Abstract
Background
A large number of studies have examined the tracking of blood pressure (BP) from childhood to adulthood, but the reported findings are inconsistent and few systematic analyses have been conducted.
Methods and Results
We conducted a systematic search of PubMed for studies that examined the tracking of BP from childhood to adulthood published between January 1970 and July 2006. From 301 retrieved papers, 50 cohort studies met our inclusion criteria and provided 617 data points (Pearson/Spearman correlation coefficients) for systolic BP (SBP) and 547 data points for diastolic BP (DBP) for our meta-analysis. Information on sample characteristics and BP measurement protocols were extracted. Fisher’s z transformation and random-effects meta-regression analysis was conducted. The reported BP tracking correlation coefficients varied from −0.12 to 0.80 for SBP and −0.16 to 0.70 for DBP, with the average of 0.38 for SBP and 0.28 for DBP. BP tracking varied significantly by baseline age and length of follow-up. The strength of BP tracking increased with baseline age by 0.012 for SBP (P< 0.001) and 0.009 for DBP (P< 0.001), and decreased with follow-up length by 0.008 for SBP (P< 0.001) and 0.005 for DBP (P< 0.001). BP tracking did not vary markedly across number of BP measurements or race/population groups.
Conclusions
Data from diverse populations show the evidence of BP tracking from childhood into adulthood is strong. Childhood BP is associated with BP in late life and early intervention is important.
Keywords: blood pressure, tracking, childhood, adulthood, meta-regression analysis
Over the past several decades, a large number of longitudinal studies have been conducted to assess blood pressure (BP) tracking over time among children and adults. These studies show that BP tracking correlations during childhood were positive, but were substantially lower than those in adulthood.1-10 Studies also reported that BP tracking was stronger for shorter follow-up periods and in older children.11-14 Some studies, such as the Bogalusa Heart Study conducted in the U.S., also investigated ethnic differences, which suggested mixed patterns.15-19 Explanations for the inconsistent findings and ethnic differences in the tracking are inconclusive. Furthermore, to our knowledge, little is known about the differences in the BP tracking patterns across populations worldwide.
There are many important public heath implications to study BP tracking from childhood to adulthood. As growing evidence indicates, hypertension, one of the major modifiable risk factors for cardiovascular disease (CVD), is established early in life.18-20 Recent studies also show that increased BP among children is related to the growing obesity epidemic.21, 22 Although the evidence of BP tracking from childhood to adulthood is rich and continues to increase, reported findings from previous studies are inconsistent; and to our knowledge, no systematic analyses have been conducted.
The present study aimed to systematically evaluate epidemiologic evidence on BP tracking from childhood to adulthood. We first performed a systematic review of studies that examined BP tracking. Then we conducted a meta-regression analysis to examine the predictors of BP tracking degree which served two goals: 1) summarize findings from different studies published over the past four decades; and 2) test the differences in the tracking by sample and study characteristics such as race/population groups, length of follow-up, and BP measurement protocols.
Methods
Literature search strategy
We conducted a comprehensive literature search of PubMed database using related key words: child, adolescent, adult, tracking, persistence, stability, maintenance, consistency, BP, hypertension, cohort, follow-up, longitudinal study, and cardiovascular disease. Related studies published between January 1970 and July 2006 were searched. Titles and abstracts of studies uncovered by the electronic searches were examined on screen first. Only studies that examined the tracking of BP from childhood to adulthood and were published in English, Chinese, and Japanese were included. This initial screening resulted in 301 studies, which were the further examined to determine if they met the criteria for inclusion in the meta-analysis described below. The full papers that met our selection criteria (see below) were carefully reviewed. Additional studies (n=6) identified in the course of reading or brought to our attention by colleagues and experts consulted were included. Studies (n=10) were also identified by searching on authors who had contributed at least one relevant article, by using the ‘related articles’ function in PubMed, and hand searching of the cross-references from retrieved articles.
Study selection criteria
Cohort studies that examined the BP tracking from childhood to adulthood and met the following criteria were included: 1) BP tracking correlation coefficients (Pearson or Spearman approach) were reported; 2) Cohorts’ baseline ages were under 18 years; 3) Sample sizes were greater than 50; and 4) Studies were published in English, Chinese, or Japanese. Tracking (correlation) coefficients ranged between −1 and 1, which measures the degree of correlation between two repeated observations over time. A value of 1 indicates perfect positive tracking and −1 means perfect negative tracking; and a value of 0 shows no tracking. Multiple follow-up studies based on the same cohort with different follow-up periods were included. We defined “data points” as the reported BP tracking correlation coefficients for each subgroup included in a certain study, ie, a study might provide different BP tracking results by sex, baseline age, and follow-up. Note that some studies only reported systolic BP (SBP) or diastolic BP (DBP) alone. Data points for SBP and DBP were analyzed separately.
Data extraction
Using a standardized data extraction form, we extracted and tabulated related data. Information extracted included first author’s name, publication year, country of data collection, sample characteristics (e.g., baseline age, sex, race/population, sample size), length of follow-up, methods of BP measurement (e.g., mercury sphygmomanometer), number of BP measurements, and outcome assessment (e.g., correlation coefficient). Based on our review of previous studies and our research interest, we chose to include sex, baseline age, length of follow-up (years), number of BP measurement, publication year (which may also help indicates possible time change), and race/population as the potential predictors of BP tracking in our meta-analysis. A dataset based on this data extraction was created using a spreadsheet program (Microsoft Excel, Microsoft Co., 2003). From 301 retrieved papers, 50 cohort studies met our inclusion criteria and provided 617 data points for SBP and 547 data point for DBP for our meta-analysis. Appendix 1 summarized those 251 papers that were reviewed but were not included in our meta-analysis.
Statistical analysis
We first created scatter plots of BP tracking correlations by length of follow-up and baseline age, respectively. Then we conducted locally weighted regression (LOWESS) as a non-parametric smoothing technique to show the relationships, with a bandwidth of 0.4.23 We also fit univariate models assessing the association between BP tracking and length of follow-up. Using the Fisher’s z transformation, we converted Pearson’s or Spearman’s BP tracking correlation coefficients to z’s to obtain approximate normality, and then calculated a mean transformed correlation weighted by the sample sizes in these studies.16, 24 We used t-test to compare the average transformed BP tracking correlation coefficients between males and females. Note that our findings with- and without the Fisher’s z transformation were similar; and reported ones are based on the transformed data when relevant.
The homogeneity of the effect size among studies was tested using Q-test. Our tests indicated heterogeneity across the studies included in our meta-analysis for SBP (Q= 847.7, P<0.001) and for DBP (Q= 384.5, P< 0.001). Thus, we fit random-effects models, which took into account the between-study variations, to study the factors that might affect BP tracking. These results were presented, but were more conservative than those based on our multiple linear regression models (Appendix 2), which found that the number of BP measurements, publication year, and race/population were also significant predictors.
In addition, using general linear models (GLM), we computed least-square means (LS means) of BP tracking correlation coefficients by length of follow-up groups (<5, 5-9, 10-14, ≥15 years) to control for baseline age. Similarly, we calculated LS means of BP tracking correlation coefficients by baseline age groups (<5, 5-9, 10-14, ≥15 years old) after adjustment for length of follow-up. Trend tests were conducted using Kruskal-Wallis test, a non-parametric approach.
Since some studies only reported BP tracking correlation coefficients for males and females combined instead of for each sex, we grouped the studies as males, females, and combined (both). To test the ethnic/population differences, we grouped the studies as European, American, Asian, and other populations. European was treated as the reference group. We further grouped American data points into three categories: General American (when the study sample could not be classified as African American or White based on reported information); White (> 80% of the study participants were white); and African American. This allowed us to further test ethnic differences in the US. In addition, if the centered publication year variable (i.e., = original publication year - 1977) rather than the original publication year was included in our models, the intercepts changed, but the other effect estimates were not affected. We also assessed publication bias by plotting sample sizes against BP tracking correlations as well as by the Begg’s adjusted rank correlation test and the Egger’s regression asymmetry test.25-27
All analyses were performed using SAS version 9.1 (SAS Institute Inc., Cary, NC) and STATA release 9. Statistical significance was set at P<0.05. The authors had full access to and take full responsibility for the integrity of the data. All authors have read and agree to the manuscript as written.
Results
Results of systematic review
Table 1 and Appendix 3 summarized the main characteristics and findings of the 50 follow-up studies on BP tracking. The majority of them (n=29, 58%) were from the US, 11 (22%) from Europe, 6 (12%) from Asia, and 4 (8%) from other populations (including Australia, Canada, Israel, and New Zealand). All of these studies included males and females. Length of follow-up ranged from 0.5 to 47 years. Reported BP tracking correlation coefficient varied from −0.12 to 0.80 for SBP and −0.16 to 0.70 for DBP, with the mean of 0.38 for SBP (SD=0.16) and 0.28 for DBP (SD=0.15). In males, the means (SD) of SBP and DBP tracking correlation coefficient were 0.39±0.15 vs. 0.29±0.16. In females, the corresponding figures were 0.38±0.15 vs. 0.26±0.15. T-test indicated no sex difference in BP tracking. Of these studies, 6% recorded BP measurements once/per visit, 22% twice, 50% ≥3 times, and 22% did not provide detailed information; 62.5% used mercury manometer, 6.3% random-zero manometer, 8.3% ultrasound device, and 4.2% automated device.
Table 1.
Summary of main characteristics of the 50 cohort studies that examined blood pressure tracking during/from childhood to adulthood
| Studies/cohorts* |
Data points§ |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SBP | DBP | Male† | Female† | ||||||||
|
|
|
|
|
||||||||
| N | % | N | % | N | % | N | % | N | % | ||
| Baseline age | < 5y | 11 | 12.2 | 85 | 13.8 | 33 | 6.0 | 8 | 3.7 | 8 | 3.7 |
| 5-9y | 31 | 34.4 | 233 | 37.8 | 214 | 39.1 | 87 | 40.7 | 87 | 40.7 | |
| 10-14y | 29 | 32.2 | 216 | 35.0 | 219 | 40.0 | 85 | 39.7 | 85 | 39.7 | |
| ≥ 15y | 19 | 21.1 | 83 | 13.5 | 81 | 14.8 | 34 | 15.9 | 34 | 15.9 | |
| Total | 90 | 100.0 | 617 | 100.0 | 547 | 100.0 | 214 | 100.0 | 214 | 100.0 | |
| Length of follow-up | <5y | 33 | 37.5 | 270 | 43.8 | 227 | 41.5 | 79 | 36.9 | 79 | 36.9 |
| 5-9y | 23 | 26.1 | 115 | 18.6 | 100 | 18.3 | 38 | 17.8 | 38 | 17.8 | |
| 10-14y | 12 | 13.6 | 84 | 13.6 | 64 | 11.7 | 39 | 18.2 | 39 | 18.2 | |
| ≥ 15y | 17 | 19.3 | 148 | 24.0 | 156 | 28.5 | 58 | 27.1 | 58 | 27.1 | |
| Total | 88 | 100.0 | 617 | 100.0 | 547 | 100.0 | 214 | 100.0 | 214 | 100.0 | |
| Publication year | 1970s | 8 | 16.0 | 110 | 17.8 | 110 | 20.1 | 44 | 20.6 | 44 | 20.6 |
| 1980s | 19 | 38.0 | 159 | 25.8 | 124 | 22.7 | 44 | 20.6 | 44 | 20.6 | |
| 1990s | 17 | 34.0 | 320 | 51.9 | 285 | 52.1 | 116 | 54.2 | 116 | 54.2 | |
| 2000s | 6 | 12.0 | 28 | 4.5 | 28 | 5.1 | 10 | 4.7 | 10 | 4.7 | |
| Total | 50 | 100.0 | 617 | 100.0 | 547 | 100.0 | 214 | 100.0 | 214 | 100.0 | |
| Race/population | European | 11 | 18.3 | 96 | 15.6 | 48 | 8.8 | 18 | 8.4 | 18 | 8.4 |
| General American | 11 | 18.3 | 48 | 7.8 | 61 | 11.2 | - | - | - | - | |
| White American | 22 | 36.7 | 336 | 54.5 | 301 | 55.0 | 130 | 60.8 | 130 | 60.8 | |
| African American | 6 | 10.0 | 55 | 8.9 | 55 | 10.1 | 27 | 12.6 | 27 | 12.6 | |
| Asian | 6 | 10.0 | 60 | 9.7 | 60 | 11.0 | 29 | 13.6 | 29 | 13.6 | |
| Other populations | 4 | 6.7 | 22 | 3.6 | 22 | 4.0 | 10 | 4.7 | 10 | 4.7 | |
| Total | 60 | 100.0 | 617 | 100.0 | 547 | 100.0 | 214 | 100.0 | 214 | 100.0 | |
SBP: systolic blood pressure; DBP: diastolic blood pressure
One study might provide multiple cohorts and data points (e.g., different baseline age, sex, follow-up, and ethnicity). Thus, the 50 cohort studies provided 90 cohorts with different baseline age groups, and 617 data points for SBP, for example. In total, 49 studies reported SBP and 47 studies reported DBP.
Data points: correlation coefficients reported for various study sample groups.
Based on SBP data.
No data available
In summary, previous longitudinal data from diverse populations have shown different BP tracking patterns. The discrepancies may be due to differences in study design, baseline age, follow-up period, measuring instruments, intra-subject variability, characteristics of study samples, and analytic methods used. Nevertheless, most studies found significant BP tracking; and some found a weaker tracking for longer follow-up periods, and a stronger tracking for SBP than for DBP, which was possibly due to the difficulties in measuring DBP in children as well as the changes in the recommendations on how to take DBP measures over time.
Results of meta-analysis
Difference by length of follow-up
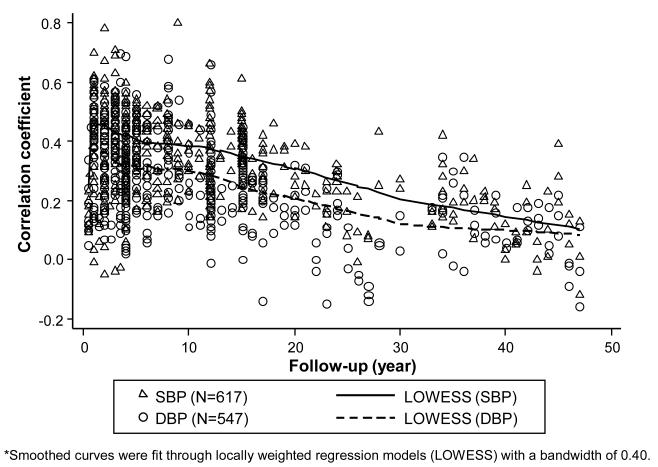
Figure 1 shows the scatter plots and smoothed curves of SBP and DBP tracking correlation coefficients against follow-up period. We observed linear relationships between the length of follow-up and SBP and DBP tracking correlation coefficient by using LOWESS approach. Our univariate models indicate a negative linear relationship between BP tracking and length of follow-up, and it was similar for SBP and DBP (β= −0.007, P<0.001; β= −0.005, P<0.001; respectively). Our predicted 5-year follow-up BP tracking correlation coefficient was 0.43 (95% confidence interval: 0.30-0.59) for SBP and 0.32 (0.17-0.49) for DBP. The corresponding figures for 10-year follow-up were 0.40 (0.27-0.56) and 0.29 (0.17-0.44), respectively.
Figure 1.
Scatter plot and smoothed curves of systolic and diastolic blood pressure (SBP/DBP) tracking correlation coefficients against follow-up period*
*Smoothed curves were fit through locally weighted regression models (LOWESS) with a bandwidth of 0.40.
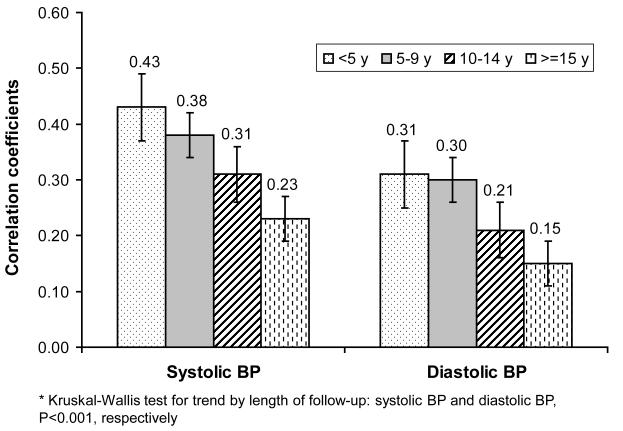
Figure 2 shows the LS means of BP tracking correlations for length of follow-up after adjustment for baseline age. LS means of tracking correlation coefficients for <5, 5-9, 10-14, ≥15 years follow-up were 0.43, 0.38, 0.31, 0.23 for SBP and 0.31, 0.30, 0.21, 0.15 for DBP, respectively. There was a clear trend of weaker SBP and DBP tracking with longer follow-up (P<0.001 for both).
Figure 2.
Least square means of blood pressure (BP) tracking correlation coefficients for length of follow-up, adjusted for baseline age*
* Kruskal-Wallis test for trend by length of follow-up: systolic BP and diastolic BP, P<0.001, respectively
Difference by baseline age
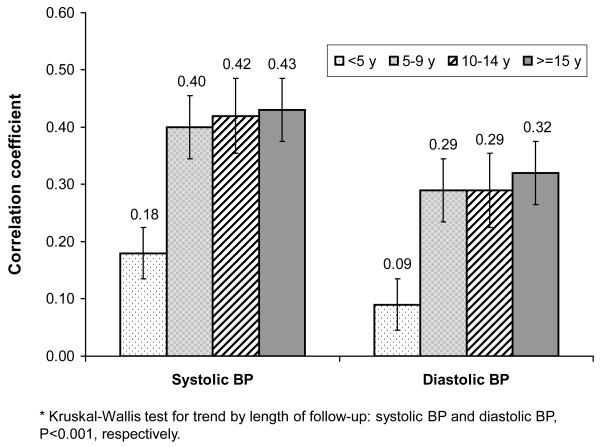
Figure 3 shows the scatter plots and smoothed curves of SBP and DBP tracking correlation coefficients against baseline age. Almost linear relationships between baseline age and SBP and DBP tracking correlation coefficient were observed. SBP seems to track better than DBP with the increase in baseline age. In cohorts with baseline ages of 8 years or younger, the strength of SBP tracking increased sharply with baseline age; among those with baseline age of 8-15 years, SBP tracking kept stable at about 0.40; and for those older than 15 years, SBP tracking increased again with age. The baseline age difference in DBP tracking differed slightly from that of SBP. Figure 4 shows the LS means of BP tracking correlation for baseline age groups adjusted for length of follow-up. SBP and DBP tracking correlations increased significantly with baseline age (both P< 0.001, respectively), in particular due to the big difference between preschool children and their older counterparts. The age differences were small after age 5.
Figure 3.
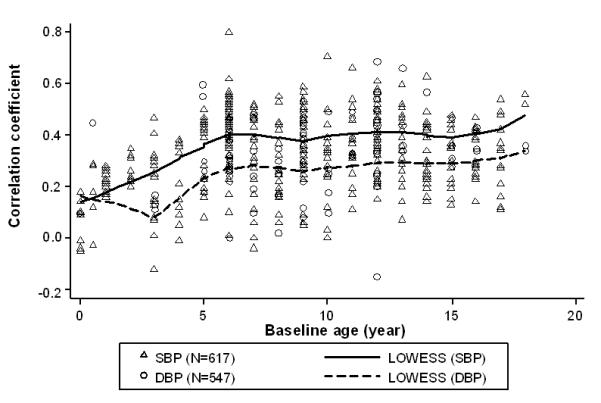
Scatter plot and smoothed curves of systolic and diastolic blood pressure (SBP/DBP) tracking correlation coefficients against baseline age*
*Smoothed curves were fit through locally weighted regression models (LOWESS) with a bandwidth of 0.40.
Figure 4.
Least square means of blood pressure (BP) tracking correlation coefficients for baseline age, adjusted for length of follow-up*
* Kruskal-Wallis test for trend by length of follow-up: systolic BP and diastolic BP, P<0.001, respectively
Random-effects meta-regression analysis
Table 2 shows the predictors of BP tracking when we treated male group as reference. Our analyses show that BP tracking varied significantly by baseline age and length of follow-up. The strength of BP tracking increased with baseline age by 0.012 for SBP (P< 0.001) and 0.009 for DBP (P< 0.001), and decreased with follow-up length by 0.008 for SBP (P< 0.001) and 0.005 for DBP (P< 0.001). Compared to one BP measurement per visit, two measurements increased observed BP tracking by 0.216 for SBP (P= 0.070) and 0.122 for DBP (P= 0.078). BP tracking did not vary markedly across race/population groups. No difference was found in BP tracking when compared different BP measurement instruments with mercury manometer, except for automated devices, which predicted higher BP tracking and it was significant for DBP (β= 0.223, P< 0.01). Although no significant gender difference was found in SBP tracking, females had weaker DBP tracking than males by 0.029 (P< 0.01). We further examined the gender difference in BP tracking by excluding those data points for pooled males and females in our models and the results were similar. Table 3 shows the findings of the analysis stratified by sex, which indicate some gender-differences in the effects of the predictors such as baseline age.
Table 2.
Random-effects multiple regression analysis: predictors of blood pressure tracking correlation coefficients, based on all available data points regardless of sex
| Predictors | SBP* (N= 617) |
DBP* (N= 547) |
|||||
|---|---|---|---|---|---|---|---|
| β | SE | P value | β | SE | P value | ||
| Intercept | −4.151 | 8.181 | 0.612 | 1.362 | 4.783 | 0.776 | |
| Sex | Male (reference) | ||||||
| Female | −0.008 | 0.011 | 0.471 | −0.029 | 0.011 | 0.009 | |
| Males and females¶ | 0.019 | 0.018 | 0.299 | −0.014 | 0.018 | 0.455 | |
| Baseline age (year) | 0.012 | 0.001 | <0.001 | 0.009 | 0.002 | <0.001 | |
| Length of follow-up (year) | −0.008 | 0.001 | <0.001 | −0.005 | 0.001 | <0.001 | |
| Number of BP measurement | Once (reference) | ||||||
| Twice | 0.216 | 0.119 | 0.070 | 0.122 | 0.069 | 0.078 | |
| Three times or more | 0.086 | 0.107 | 0.417 | 0.082 | 0.059 | 0.169 | |
| Unknown† | 0.060 | 0.127 | 0.634 | −0.007 | 0.074 | 0.928 | |
| Publication year (year) | 0.002 | 0.004 | 0.586 | 0.001 | 0.002 | 0.814 | |
| Race/population | European (reference) | ||||||
| General American | 0.095 | 0.088 | 0.280 | 0.055 | 0.061 | 0.367 | |
| White American | 0.035 | 0.079 | 0.655 | −0.044 | 0.052 | 0.399 | |
| African American | 0.049 | 0.080 | 0.542 | −0.013 | 0.055 | 0.818 | |
| Asian | −0.061 | 0.095 | 0.517 | −0.074 | 0.058 | 0.203 | |
| Other‡ | −0.166 | 0.117 | 0.156 | −0.179 | 0.072 | 0.013 | |
| BP measurement technique | Mercury manometer (reference) | ||||||
| Random-zero manometer | 0.018 | 0.111 | 0.873 | 0.013 | 0.063 | 0.841 | |
| Ultrasound device | −0.197 | 0.099 | 0.046 | −0.065 | 0.063 | 0.304 | |
| Automated device | 0.071 | 0.142 | 0.617 | 0.223 | 0.080 | 0.005 | |
| Unknown† | −0.001 | 0.079 | 0.994 | 0.032 | 0.053 | 0.546 | |
| R2 | 0.480 | 0.472 | |||||
SBP: systolic blood pressure; DBP: diastolic blood pressure. Multiple linear regression models were fit for SBP and DBP, respectively.
Some studies reported the overall tracking correlation coefficient for males and females combined.
Unknown: no detailed information available.
Other: Australia, Canada, Israel, and New
Table 3.
Random-effects multiple regression analysis: predictors of blood pressure (BP) tracking correlation coefficients, stratified by sex
| Predictors | SBP |
DBP |
|||||
|---|---|---|---|---|---|---|---|
| β | SE | P value | β | SE | P value | ||
| Male (N) | 214 | 205 | |||||
| Intercept | 5.375 | 5.049 | 0.287 | −0.953 | 5.457 | 0.861 | |
| Baseline age (y) | 0.003 | 0.002 | 0.285 | 0.004 | 0.003 | 0.171 | |
| Length of follow-up (y) | −0.007 | 0.001 | <0.001 | −0.008 | 0.002 | <0.001 | |
| number of BP measurement | Once (reference) | ||||||
| Twice | 0.139 | 0.059 | 0.019 | 0.142 | 0.061 | 0.021 | |
| Three times or more | 0.104 | 0.045 | 0.020 | 0.089 | 0.048 | 0.062 | |
| Unknown | 0.224 | 0.079 | 0.005 | 0.023 | 0.081 | 0.775 | |
| Publication year (y) | −0.003 | 0.003 | 0.324 | 0.001 | 0.003 | 0.817 | |
| Ethnicity/population | European (reference) | ||||||
| General American | − | − | − | 0.087 | 0.145 | 0.548 | |
| White American | 0.011 | 0.059 | 0.851 | −0.024 | 0.064 | 0.703 | |
| African American | 0.057 | 0.065 | 0.380 | 0.032 | 0.069 | 0.645 | |
| Asian | 0.004 | 0.061 | 0.949 | −0.114 | 0.063 | 0.071 | |
| Other | −0.234 | 0.081 | 0.004 | −0.209 | 0.083 | 0.012 | |
| BP measurement technique | Mercury manometer (reference) | ||||||
| Random-zero manometer | −0.136 | 0.086 | 0.115 | −0.076 | 0.088 | 0.386 | |
| Ultrasound device | 0.221 | 0.099 | 0.026 | −0.061 | 0.102 | 0.550 | |
| Automated device | 0.215 | 0.089 | 0.017 | 0.243 | 0.092 | 0.008 | |
| Unknown† | −0.057 | 0.051 | 0.256 | −0.013 | 0.062 | 0.829 | |
| R2 | 0.486 | 0.448 | |||||
| Female (N) | 214 | 204 | |||||
| Intercept | 1.613 | 6.837 | 0.813 | 5.787 | 6.415 | 0.367 | |
| Baseline age (y) | 0.012 | 0.002 | <0.001 | 0.010 | 0.003 | <0.001 | |
| Length of follow-up (y) | −0.009 | 0.001 | <0.001 | −0.003 | 0.001 | 0.021 | |
| number of BP measurement | Once (reference) | ||||||
| Twice | 0.079 | 0.095 | 0.407 | 0.127 | 0.084 | 0.130 | |
| Three times or more | 0.069 | 0.075 | 0.354 | 0.093 | 0.066 | 0.163 | |
| Unknown | −0.017 | 0.108 | 0.875 | −0.035 | 0.098 | 0.720 | |
| Publication year (y) | −0.001 | 0.003 | 0.860 | −0.003 | 0.003 | 0.383 | |
| Ethnicity/population | European (reference) | ||||||
| General American | − | − | − | − | − | − | |
| White American | −0.080 | 0.079 | 0.313 | −0.059 | 0.075 | 0.433 | |
| African American | −0.050 | 0.083 | 0.546 | −0.040 | 0.078 | 0.611 | |
| Asian | −0.104 | 0.082 | 0.209 | −0.093 | 0.075 | 0.215 | |
| Other | −0.184 | 0.099 | 0.064 | −0.122 | 0.092 | 0.186 | |
| BP measurement technique | Mercury manometer (reference) | ||||||
| Random-zero manometer | −0.083 | 0.107 | 0.441 | −0.094 | 0.099 | 0.343 | |
| Ultrasound device | 0.197 | 0.140 | 0.159 | 0.013 | 0.125 | 0.917 | |
| Automated device | 0.082 | 0.114 | 0.470 | 0.228 | 0.105 | 0.030 | |
| Unknown† | 0.002 | 0.067 | 0.977 | 0.108 | 0.071 | 0.128 | |
| R2 | 0.468 | 0.421 | |||||
SBP: systolic blood pressure; DBP: diastolic blood pressure. Separate multiple linear regression models were fit for SBP and DBP, respectively.
Unknown: no detailed information available.
Other: Australia, Canada, Israel, and New Zealand.
No data available
Publication bias
We examined the potential publication bias by plotting sample sizes against SBP and DBP tracking correlation coefficients among studies in our meta-regression analysis (Appendix 4). There was no evidence that suggested publication bias. Furthermore, neither Begg’s adjusted rank correlation test nor Egger’s regression asymmetry test was significant for SBP or for DBP.
Discussion
The tracking of BP over time has intrigued epidemiologists both as a measure of the genetic-environmental interaction and as a way of identifying high-risk individuals.28-30 A large number of studies have shown different degree of BP tracking from childhood to adulthood. Our review and meta-analysis, based on cohort studies in diverse pediatric populations, demonstrates a moderate BP tracking between childhood and adulthood. Overall, the average of reported tracking correlation is greater for SBP than for DBP. Sex, baseline age, and length of follow-up are significant predictors of BP tracking.
Sex difference
Although our findings indicate little sex difference in SBP tracking, males have a stronger DBP tracking than females. The average of SBP tracking correlation was 0.39 in males vs. 0.38 in females, while for DBP, it was 0.29 vs. 0.26. Previous studies have reported conflicting findings. For example, the Dormont High School Follow-up Study found that SBP tracking was stronger in males than females, 0.27 vs. 0.24 over a 30-year follow-up.10 The Muscatine Study found that females had stronger DBP tracking than males in some age groups, but found no sex difference in other age groups.8 A previous 1995 review found no sex difference in BP tracking from childhood to adulthood.31 Further research is needed to help explain the sex difference in BP tracking we observed.
Baseline age
Our meta-analysis shows that BP tracking appears to increase with baseline age, i.e., older children have a strong BP tracking into adulthood. Compared to cohorts whose baseline ages were <5 years, those aged ≥15 years had stronger tracking correlation (0.18 vs. 0.43 for SBP, and 0.09 vs. 0.32 for DBP). Several studies suggest that BP tracking starts at very young ages and increases with age.32, 33 For example, one study measured BP in 1797 infants aged 4 days and then repeated at 6 weeks, 6 months, 1 year, and thereafter yearly until 4 years of age.32 During infancy the tracking correlations were weak (most <0.20). As children grew older the tracking became stronger.32 In the Bogalusa Study, children aged 10-14 years were found to have stronger BP tracking than those aged 5-9 years.18 Baseline age seems to be an important determinant of BP tracking. Difficulties in making accurate measurements of BP among younger children may result in poor BP tracking. This may also be true for the weaker tracking of DBP.
Length of follow-up
Our predicted 5-year follow-up tracking correlation coefficient was 0.42 for SBP and 0.32 for DBP. For 10-year follow-up, the corresponding figures were 0.38 and 0.29, respectively. For less than 5-year follow-up, age-adjusted means of tracking correlation was 0.43 for SBP and 0.31 for DBP, respectively. The US Dormont High School Follow-up Study provided long-term BP tracking data in a cohort of 86 males and 116 females.10 Correlations for SBP were 0.42 in males vs. 0.39 in females between baseline age 17 and 34 years at the follow-up, and 0.27 vs. 0.24 between baseline age 17 and 47 years at the follow-up.
Number of BP measurements
Our meta-analysis using the random effect models shows that taking multiple BP measurements per visit only increased BP tracking marginally, but our unreported analysis using linear regression analysis showed a clear relationship between the number of BP measurements and BP tracking correlation. The random effect models may be more conservative. Because of BP variability, it has been argued that multiple visits are more important than multiple readings per visit in children and adolescents, and more than three measurements per visit may not be needed.34 We could not evaluate the impact of multiple visits on BP tracking in our meta-analysis because of the limited available data.
Ethnic/population difference
Grouping the related published studies into several large ethnic and country categories, we attempted to examine the between-population group differences in BP tracking. Compared to Europeans, African Americans and white American did not have stronger SBP tracking. The Bogalusa Heart Study found similar BP tracking in African American and white ethnic groups, with SBP ranging from 0.38 to 0.50 for African American children and 0.36 to 0.49 for white children, while DBP tracking ranging from 0.19 to 0.41 for African American children and 0.26 to 0.42 for white children.18 However, some studies have reported ethnic differences in BP tracking, such as the CATCH study.29 African American children displayed stronger tracking than white children. It remains inconclusive what factors have contributed to these differences.
BP tracking was suggested to be related to genetic, biological, behavioral, environmental, and social determinants.19, 35 Although our study cannot test these, we suspect that several factors may affect BP tracking from childhood to adulthood. First, previous studies have reported that lower birth weight is associated with higher subsequent SBP in children and adults, although findings have not been consistent in all populations.36-41 This association might be an example of ’programming’, a view that was partly supported by the existence of tracking of BP in children.36 A birth cohort study of 3634 men and women born in Britain in 1946 was conducted, and BP was measured at ages 36, 43, and 53 years. A consistent negative association between birthweight and SBP was noted from age 36 to 53 years. Among 25,000 UK men and women, small or disproportionate (thin or short) at birth had high rates of high BP in middle age.42
Second, overweight and weight change are likely to affect BP tracking. Some studies found that tracking of SBP through adolescence to early adulthood was influenced by overweight and weight change.43 Accelerated weight gain in early childhood may increase the risk of elevated BP in later life.40 The influence of weight change on BP tracking emphasizes the importance of weight control in childhood and adolescence. Maintenance of normal weight gain in childhood may prevent clustering of hypertension and CVD risk factors in adulthood. On the other hand, it is possible that the growing obesity epidemic might have weakened the BP tracking observed in some recent studies where the study samples are affected.
An important strength of our meta-analysis is that it is based on a large number of cohort studies conducted in different countries published since 1970s. A total of 617 data points for SBP and 547 data points for DBP provided in 50 studies were used. The systematic and quantitative assessment provides strong evidence to help quantify the degree of BP tracking from childhood to adulthood. In addition, we applied several statistical analysis approaches to examine the factors that may affect BP tracking. On the other hand, our meta-analysis has some limitations, which are common to these types of studies, such as potential selection bias and cannot study additional predictors or adjust for some potential confounders. For example, we cannot have access to the original individual-level BP data. Individuals’ baseline BP levels and their treatment of elevated BP may affect BP tracking to older ages. However, these could not be tested in our meta-analysis. Second, a large proportion of the related studies are based on data from the US. The findings may not be generalizable to other populations that have different genetic background or environmental characteristics. Third, most of these studies are based on data published in the 1990s, but not more recent studies, which used different statistical analyses (e.g., did not report Spearman or Pearson correlation coefficients) and thus could not be included in our meta-analysis. Fourth, due to the limited available information reported in the selected studies and the scope of the present study, we could not provide a through explanation of the observed between-population differences. In addition, one may be concerned as we used more than one data point from some cohort studies in which different follow-up periods or age groups were included, but such data in fact can help better test their influences on BP tracking. Our models have controlled for the possible dependence between multiple data points from the same cohort.
In conclusion, based on studies from diverse populations, our meta-analysis reinforces the concept that BP tracks from childhood to adulthood and that an elevated BP in childhood is likely to help predict adult hypertension. Future studies should focus on the determinants of BP tracking across sex and ethnic/ population groups. Longitudinal studies are needed to identify the determinants of BP tracking in infancy, childhood, and adolescence. The persisting high rates of CVD in developed countries and the growing CVD and hypertension epidemic in developing countries, along with the growing global childhood obesity epidemic all support the importance for continuing research and attention on BP tracking.44, 45
Supplementary Material
CLINICAL PERSPECTIVE.
Previously a large number of studies have examined blood pressure (BP) tracking over time among children and adults. Although rich evidence supports BP tracking from childhood to adulthood, the reported findings are inconsistent. No systematic analyses have been conducted to examine the consistence of findings from different studies or to test the differences across populations worldwide. Based on a systematic search of PubMed for studies that examined the BP tracking from childhood to adulthood published between 1970 and 2006, we conducted a meta-analysis of findings from 50 cohort studies, which provided approximately 600 data points (i.e., correlation coefficients) for systolic BP (SBP) and diastolic BP (DBP) tracking, respectively. BP tracking was stronger for SBP, but did not vary significantly by the number of BP measurements taken per visit or across race/populations. SBP tracking did not vary significantly by sex, but females had weaker DBP tracking than boys. The reported BP tracking coefficients varied from −0.12 to 0.80 for SBP and −0.16 to 0.70 for DBP. The average was 0.38 for SBP and 0.28 for DBP. The strength of BP tracking increased with baseline age by 0.012 for SBP and 0.009 for DBP, and decreased with follow-up by 0.008 for SBP and 0.005 for DBP. Based on studies from diverse populations, our meta-analysis reinforces the concepts that BP tracks from childhood to adulthood and that an elevated BP in childhood is likely to predict adult hypertension. Childhood BP is associated with BP in late life and early intervention is important.
Acknowledgements
We would like to thank Drs. May Beydoun and Yiqing Song for their assistance in conducting some related statistical analysis.
Sources of Funding The study was supported in part by the Johns Hopkins University Bloomberg School of Public Health and the National Institutes of Health (NIH)/National Institute of Diabetes & Digestive & Kidney Diseases (NIDDK) (R01 DK63383).
Footnotes
Conflict of Interest Disclosures None.
References
- 1.Rosner B, Hennekens CH, Kass EH, Miall WE. Age-specific correlation analysis of longitudinal blood pressure data. Am J Epidemiol. 1977;106:306–313. doi: 10.1093/oxfordjournals.aje.a112466. [DOI] [PubMed] [Google Scholar]
- 2.Clarke WR, Schrott HG, Leaverton PE, Connor WE, Lauer RM. Tracking of blood lipids and blood pressures in school age children: the Muscatine study. Circulation. 1978;58:626–634. doi: 10.1161/01.cir.58.4.626. [DOI] [PubMed] [Google Scholar]
- 3.Zinner SH, Margolius HS, Rosner B, Kass EH. Stability of blood pressure rank and urinary kallikrein concentration in childhood: an eight-year follow-up. Circulation. 1978;58:908–915. doi: 10.1161/01.cir.58.5.908. [DOI] [PubMed] [Google Scholar]
- 4.Voors AW, Webber LS, Berenson GS. Time course studies of blood pressure in children--the Bogalusa Heart Study. Am J Epidemiol. 1979;109:320–334. doi: 10.1093/oxfordjournals.aje.a112685. [DOI] [PubMed] [Google Scholar]
- 5.Webber LS, Cresanta JL, Voors AW, Berenson GS. Tracking of cardiovascular disease risk factor variables in school-age children. J Chronic Dis. 1983;36:647–660. doi: 10.1016/0021-9681(83)90081-4. [DOI] [PubMed] [Google Scholar]
- 6.Berenson GS, Cresanta JL, Webber LS. High blood pressure in the young. Annu Rev Med. 1984;35:535–560. doi: 10.1146/annurev.me.35.020184.002535. [DOI] [PubMed] [Google Scholar]
- 7.Michels VV, Bergstralh EJ, Hoverman VR, O’Fallon WM, Weidman WH. Tracking and prediction of blood pressure in children. Mayo Clin Proc. 1987;62:875–881. doi: 10.1016/s0025-6196(12)65041-1. [DOI] [PubMed] [Google Scholar]
- 8.Lauer RM, Clarke WR. Childhood risk factors for high adult blood pressure: the Muscatine Study. Pediatrics. 1989;84:633–641. [PubMed] [Google Scholar]
- 9.Nelson MJ, Ragland DR, Syme SL. Longitudinal prediction of adult blood pressure from juvenile blood pressure levels. Am J Epidemiol. 1992;136:633–645. doi: 10.1093/oxfordjournals.aje.a116543. [DOI] [PubMed] [Google Scholar]
- 10.Yong LC, Kuller LH. Tracking of blood pressure from adolescence to middle age: the Dormont High School Study. Prev Med. 1994;23:418–426. doi: 10.1006/pmed.1994.1057. [DOI] [PubMed] [Google Scholar]
- 11.Lauer RM, Burns TL, Clarke WR, Mahoney LT. Childhood predictors of future blood pressure. Hypertension. 1991;18:I74–81. doi: 10.1161/01.hyp.18.3_suppl.i74. [DOI] [PubMed] [Google Scholar]
- 12.Hait HI, Lemeshow S, Rosenman KD. A longitudinal study of blood pressure in a national survey of children. Am J Public Health. 1982;72:1285–1287. doi: 10.2105/ajph.72.11.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suh I, Nam CM, Jee SH, Kim SI, Lee KH, Kim HC, Kim CS. Twelve-year tracking of blood pressure in Korean school children: the Kangwha Study. Yonsei Med J. 1999;40:383–387. doi: 10.3349/ymj.1999.40.4.383. [DOI] [PubMed] [Google Scholar]
- 14.de Swiet M, Fayers P, Shinebourne EA. Systolic blood pressure in a population of infants in the first year of life: the Brompton study. Pediatrics. 1980;65:1028–1035. [PubMed] [Google Scholar]
- 15.Rosner B, Cook NR, Evans DA, Keough ME, Taylor JO, Polk BF, Hennekens CH. Reproducibility and predictive values of routine blood pressure measurements in children. Comparison with adult values and implications for screening children for elevated blood pressure. Am J Epidemiol. 1987;126:1115–1125. doi: 10.1093/oxfordjournals.aje.a114750. [DOI] [PubMed] [Google Scholar]
- 16.Gillman MW, Rosner B, Evans DA, Keough ME, Smith LA, Taylor JO, Hennekens CH. Use of multiple visits to increase blood pressure tracking correlations in childhood. Pediatrics. 1991;87:708–711. [PubMed] [Google Scholar]
- 17.Manatunga AK, Jones JJ, Pratt JH. Longitudinal assessment of blood pressures in black and white children. Hypertension. 1993;22:84–89. doi: 10.1161/01.hyp.22.1.84. [DOI] [PubMed] [Google Scholar]
- 18.Bao W, Threefoot SA, Srinivasan SR, Berenson GS. Essential hypertension predicted by tracking of elevated blood pressure from childhood to adulthood: the Bogalusa Heart Study. Am J Hypertens. 1995;8:657–665. doi: 10.1016/0895-7061(95)00116-7. [DOI] [PubMed] [Google Scholar]
- 19.Dekkers JC, Snieder H, Van Den Oord EJ, Treiber FA. Moderators of blood pressure development from childhood to adulthood: a 10-year longitudinal study. J Pediatr. 2002;141:770–779. doi: 10.1067/mpd.2002.128113. [DOI] [PubMed] [Google Scholar]
- 20.Lane DA, Gill P. Ethnicity and tracking blood pressure in children. J Hum Hypertens. 2004;18:223–228. doi: 10.1038/sj.jhh.1001674. [DOI] [PubMed] [Google Scholar]
- 21.Chiolero A, Cachat F, Burnier M, Paccaud F, Bovet P. Prevalence of hypertension in schoolchildren based on repeated measurements and association with overweight. J Hypertens. 2007;25:2209–2217. doi: 10.1097/HJH.0b013e3282ef48b2. [DOI] [PubMed] [Google Scholar]
- 22.Din-Dzietham R, Liu Y, Bielo MV, Shamsa F. High blood pressure trends in children and adolescents in national surveys, 1963 to 2002. Circulation. 2007;116:1488–1496. doi: 10.1161/CIRCULATIONAHA.106.683243. [DOI] [PubMed] [Google Scholar]
- 23.Schimek MG. Smoothing and regression: approaches, computations and application. John Wiley & Sons; New York: 2000. [Google Scholar]
- 24.Donner A, Rosner B. On inferences concerning a common correlation coefficient. Appl Statist. 1980;29:69–72. [Google Scholar]
- 25.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 26.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Egger M, Smith GD. Bias in location and selection of studies. BMJ. 1998;316:61–66. doi: 10.1136/bmj.316.7124.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levin SE. Significance of hypertension in children. Clin Cardiol. 1983;6:373–377. doi: 10.1002/clc.4960060803. [DOI] [PubMed] [Google Scholar]
- 29.Kelder SH, Osganian SK, Feldman HA, Webber LS, Parcel GS, Leupker RV, Wu MC, Nader PR. Tracking of physical and physiological risk variables among ethnic subgroups from third to eighth grade: the Child and Adolescent Trial for Cardiovascular Health cohort study. Prev Med. 2002;34:324–333. doi: 10.1006/pmed.2001.0990. [DOI] [PubMed] [Google Scholar]
- 30.Kupper N, Ge D, Treiber FA, Snieder H. Emergence of novel genetic effects on blood pressure and hemodynamics in adolescence: the Georgia Cardiovascular Twin Study. Hypertension. 2006;47:948–954. doi: 10.1161/01.HYP.0000217521.79447.9a. [DOI] [PubMed] [Google Scholar]
- 31.Woelk G. Blood pressure tracking from child to adulthood: a review. Cent Afr J Med. 1994;40:163–169. [PubMed] [Google Scholar]
- 32.de Swiet M, Fayers P, Shinebourne EA. Blood pressure in first 10 years of life: the Brompton study. BMJ. 1992;304:23–26. doi: 10.1136/bmj.304.6818.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zinner SH, Rosner B, Oh W, Kass EH. Significance of blood pressure in infancy. Familial aggregation and predictive effect on later blood pressure. Hypertension. 1985;7:411–416. [PubMed] [Google Scholar]
- 34.Gillman MW, Cook NR. Blood pressure measurement in childhood epidemiological studies. Circulation. 1995;92:1049–1057. doi: 10.1161/01.cir.92.4.1049. [DOI] [PubMed] [Google Scholar]
- 35.Rice T, Rao R, Perusse L, Bouchard C, Rao DC. Tracking of familial resemblance for resting blood pressure over time in the Quebec Family Study. Hum Biol. 2000;72:415–431. [PubMed] [Google Scholar]
- 36.Lucas A, Morley R. Does early nutrition in infants born before term programme later blood pressure? BMJ. 1994;309:304–308. doi: 10.1136/bmj.309.6950.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matthes JW, Lewis PA, Davies DP, Bethel JA. Relation between birth weight at term and systolic blood pressure in adolescence. BMJ. 1994;308:1074–1077. doi: 10.1136/bmj.308.6936.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hardy R, Kuh D, Langenberg C, Wadsworth ME. Birthweight, childhood social class, and change in adult blood pressure in the 1946 British birth cohort. Lancet. 2003;362:1178–1183. doi: 10.1016/S0140-6736(03)14539-4. [DOI] [PubMed] [Google Scholar]
- 39.Huxley RR, Shiell AW, Law CM. The role of size at birth and postnatal catch-up growth in determining systolic blood pressure: a systematic review of the literature. J Hypertens. 2000;18:815–831. doi: 10.1097/00004872-200018070-00002. [DOI] [PubMed] [Google Scholar]
- 40.Law CM, Shiell AW, Newsome CA, Syddall HE, Shinebourne EA, Fayers PM, Martyn CN, de Swiet M. Fetal, infant, and childhood growth and adult blood pressure: a longitudinal study from birth to 22 years of age. Circulation. 2002;105:1088–1092. doi: 10.1161/hc0902.104677. [DOI] [PubMed] [Google Scholar]
- 41.Milligan RA, Burke V, Dunbar DL, Spencer M, Balde E, Beilin LJ, Gracey MP. Associations between lifestyle and cardiovascular risk factors in 18-year-old Australians. J Adolesc Health. 1997;21:186–195. doi: 10.1016/S1054-139X(97)00006-2. [DOI] [PubMed] [Google Scholar]
- 42.Godfrey KM, Barker DJ. Fetal nutrition and adult disease. Am J Clin Nutr. 2000;71:1344S–1352S. doi: 10.1093/ajcn/71.5.1344s. [DOI] [PubMed] [Google Scholar]
- 43.Burke V, Beilin LJ, Dunbar D. Tracking of blood pressure in Australian children. J Hypertens. 2001;19:1185–1192. doi: 10.1097/00004872-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 44.McCarron P, Smith GD, Okasha M. Secular changes in blood pressure in childhood, adolescence and young adulthood: systematic review of trends from 1948 to 1998. J Hum Hypertens. 2002;16:677–689. doi: 10.1038/sj.jhh.1001471. [DOI] [PubMed] [Google Scholar]
- 45.Wang Y, Mi J, Shan XY, Wang QJ, Ge KY. Is China facing an obesity epidemic and the consequences? The trends in obesity and chronic disease in China. Int J Obes (Lond) 2007;31:177–188. doi: 10.1038/sj.ijo.0803354. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.