc | 4 (20.0); A (2), B1, D | 6 (14.0); A (2), B1 (3), D | 7 (16.3); A (3), B1 (3), D | 6 (16.2); A (2), B2 (4) | 1 (6.3); D | 24 (15.1); A (9), B1 (7), B2 (4), D (4) |
| Totald | 20 | 48 | 43 | 44 | 16 | 171 |
P values were determined by chi-squared tests with Yates continuity correction or Fisher's exact probability test; * and *** indicate significant differences among cattle versus swine and among healthy carriers versus patients at P < 0.05 and P < 0.001, respectively.
Numbers in parentheses indicate the number of strains.
UT, untypeable.
Five bovine strains and seven strains from healthy carriers showed positive reactions with two sets of intimin primers simultaneously.
Some of the EPEC strains could be assigned to specific subtypes based on their intimin types and phylogenetic groups. When the prevalence of each subtype of the intimin phylogroup was statistically compared based on the source of the isolates, β1-B1 was most prevalent among bovine strains (26%; P = 0.0017), followed by θ/γ2-B1 (19%), νR/ε2-B1 (14%; P = 0.013), ε1-B1 (12%; P = 0.028), and ζ-B1 (12%; P = 0.028) (Table 3); β1-B1 was also significantly more prevalent among patients (25%; P = 0.025) than among healthy carriers. In contrast, the intimin δ/κ/β2O-phylogroups A (δ/κ/β2O-A) (P = 0.00024), θ/γ2-A, and θ/γ2-B1 were present among 51% of EPEC strains from swine feces.
Most of the intimin δ/κ/β2O strains (80%) belonged to phylogroup A and were from swine feces (11 strains of phylogroup A) and healthy carriers (1 strain of phylogroup A and 3 strains of phylogroup B2). All of the intimin νR/ε2 strains (six bovine strains and a healthy carrier strain) belonged to phylogroup B1, and all of the intimin α1 strains (one strain from swine feces, one from foods, and three from healthy carriers), ξR/β2B strains (one strain from foods, four strains from healthy carriers, and one from patients), η strains (two from healthy carriers), and a μB strain from healthy carriers belonged to phylogroup B2.
A total of 12 strains (7%; five bovine strains and seven strains from healthy carriers) showed positive reactions with two sets of typing primers. Three O153 and two O-untypeable strains from bovine feces and one O168 strain from healthy carriers were ε1 and νR/ε2 positive. For the other six strains from healthy carriers, one O27 strain was θ/γ2 and ι1 positive; one O74 strain was θ/γ2 and γ1 positive; one O124 strain was θ/γ2 and α2 positive; one O128 and another O-untypeable strain were ε1 and η positive; and one O-untypeable strain was α1 and ι1 positive. Although it was not elucidated whether this double positivity was the result of nonspecific reactions or reflected the presence of double eae genes, these strains were counted twice for both positive genes.
Virulence profiles.
According to the scheme of Afset et al. (16), 159 strains of EPEC were assigned to three virulence groups (Table 4; also see Table S1 in the supplemental material). The virulence groups Ia and Ib were significantly more frequent among strains from cattle than among porcine strains. Similarly, virulence group Ia was detected significantly more frequently among strains from patients than among strains from healthy carriers. Organisms belonging to virulence group II and the untypeable group were significantly more prevalent among porcine strains than among bovine strains. Twenty-five strains did not belong to either of the two main virulence groups.
Table 4.
Prevalence of each virulence group of aEPEC isolated from different sourcesa
| Source | No. (%) of strains in virulence group: |
Subtotal | |||
|---|---|---|---|---|---|
| Ia | Ib | II | None | ||
| Cattle | 8** (18.6) | 31*** (72.1) | 3 (7.0) | 1 (2.3) | 43 |
| Swine | 0 | 14 (32.6) | 18*** (41.9) | 11** (25.6) | 43 |
| Foods | 1 (5.0) | 8 (40.0) | 5 (25.0) | 6 (30.0) | 20 |
| Healthy carriers | 2 (5.4) | 8 (21.6) | 20* (54.1) | 7 (18.9) | 37 |
| Patients | 7** (43.8) | 6 (37.5) | 3 (18.8) | 0 | 16 |
| Total | 18 | 67 | 49 | 25 | 159 |
P values were determined by chi-squared tests with Yates continuity correction or Fisher's exact probability test; *, **, and *** indicate significantly higher numbers of strains at P < 0.05, P < 0.01, and P < 0.001, respectively, between cattle and swine or patients and healthy carriers.
A total of 18 strains were present in group I, and 11 strains (61%) belonged to phylogenetic group B1 (see Table S1 in the supplemental material). Sixty-seven strains were present in group Ib, and 52 strains (78%) were present in phylogenetic group B1. In contrast, the 49 strains in virulence group II comprised 21 strains (43%) from phylogenetic group A and 28 strains (57%) from group B2. Twenty-five strains did not fit into any virulence group. These untypeable strains belonged to four phylogenetic groups; however, group A (16 strains; 64%) and group B2 (six strains; 24%) were major constituents.
Adherence to HEp-2 cells.
One hundred thirty EPEC strains (82%) showed the localized adherence (LA) pattern, whereas localized adherence/aggregative adherence (LA/AA) (one strain from pig, one from patients), localized adherence/diffuse adherence (LA/DA) (two strains from patients), and diffuse adherence (DA) (one strain from pig, one from healthy carriers, two from patients) patterns were also observed (Table 5). Nineteen strains (12%) did not adhere to HEp-2 cells in a 6-h adherence assay. Two strains (1.3%) from swine were confirmed as promoting cell detachment after six repeated adherence tests.
Table 5.
Number of EPEC strains with HEp-2 adhesion patterns from different sources
| Source | No. of strains froma: |
Subtotal | |||||
|---|---|---|---|---|---|---|---|
| LA | DA | LA/DA | LA/AA | DE | NA | ||
| Cattle | 41 | 0 | 0 | 0 | 0 | 2 | 43 |
| Swine | 36 | 1 | 0 | 1 | 2 | 3 | 43 |
| Subtotal | 77*/*** | 1 | 0 | 1 | 2 | 5 | 86 |
| Food | 16 | 0 | 0 | 0 | 0 | 4 | 20 |
| Healthy carriers | 28 | 1 | 0 | 0 | 0 | 8† | 37 |
| Patients | 9 | 2 | 2# | 1 | 0 | 2 | 16 |
| Total | 130 | 4 | 2 | 2 | 2 | 19 | 159 |
LA, localized adherence; DA, diffuse adherence; LA/DA, localized adherence/diffuse adherence; LA/AA, localized adherence/aggregative adherence; DE, detachment; NA, nonadherence. P values were determined by chi-squared tests with Yates continuity correction or Fisher's exact probability test; * and *** indicate significantly more strains among domestic animals versus healthy carriers and patients, at P < 0.05 and P < 0.001, respectively, # indicates significantly more strains among patients versus healthy carriers at P < 0.05, and † indicates significantly more strains among healthy carriers versus domestic animals at P < 0.01.
LA was more prevalent among animal strains (90%) than among strains from humans (70%; P < 0.01), and prevalence of nonadherent strains was significantly higher among humans (19%) than among domestic animals (6%; P = 0.016). However, no significant differences in adherence were observed between the strains from patients and those of healthy carriers. No significant differences in adherence ability were observed between bfpA-positive EPEC and bfpA-negative strains (P = 0.82) (data not shown).
DISCUSSION
It remains to be clarified whether all of the eae-possessing E. coli strains are enteropathogenic in humans (27, 28). In this study, we attempted to discriminate between EPEC isolated from diarrheal patients and microorganisms isolated from food, animals, and healthy individuals. To our knowledge, this is the first study to simultaneously perform phylogenetic grouping, intimin typing, and virulence profiling of both human strains and strains isolated from animals and food. Our EPEC strains included serogroups O55, O157, and O119, which are the main EPEC serotypes (29). We cannot confirm whether the three strains of O157, one from a patient and two from cattle, were originally EHEC, although they possessed no Shiga toxin genes at their isolation. Although O antigen grouping could not provide useful information to distinguish patient EPEC from other EPEC strains, molecular epidemiological grouping could be effective for this purpose.
Phylogenetic grouping revealed that group A is prevalent in swine while group B1 is prevalent in cattle; these findings are concordant with the observations of Baldy-Chudzik et al., who reported the prevalence of group B1 in herbivorous animals and the prevalence of group A in carnivorous and omnivorous animals (30). The prevalence of groups B2 and A in healthy individuals was also similar to the findings of Escobar-Páramo et al. (31). In contrast, the strains isolated from patients belonged to groups B1 and D. These findings suggest cattle as a major source of diarrheagenic strains in humans, particularly of group B1.
Afset et al. developed a virulence profiling scheme and showed its epidemiological significance (16). In this study, we utilized their scheme, but PCR was used instead of oligonucleotide microarray. Our virulence profiling also supports the finding that cattle are the source of diarrheagenic EPEC, in addition to the phylogenetic and intimin typing data. Combined use of phylogenetic grouping and virulence profiles confirmed that groups B1 and D and virulence group Ia were specific among patients and cattle. Virulence group II was prevalent among swine and healthy individuals; however, group B2 was common in healthy individuals while group A was common in swine, and this finding is concordant with a previous report that used a microarray (32). These findings could be due to the fact that aEPEC of group Ia has efa1 (lifA) instead of bfp, as these genes are implicated in the adherence to aEPEC to epithelial cells (28, 33).
Through the simultaneous analysis of intimin types and phylogenetic groups, we found that several intimin subtypes belonged to specific phylogenetic groups. Intimin type β1 was prevalent among the strains, particularly in phylogenetic group B1 and virulence group I; similarly, intimin type γ1 was found in phylogenetic group D and virulence group Ia. This is also concordant with a previous report in which most intimin β strains belonged to phylogenetic groups A and B1 (34). As these aEPEC strains were from patients and cattle, the organisms must be diarrheagenic in humans and be carried by beef products; they were also detected in three food samples. Strains of θ/γ2 belonged to phylogenetic group B1. However, most of these belonged to virulence group Ib and were often observed among healthy individuals rather than patients. All strains of intimin type α1, ξR/β2B, η, and μB belonged to phylogenetic group B2 in this study, and most of these were in virulence group II; this finding is similar to those of previous reports in which all intimin α and ξ strains belonged to phylogenetic group B2 (34, 35).
Thus, combined use of phylogenetic grouping and intimin typing or virulence grouping is able to distinguish human diarrheagenic strains among aEPEC isolates. Intimin mediates the intimate bacterial attachment to the host cell surface of EPEC. EPEC strains from patients reportedly possess intimin prevalence similar to that of STEC strains, particularly those recovered from outbreaks of hemolytic-uremic syndrome (HUS) and hemorrhagic colitis (HC); intimin β1, γ1, and θ/γ2 were the most prevalent subtypes of eae-possessing STEC (36, 37). In our study, intimin β1 (31%) was the most common subtype of aEPEC strains from patients, and it was significantly more prevalent than strains from healthy carriers, although no significant differences were observed between the isolation rates of γ1 and θ/γ2 EPEC among patients and among healthy carriers.
EPEC strains isolated from healthy carriers showed a diversity of intimin types compared to strains from foods, domestic animals, and patients. Intimin α2, η, and μB were detected in only five human-derived EPEC strains. Previously, intimin α2, η, and μ were detected mainly in human EPEC strains (36, 38–40), with the exception of one intimin α2 strain from cat (40) and one intimin η2 strain from cattle (41). Humans also appear to be a reservoir of the aEPEC possessing these intimin types in Japan. However, avian pathogenic E. coli (APEC) possessing eae is highly prevalent in chickens (42, 43) and tends to belong to phylogenetic group B2 (44). A future investigation will be necessary to determine whether the variety of aEPEC strains of the phylogenetic group B2 isolated from healthy individuals is from poultry origins.
In addition to healthy carriers, intimin δ/κ/β2O strains were found only in swine feces. However, swine strains of intimin δ/κ/β2O were of phylogenetic group A, while intimin δ/κ/β2O strains from healthy carriers belonged to phylogenetic groups A and B2. Intimin δ, κ, β2, and β2/δ subtypes were reportedly detected in cattle and sheep (phylogenetic group not known), dogs and cats (phylogenetic group A), and diarrheal children (phylogenetic group not known) (34, 38, 41, 45). These results suggest that swine and pets are a reservoir of δ/κ/β2O-A strains, while humans are a reservoir of δ/κ/β2O-B2 EPEC.
The ξR/β2B-B2 strains were found in one ocean fish sample, one patient, and four healthy carriers. The fish may have been contaminated at the market, as ruminants are a potential reservoir of ξR/β2B-B2 strains (41, 45). On the other hand, intimin ε1, ζ, and ι1 strains are not associated with specific phylogenetic groups or sources, while intimins μR/ι2, λ, νB, and ξB were not detected in this study or in the study of Blanco et al. (13). Few studies have reported EPEC with intimins μ, ι2, λ, ν, and ξ. Two intimin μ and one intimin λ strain from children (39), one intimin λ strain from a diarrheal child (46), two intimin ξ strains from goose (34), and two intimin ι2 and three ν strains from cattle (45) were detected in Brazil, India, the United States, and New Zealand, respectively. One intimin ξ strain from cattle was STEC (47). The intimin μR/ι2, λ, νB, and ξB EPEC strains do not appear to be prevalent in humans or domestic animals in Osaka, Japan.
Nonadherent EPEC strains were isolated from healthy carriers more frequently than from domestic animals (P < 0.01). However, Fisher's exact test showed no significant differences between domestic animals and patients. This finding also supports the notion that domestic animals are the reservoirs and sources of EPEC infection in humans, as previously suggested (48). The EPEC group mainly isolated from healthy individuals may be part of human commensal flora and is unlikely to be enteropathogenic in humans.
According to the definition of typical and atypical EPEC, a total of 6 strains (2.5%) were first identified as typical EPEC based on their possessing bfp, although none of these was isolated from patients. In tEPEC, the per operon located on the EPEC adherence factor plasmid is known to be a positive regulator for the LEE genes (49). As our bfp-positive strains were per negative and, unlike tEPEC, did not show typical localized adhesion to HEp-2 cells in 3 h, we assigned these strains to aEPEC; bfp is unlikely to be a decisive marker to identify highly virulent tEPEC strains in Japan. The results are similar to recent reports in which aEPEC is an emerging DEC pathotype (28).
EPEC is the most well-known category of DEC; however, recent isolates are atypical EPEC, and its etiological role remains controversial. It is difficult to judge whether aEPEC isolates are causative agents in sporadic patient cases or serious hazards in food hygiene. The present study suggests that aEPEC, particularly of phylogenetic group B1 or D, virulence group Ia, or intimin type β1 or γ1, induce diarrhea in humans. To conveniently screen for aEPEC strains that are diarrheagenic to humans, phylogenetic grouping is the first choice, and combined use with intimin typing or virulence grouping would further assist in estimating the diarrheagenicity of aEPEC strains. Alternating the full scheme of Afset et al. (16) or intimin typing, PCRs for efa1 (lifA) and intimin types β1 and γ1 could be used to identify the most etiologically important aEPEC strains.
Supplementary Material
ACKNOWLEDGMENTS
This study was partially supported by a grant from the Ministry of Health, Labor, and Welfare of Japan and a Japan-Korea Joint Research Project from the Japan Society for the Promotion of Science.
Footnotes
Published ahead of print 7 December 2012
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.03380-12.
REFERENCES
- 1. Scaletsky IC, Fabbricotti SH, Silva SO, Morais MB, Fagundes-Neto U. 2002. HEp-2-adherent Escherichia coli strains associated with acute infantile diarrhea, Sao Paulo, Brazil. Emerg. Infect. Dis. 8:855–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Moon HW, Whipp SC, Argenzio RA, Levine MM, Giannella RA. 1983. Attaching and effacing activities of rabbit and human enteropathogenic Escherichia coli in pig and rabbit intestines. Infect. Immun. 41:1340–1351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Castillo A, Eguiarte LE, Souza V. 2005. A genomic population genetics analysis of the pathogenic enterocyte effacement island in Escherichia coli: the search for the unit of selection. Proc. Natl. Acad. Sci. U. S. A. 102:1542–1547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Girón JA, Ho AS, Schoolnik GK. 1991. An inducible bundle-forming pilus of enteropathogenic Escherichia coli. Science 254:710–713 [DOI] [PubMed] [Google Scholar]
- 5. Nataro JP, Baldini MM, Kaper JB, Black RE, Bravo N, Levine MM. 1985. Detection of an adherence factor of enteropathogenic Escherichia coli with a DNA probe. J. Infect. Dis. 152:560–565 [DOI] [PubMed] [Google Scholar]
- 6. Campos LC, Franzolin MR, Trabulsi LR. 2004. Diarrheagenic Escherichia coli categories among the traditional enteropathogenic E. coli O serogroups–a review. Mem. Inst. Oswaldo Cruz 99:545–552 [DOI] [PubMed] [Google Scholar]
- 7. Ochoa TJ, Barletta F, Contreras C, Mercado E. 2008. New insights into the epidemiology of enteropathogenic Escherichia coli infection. Trans. R. Soc. Trop. Med. Hyg. 102:852–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Trabulsi LR, Keller R, Tardelli Gomes TA. 2002. Typical and atypical enteropathogenic Escherichia coli. Emerg. Infect. Dis. 8:508–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nguyen RN, Taylor LS, Tauschek M, Robins-Browne RM. 2006. Atypical enteropathogenic Escherichia coli infection and prolonged diarrhea in children. Emerg. Infect. Dis. 12:597–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Forestier C, Meyer M, Favre-Bonte S, Rich C, Malpuech G, Le Bouguenec C, Sirot J, Joly B, De Champs C. 1996. Enteroadherent Escherichia coli and diarrhea in children: a prospective case-control study. J. Clin. Microbiol. 34:2897–2903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fujihara S, Arikawa K, Aota T, Tanaka H, Nakamura H, Wada T, Hase A, Nishikawa Y. 2009. Prevalence and properties of diarrheagenic Escherichia coli among healthy individuals in Osaka City, Japan. Jpn. J. Infect. Dis. 62:318–323 [PubMed] [Google Scholar]
- 12. Meraz IM, Arikawa K, Nakamura H, Ogasawara J, Hase A, Nishikawa Y. 2007. Association of IL-8-inducing strains of diffusely adherent Escherichia coli with sporadic diarrheal patients with less than 5 years of age. Braz. J. Infect. Dis. 11:44–49 [DOI] [PubMed] [Google Scholar]
- 13. Blanco M, Schumacher S, Tasara T, Zweifel C, Blanco JE, Dahbi G, Blanco J, Stephan R. 2005. Serotypes, intimin variants and other virulence factors of eae positive Escherichia coli strains isolated from healthy cattle in Switzerland. Identification of a new intimin variant gene (eae-eta2). BMC Microbiol. 5:23 doi:10.1186/1471-2180-5-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Torres AG, Zhou X, Kaper JB. 2005. Adherence of diarrheagenic Escherichia coli strains to epithelial cells. Infect. Immun. 73:18–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Clermont O, Bonacorsi S, Bingen E. 2000. Rapid and simple determination of the Escherichia coli phylogenetic group. Appl. Environ. Microbiol. 66:4555–4558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Afset JE, Bruant G, Brousseau R, Harel J, Anderssen E, Bevanger L, Bergh K. 2006. Identification of virulence genes linked with diarrhea due to atypical enteropathogenic Escherichia coli by DNA microarray analysis and PCR. J. Clin. Microbiol. 44:3703–3711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hidaka A, Hokyo T, Arikawa K, Fujihara S, Ogasawara J, Hase A, Hara-Kudo Y, Nishikawa Y. 2009. Multiplex real-time PCR for exhaustive detection of diarrhoeagenic Escherichia coli. J. Appl. Microbiol. 106:410–420 [DOI] [PubMed] [Google Scholar]
- 18. Wang L, Wakushima M, Kamata Y, Nishikawa Y. 2011. Exhaustive isolation of diarrhoeagenic Escherichia coli by a colony hybridization method using hydrophobic grid-membrane filters in combination with multiplex real-time PCR. Lett. Appl. Microbiol. 53:264–270 [DOI] [PubMed] [Google Scholar]
- 19. Nishikawa Y, Zhou Z, Hase A, Ogasawara J, Kitase T, Abe N, Nakamura H, Wada T, Ishii E, Haruki K, Surveillance Team 2002. Diarrheagenic Escherichia coli isolated from stools of sporadic cases of diarrheal illness in Osaka City, Japan between 1997 and 2000: prevalence of enteroaggregative E. coli heat-stable enterotoxin 1 gene-possessing E. coli. Jpn. J. Infect. Dis. 55:183–190 [PubMed] [Google Scholar]
- 20. Nicholls L, Grant TH, Robins Browne RM. 2000. Identification of a novel genetic locus that is required for in vitro adhesion of a clinical isolate of enterohaemorrhagic Escherichia coli to epithelial cells. Mol. Microbiol. 35:275–288 [DOI] [PubMed] [Google Scholar]
- 21. Paton AW, Paton JC. 1998. Detection and characterization of Shiga toxigenic Escherichia coli by using multiplex PCR assays for stx1, stx2, eaeA, enterohemorrhagic E. coli hlyA, rfbO111, and rfbO157. J. Clin. Microbiol. 36:598–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Friedrich AW, Kock R, Bielaszewska M, Zhang W, Karch H, Mathys W. 2005. Distribution of the urease gene cluster among and urease activities of enterohemorrhagic Escherichia coli O157 isolates from humans. J. Clin. Microbiol. 43:546–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Niu C, Wang S, Lu C. 2012. Development and evaluation of a dot blot assay for rapid determination of invasion-associated gene ibeA directly in fresh bacteria cultures of E. coli. Folia Microbiol. (Praha) 57:557–561 [DOI] [PubMed] [Google Scholar]
- 24. Nara JM, Cianciarullo AM, Culler HF, Bueris V, Horton DS, Menezes MA, Franzolin MR, Elias WP, Piazza RM. 2010. Differentiation of typical and atypical enteropathogenic Escherichia coli using colony immunoblot for detection of bundle-forming pilus expression. J. Appl. Microbiol. 109:35–43 [DOI] [PubMed] [Google Scholar]
- 25. Nishikawa Y, Scotland SM, Smith HR, Willshaw GA, Rowe B. 1995. Catabolite repression of the adhesion of Vero cytotoxin-producing Escherichia coli of serogroups O157 and O111. Microb. Pathog. 18:223–229 [DOI] [PubMed] [Google Scholar]
- 26. Goncalves AG, Campos LC, Gomes TA, Rodrigues J, Sperandio V, Whittam TS, Trabulsi LR. 1997. Virulence properties and clonal structures of strains of Escherichia coli O119 serotypes. Infect. Immun. 65:2034–2040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schmidt MA. 2010. LEEways: tales of EPEC, ATEC and EHEC. Cell. Microbiol. 12:1544–1552 [DOI] [PubMed] [Google Scholar]
- 28. Hernandes RT, Elias WP, Vieira MA, Gomes TA. 2009. An overview of atypical enteropathogenic Escherichia coli. FEMS Microbiol. Lett. 297:137–149 [DOI] [PubMed] [Google Scholar]
- 29. Scaletsky IC, Souza TB, Aranda KR, Okeke IN. 2010. Genetic elements associated with antimicrobial resistance in enteropathogenic Escherichia coli (EPEC) from Brazil. BMC Microbiol. 10:25 doi:10.1186/1471-2180-10-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Baldy-Chudzik K, Mackiewicz P, Stosik M. 2008. Phylogenetic background, virulence gene profiles, and genomic diversity in commensal Escherichia coli isolated from ten mammal species living in one zoo. Vet. Microbiol. 131:173–184 [DOI] [PubMed] [Google Scholar]
- 31. Escobar-Paramo P, Grenet K, Le Menac'h A, Rode L, Salgado E, Amorin C, Gouriou S, Picard B, Rahimy MC, Andremont A, Denamur E, Ruimy R. 2004. Large-scale population structure of human commensal Escherichia coli isolates. Appl. Environ. Microbiol. 70:5698–5700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Afset JE, Anderssen E, Bruant G, Harel J, Wieler L, Bergh K. 2008. Phylogenetic backgrounds and virulence profiles of atypical enteropathogenic Escherichia coli strains from a case-control study using multilocus sequence typing and DNA microarray analysis. J. Clin. Microbiol. 46:2280–2290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Badea L, Doughty S, Nicholls L, Sloan J, Robins-Browne RM, Hartland EL. 2003. Contribution of Efa1/LifA to the adherence of enteropathogenic Escherichia coli to epithelial cells. Microb. Pathog. 34:205–215 [DOI] [PubMed] [Google Scholar]
- 34. Ishii S, Meyer KP, Sadowsky MJ. 2007. Relationship between phylogenetic groups, genotypic clusters, and virulence gene profiles of Escherichia coli strains from diverse human and animal sources. Appl. Environ. Microbiol. 73:5703–5710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Escobar-Paramo P, Clermont O, Blanc-Potard AB, Bui H, Le Bouguenec C, Denamur E. 2004. A specific genetic background is required for acquisition and expression of virulence factors in Escherichia coli. Mol. Biol. Evol. 21:1085–1094 [DOI] [PubMed] [Google Scholar]
- 36. Oswald E, Schmidt H, Morabito S, Karch H, Marches O, Caprioli A. 2000. Typing of intimin genes in human and animal enterohemorrhagic and enteropathogenic Escherichia coli: characterization of a new intimin variant. Infect. Immun. 68:64–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Torres AG, Blanco M, Valenzuela P, Slater TM, Patel SD, Dahbi G, Lopez C, Barriga XF, Blanco JE, Gomes TA, Vidal R, Blanco J. 2009. Genes related to long polar fimbriae of pathogenic Escherichia coli strains as reliable markers to identify virulent isolates. J. Clin. Microbiol. 47:2442–2451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Beutin L, Marches O, Bettelheim KA, Gleier K, Zimmermann S, Schmidt H, Oswald E. 2003. HEp-2 cell adherence, actin aggregation, and intimin types of attaching and effacing Escherichia coli strains isolated from healthy infants in Germany and Australia. Infect. Immun. 71:3995–4002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Scaletsky IC, Aranda KR, Souza TB, Silva NP. 2010. Adherence factors in atypical enteropathogenic Escherichia coli strains expressing the localized adherence-like pattern in HEp-2 cells. J. Clin. Microbiol. 48:302–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Moura RA, Sircili MP, Leomil L, Matte MH, Trabulsi LR, Elias WP, Irino K, Pestana de Castro AF. 2009. Clonal relationship among atypical enteropathogenic Escherichia coli strains isolated from different animal species and humans. Appl. Environ. Microbiol. 75:7399–7408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Blanco JE, Blanco M, Alonso MP, Mora A, Dahbi G, Coira MA, Blanco J. 2004. Serotypes, virulence genes, and intimin types of Shiga toxin (verotoxin)-producing Escherichia coli isolates from human patients: prevalence in Lugo, Spain, from 1992 through 1999. J. Clin. Microbiol. 42:311–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Alonso MZ, Padola NL, Parma AE, Lucchesi PM. 2011. Enteropathogenic Escherichia coli contamination at different stages of the chicken slaughtering process. Poult. Sci. 90:2638–2641 [DOI] [PubMed] [Google Scholar]
- 43. Oh JY, Kang MS, An BK, Shin EG, Kim MJ, Kim YJ, Kwon YK. 2012. Prevalence and characteristics of intimin-producing Escherichia coli strains isolated from healthy chickens in Korea. Poult. Sci. 91:2438–2443 [DOI] [PubMed] [Google Scholar]
- 44. Mora A, Lopez C, Dabhi G, Blanco M, Blanco JE, Alonso MP, Herrera A, Mamani R, Bonacorsi S, Moulin-Schouleur M, Blanco J. 2009. Extraintestinal pathogenic Escherichia coli O1:K1:H7/NM from human and avian origin: detection of clonal groups B2 ST95 and D ST59 with different host distribution. BMC Microbiol. 9:132 doi:10.1186/1471-2180-9-132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cookson AL, Bennett J, Thomson-Carter F, Attwood GT. 2007. Intimin subtyping of Escherichia coli: concomitant carriage of multiple intimin subtypes from forage-fed cattle and sheep. FEMS Microbiol. Lett. 272:163–171 [DOI] [PubMed] [Google Scholar]
- 46. Ghosh PK, Ali A. 2010. Isolation of atypical enteropathogenic Escherichia coli from children with and without diarrhoea in Delhi and the National Capital Region, India. J. Med. Microbiol. 59:1156–1162 [DOI] [PubMed] [Google Scholar]
- 47. Blanco M, Blanco JE, Mora A, Dahbi G, Alonso MP, Gonzalez EA, Bernardez MI, Blanco J. 2004. Serotypes, virulence genes, and intimin types of Shiga toxin (verotoxin)-producing Escherichia coli isolates from cattle in Spain and identification of a new intimin variant gene (eae-xi). J. Clin. Microbiol. 42:645–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Krause G, Zimmermann S, Beutin L. 2005. Investigation of domestic animals and pets as a reservoir for intimin (eae) gene positive Escherichia coli types. Vet. Microbiol. 106:87–95 [DOI] [PubMed] [Google Scholar]
- 49. Iyoda S, Watanabe H. 2004. Positive effects of multiple pch genes on expression of the locus of enterocyte effacement genes and adherence of enterohaemorrhagic Escherichia coli O157:H7 to HEp-2 cells. Microbiology 150:2357–2371 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.