Abstract
Immunotherapy for solid tumors has shown promise in preclinical as well as early clinical studies. However, its efficacy remains limited. The hindrance to achieving objective, long-lasting therapeutic responses in solid tumors is, in part, mediated by the dynamic nature of the tumor and its complex microenvironment. Tumor-directed therapies fail to eliminate components of the microenvironment, which can reinstate a tumorigenic milieu and contribute to recurrence. Cancer-associated fibroblasts (CAFs) form the most preponderant cell type in the solid tumor microenvironment. Given their pervasive role in facilitating tumor growth and metastatic dissemination, CAFs have emerged as attractive therapeutic targets in the tumor microenvironment. In this article, we highlight the cross-talk between CAFs and cancer cells, and discuss how targeting CAFs has the potential to improve current immunotherapy approaches for cancer.
Keywords: cancer-associated fibroblasts, FAP-directed therapies, fibroblast activation protein, immunotherapy, tumor microenvironment
The importance of the microenvironment in providing tumorigenic signals first came to light in 1889, when Stephen Paget proposed his ‘seed and soil’ hypothesis. Contrary to the then accepted notion that the site of secondary metastasis was simply random, Paget proposed that the tissue microenvironment or ‘the soil’ dictated the affinity of disseminated cancer cells to certain organs versus others [1]. Since then, studies have unraveled the complex interactions between cancer cells and the host microenvironment that govern every facet of tumorigenesis and metastatic spread. The microenvironment is highly heterogeneous, where a multitude of cells secrete autocrine and paracrine factors and form a labyrinth of signaling networks. Cancer-associated fibroblasts (CAFs) form the nexus of this labyrinth and regulate the cross-talk between cancer cells and components of the host milieu. CAFs contribute to desmoplasia, which not only increases tumor growth but also results in profound therapeutic resistance [2]. In this article, we will review the role of CAFs in tumor progression and their emergence as novel targets for immunotherapy.
The origin of CAFs
The term CAF is used to describe cells that are morphologically and functionally distinct from their resting or quiescent counterparts. Elongated into a spindle shape by the acquisition of contractile stress fibers, CAFs remain perpetually activated resulting in the ‘wounds that never heal’ phenotype that is associated with most cancers. Additionally, they express markers like the actin isoforms α-SMA and γ-SMA; paladin, vimentin, endosialin, podoplanin, FSP-1 and FAP [3]. There are those that arise from senescent fibroblasts as a result of chronic inflammation or an accumulation of free radicals. These primed fibroblasts can easily convert benign lesions into malignant tumors explaining the increased incidence of certain cancers with age [4]. Chronic inflammation concomitantly induces somatic mutations in the epithelial compartment resulting in activation of proto-oncogenes and this further drives tumorigenesis [5]. Inevitably, the expanding tumor expropriates other normal tissue fibroblasts either by inducing somatic mutations [6,7] or by epigenetic means [8] to meet its growing demands. The cancer cells also commandeer other components in the microenvironment, including secondary epithelia [9] and endothelial cells [10], to transdifferentiate into CAFs. An influx of bone marrow-derived cells provides yet another source [11], allowing further accumulation of CAFs in the microenvironment. These seemingly disparate sources highlight the exigency of the growing malignancy for CAF-mediated support. Consequently, the cancer cells and CAFs enter into a symbiotic relationship that initiates an irreversible chain of events leading to their activation and growth.
Activation of CAFs
Once ensconced in to the tumor microenvironment, fibroblasts commence a differentiation pathway, which is in part evoked by the inflammatory milieu, which closely resembles what is seen during wound healing. In the first stage, fibroblasts differentiate into proto-myofibroblasts that are characterized by the formation of stress fibers. These proto-myofibroblasts generate mechanical tension in the extracellular matrix (ECM), which activates TGF-β1 [12]. In the second stage, TGF-β1 causes trans-differentiation of proto-myofibroblasts into myofibroblasts or CAFs, which can be recognized by the neo-expression of α-SMA [13]. This differentiation event is potentiated by downstream effectors of TGF-β1, which include heparin sulfate and decorin [14]. The neighboring cancer cells are also inextricably exposed to these growth factors, facilitating their rapid proliferation. To sustain CAF-mediated support for the growing tumor mass, cancer cells bring in additional reinforcements. Macrophages infiltrating the tumor for example, secrete PDGF and TGF-β1 [15]. Accumulating TGF-β1 can then transform resident endothelial cells [10] and infiltrating mesenchymal stromal cells [16] into CAFs. Additionally, some cancer cells undergo epithelial-to-mesenchymal transition (EMT) to generate a small proportion of supportive fibroblasts [17]. Once induced, CAFs deposit increasing amounts of stiff fibrillar ECM composed of collagen [18] and fibronectin splice variants [19]. Contraction of the ECM by CAFs causes compression of the granulation tissue. As a result, pools of soluble factors released by CAFs accumulate in these compressed areas allowing further differentiation and growth. However, unlike their counterparts in wound healing, CAFs do not undergo apoptosis when epithelization is completed. Instead, they form an irreversibly activated stroma, which contributes to tumor growth and metastatic dissemination.
Functions of CAFs
CAFs support tumor growth
Following activation, CAFs revert to a more fetal phenotype and display an elevated migratory potential along with the ability to secrete a gamut of soluble factors [20]. Some of these factors, like TGF-β1, function in an autocrine manner to sustain fibrogenesis. Others, like HGF [21], IGF-1 [22], FGF [23] and PGE-2 [24] act on neighboring cancer cells and promote rapid proliferation. Cancer cells reciprocate by secreting soluble factors like PDGF [25], IL-1α [26] and IL-6 [27] to name a few, which further activate CAFs. The fibrillar ECM deposited by CAFs aid in this process by binding and distributing soluble factors to different cell types [28]. An autocrine cytokine and chemokine network secreted by CAFs causes infiltration of inflammatory cells and promotes neoangiogenesis [29]. SDF-1/CXCL12 [30], VEGF [31] and PDGF produced by CAFs [32], for example, recruit bone marrow-derived endothelial progenitor cells to the tumor and promote angiogenesis. Blood vessels act as conduits for shuttling in nutrients required for tumor expansion. Sites distant to blood vessels however, become hypoxic and undergo necrosis [33]. To perpetuate the growth of cancer cells in these areas, CAFs undergo autophagy/mitophagy and generate fuel (L-lactate and ketone bodies) for their consumption [34]. Cancer cells then adopt a mesenchymal phenotype characterized by enhanced migratory and invasive capabilities via EMT [35]. This event is sustained by paracrine factors produced by CAFs, and marks the transition of a potentially malignant lesion to an aggressive and invasive carcinoma.
CAFs contribute to the immunosuppressive tumor microenvironment
During initial stages of neoplasia, CAFs recruit T cells to the tumor by secreting chemokines [36] and promote chronic inflammation by sustaining proliferation even in the absence of antigen stimulation [37]. Alternatively, this pro-tumorigenic inflammatory milieu is maintained by severely inhibiting antigen-specific T-cell responses. These T cells fail to control tumor progression because of both extrinsic and intrinsic factors that are manipulated by CAFs [38]. By secreting a gamut of paracrine factors, CAFs modulate the microenvironment to a largely Th2 phenotype, which inhibits effector T-cell function and promotes tumor growth [39]. A variety of immune cells aid in this process. In the presence of CAFs, monocytes for example, differentiate into a distinct M2 polarized macrophage population called tumor-associated macrophages. Tumor-associated macrophages in turn have poor antigen-presenting capacity and further suppress Th1-adaptive immune responses [40]. For example, in pancreatic cancer, the Th2 phenotype is largely maintained by the activation and maturation of myeloid dendritic cells (DCs) in the presence of CAF-secreted thymic stromal lymphopoietin (TSLP). These DCs subsequently migrate to the draining lymph nodes and activate CD4+ T cells towards a Th2 phenotype [41]. This immunosuppressive, pro-tumorigenic microenvironment is further bolstered by the recruitment of myeloid-derived suppressor cells and Tregs by CAFs. In the presence of Th2 cytokines, like IL-4, IL-10 and IL-13, myeloid cells fail to differentiate and adopt a suppressive phenotype giving rise to the myeloid-derived suppressor cell population. These myeloid-derived suppressor cells secrete immunosuppressive cytokines, reactive oxygen species and nitric oxide NO [42]. Similarly, naturally occurring Tregs expand in the presence of CAF-secreted factors like TGF-β [43] and indoleamine 2,3 dioxygenase [44]. These regulatory cells greatly suppress the activity of effector T cells allowing tumor progression. CAFs also contribute to increasing tumor fibrosis, which inhibits the migration of T cells into the bulk of the tumor, relegating them to the periphery. Furthermore, through a contact-dependent mechanism, CAFs significantly inhibit the proliferation of activated T cells by disrupting their IL-2 production [45]. TGF-β1 secreted by CAFs, inhibits CD4+ T cells by disrupting IL-2 Itk phosphorylation and nuclear factor of activated T-cells (NFAT) translocation, both essential for its differentiation and function [46]. Additionally, CAFs express programmed death ligands 1 and 2 (PD-L1 and PD-L2), which can interact with the programmed death 1 receptor (PD-1) on T cells and inhibit their function [47]. Finally, in order to evade the NK cells, CAFs secrete indoleamine 2,3 dioxygenase and prostaglandin-E2 (PGE2), which severely inhibits both the acquisition of activating receptors as well as the release of cytotoxic granules by these cells [48]. CAFs are therefore the architects of the tumor inflammatory milieu and subvert the anti-tumor responses of T cells to facilitate tumor growth and metastasis.
CAFs promote invasion & metastasis
The defining event that signifies cancer progression to a malignant state involves the disruption of basement membrane. This is assisted by matrix metalloproteinases (MMPs) that are secreted by CAFs. MMPs degrade the ECM that forms the basement membrane, freeing the cancer cells from their tight quarters [49]. This is associated with a rapid increase in reactive oxygen species, which induces genomic instability in the cancer cells and bolsters EMT [50]. Detachment from the ECM is however known to induce anoikis, a form of programmed cell death [51]. CAFs secrete HGF, which subsequently induces activation of the PI3K signaling cascade in the cancer cells and renders them resistant to anoikis [52]. CAFs then generate tracks in the ECM and lead a small minority of motile cancer cells through the breached basement membrane [53]. These motile cancer cells share properties with somatic or embryonic stem cells and are selected for by CAFs during EMT [54]. Once they enter the underlying connective tissue, the leading CAFs secrete TGF-β and subvert normal fibroblasts to boost their motility [55]. This entourage may then follow an as yet unidentified chemokine path to reach a specific secondary site. Upon arrival, CAFs colonize the site and provide a growth advantage to their accompanying cancer cells. The metastatic cells and CAFs then co-evolve and reinstate their reciprocal relationship as seen in the primary tumor.
CAFs have a permeating role in all aspects of cancer progression. It is therefore not entirely surprising that a significant prognostic value is attributed to them. Excessive desmoplasia in normal breasts for example is an independent risk factor for subsequent mammary cancer development [56]. When formed, invasive tumors are typified by extensive fibrosis, which is associated with preoperative chemoresistance and shorter disease-free survival [57]. Furthermore, CAF activation when directly assessed by histological staining of specific activation markers like FAP [58], αSMA [59] and podoplanin [60] is often correlated with poor prognosis. These findings corroborate the role of CAFs in cancer development, making them excellent therapeutic targets.
Targeting CAFs
Historically, cancer treatments have largely focused on targeting the tumor discounting the stroma as an innocent bystander. It is now accepted that a permissive stroma composed of activated CAFs can revive dormant tumorigenic cells and establish cancer. Indeed, several studies have highlighted that co-targeting tumor and stromal cells is necessary to eradicate large solid tumors, and that killing of the tumor stroma allows the eradication of tumor cells that have downregulated the expression of the targeted antigen (antigen loss variants) by an antigen-independent mechanism [61,62]. CAFs also mediate resistance to conventional therapeutic regimens indirectly by secreting a host of soluble factors. Through the secretion of TGF-β, CAFs lay out an ECM that juxtaposes different cell types and mediates cell adhesion-mediated drug resistance [63]. Additionally, the rigid collagen fibers of the ECM inhibit the transport of macromolecules into the tumor. This is further exacerbated by elevated interstitial fluid pressure, which causes blood vessel collapse, further reducing drug access to the tumors. A combination of leaky blood vessels and ECM remodeling by CAFs contribute to this increased interstitial fluid pressure [64]. Growth factors released by CAFs induce rapid proliferation of the tumor cells, which expand beyond the purview of existing blood vessels. Hypoxia sets into these areas and decreases the effects of therapeutic regimens that are oxygen or free radical-dependent [65]. Increasing hypoxia also induces genetic instability in cancer cells selecting for highly aggressive and resistant clones. These clones often express stem cell markers like Sca-1, CD133, Oct-3/4 and multidrug resistance efflux pumps [66]. Such self-renewing populations of malignant cells escape cytotoxic effects, recruit CAFs and other components of the tumor stroma and reinstate tumorigenesis, which accounts for recurrence. A durable therapeutic response therefore necessitates targeting stromal elements like CAFs in addition to tumor cells. This hypothesis has garnered support from immunotherapeutic studies, which showed that successful eradication of large solid tumors required co-targeting of cancer cells and stromal elements [62].
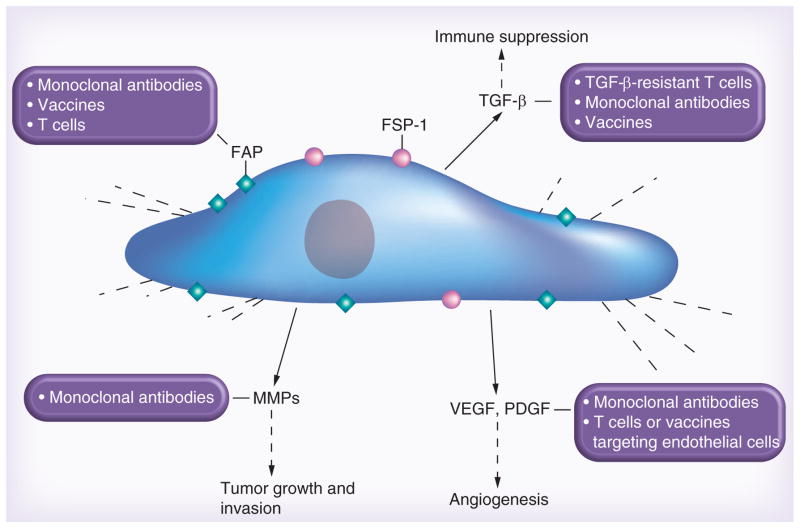
CAFs express a range of molecules that could serve as targets for immunotherapies including MMPs and FAP-α [67,68]. Of these, FAP has emerged over the last decade as the most promising immunotherapeutic target. Besides destruction of CAFs, counteracting tumor-promoting factors secreted by CAFs also has the potential to increase the efficacy of immunotherapies (Figure 1). In the remaining review, we will discuss FAP-targeted therapies and review strategies to counteract CAFs’ tumor-promoting factors.
Figure 1. Immunotherapy targeting cancer-associated fibroblasts.
Upon activation, cancer-associated fibroblasts express markers like FAP, ED-FN, FSP-1, SMA and vimentin. They secrete cytokines (TGF-β), proteases (matrix metalloproteinases) and growth factors (VEGF, PDGF) among others, which directly and indirectly contribute to tumor growth and metastasis. Either direct targeting of cancer-associated fibroblasts or targeting their secreted paracrine factors through various immunotherapeutic modalities can greatly improve therapeutic response.
ED-FN: ED splice variant of fibronectin; MMP: Matrix metalloproteinase; SMA: Smooth muscle actin.
Targeting FAP
FAP was first isolated from reactive stroma in human sarcomas [69–71]. Its expression was subsequently compartmentalized to reactive fibroblasts in >90% of solid tumors, including breast, prostate, colorectal and pancreatic tumors [72]. As a member of the dipeptidyl peptidase family, FAP retains both collagenolytic and gelatinase activity [73]. While it is purported to cleave ECM and aid in invasion [74], its exact biological remains ill defined. Overexpression of FAP increases tumor incidence, growth and microvessel density in experimental tumor models [75]. Preclinical studies targeting FAP in the stroma have been made possible by the finding that murine stromal cells, expressing murine FAP, infiltrate human tumor xenografts in immunodeficient mice [76]. Selectively depleting FAP+ CAFs in a transgenic mouse model resulted in a cytokine-mediated hypoxic necrosis of the tumor and the stroma, and allowed for immounological tumor control, highlighting the central role of CAFs in the tumor microenvironment [77]. Anti-tumor responses have also been seen in studies that have employed the pharmacological FAP inhibitor PT-630 [78] and the FAP-activated promelittin protoxin [79]. These studies mark the advent of exciting therapeutic strategies that have the potential to improve current immunotherapeutic treatments.
FAP-specific monoclonal antibodies
Two clinical studies have been conducted with FAP monoclonal antibodies (MAbs) [80,81]. In the first study, 17 patients with hepatic colon cancer metastases received iodine 131-labeled FAP-specific F19 MAbs (131I-F19) 7–8 days prior to surgery. Fifteen of 17 patients were also imaged by single-photon emission tomography. No toxicity was observed and the smallest lesion that could be detected was 1 cm in diameter. Preferential uptake of 131I-F19 was confirmed by surgery. The half-life of 131I-F19 was approximately 38 h, which is comparable to other murine MAbs that do not bind normal tissue antigens [82]. Subsequently, one Phase I study was conducted with humanized F19 MAb (sibrotuzumab) [81]. Twenty six patients received weekly injections of up to 50 mg/m2 sibrotuzumab for 12 weeks with pharmacokinetic studies being performed in weeks 1, 5, and 9 with 131I-labeled sibrotuzumab. Infusions were well tolerated and only one dose-limiting toxicity (back pain) was observed. While this study confirmed the preferential uptake of FAP-specific MAbs into tumor tissue, no objective clinical response was observed [81]. Since the tissue distribution of FAP-specific MAbs was encouraging, investigators have explored the use of these MAbs to deliver therapeutics to tumor sites. In preclinical studies a FAP-specific maytansinoid MAb conjugate has shown potent anti-tumor activity in several murine xenograft models without any side effects [83]. In addition, FAP-specific single-chain variable fragments have been used to deliver lipidcoated nanoparticles or TNF to FAP+ cells [84,85]. Anti-FAP fusion proteins have also been developed and tested in preclinical models. For example, a FasL fusion protein (sc40-FasL) induced apoptosis and prevented FAP+ tumor growth [86], and an anti-FAP-murine glucocorticoid-induced TNF receptor ligand (mGITRL) fusion protein induced significant co-stimulation of CD8− and CD4+ T cells resulting in enhanced anti-tumor effects, upon dimerization with both murine GITR and FAP [87].
FAP-targeted vaccines
Several groups have explored the use of FAP vaccines [39,88–90]. In one study, investigators showed that mice vaccinated with DCs loaded with mRNA encoding FAP decreased tumor growth in several immune-competent animal models [88]. Anti-tumor activity was dependent on the presence of CD4+ and CD8+ T cells. Similar results were obtained with a plasmid DNA vaccine [90]. Loeffler and colleagues evaluated a FAP-targeted Salmonella vaccine in murine models [89]. While the anti-tumor activity of this vaccine was modest, vaccination reduced tumor collagen content facilitating both the diffusion of small molecules as well as the convection of larger molecules. Combining FAP vaccination with doxorubicin resulted in a significant increase in overall survival when compared with mice treated with vaccine or doxorubicin alone. In another study, the same group of investigators examined the effects of the vaccine on the tumor microenvironment in the 4T1 breast cancer models [39]. FAP vaccination reversed the immunosuppressive microenvironment and decreased tumor angiogenesis, indicating that combining CAF-targeted immunotherapies could enhance immunotherapeutic approaches that target tumor cells.
FAP-specific T-cell therapies
Limited information is available in regards to the adoptive immunotherapy of FAP-specific T cells. In one study, adoptively transferred splenocytes from FAP-vaccinated mice significantly decreased tumor growth [90]. Fassnacht and colleagues determined the ability to generate CAF-specific T cells ex vivo in humans by targeting FAP or MMPs expressed by CAFs [67]. Using a DC stimulation protocol FAP-specific T cells could be activated in six out of six donors, where has MMP9-specific T cells in one out of four, and MMP14-specific T cells in three out of five, indicating that it is feasible to induce CAF-specific T-cell responses in humans. Besides using conventional APCs to generate CAF-specific T cells, the genetic modification of T cells with chimeric antigen receptors is an alternative strategy to generate CAF-specific T cells [91]. We have generated a FAP-specific chimeric antigen receptors and initial evaluation in preclinical models is promising [92].
Targeting TGF-β
TGF-β1 is a potent fibrogenic cytokine that stimulates CAF differentiation and growth. Once activated, CAFs themselves secrete TGF-β1, creating an autocrine loop to sustain their differentiated phenotype. Accumulating TGF-β1 in the tumor microenvironment upregulates growth factors like VEGF [93] and CTGF [94] that allow tumor proliferation and angiogenesis. TGF-β1 also mediates immunosupression, ECM degradation and tumor cell invasion [95]. Preclinical studies using a TGF-β2 antisense-modified allogeneic tumor cell vaccine showed improved survival in mice with intracranial gliomas [96]. Similar results were seen in patients with non-small cell lung cancer in a Phase II dose study using a TGF-β2 antisense gene-modified tumor cell vaccine (belagenpumatucel-L) [97]. In addition, a small molecule TGF-β1 kinase inhibitor (LY2109761) [98] has shown promise in preclinical studies. While promising, there are safety concerns in regards to systemic treatment with TGF-β antagonists, mainly because of its pleiotropic effects on tissue remodeling, immunomodulation and cancer development [99,100]. A solution to this conundrum for the adoptive immunotherapy of malignancies is the use of T cells that are resistant to the immunosuppressive effects of TGF-β. Expression of a dominant-negative TGF-β receptor in human T cells rendered them resistant to the immunosuppressive effects of TGF-β in preclinical models [101,102] and a clinical study evaluating their safety and efficacy is in progress.
Targeting MMPs
MMPs are proteolytic enzymes that are ubiquitously produced by several different cell types in the tumor microenvironment. CAFs form the major source of MMPs (particularly MMP2 and MMP9), which cleave ECM, facilitating cell migration, invasion and metastasis. MMPs also regulate the bioavailability of soluble factors by cleaving and activating their cell-bound latent forms [49]. MMP promote the immunosuppressive tumor environment by proteolytically activating TGF-β [103] and degrading IL-2 receptor-β resulting in inhibition of intratumoral T-cell proliferation [49]. Blocking MMP synthesis or its activity should counter these effects and have the potential to enhance immunotherapy for cancer. Tetracycline analogs like metastat have the ability to inhibit the synthesis and activity of MMPs, and are in Phase II clinical trials for Kaposi’s sarcoma and brain tumors [104]. Peptido- and non-peptidomimetics like marimastat and prinomastat are another class of MMP inhibitors, which despite promising results in preclinical studies, have failed to show beneficial effects as single agents in clinical trials. Dose-limiting toxicities and narrow therapeutic windows have been attributed to this failure [49]. Nevertheless, combining MMP inhibitors with cancer immunotherapies warrants further active exploration due to their role in shaping the immunosuppressive tumor microenvironment.
Targeting growth factors & their receptors
CAFs produce an array of growth factors, including PDGF and VEGF, which mediate tumor growth, angiogenesis, invasion and dissemination. Small molecule inhibitors that target multiple growth factor signaling pathways like sunitinib (PDGFR, VEGFR, KIT and FLT-3) [105], sorafenib (PDGFR, VEGFR, c-Raf-1, B-raf) [106], leflunomide (PDGFR, VEGFR, FGFR) [105] have the potential to improve cancer immunotherapies and have been recently reviewed elsewhere [107]. Besides small molecule inhibitors, combining VEGF MAbs with the adoptive transfer of tumor-specific T cells increased their anti-tumor activity in preclinical animal models [108].
Conclusion & future perspective
The tumor-associated stroma is no longer considered an innocent bystander, but a key player in cancer initiation and progression. CAFs, the principle components of the tumor stroma, play a central role in tumorigenesis, growth and metastasis. CAFs also contribute to the immunosuppressive tumor microenvironment and thus are excellent therapeutic targets to improve current immunotherapy approaches for cancer. In addition, their genetic stability bodes well for a durable therapeutic response with very low probability of acquired resistance. CAF destruction can result in reversal of the immunosuppressive milieu and decrease in fibrosis allowing for better infiltration and expansion of effector T cells as well as APCs. Therapeutic regimens targeting growth factors secreted by CAFs or CAFs themselves in combination with therapies that directly target malignant cells have shown promise in preclinical studies. While clinical studies are underway that evaluate the combination of kinase inhibitors, such as imatinib, and cancer vaccines, there is currently no clinical study evaluating the safety and efficacy of CAF-specific T-cell therapies or vaccines. Integrating CAF-targeted therapies into cancer immunotherapies might help the ‘magic bullet’ to live up to its promise.
Executive summary.
Eradication of large tumors requires the destruction of the tumor stroma in preclinical animal models.
Cancer-associated fibroblasts (CAFs) form the most preponderant cell type in the tumor stroma.
CAFs play a central role in tumorigenesis, tumor growth and metastasis.
CAFs contribute to the immunosuppressive tumor microenvironment.
CAF-targeted vaccines reverse the immunosuppressive tumor microenvironment and decrease the collagen content of tumors resulting in anti-tumor effects.
Targeting CAFs in addition to cancer cells has the potential to improve cancer immunotherapies.
Footnotes
For reprint orders, please contact: reprints@futuremedicine.com
Financial & competing interests disclosure
The authors were supported by grants from the Department of Defense (DAMD W81XWH-10-1-0281) and the NIH (1R01CA148748-01A1). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Paget S. The distribution of secondary growth in cancer of the breast. Lancet. 1889;1:571–573. [PubMed] [Google Scholar]
- 2.Mueller MM, Fusenig NE. Friends or foes – bipolar effects of the tumour stroma in cancer. Nat Rev Cancer. 2004;4(11):839–849. doi: 10.1038/nrc1477. [DOI] [PubMed] [Google Scholar]
- 3.De WO, Demetter P, Mareel M, Bracke M. Stromal myofibroblasts are drivers of invasive cancer growth. Int J Cancer. 2008;123(10):2229–2238. doi: 10.1002/ijc.23925. [DOI] [PubMed] [Google Scholar]
- 4.Krtolica A, Parrinello S, Lockett S, Desprez PY, Campisi J. Senescent fibroblasts promote epithelial cell growth and tumorigenesis: a link between cancer and aging. Proc Natl Acad Sci USA. 2001;98(21):12072–12077. doi: 10.1073/pnas.211053698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gonda TA, Tu S, Wang TC. Chronic inflammation, the tumor microenvironment and carcinogenesis. Cell Cycle. 2009;8(13):2005–2013. doi: 10.4161/cc.8.13.8985. [DOI] [PubMed] [Google Scholar]
- 6.Kurose K, Gilley K, Matsumoto S, Watson PH, Zhou XP, Eng C. Frequent somatic mutations in PTEN and TP53 are mutually exclusive in the stroma of breast carcinomas. Nat Genet. 2002;32(3):355–357. doi: 10.1038/ng1013. [DOI] [PubMed] [Google Scholar]
- 7.Hill R, Song Y, Cardiff RD, van Dyke T. Selective evolution of stromal mesenchyme with p53 loss in response to epithelial tumorigenesis. Cell. 2005;123(6):1001–1011. doi: 10.1016/j.cell.2005.09.030. [DOI] [PubMed] [Google Scholar]
- 8.Hu M, Yao J, Cai L, et al. Distinct epigenetic changes in the stromal cells of breast cancers. Nat Genet. 2005;37(8):899–905. doi: 10.1038/ng1596. [DOI] [PubMed] [Google Scholar]
- 9.Radisky DC, Kenny PA, Bissell MJ. Fibrosis and cancer: do myofibroblasts come also from epithelial cells via EMT? J Cell Biochem. 2007;101(4):830–839. doi: 10.1002/jcb.21186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zeisberg EM, Potenta S, Xie L, Zeisberg M, Kalluri R. Discovery of endothelial to mesenchymal transition as a source for carcinoma-associated fibroblasts. Cancer Res. 2007;67(21):10123–10128. doi: 10.1158/0008-5472.CAN-07-3127. [DOI] [PubMed] [Google Scholar]
- 11.Direkze NC, Hodivala-Dilke K, Jeffery R, et al. Bone marrow contribution to tumor-associated myofibroblasts and fibroblasts. Cancer Res. 2004;64(23):8492–8495. doi: 10.1158/0008-5472.CAN-04-1708. [DOI] [PubMed] [Google Scholar]
- 12.Wipff PJ, Rifkin DB, Meister JJ, Hinz B. Myofibroblast contraction activates latent TGF-β1 from the extracellular matrix. J Cell Biol. 2007;179(6):1311–1323. doi: 10.1083/jcb.200704042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leask A, Abraham DJ. TGF-β signaling and the fibrotic response. FASEB J. 2004;18(7):816–827. doi: 10.1096/fj.03-1273rev. [DOI] [PubMed] [Google Scholar]
- 14.Powell DW, Adegboyega PA, Di Mari JF, Mifflin RC. Epithelial cells and their neighbors I. Role of intestinal myofibroblasts in development, repair, and cancer. Am J Physiol Gastrointest Liver Physiol. 2005;289(1):G2–G7. doi: 10.1152/ajpgi.00075.2005. [DOI] [PubMed] [Google Scholar]
- 15.Ostman A, Heldin CH. PDGF receptors as targets in tumor treatment. Adv Cancer Res. 2007;97:247–274. doi: 10.1016/S0065-230X(06)97011-0. [DOI] [PubMed] [Google Scholar]
- 16.Spaeth EL, Dembinski JL, Sasser AK, et al. Mesenchymal stem cell transition to tumor-associated fibroblasts contributes to fibrovascular network expansion and tumor progression. PLoS One. 2009;4(4):e4992. doi: 10.1371/journal.pone.0004992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iwano M, Plieth D, Danoff TM, Xue C, Okada H, Neilson EG. Evidence that fibroblasts derive from epithelium during tissue fibrosis. J Clin Invest. 2002;110(3):341–350. doi: 10.1172/JCI15518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Faouzi S, Le BB, Neaud V, et al. Myofibroblasts are responsible for collagen synthesis in the stroma of human hepatocellular carcinoma: an in vivo and in vitro study. J Hepatol. 1999;30(2):275–284. doi: 10.1016/s0168-8278(99)80074-9. [DOI] [PubMed] [Google Scholar]
- 19.Serini G, Bochaton-Piallat ML, Ropraz P, et al. The fibronectin domain ED-A is crucial for myofibroblastic phenotype induction by transforming growth factor-β1. J Cell Biol. 1998;142(3):873–881. doi: 10.1083/jcb.142.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang HY, Chi JT, Dudoit S, et al. Diversity, topographic differentiation, and positional memory in human fibroblasts. Proc Natl Acad Sci USA. 2002;99(20):12877–12882. doi: 10.1073/pnas.162488599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grugan KD, Miller CG, Yao Y, et al. Fibroblast-secreted hepatocyte growth factor plays a functional role in esophageal squamous cell carcinoma invasion. Proc Natl Acad Sci USA. 2010;107(24):11026–11031. doi: 10.1073/pnas.0914295107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li G, Satyamoorthy K, Herlyn M. N-cadherin-mediated intercellular interactions promote survival and migration of melanoma cells. Cancer Res. 2001;61(9):3819–3825. [PubMed] [Google Scholar]
- 23.Franco OE, Jiang M, Strand DW, et al. Altered TGF-β signaling in a subpopulation of human stromal cells promotes prostatic carcinogenesis. Cancer Res. 2011;71(4):1272–1281. doi: 10.1158/0008-5472.CAN-10-3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rudnick JA, Arendt LM, Klebba I, et al. Functional heterogeneity of breast fibroblasts is defined by a prostaglandin secretory phenotype that promotes expansion of cancer-stem like cells. PLoS One. 2011;6(9):e24605. doi: 10.1371/journal.pone.0024605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Skobe M, Fusenig NE. Tumorigenic conversion of immortal human keratinocytes through stromal cell activation. Proc Natl Acad Sci USA. 1998;95(3):1050–1055. doi: 10.1073/pnas.95.3.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tjomsland V, Spangeus A, Valila J, et al. Interleukin 1α sustains the expression of inflammatory factors in human pancreatic cancer microenvironment by targeting cancer-associated fibroblasts. Neoplasia. 2011;13(8):664–675. doi: 10.1593/neo.11332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hugo HJ, Lebret S, Tomaskovic-Crook E, et al. Contribution of fibroblast and mast cell (afferent) and tumor (efferent) IL-6 effects within the tumor microenvironment. Cancer Microenviron. 2012;5(1):83–93. doi: 10.1007/s12307-012-0098-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hynes RO. The extracellular matrix: not just pretty fibrils. Science. 2009;326(5957):1216–1219. doi: 10.1126/science.1176009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Erez N, Truitt M, Olson P, Arron ST, Hanahan D. Cancer-associated fibroblasts are activated in incipient neoplasia to orchestrate tumor-promoting inflammation in an NF-κB-dependent manner. Cancer Cell. 2010;17(2):135–147. doi: 10.1016/j.ccr.2009.12.041. [DOI] [PubMed] [Google Scholar]
- 30.Orimo A, Gupta PB, Sgroi DC, et al. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121(3):335–348. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 31.Fukumura D, Xavier R, Sugiura T, et al. Tumor induction of VEGF promoter activity in stromal cells. Cell. 1998;94(6):715–725. doi: 10.1016/s0092-8674(00)81731-6. [DOI] [PubMed] [Google Scholar]
- 32.Crawford Y, Kasman I, Yu L, et al. PDGF-C mediates the angiogenic and tumorigenic properties of fibroblasts associated with tumors refractory to anti-VEGF treatment. Cancer Cell. 2009;15(1):21–34. doi: 10.1016/j.ccr.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 33.Powathil G, Kohandel M, Milosevic M, Sivaloganathan S. Modeling the spatial distribution of chronic tumor hypoxia: implications for experimental and clinical studies. Comput Math Methods Med. 2012:410602. doi: 10.1155/2012/410602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pavlides S, Tsirigos A, Migneco G, et al. The autophagic tumor stroma model of cancer: role of oxidative stress and ketone production in fueling tumor cell metabolism. Cell Cycle. 2010;9(17):3485–3505. doi: 10.4161/cc.9.17.12721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cannito S, Novo E, Compagnone A, et al. Redox mechanisms switch on hypoxia-dependent epithelial-mesenchymal transition in cancer cells. Carcinogenesis. 2008;29(12):2267–2278. doi: 10.1093/carcin/bgn216. [DOI] [PubMed] [Google Scholar]
- 36.Taub DD, Turcovski-Corrales SM, Key ML, Longo DL, Murphy WJ. Chemokines and T lymphocyte activation: I. β chemokines costimulate human T lymphocyte activation in vitro. J Immunol. 1996;156(6):2095–2103. [PubMed] [Google Scholar]
- 37.Sturm A, Krivacic KA, Fiocchi C, Levine AD. Dual function of the extracellular matrix: stimulatory for cell cycle progression of naive T cells and antiapoptotic for tissue-derived memory T cells. J Immunol. 2004;173(6):3889–3900. doi: 10.4049/jimmunol.173.6.3889. [DOI] [PubMed] [Google Scholar]
- 38.Barnas JL, Simpson-Abelson MR, Yokota SJ, Kelleher RJ, Bankert RB. T cells and stromal fibroblasts in human tumor microenvironments represent potential therapeutic targets. Cancer Microenviron. 2010;3(1):29–47. doi: 10.1007/s12307-010-0044-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liao D, Luo Y, Markowitz D, Xiang R, Reisfeld RA. Cancer associated fibroblasts promote tumor growth and metastasis by modulating the tumor immune microenvironment in a 4T1 murine breast cancer model. PLoS One. 2009;4(11):e7965. doi: 10.1371/journal.pone.0007965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chomarat P, Banchereau J, Davoust J, Palucka AK. IL-6 switches the differentiation of monocytes from dendritic cells to macrophages. Nat Immunol. 2000;1(6):510–514. doi: 10.1038/82763. [DOI] [PubMed] [Google Scholar]
- 41.Protti MP, De ML. Cross-talk within the tumor microenvironment mediates Th2-type inflammation in pancreatic cancer. Oncoimmunology. 2012;1(1):89–91. doi: 10.4161/onci.1.1.17939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shojaei F, Zhong C, Wu X, Yu L, Ferrara N. Role of myeloid cells in tumor angiogenesis and growth. Trends Cell Biol. 2008;18(8):372–378. doi: 10.1016/j.tcb.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 43.Nizar S, Meyer B, Galustian C, Kumar D, Dalgleish A. T regulatory cells, the evolution of targeted immunotherapy. Biochim Biophys Acta. 2010;1806(1):7–17. doi: 10.1016/j.bbcan.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 44.Munn DH, Mellor AL. Indoleamine 2,3-dioxygenase and tumor-induced tolerance. J Clin Invest. 2007;117(5):1147–1154. doi: 10.1172/JCI31178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pinchuk IV, Saada JI, Beswick EJ, et al. PD-1 ligand expression by human colonic myofibroblasts/fibroblasts regulates CD4+ T-cell activity. Gastroenterology. 2008;135(4):1228–1237. doi: 10.1053/j.gastro.2008.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen CH, Seguin-Devaux C, Burke NA, et al. Transforming growth factor β blocks Tec kinase phosphorylation, Ca2+ influx, and NFATc translocation causing inhibition of T cell differentiation. J Exp Med. 2003;197(12):1689–1699. doi: 10.1084/jem.20021170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nazareth MR, Broderick L, Simpson-Abelson MR, Kelleher RJ, Yokota SJ, Bankert RB. Characterization of human lung tumor-associated fibroblasts and their ability to modulate the activation of tumor-associated T cells. J Immunol. 2007;178(9):5552–5562. doi: 10.4049/jimmunol.178.9.5552. [DOI] [PubMed] [Google Scholar]
- 48.Balsamo M, Scordamaglia F, Pietra G, et al. Melanoma-associated fibroblasts modulate NK cell phenotype and antitumor cytotoxicity. Proc Natl Acad Sci USA. 2009;106(49):20847–20852. doi: 10.1073/pnas.0906481106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2(3):161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 50.Toullec A, Gerald D, Despouy G, et al. Oxidative stress promotes myofibroblast differentiation and tumour spreading. EMBO Mol Med. 2010;2(6):211–230. doi: 10.1002/emmm.201000073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Marastoni S, Ligresti G, Lorenzon E, Colombatti A, Mongiat M. Extracellular matrix: a matter of life and death. Connect Tissue Res. 2008;49(3):203–206. doi: 10.1080/03008200802143190. [DOI] [PubMed] [Google Scholar]
- 52.Kanayama S, Yamada Y, Kawaguchi R, Tsuji Y, Haruta S, Kobayashi H. Hepatocyte growth factor induces anoikis resistance by up-regulation of cyclooxygenase-2 expression in uterine endometrial cancer cells. Oncol Rep. 2008;19(1):117–122. [PubMed] [Google Scholar]
- 53.Gaggioli C, Hooper S, Hidalgo-Carcedo C, et al. Fibroblast-led collective invasion of carcinoma cells with differing roles for RhoGTPases in leading and following cells. Nat Cell Biol. 2007;9(12):1392–1400. doi: 10.1038/ncb1658. [DOI] [PubMed] [Google Scholar]
- 54.Mani SA, Guo W, Liao MJ, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133(4):704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stuelten CH, Busch JI, Tang B, et al. Transient tumor-fibroblast interactions increase tumor cell malignancy by a TGF-β mediated mechanism in a mouse xenograft model of breast cancer. PLoS One. 2010;5(3):e9832. doi: 10.1371/journal.pone.0009832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Boyd NF, Guo H, Martin LJ, et al. Mammographic density and the risk and detection of breast cancer. N Engl J Med. 2007;356(3):227–236. doi: 10.1056/NEJMoa062790. [DOI] [PubMed] [Google Scholar]
- 57.Cohen SJ, Alpaugh RK, Palazzo I, et al. Fibroblast activation protein and its relationship to clinical outcome in pancreatic adenocarcinoma. Pancreas. 2008;37(2):154–158. doi: 10.1097/MPA.0b013e31816618ce. [DOI] [PubMed] [Google Scholar]
- 58.Shi M, Yu DH, Chen Y, et al. Expression of fibroblast activation protein in human pancreatic adenocarcinoma and its clinicopathological significance. World J Gastroenterol. 2012;18(8):840–846. doi: 10.3748/wjg.v18.i8.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fujita H, Ohuchida K, Mizumoto K, et al. α-smooth muscle actin expressing stroma promotes an aggressive tumor biology in pancreatic ductal adenocarcinoma. Pancreas. 2010;39(8):1254–1262. doi: 10.1097/MPA.0b013e3181dbf647. [DOI] [PubMed] [Google Scholar]
- 60.Schoppmann SF, Berghoff A, Dinhof C, et al. Podoplanin-expressing cancer-associated fibroblasts are associated with poor prognosis in invasive breast cancer. Breast Cancer Res Treat. 2012;134(1):237–244. doi: 10.1007/s10549-012-1984-x. [DOI] [PubMed] [Google Scholar]
- 61▪▪.Spiotto MT, Rowley DA, Schreiber H. Bystander elimination of antigen loss variants in established tumors. Nat Med. 2004;10(3):294–298. doi: 10.1038/nm999. Demonstrates the importance of targeting tumor stroma to erradicate large tumors and prevent immune escape. [DOI] [PubMed] [Google Scholar]
- 62.Zhang B, Bowerman NA, Salama JK, et al. Induced sensitization of tumor stroma leads to eradication of established cancer by T cells. J Exp Med. 2007;204(1):49–55. doi: 10.1084/jem.20062056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sethi T, Rintoul RC, Moore SM, et al. Extracellular matrix proteins protect small cell lung cancer cells against apoptosis: a mechanism for small cell lung cancer growth and drug resistance in vivo. Nat Med. 1999;5(6):662–668. doi: 10.1038/9511. [DOI] [PubMed] [Google Scholar]
- 64.Heldin CH, Rubin K, Pietras K, Ostman A. High interstitial fluid pressure – an obstacle in cancer therapy. Nat Rev Cancer. 2004;4(10):806–813. doi: 10.1038/nrc1456. [DOI] [PubMed] [Google Scholar]
- 65.Brown JM, Giaccia AJ. The unique physiology of solid tumors: opportunities (and problems) for cancer therapy. Cancer Res. 1998;58(7):1408–1416. [PubMed] [Google Scholar]
- 66.Liang D, Ma Y, Liu J, et al. The hypoxic microenvironment upgrades stem-like properties of ovarian cancer cells. BMC Cancer. 2012;12:201. doi: 10.1186/1471-2407-12-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fassnacht M, Lee J, Milazzo C, et al. Induction of CD4+ and CD8+ T-cell responses to the human stromal antigen, fibroblast activation protein: implication for cancer immunotherapy. Clin Cancer Res. 2005;11(15):5566–5571. doi: 10.1158/1078-0432.CCR-05-0699. [DOI] [PubMed] [Google Scholar]
- 68.Ostman A, Augsten M. Cancer-associated fibroblasts and tumor growth – bystanders turning into key players. Curr Opin Genet Dev. 2009;19(1):67–73. doi: 10.1016/j.gde.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 69▪.Rettig WJ, Garin-Chesa P, Beresford HR, Oettgen HF, Melamed MR, Old LJ. Cell-surface glycoproteins of human sarcomas: differential expression in normal and malignant tissues and cultured cells. Proc Natl Acad Sci USA. 1988;85(9):3110–3114. doi: 10.1073/pnas.85.9.3110. Identifies FAP in the tumor stroma. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rettig WJ, Su SL, Fortunato SR, et al. Fibroblast activation protein: purification, epitope mapping and induction by growth factors. Int J Cancer. 1994;58(3):385–392. doi: 10.1002/ijc.2910580314. [DOI] [PubMed] [Google Scholar]
- 71.Park JE, Lenter MC, Zimmermann RN, Garin-Chesa P, Old LJ, Rettig WJ. Fibroblast activation protein, a dual specificity serine protease expressed in reactive human tumor stromal fibroblasts. J Biol Chem. 1999;274(51):36505–36512. doi: 10.1074/jbc.274.51.36505. [DOI] [PubMed] [Google Scholar]
- 72.Garin-Chesa P, Old LJ, Rettig WJ. Cell surface glycoprotein of reactive stromal fibroblasts as a potential antibody target in human epithelial cancers. Proc Natl Acad Sci USA. 1990;87(18):7235–7239. doi: 10.1073/pnas.87.18.7235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kelly T. Fibroblast activation protein-α and dipeptidyl peptidase IV (CD26): cell-surface proteases that activate cell signaling and are potential targets for cancer therapy. Drug Res Updat. 2005;8(1–2):51–58. doi: 10.1016/j.drup.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 74.Chen WT, Kelly T. Seprase complexes in cellular invasiveness. Cancer Metastasis Rev. 2003;22(2–3):259–269. doi: 10.1023/a:1023055600919. [DOI] [PubMed] [Google Scholar]
- 75.Brennen WN, Isaacs JT, Denmeade SR. Rationale behind targeting fibroblast activation protein-expressing carcinoma-associated fibroblasts as a novel chemotherapeutic strategy. Mol Cancer Ther. 2012;11(2):257–266. doi: 10.1158/1535-7163.MCT-11-0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cheng JD, Valianou M, Canutescu AA, et al. Abrogation of fibroblast activation protein enzymatic activity attenuates tumor growth. Mol Cancer Ther. 2005;4(3):351–360. doi: 10.1158/1535-7163.MCT-04-0269. [DOI] [PubMed] [Google Scholar]
- 77▪▪.Kraman M, Bambrough PJ, Arnold JN, et al. Suppression of antitumor immunity by stromal cells expressing fibroblast activation protein-α. Science. 2010;330(6005):827–830. doi: 10.1126/science.1195300. Paper highlights the critical role of FAP+ cancer-associated fibroblasts in tumor growth and progression. [DOI] [PubMed] [Google Scholar]
- 78.Santos AM, Jung J, Aziz N, Kissil JL, Pure E. Targeting fibroblast activation protein inhibits tumor stromagenesis and growth in mice. J Clin Invest. 2009;119(12):3613–3625. doi: 10.1172/JCI38988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.LeBeau AM, Brennen WN, Aggarwal S, Denmeade SR. Targeting the cancer stroma with a fibroblast activation protein-activated promelittin protoxin. Mol Cancer Ther. 2009;8(5):1378–1386. doi: 10.1158/1535-7163.MCT-08-1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Welt S, Divgi CR, Scott AM, et al. Antibody targeting in metastatic colon cancer: a Phase I study of monoclonal antibody F19 against a cell-surface protein of reactive tumor stromal fibroblasts. J Clin Oncol. 1994;12(6):1193–1203. doi: 10.1200/JCO.1994.12.6.1193. [DOI] [PubMed] [Google Scholar]
- 81▪.Scott AM, Wiseman G, Welt S, et al. A Phase I dose-escalation study of sibrotuzumab in patients with advanced or metastatic fibroblast activation protein-positive cancer. Clin Cancer Res. 2003;9(5):1639–1647. Clinical study with a humanized FAP-specific monoclonal antibody. [PubMed] [Google Scholar]
- 82.Tanswell P, Garin-Chesa P, Rettig WJ, et al. Population pharmacokinetics of antifibroblast activation protein monoclonal antibody F19 in cancer patients. Br J Clin Pharmacol. 2001;51(2):177–180. doi: 10.1111/j.1365-2125.2001.01335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ostermann E, Garin-Chesa P, Heider KH, et al. Effective immunoconjugate therapy in cancer models targeting a serine protease of tumor fibroblasts. Clin Cancer Res. 2008;14(14):4584–4592. doi: 10.1158/1078-0432.CCR-07-5211. [DOI] [PubMed] [Google Scholar]
- 84.Wuest T, Gerlach E, Banerjee D, Gerspach J, Moosmayer D, Pfizenmaier K. TNF-selectokine: a novel prodrug generated for tumor targeting and site-specific activation of tumor necrosis factor. Oncogene. 2002;21(27):4257–4265. doi: 10.1038/sj.onc.1205193. [DOI] [PubMed] [Google Scholar]
- 85.Messerschmidt SK, Musyanovych A, Altvater M, et al. Targeted lipid-coated nanoparticles: delivery of tumor necrosis factor-functionalized particles to tumor cells. J Control Release. 2009;137(1):69–77. doi: 10.1016/j.jconrel.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 86.Samel D, Muller D, Gerspach J, et al. Generation of a FasL-based proapoptotic fusion protein devoid of systemic toxicity due to cell-surface antigen-restricted activation. J Biol Chem. 2003;278(34):32077–32082. doi: 10.1074/jbc.M304866200. [DOI] [PubMed] [Google Scholar]
- 87.Burckhart T, Thiel M, Nishikawa H, et al. Tumor-specific crosslinking of GITR as costimulation for immunotherapy. J Immunother. 2010;33(9):925–934. doi: 10.1097/CJI.0b013e3181f3cc87. [DOI] [PubMed] [Google Scholar]
- 88.Lee J, Fassnacht M, Nair S, Boczkowski D, Gilboa E. Tumor immunotherapy targeting fibroblast activation protein, a product expressed in tumor-associated fibroblasts. Cancer Res. 2005;65(23):11156–11163. doi: 10.1158/0008-5472.CAN-05-2805. [DOI] [PubMed] [Google Scholar]
- 89▪▪.Loeffler M, Kruger JA, Niethammer AG, Reisfeld RA. Targeting tumor-associated fibroblasts improves cancer chemotherapy by increasing intratumoral drug uptake. J Clin Invest. 2006;116(7):1955–1962. doi: 10.1172/JCI26532. Demonstrates that FAP vaccines improves chemotherapy in preclincial animal models. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wen Y, Wang CT, Ma TT, et al. Immunotherapy targeting fibroblast activation protein inhibits tumor growth and increases survival in a murine colon cancer model. Cancer Sci. 2010;101(11):2325–2332. doi: 10.1111/j.1349-7006.2010.01695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sadelain M, Riviere I, Brentjens R. Targeting tumours with genetically enhanced T lymphocytes. Nat Rev Cancer. 2003;3(1):35–45. doi: 10.1038/nrc971. [DOI] [PubMed] [Google Scholar]
- 92.Kakarla S, Wang L, Rowley D, Pfizenmaier K, Gottschalk S. Improving T-cell immunotherapies for solid tumors by targeting the tumor stroma. Biol Blood Marrow Trans. 2011;17(2 Suppl 1):S270. [Google Scholar]
- 93.Kobayashi T, Liu X, Wen FQ, et al. Smad3 mediates TGF-β1 induction of VEGF production in lung fibroblasts. Biochem Biophys Res Commun. 2005;327(2):393–398. doi: 10.1016/j.bbrc.2004.12.032. [DOI] [PubMed] [Google Scholar]
- 94.Chen MM, Lam A, Abraham JA, Schreiner GF, Joly AH. CTGF expression is induced by TGF-β in cardiac fibroblasts and cardiac myocytes: a potential role in heart fibrosis. J Mol Cell Cardiol. 2000;32(10):1805–1819. doi: 10.1006/jmcc.2000.1215. [DOI] [PubMed] [Google Scholar]
- 95.Yingling JM, Blanchard KL, Sawyer JS. Development of TGF-β signalling inhibitors for cancer therapy. Nat Rev Drug Discov. 2004;3(12):1011–1022. doi: 10.1038/nrd1580. [DOI] [PubMed] [Google Scholar]
- 96.Fakhrai H, Dorigo O, Shawler DL, et al. Eradication of established intracranial rat gliomas by transforming growth factor β antisense gene therapy. Proc Natl Acad Sci USA. 1996;93(7):2909–2914. doi: 10.1073/pnas.93.7.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nemunaitis J, Dillman RO, Schwarzenberger PO, et al. Phase II study of belagenpumatucel-L, a transforming growth factor β-2 antisense gene-modified allogeneic tumor cell vaccine in non-small-cell lung cancer. J Clin Oncol. 2006;24(29):4721–4730. doi: 10.1200/JCO.2005.05.5335. [DOI] [PubMed] [Google Scholar]
- 98.Flechsig P, Dadrich M, Bickelhaupt S, et al. LY2109761 attenuates radiation-induced pulmonary murine fibrosis via reversal of TGF-β and BMP associated proinflammatory and proangiogenic signals. Clin Cancer Res. 2012;18(13):3616–3627. doi: 10.1158/1078-0432.CCR-11-2855. [DOI] [PubMed] [Google Scholar]
- 99.Akhurst RJ. TGF-β antagonists: why suppress a tumor suppressor? J Clin Invest. 2002;109(12):1533–1536. doi: 10.1172/JCI15970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Akhurst RJ. TGF β signaling in health and disease. Nat Genet. 2004;36(8):790–792. doi: 10.1038/ng0804-790. [DOI] [PubMed] [Google Scholar]
- 101.Bollard CM, Rossig C, Calonge MJ, et al. Adapting a transforming growth factor β-related tumor protection strategy to enhance antitumor immunity. Blood. 2002;99(9):3179–3187. doi: 10.1182/blood.v99.9.3179. [DOI] [PubMed] [Google Scholar]
- 102.Lacuesta K, Buza E, Hauser H, et al. Assessing the safety of cytotoxic T lymphocytes transduced with a dominant negative transforming growth factor-β receptor. J Immun. 2006;29(3):250–260. doi: 10.1097/01.cji.0000192104.24583.ca. [DOI] [PubMed] [Google Scholar]
- 103.Yu Q, Stamenkovic I. Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-β and promotes tumor invasion and angiogenesis. Genes Dev. 2000;14(2):163–176. [PMC free article] [PubMed] [Google Scholar]
- 104.Hidalgo M, Eckhardt SG. Development of matrix metalloproteinase inhibitors in cancer therapy. J Natl Cancer Inst. 2001;93(3):178–193. doi: 10.1093/jnci/93.3.178. [DOI] [PubMed] [Google Scholar]
- 105.Steeghs N, Nortier JW, Gelderblom H. Small molecule tyrosine kinase inhibitors in the treatment of solid tumors: an update of recent developments. Ann Surg Oncol. 2007;14(2):942–953. doi: 10.1245/s10434-006-9227-1. [DOI] [PubMed] [Google Scholar]
- 106.Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356(2):125–134. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 107.Vanneman M, Dranoff G. Combining immunotherapy and targeted therapies in cancer treatment. Nat Rev Cancer. 2012;12(4):237–251. doi: 10.1038/nrc3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Shrimali RK, Yu Z, Theoret MR, Chinnasamy D, Restifo NP, Rosenberg SA. Antiangiogenic agents can increase lymphocyte infiltration into tumor and enhance the effectiveness of adoptive immunotherapy of cancer. Cancer Res. 2010;70(15):6171–6180. doi: 10.1158/0008-5472.CAN-10-0153. [DOI] [PMC free article] [PubMed] [Google Scholar]