Abstract
Purpose
Breast cancer patients often experience a decline in physical functioning following cancer diagnosis. Although most patients recover after treatment, some patients do not. These changes may be magnified in older women with comorbid conditions and could impact survival outcomes.
Methods
We used longitudinal data from a prospective cohort study of women 65+ years of age, recruited shortly after diagnosis of early stage breast cancer, to examine changes in self-reported physical functioning measured with the Physical Function Index (PF-10) of the Medical Outcomes Study Short Form-36 (SF-36). Outcomes were constructed for small (0.2 SD), medium (0.5 SD), and large (0.8 SD) declines in the PF-10 measurement over two intervals 1) 3 to 15 months following cancer diagnosis, encompassing treatment and early recovery, and 2) 3 to 27 months following cancer diagnosis, in order to detect sustained recovery versus persistent decline. Cox-proportional hazards regression was used to examine association between survival and decline in PF-10 scores.
Results
A large (> 0.8 SD) decline in PF-10 scores from 3 to 27 months predicted shorter 10 year survival (HR=1.34, 95% CI 1.1–1.6). Persistent decline at 27 months was associated with less education, higher baseline PF-10, increased comorbidity, and higher body mass index.
Conclusions
Older women with breast cancer who experience a large and persistent decline in PF-10 are at increased mortality risk. Future research should examine the value of clinical assessment of physical function as a marker for mortality and test interventions to prevent decline in physical function to improve post-treatment survival outcomes.
Keywords: functional status, breast cancer, survival, aging
INTRODUCTION
Breast cancer is the most common female cancer, with over 200,000 women diagnosed each year in the Unites States [1], including a large proportion (43%) who are over the age of 65 years. Women with early stage breast cancer can anticipate excellent survival rates similar to age-matched women [1]. However, treatment modalities, including surgery, radiotherapy, axillary node dissection, chemotherapy, and endocrine therapy, can cause long-term treatment-related effects that may persist for up to 20 years following treatment [2–5]. Older breast cancer survivors are particularly vulnerable, as cancer and its treatments usually co-occur with comorbid conditions that have an impact on functional status [6,7]. Identifying patterns of functional status decline and risk factors for persistent functional decline after breast cancer treatment may inform the design of interventions to improve health outcomes in older women with breast cancer.
Disability and loss of independence result in a large economic toll on all cancer survivors, making long term health outcomes a very important focus in oncology [8,9]. Functional status measures, including activities of daily living and mobility, are important predictors of survival in the general population of community dwelling older persons, and may be even more relevant for older women with breast cancer [10]. The rate of functional decline is dramatically accelerated in patients over the age of 65 years with cancer [11], and older cancer survivors report more functional impairment than individuals who have not had cancer [12].
Breast cancer patients commonly report a decline in physical functioning in the year following a cancer diagnosis, primarily due to the effects of primary treatment [13]. While the majority of patients are expected to recover their physical functioning capacity in the months following the completion of therapy, some do not recover fully or decline even further [6]. To develop a better understanding of the impact of persistent decline in physical functioning after breast cancer in older women, we used data from a prospective cohort of women diagnosed with breast cancer at age 65 years or older [14]. We hypothesized that decline in self-reported physical functioning that persisted two years after breast cancer diagnosis would predict survival. This paper describes our findings and links persistent decline in physical functioning over the two years after diagnosis to greater all cause mortality.
METHODS
Study population
Data from a previously reported study, described in detail elsewhere, were used for this study [14]. Briefly, women aged 65 years or older diagnosed with early stage breast cancer (stage I with tumor diameter ≥ 1 cm, stage II, or stage IIIA) between 1996 and 1999 at 1 of 61 hospitals in Rhode Island, North Carolina, Minnesota, or Los Angeles, were identified through tumor registries and hospital pathology reports. Physicians gave permission to contact women (n=1,621), and invitations were sent to participate in the study. Additional entry criteria included the following: (1) no prior history of primary breast cancer, (2) no simultaneously diagnosed primary tumor at another anatomic site, (3) English-speaking or with an available translator, and (4) competent for interview with satisfactory hearing or with an available proxy respondent. Women had to be enrolled within 5 months of the date of their breast cancer surgery. Of the 1,621 women who were invited, 865 consented to participate in the study and were subsequently enrolled; however, only 689 completed the baseline telephone survey at 3 months post-diagnosis that captured self-reported information needed for this study.
Physical functioning outcome
Self-reported physical functioning was measured with the 10-item Physical Function Index (PF-10) of the Medical Outcomes Study Short Form-36 [15,16]. Decline in PF-10 was measured using longitudinal assessments obtained at 15 and 27 months following diagnosis, and a retrospective rating of pre-diagnosis PF-10 function that was obtained at 3 months after diagnosis (3-month PF-10).
Decline in PF-10 was examined between 2 different assessment time intervals: 1) 3 and 15 months, and 2) 3 and 27 months. This choice was based on our hypothesis that a decline that persisted over the longer interval of time would be more informative, as patients might exhibit transient decline followed by stabilization or return to pre-treatment status.
Survival analysis
Women were enrolled between December 1996 and September 1999 and date of death was obtained from the National Death Index examined in February 2010 available through 2008. We examined the difference in PF-10 during the two intervals as a continuous predictor of survival. PF-10 decline was also examined as a binary predictor in order to examine for a non-linear relationship with survival. To identify a meaningful amount of decline in PF-10 score, cut points were defined using standard population norms from a group of 413 women over the age of 65 who completed the SF-36 Health Survey, including mean 61.86 and SD 28.95 [15, 16]. A 10-point difference in the SF-36 scores can be considered clinically meaningful [17]. Binary outcomes were constructed for small (0.2 SD = 5.79 points), medium (0.5 SD = 14.475 points), and large (0.8 SD = 23.16 points) amounts of decline in the PF-10 measurement over the designated intervals, based on the method of Cohen for calculating effect sizes [18].
Independent variables
Variables used to predict decline and survival included demographic, health-related, and cancer-related factors. Demographic variables included age at diagnosis (categories of 65–69 years, 70–79 years, and 80 years and above), race (Caucasian versus other), education (less than high school vs. high school graduate vs. more than high school graduate), marital status (married versus unmarried), and site (Rhode Island, Minnesota, North Carolina, and Los Angeles). Health variables included body mass index (BMI) treated as a continuous variable, Charlson Comorbidity Index [19] (extracted from medical records) treated as a discrete variable (sum of conditions), self-reported exercise 3 months after diagnosis (0 = no, 1 = yes), tobacco smoking history (0 = none, 1= ever smoked), and self-rated health before diagnosis treated as a dichotomous variable (0 = excellent, very good, or good, 1 = fair or poor). Tumor characteristics included stage at diagnosis (stage I, IIA, IIB, or IIIA) and estrogen receptor status. Additional variables that were examined in preliminary analyses but not found significantly associated with PF-10 or survival in multivariate models included treatment (type of surgery, chemotherapy, radiation, and endocrine therapy), a five-question Mental Health Inventory general measure of emotional health (MHI5, scaled 0 to 100, higher scores reflect better function) from the Medical Outcomes Study–Short Form (MOS SF-36) [15], and the psychosocial and medical interactions subscales from the Cancer Rehabilitation Evaluations System – Short-Form (CARES-SF) [20].
Imputation of missing data
Data were available from 689, 491, and 451 women at the 3-, 15-, and 27-month interviews, respectively, leading to potential bias in summary statistics generated by the complete-case data analysis. Of the 198 with missing data at 15 months, 13 women returned to the study at 27 months. Missing data at 15 and 27 months were attributable to 1 and 3 breast cancer deaths and 1 and 4 deaths from competing causes, respectively. Data exploration revealed that demographic and medical characteristics of dropouts were similar to those of decliners, although decliners were younger and had higher baseline PF-10 (see Table 1). We estimated the need for a sample of 752 individuals, using Cox proportional hazards analysis, to detect a hazard ration difference of 1.2 with 80% power. Based on preliminary findings of a significant association between survival and persistent decline in physiological functioning between 3 and 27 months in the subset of study participants who are present at the 27 month visit, we wished to examine this relation more closely using the full sample enrolled in the study at 3 months (N=689). Therefore, data imputation was required to have an adequate sample, given the missing data bias, and limited sample of complete data. To address this issue, we used the method of multiple imputation [21–22] with 5 complete data sets created by imputation under a multivariate normal model that incorporated PF-10 at the 3 month visit, as well as PF-10 difference between 3rd and 15th month, and the PF-10 difference between the 3rd and 27th month, along with fixed covariates including the demographic variables, health variables, and tumor characteristics described above. Imputation was performed with survival time in the model, using a multivariate normal model [23] with adaptive rounding postimputation for binary measures [24]. Thirty five individuals with survival less than 27 months were excluded from subsequent analyses. Estimates of parameters of interest were obtained by averaging across the 5 results generated by analyzing each imputed data set separately, with Wald-type confidence intervals calculated from the multiply imputed data using well-established combination rules [22, 25].
Table 1.
Characteristics of the study sample.
| 3 months | Present at 27 months | Dropout at 27 months | p1 | p2 | ||
|---|---|---|---|---|---|---|
| Δ > 0.8 SD | Δ ≤ 0.8 SD | |||||
| (N=689) | (N=106) | (N=345) | (N=238) | |||
| N (%) | N (%) | N (%) | N (%) | |||
| Demographic characteristics | ||||||
| Age (y) | ||||||
| 65 to 69 | 176 (26) | 20 (19) | 98 (28) | 58 (24) | 0.15 | 0.05 |
| 70 to 79 | 383 (56) | 68 (64) | 197 (57) | 118 (50) | ||
| 80+ | 129 (19) | 18 (17) | 50 (14) | 62 (26) | ||
| Ethnicity | ||||||
| White | 642 (93) | 99 (93) | 327 (95) | 217 (91) | 0.76 | 0.63 |
| Nonwhite | 46 (7) | 7 (7) | 18 (5) | 21 (9) | ||
| Marital status | ||||||
| Married | 311 (45) | 43 (40) | 180 (52) | 88 (37) | 0.11 | 0.77 |
| Widowed | 277 (40) | 46 (43) | 118 (34) | 113 (48) | ||
| Other | 101 (15) | 17 (16) | 47 (14) | 37 (16) | ||
| Education | ||||||
| < HS | 127 (18) | 26 (24) | 43 (12) | 58 (24) | 0.01 | 0.21 |
| HS | 240 (35) | 29 (27) | 126 (37) | 85 (36) | ||
| > HS | 321 (47) | 51 (48) | 175 (51) | 95 (40) | ||
| Missing | 1 (0.1) | 0 | 1 (0.3) | 0 | ||
| Study site | ||||||
| Rhode Island | 174 (25) | 33 (31) | 81 (23) | 60 (25) | 0.36 | 0.42 |
| North Carolina | 173 (25) | 24 (23) | 83 (24) | 66 (28) | ||
| Minnesota | 190 (28) | 24 (23) | 101 (29) | 65 (27) | ||
| Los Angeles | 152 (22) | 25 (24) | 80 (23) | 47 (20) | ||
| Health status characteristics | ||||||
| Comorbidity index (Charlson score) | ||||||
| 0 | 390 (57) | 62 (58) | 212 (61) | 116 (49) | <0.01 | 0.19 |
| 1 | 248 (36) | 38 (36) | 111 (32) | 99 (42) | ||
| > 1 | 51 (7) | 6 (6) | 22 (6) | 23 (10) | ||
| Tobacco smoking | ||||||
| Ever smoked | 295 (48) | 55 (52) | 152 (44) | 68 (29) | 0.19 | 0.95 |
| Currently smoking | 55 (19) | 7 (7) | 30 (9) | 18 (8) | 0.34 | 0.34 |
| Body mass index, Mean (SD) | 26 (5.4) | 27 (5) | 25 (5) | 26 (6) | 0.04 | 0.28 |
| MHI-5, Mean (SD) | 81 (18) | 85 (17) | 88 (16) | 84 (20) | 0.33 | 0.07 |
| Baseline PF-10, Mean (SD) | 79 (25) | 88 (13) | 79 (25) | 74 (29) | <0.01 | <0.01 |
| CARES-SF psychosocial, Mean (SD) | 79 (15) | 78 (14) | 80 (16) | 77 (16) | 0.17 | 0.63 |
| Current exercise | ||||||
| Yes | 350 (51) | 48 (45) | 211 (61) | 91 (38) | 0.01 | 0.17 |
| No | 262 (38) | 56 (53) | 133 (38) | 73 (31) | ||
| missing | 77 (11) | 2 (2) | 1 (0.3) | 74 (31) | ||
| Health before diagnosis | ||||||
| Excellent | 137 (20) | 14 (13) | 83 (24) | 40 (17) | 0.19 | 0.09 |
| Very good | 261 (38) | 49 (46) | 137 (40) | 75 (32) | ||
| Good | 182 (26) | 27 (25) | 80 (23) | 75 (32) | ||
| Fair | 77 (11) | 13 (12) | 33 (10) | 31 (13) | ||
| Poor | 31 (4) | 3 (3) | 12 (3) | 16 (7) | ||
| Don’t know | 1 (0.1) | 0 | 0 | 1 (0.4) | ||
| Breast cancer characteristics | ||||||
| AJCC tumor stage | ||||||
| I | 351 (51) | 52 (49) | 182 (53) | 117 (49) | 0.82 | 0.78 |
| IIA | 207 (30) | 36 (34) | 101 (29) | 70 (29) | ||
| IIB | 103 (15) | 14 (13) | 50 (14) | 39 (16) | ||
| IIIA | 27 (4) | 4 (4) | 12 (3) | 11 (5) | ||
| Estrogen receptor status | ||||||
| Positive | 510 (74) | 86 (81) | 255 (74) | 169 (71) | 0.20 | 0.07 |
| Negative | 169 (25) | 19 (18) | 84 (24) | 66 (28) | ||
| missing | 10 (1) | 1 (1) | 6 (2) | 3 (1) | ||
| Surgery type | ||||||
| Mastectomy | 433 (51) | 54 (51) | 167 (48) | 128 (54) | 0.56 | 0.26 |
| Breast conserving surgery | 418 (49) | 48 (45) | 174 (50) | 107 (45) | ||
| missing | 11 (1) | 4 (4) | 4 (1) | 3 (1) | ||
| Chemotherapy | 134 (19) | 29 (27) | 75 (22) | 30 (13) | 0.31 | 0.10 |
| Radiation therapy | 334 (48) | 46 (43) | 177 (51) | 111 (47) | 0.25 | 0.72 |
| Axillary dissection | 580 (84) | 92 (87) | 298 (86) | 190 (80) | 0.96 | 0.16 |
| Tamoxifen | 453 (66) | 70 (66) | 226 (66) | 157 (66) | 0.99 | 0.91 |
p-values p1 comparing decliners to non-decliners; p2 comparing dropouts to decliners. Δ = difference in PF-10 from baseline to 27 months after diagnosis.
Statistical Analysis
Analyses were performed using R statistical software. Multivariate Cox proportional hazards analysis was used to examine both 1) retrospective PF-10 reported 3 months after diagnosis, and 2) decline in PF-10, as predictors of survival. Models were constructed to adjust for those covariates shown to have an effect on survival in minimally adjusted models not including 3-month PF-10 as a covariate, and those known to impact survival in early stage breast cancer. Independent variables included demographics, tumor and treatment characteristics, health status variables, and comorbidity described earlier. We explored the association of change in PF-10 with survival in models adjusted for 1) demographic variables and baseline PF-10, 2) tumor characteristics and health characteristics, and 3) self-rated health before diagnosis (fully-adjusted models). We then examined for nonlinear effects of PF-10 on survival, by exploring whether small, medium, and large amounts of early and persistent decline in PF-10 had an impact on survival. Five separate Cox models were obtained, one for each of five imputations, and the results were combined to yield a single parameter estimate and standard error, accounting for the within- and between-imputation variability.
Multiple linear regression analysis was used to examine what factors were associated with persistent decline in PF-10 in the 27 months following cancer diagnosis. Models were adjusted for 1) demographic variables and 3-month PF-10, 2) tumor characteristics, comorbidity, and self-rated health before diagnosis, and 3) additional health-related variables.
RESULTS
Characteristics of Study Participants by Functional Status
Table 1 reveals characteristics of the study population at 3-month assessment, and reflects data before imputation was performed. For the 689 women assessed 3 months after diagnosis, mean retrospective pre-diagnosis PF-10 was 79 (SD 25). The mean PF-10 in our study sample is higher than that of the general population of women over the age of 65 years reported in the SF-36 Health Survey Manual [16], likely representing the selective nature of women participating in the study, including relatively few comorbid conditions. A large proportion of the participants were Caucasian (93%), married (45%), with at least a high school education (47%). Few of the participants had a score of more than 1 on the Charlson Comorbidity Index (7%) and 36% had a score of 1. 48% of the cohort had a history of ever smoking, while 19% were currently smoking. Half of women (51%) reported that they were currently exercising, and 84% of the patients reported good, very good or excellent health before diagnosis. Most women had stage I (51%) or IIA (30%) disease, and tumors that were estrogen receptor positive (74%). About half of the patients received mastectomy with the remainder receiving breast conservation surgery plus radiation. 19% of patients received adjuvant chemotherapy and 66% of patients received adjuvant endocrine therapy.
106 women who provided data at 27 months experienced a large decline (> 0.8 SD) in PF-10 from 3 to 27 months following breast cancer diagnosis. In Table 1, we compare the baseline characteristics of patients with a large persistent decline compared to those who did not experience a decline and with those who dropped out from the study at 27 months (using data before imputation). Women with a large persistent decline in PF-10 were significantly less educated (p=0.01), less likely to exercise (45 versus 61%, p=0.01), were more likely to have Charlson score at least 1 (42 versus 38%, p<0.01), and had higher BMI (mean 27 versus 25, p = 0.04) and 3-month PF-10 (88 versus 79, p <0.01) than those without large decline. Comparing those who experienced large decline to those who dropped out of the study at 27 months, dropouts were older than decliners (borderline p=0.05), and decliners had much higher 3-month PF-10 (88 versus 74, p<0.01). No other differences in health characteristics and tumor characteristics were identified between decline and dropout groups.
Patterns of Decline in Physical Functioning
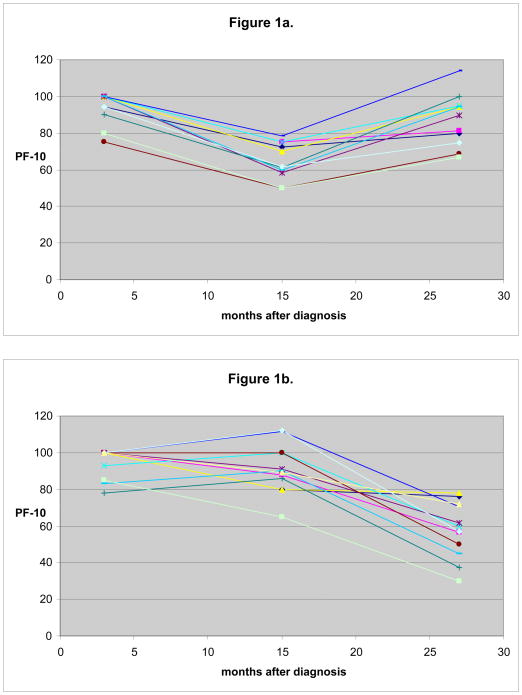
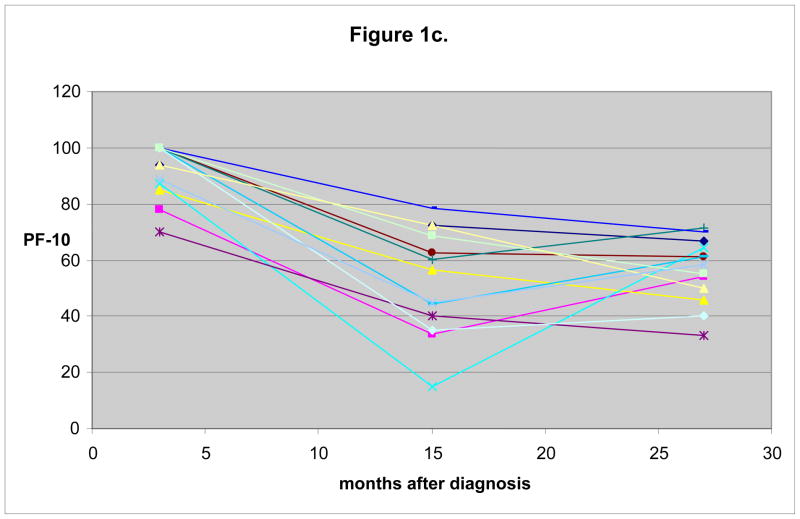
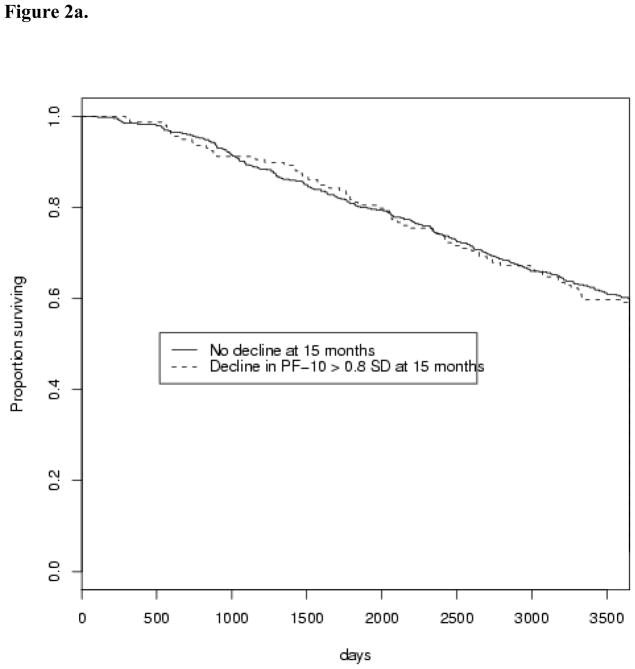
Figure 1 displays example patterns of decline in PF-10 over 27 months following diagnosis, grouped based on functional decline. Trajectories of PF-10 from 3 to 27 months were examined using manually selected individuals to identify sample patterns that occur for 3 groups defined with respect to large decline (>0.8 SD) in the early (3 to 15 months) and persistent (3 to 27 months) intervals. The 3 groups included: 1) women with an initial large decline from 3 to 15 months, and who recovered before 27 months (Figure 1a), 2) those without large decline between 3 and 15 months, but with either a large decline between 15 and 27 months or some decline in both intervals that sums to a large decline between 3 and 27 months (Figure 1b), and 3) women who experienced a large decline from 3 to 15 months and either continued to decline or did not recover in the second interval (Figure 1c). For simplicity of analysis, we group together the individuals who experience decline between 3 and 27 months (depicted in Figure 1b and 1c) as those who experience a persistent decline and compare them with those who experience early decline and recover (depicted in Figure 1a).
Fig. 1.
Example patterns of individual decline in physical functioning over 27 months after breast cancer diagnosis. Sample trajectories from 30 manually selected individuals from each of three categories (10 individuals in each category). In Figure 1a, individuals are shown who do experience > 0.8 SD decline from 3 to 15 months, but this decline does not persist at 27 months. Figure 1b shows those who do not experience a significant decline initially, but who do experience ≥ 0.8 SD decline from 3 to 27 months. Those individuals who experience decline both from 3 to 15 months and a persistence of this decline at 27 months are shown in Figure 1c.
Predictors of Persistent Decline
Table 2 reveals factors associated with change in PF-10 from 3 to 27 months following cancer diagnosis. In initial models less education (β=−6.5, 95% CI −11 to −1.6 for high school education) and higher 3-month PF-10 (β = 19 with 95% CI 13–28 and 17 with 95% CI 13–22 for those with 3-month PF-10 between 75 and 85 and >85, respectively) were associated with persistent decline. In models adjusted for tumor characteristics, Charlson Comorbidity Index, and self-rated health before diagnosis, persistent decline in PF-10 at 27 months after diagnosis was associated with Charlson Comorbidity Index (β = 1.4, 95% CI 0.10–2.7) in addition to lower education and higher 3-month PF-10. In full models adjusted for marital status, BMI, and other health characteristics, higher BMI was associated with persistent decline in PF-10 at 27 months with borderline statistical significance (β = 0.59, 95% CI −0.01–1.2).
Table 2.
Factors associated with functional decline at 27 months after cancer diagnosis.
| Model 1* | Model 2** | Model 3*** | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Age (y) | β | 95% CI | p | β | 95% CI | p | β | 95% CI | p |
| 65 to 69 | Reference | Reference | Reference | ||||||
| 70 to 79 | −0.57 | (−4.6, 3.5) | 0.78 | −0.80 | (−4.8, 3.2) | 0.69 | −0.72 | (−4.6, 3.2) | 0.72 |
| 80+ | 5.1 | (−0.2, 11.1) | 0.06 | 4.3 | (−1.9, 11) | 0.17 | 5.2 | (−0.84, 11) | 0.09 |
| Ethnicity | |||||||||
| White | Reference | Reference | Reference | ||||||
| Nonwhite | −1.5 | (−1.1,11.2) | 0.10 | −2.0 | (−9.7, 5.7) | 0.61 | −3.3 | (−11, 4.1) | 0.38 |
| Education | |||||||||
| < HS | Reference | Reference | Reference | ||||||
| HS | −2.3 | (−8.1, 3.5) | 0.43 | −2.8 | (−8.7, 3.1) | 0.34 | −2.2 | (−8.0, 3.7) | 0.46 |
| > HS | −6.5 | (−11, −1.6) | 0.01 | −7.1 | (−12, −2.3) | <0.01 | −6.1 | (−11, −1.3) | 0.01 |
| Study site | |||||||||
| Los Angeles | Reference | Reference | Reference | ||||||
| Rhode Island | 1.2 | (−5.6, 8.0) | 0.73 | 0.01 | (−6.4, 6.4) | 0.998 | 0.33 | (−5.8, 6.5) | 0.92 |
| Minnesota | −1.6 | (−9.3, 6.1) | 0.66 | −2.6 | (−9.8, 4.5) | 0.45 | −2.0 | (−9.2, 5.2) | 0.58 |
| North Carolina | −1.7 | (−7.9, 4.5) | 0.58 | −1.4 | (−7.6, 4.8) | 0.66 | −0.68 | (−7.1, 5.7) | 0.83 |
| 3 month PF-10 | |||||||||
| < 75 | Reference | Reference | Reference | ||||||
| 75 – 85 | 19 | (13, 25) | <0.01 | 20 | (14, 25) | <0.01 | 21 | (15, 26) | <0.01 |
| > 85 | 17 | (13, 22) | <0.01 | 19 | (15, 24) | <0.01 | 22 | (18, 27) | <0.01 |
| AJCC tumor stage | |||||||||
| I | Reference | Reference | Reference | ||||||
| IIA | - | - | - | 2.8 | (−1.6, 7.3) | 0.20 | 2.1 | (−2.1, 6.4) | 0.32 |
| IIB | - | - | - | 2.6 | (−2.7, 7.8) | 0.34 | 1.9 | (−3.2, 7.0) | 0.47 |
| IIIA | - | - | - | −1.5 | (−12, 9.4) | 0.78 | −2.8 | (−14, 8.3) | 0.62 |
| Estrogen receptor status | |||||||||
| Negative | Reference | Reference | Reference | ||||||
| Positive | - | - | - | 3.7 | 0.15 | (−1.3, 8.7) | 3.4 | (−1.4, 8.2) | 0.16 |
| Charlson score | - | - | - | 1.4 | 0.04 | (0.10, 2.7) | 1.1 | (−0.18, 2.4) | 0.09 |
| Health before diagnosis | - | - | - | 0.65 | 0.81 | (−4.6, 5.9) | 0.92 | (−4.4, 6.2) | 0.73 |
| Marital status | |||||||||
| Not married | Reference | Reference | Reference | ||||||
| Married | - | - | - | - | - | −3.5 | (−7.5, 0.49) | 0.08 | |
| Body mass index | - | - | - | - | - | 0.59 | (−0.01, 1.2) | 0.05 | |
| Exercise | - | - | - | - | - | −3.3 | (−7.6, 1.1) | 0.14 | |
| Ever smoked | - | - | - | - | - | 2.6 | (−1.6, 6.9) | 0.22 | |
All models adjusted for age, ethnicity, education, and site.
Models 2 and 3 adjusted for estrogen receptor status, comorbidity index, baseline self-rated health.
Model 3 adjusted for marital status, body mass index, and tobacco smoking.
Decline in Self-reported Physical Functioning and Survival
Of the 689 women who provided data for the 3 month initial survey, there were 247 deaths, with 91 deaths attributable to breast cancer and 156 deaths attributable to competing causes. Change in PF-10 from 3 months to 27 months after diagnosis was significantly associated with shorter survival, both in minimally adjusted models and after adjustment for factors known to influence survival (see Table 3). Factors associated with shorter survival included 3-month PF-10 (HR=0.92 for every 10 points in PF-10 above the mean, 95% CI 0.89–0.95), higher stage (HR=1.70 for stage 3a, 95% CI 1.1–2.6), and poorer self-rated health before diagnosis (HR=1.29, 95% CI 1.0–1.6). A 10-point decline in PF-10 from 3 months to 27 months confers a hazard ratio of 1.06 (95% CI 1.0–1.1) and a 25-point decline over this period confers a hazard ratio of 1.16 (95% CI 1.1–1.3). In contrast, change in PF-10 from 3 to 15 months after diagnosis was not significantly associated with survival (HR=1.03 for 10-point decline, 95% CI 0.99–1.1). In our multivariate model predicting survival, we have adjusted for self-rated health at baseline and self-reported baseline physical functioning by introducing these as covariates in the model. Decline remains significantly associated with survival while adjusting for these factors. The value of adding decline is exhibited by comparing the variance explained by proportional hazards models with and without decline as a predictor. On average, models that include SRH and decline explain 9.2% of the variance, whereas models adjusted for decline and not SRH exaplain 8.7% of the variance, and models that are adjusted for SRH and not decline explain 7.2% of the variance.
Table 3.
Amount of decline in self-reported physical functioning over 2 years following cancer diagnosis predicts 10-year survival.
| Decline from baseline to 15 months | Decline from baseline to 27 months | |||||||
|---|---|---|---|---|---|---|---|---|
| β | HR | 95% CI | p | β | HR | 95% CI | p | |
| Age (y) | ||||||||
| 65 to 69 | Reference | Reference | ||||||
| 70 to 79 | 0.045 | 1.05 | (0.87, 1.3) | 0.64 | 0.055 | 1.06 | (0.88, 1.3) | 0.57 |
| 80+ | 0.27 | 1.31 | (1.02, 1.7) | 0.035 | 0.26 | 1.30 | (1.01, 1.7) | 0.041 |
| Ethnicity | ||||||||
| White | Reference | Reference | ||||||
| Nonwhite | −0.036 | 0.96 | (0.69, 1.3) | 0.83 | 0.023 | 1.02 | (0.73, 1.4) | 0.89 |
| Education | ||||||||
| < HS | Reference | Reference | ||||||
| HS | −0.072 | 0.93 | (0.74, 1.2) | 0.54 | −0.059 | 0.94 | (0.75, 1.2) | 0.62 |
| > HS | −0.13 | 0.88 | (0.70, 1.1) | 0.26 | −0.095 | 0.91 | (0.72, 1.1) | 0.42 |
| Study site | ||||||||
| LA | Reference | Reference | ||||||
| RI | −0.012 | 0.99 | (0.77, 1.3) | 0.92 | −0.033 | 0.97 | (0.75, 1.2) | 0.79 |
| MN | 0.17 | 1.19 | (0.94, 1.5) | 0.16 | 0.17 | 1.18 | (0.94, 1.5) | 0.16 |
| NC | 0.18 | 1.20 | (0.95, 1.5) | 0.13 | 0.18 | 1.20 | (0.95, 1.5) | 0.12 |
| 3 month PF-10 | −0.007 | 0.99 | (0.99, 1.0) | <0.01 | −0.008 | 0.99 | (0.99, 1.0) | <0.01 |
| AJCC tumor stage | ||||||||
| I | Reference | Reference | ||||||
| IIA | 0.083 | 1.09 | (0.91, 1.3) | 0.37 | 0.074 | 1.08 | (0.90, 1.3) | 0.42 |
| IIB | 0.15 | 1.16 | (0.92, 1.5) | 0.21 | 0.15 | 1.16 | (0.92, 1.5) | 0.20 |
| IIIA | 0.50 | 1.65 | (1.1, 2.5) | 0.02 | 0.53 | 1.70 | (1.1, 2.6) | 0.01 |
| Estrogen receptor status | ||||||||
| Negative | Reference | Reference | ||||||
| Positive | −0.083 | 0.92 | (0.76, 1.1) | 0.38 | −0.089 | 0.92 | (0.76, 1.1) | 0.35 |
| Charlson score | 0.041 | 1.04 | (0.99, 1.1) | 0.12 | 0.035 | 1.04 | (0.98, 1.1) | 0.19 |
| Health before diagnosis | 0.29 | 1.34 | (1.0, 1.7) | 0.02 | 0.26 | 1.29 | (1.0, 1.6) | 0.03 |
| Decline in PF-10 | 0.003 | 1.00 | (0.999, 1.01) | 0.11 | 0.006 | 1.01 | (1.00, 1.01) | <0.01 |
Models adjusted for age, ethnicity, education, site, baseline PF-10, stage, ER status, Charlson score, and health before diagnosis (SRH).
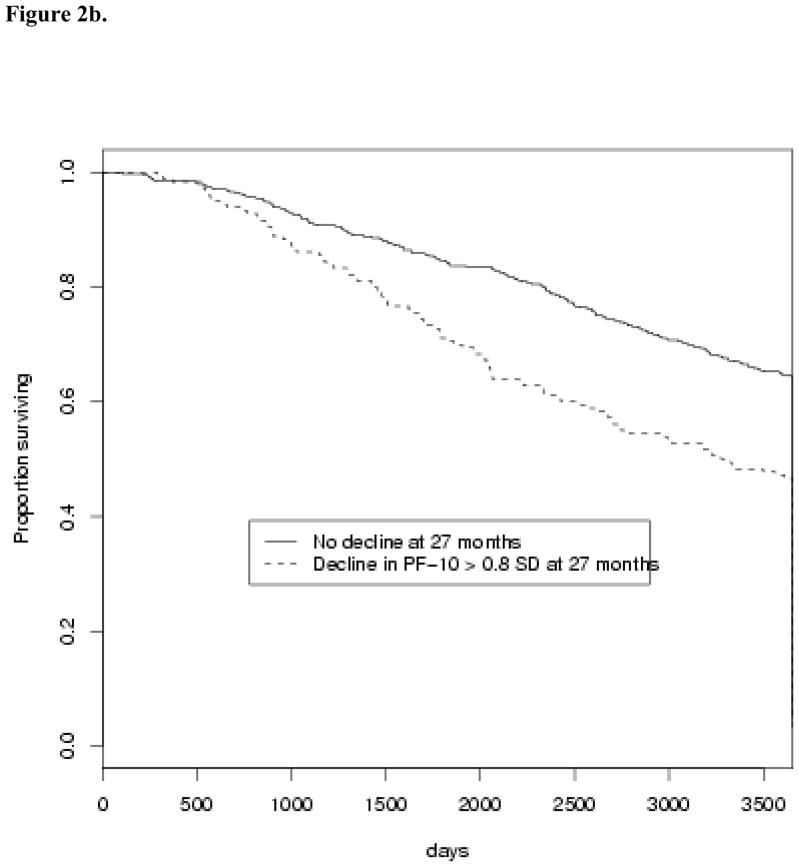
When we examine small (> 0.2 SD), medium (> 0.5 SD), and large (> 0.8 SD) sizes of decline in PF-10 over the early (3 to 15 months after diagnosis) and longer (3 to 27 months after diagnosis) intervals, both medium and large decline over the longer interval were associated with shorter survival (Table 4). This effect persists after adjustment for factors known to affect survival, with HR=1.22 for medium (95% CI 1.0–1.5) and HR=1.34 for large (95% CI 1.1–1.6) decline in PF-10 from 3 to 27 months. Figure 2 reveals Kaplan-Meier survival curves according to the presence of large (> 0.8 SD) decline in PF-10. While a clear difference in survival is observed for large decline from 3 to 27 months (Figure 2b, Table 4), survival was not significantly affected by any amount of decline in PF-10 from 3 to 15 months (Figure 2a, Table 4).
Table 4.
Association between survival and amount of decline in physical functioning over 2 years following cancer diagnosis.
| Small (0.2 SD) | Medium (0.5 SD) | Large (0.8 SD) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | HR | 95% CI | p | |
| Early Decline (baseline to 15 months) | |||||||||
| Reference | 1 | - | - | 1 | - | - | 1 | - | - |
| Model 1 | 1.13 | (0.95, 1.3) | 0.18 | 1.07 | (0.88, 1.3) | 0.50 | 1.10 | (0.88, 1.4) | 0.42 |
| Model 2 | 1.12 | (0.94, 1.3) | 0.21 | 1.06 | (0.87, 1.3) | 0.58 | 1.08 | (0.86, 1.4) | 0.49 |
| Model 3 | 1.11 | (0.94, 1.3) | 0.22 | 1.06 | (0.88, 1.3) | 0.57 | 1.10 | (0.88, 1.4) | 0.41 |
| Persistent Decline (baseline to 27 months) | |||||||||
| Reference | 1 | - | - | 1 | - | - | 1 | - | - |
| Model 1 | 1.11 | (0.93, 1.3) | 0.25 | 1.21 | (1.0, 1.5) | 0.040 | 1.35 | (1.1, 1.7) | 0.004 |
| Model 2 | 1.09 | (0.91, 1.3) | 0.36 | 1.22 | (1.0, 1.5) | 0.039 | 1.36 | (1.1, 1.7) | 0.004 |
| Model 3 | 1.08 | (0.90, 1.3) | 0.42 | 1.22 | (1.0, 1.5) | 0.038 | 1.34 | (1.1, 1.6) | 0.006 |
Model 1 – adjusted for age, ethnicity, education, and site.
Model 2 – adjusted for above plus stage, ER status, Charlson score, and baseline PF-10.
Model 3 – adjusted for above plus self-rated heath.
Fig. 2.
Effects of early decline (Figure 2a) and persistent decline (Figure 2b) on overall survival. A large (> 0.8 SD) decline in PF-10 between 3 months and 15 months after diagnosis is not associated with survival (HR=1.11 [0.87–1.42]), whereas a large decline in PF-10 between 3 and 27 months after diagnosis is associated with significantly shorter survival (HR=1.38 [1.13–1.68]).
DISCUSSION
We found that a large, persistent decline in self-rated PF-10 in the two years after breast cancer diagnosis was associated with all-cause mortality 10 years later in older women. This effect remains significant after adjustment for factors known to be associated with lower functional status and survival, including older age and poor self-rated health. Furthermore, we identified baseline characteristics, including less education, increased comorbidity, and BMI that may be useful in identifying a subpopulation of women at risk for functional decline and reduced survival time.
Following completion of adjuvant treatment, breast cancer survivors experience diminished quality of life and self-reported functional limitations [26–31]. Functional limitations following initial treatment for breast cancer in women of all ages substantially impacts overall survival [26, 27]. In this analysis, we find that the patterns of decline in physical function and its persistence in women over the age of 65 years adds further information in predicting survival.
This selected, volunteer sample had a mean pre-diagnosis retrospective rate of PF-10 at 3-months (79 +/− 25) that was significantly higher than that of the general population of women over the age of 65 years (61.86 +/− 28.95), as reported in literature [16]. Higher values for 3-month PF-10 suggests that our study population was healthier and higher functioning, on average, reflecting selection by both physicians and subjects. Notably, the parent study had more difficulty recruiting the oldest patients who were potentially eligible [14]. Likewise, the proportion of patients whose physicians did not allow them to be contacted increased with advancing age [14]. In addition, our cohort had higher education levels and socioeconomic status than the general population, both of which are also associated with better functional status. Modest declines in self-reported PF-10 may significantly impact the health and well-being of this highly functional older population. A higher PF-10 prior to diagnosis was associated with a greater likelihood of persistent decline at 27 months after diagnosis. This may represent a ceiling effect where the individuals who start out the highest are much more likely to experience a large decline than those who do not have as much to lose. Other explanations include regression to the mean, inflated perceptions of physical function prior to the breast cancer diagnosis, and/or the greater impact of breast cancer and its treatment on very high functioning older women.
Limitations of our study include response bias, and the diminishing numbers of individuals participating in the study at each follow up interval. To account for this, we used data imputation to perform our analysis. Individuals who had available data and experienced decline had similar baseline characteristics to those who dropped out at 27 months. While we did find an association between a large decline in PF-10 and survival, our analysis was limited by a small sample size and low death rate.
Type and extent of treatment received are well known to be closely related to both functional status, stage of disease, and survival. Survival analyses presented in our study do not include treatment as a covariate. Stage and treatments received and completed, including chemotherapy, radiation, tamoxifen prescribed at 3 months and 36 months, and type of surgery were included as covariates in the imputation. In preliminary analyses, treatment received did not predict survival when adjusting for stage. Therefore, stage was included in our analyses rather than treatment received. We also examined for an association between chemotherapy received and decline in self-reported PF-10 using chi-square analyses. Completion of chemotherapy was not associated with early decline or persistent decline in our population, and therefore is unlikely to be confounding the association of decline in physical functioning with survival.
Response shifts are important in considering longitudinal change in self-reported outcomes. We examine the change in PF-10 that occurs in relation to a retrospective rating 3 months following diagnosis in which the patient was asked about physical functioning right before her breast cancer was diagnosed. Women with more aggressive and higher stage of disease may undergo more debilitating treatments and may therefore have higher retrospective ratings, suggesting that response shift might account for our findings. However, both of our decline variables (early decline and persistent decline) use the same baseline assessment and only persistent decline is associated with survival. Furthermore, we adjust for stage in our analyses, and persistent decline predicts survival after adjustment for stage. Response shift may also play a role in the finding that our study population has a high baseline average PF-10 (78.64), relative to the population average (61.86). However, the average PF-10 remains elevated in follow up assessments (70.64 at 15 months, 70.43 at 27 months, 67.38 at 39 months, 68.14 at 51 months), suggesting that our study population represents a physically robust population.
Chronic inflammation may drive the link between persistent decline and long term survival in breast cancer survivors. We hypothesize that early decline is caused by cancer and its treatment, while persistent decline is related to host factors such as chronic disease and inflammation. There is a strong link between inflammation, disease burden, and functional decline and disability in general populations of older adults [32–37]. In turn, inflammation and disease play a role in fatigue and functional decline in cancer survivors of all ages [38]. Aging and cancer may synergistically worsen inflammation and accompanying disease burden and these factors may underlie the link between functional decline and mortality observed in our study and others [27]. Identifying inflammatory biomarkers associated with functional decline may add important prognostic information in the care of older breast cancer survivors. Future studies should also examine associations between decline in physical functioning and breast cancer-specific survival, where both early and persistent decline may play a role.
We conclude that decline in self-reported PF-10 that persisted over two years following breast cancer diagnosis was predictive of poorer 10-year survival in older breast cancer survivors. Future research should determine whether early recognition of decline in physical functioning can prospectively improve survival outcomes, and coordination of care between primary care physicians and oncologists may optimize management of comorbid conditions [39]. Physical activity and health education interventions have been shown to improve physical performance scores in sedentary older adults at risk of disability [40]. Further research should address whether exercise interventions that improve physical function or diet and smoking cessation interventions have an impact on patterns of decline in physical functioning specifically in older cancer survivors.
Acknowledgments
We would like to thank Dr. Tom Belin for valuable input on our analysis. This research was supported by a career development award from The ASCO Cancer Foundation and the Breast Cancer Research Foundation, as well as by grants from the Breast Cancer Research Foundation and Susan G. Komen for the Cure to Dr. Ganz.
References
- 1.American Cancer Society. Breast Cancer Facts and Figures. 2010. [Google Scholar]
- 2.Ganz PA, Hahn EE. Implementing a survivorship care plan for patients with breast cancer. J Clin Oncol. 2008;26:759–67. doi: 10.1200/JCO.2007.14.2851. [DOI] [PubMed] [Google Scholar]
- 3.Garman KS, Pieper CF, Seo P, et al. Function in elderly cancer survivors depends on comorbidities. J Gerontol A Biol Sci Med Sci. 2003:M1119–24. doi: 10.1093/gerona/58.12.m1119. [DOI] [PubMed] [Google Scholar]
- 4.Hewitt M, Rowland JH, Yancik R. Cancer survivors in the United States: Age, health and disability. J Gerontol A Biol Sci Med Sci. 2003;58:82–91. doi: 10.1093/gerona/58.1.m82. [DOI] [PubMed] [Google Scholar]
- 5.Kornblith AB, Herndon JE, II, Weiss RB, et al. Long-term adjustment of survivors of early-stage breast carcinoma, 20 years after adjuvant chemotherapy. Cancer. 2003;98:679–89. doi: 10.1002/cncr.11531. [DOI] [PubMed] [Google Scholar]
- 6.Ganz PA, Kwan L, Stanton AL, et al. Physical and psychosocial recovery in the year after primary treatment of breast cancer. J Clin Oncol. 2011;29:1101–9. doi: 10.1200/JCO.2010.28.8043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deimling GT, Arendt JA, Kypriotakis G, Bowman KF. Functioning of older, long-term cancer survivors: the role of cancer and comorbidities. J Am Geriatr Soc. 2009;57:S289–92. doi: 10.1111/j.1532-5415.2009.02515.x. [DOI] [PubMed] [Google Scholar]
- 8.Cohen HJ. Functional assessment and the cancer survivor: something old, something new. J Natl Cancer Inst. 2010;102:1450–1. doi: 10.1093/jnci/djq365. [DOI] [PubMed] [Google Scholar]
- 9.Extermann M. Interaction between comorbidity and cancer. Cancer Control. 2007;14:13–22. doi: 10.1177/107327480701400103. [DOI] [PubMed] [Google Scholar]
- 10.Keeler E, Guralnik JM, Tian H, Wallace RB, Reuben DB. The impact of functional status on life expectancy in older persons. J Gerontol A Biol Sci Med Sci. 2010;65:727–33. doi: 10.1093/gerona/glq029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lunney JR, Lynn J, Foley DJ, et al. Patterns of functional decline at the end of life. JAMA. 2003;289:2387–92. doi: 10.1001/jama.289.18.2387. [DOI] [PubMed] [Google Scholar]
- 12.Sweeney C, Schmitz KH, Lazovich D, et al. Functional limitations in elderly female cancer survivors. J Nat Cancer Inst. 2006;98:521–9. doi: 10.1093/jnci/djj130. [DOI] [PubMed] [Google Scholar]
- 13.Ganz PA, Kwan L, Stanton AL, et al. Quality of Life at the End of Primary Treatment of Breast Cancer: First Results From the Moving Beyond Cancer Randomized Trial. J Natl Cancer Inst. 2004;96:376–87. doi: 10.1093/jnci/djh060. [DOI] [PubMed] [Google Scholar]
- 14.Silliman RA, Guadagnoli E, Rakowski W, et al. Adjuvant tamoxifen prescription in women 65 years and older with primary breast cancer. J Clin Oncol. 2002;20:2680–8. doi: 10.1200/JCO.2002.08.137. [DOI] [PubMed] [Google Scholar]
- 15.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36): I. Conceptual framework and item selection. Med Care. 1992;30:473–83. [PubMed] [Google Scholar]
- 16.Ware JE. SF-36 Health Survey Manual and Interpretation Guide. Boston: Nimrod Press; 1993. [Google Scholar]
- 17.Biorner JB, Wallenstein GV, Martin MC, et al. Interpreting score differences in the SF-36 Vitality scale: using clinical conditions and functional outcomes to define the minimally important difference. Curr Med Res Opin. 2007;23:731–9. doi: 10.1185/030079907x178757. [DOI] [PubMed] [Google Scholar]
- 18.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 19.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 20.Schag CA, Ganz PA, Heinrich RL. Cancer Rehabilitation Evaluation System–short form (CARES-SF): A cancer specific rehabilitation and quality of life instrument. Cancer. 1991;68:1406–1413. doi: 10.1002/1097-0142(19910915)68:6<1406::aid-cncr2820680638>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 21.Schafer JL, Olsen MK. Multiple imputation for multivariate missing-data problems: A data analysts’s perspective. Multivariate Behav Res. 1998;33:545–71. doi: 10.1207/s15327906mbr3304_5. [DOI] [PubMed] [Google Scholar]
- 22.Rubin DB. Multiple Imputation for Non-Response in Surveys. New York, NY: John Wiley & Sons, Inc; 1987. [Google Scholar]
- 23.Schafer JL. [Accessed August 10, 2007];NORM. 1999 http://www.stat.psu.edu/~jls/misoftwa.html.
- 24.Bernaards CA, Belin TR, Schafer JL. Robustness of a multivariate normal approximation for imputation of incomplete binary data. Stat Med. 2007;26:1368–1382. doi: 10.1002/sim.2619. [DOI] [PubMed] [Google Scholar]
- 25.Schafer JL. Analysis of Incomplete Multivariate Data. London, England: CRC Press; 1997. pp. 147–192. [Google Scholar]
- 26.Arndt V, Merx H, Sturmer T, Stegmaier C, Ziegler H, Brenner H. Age-specific detriments to quality of life among breast cancer patients one year after diagnosis. Eur J Cancer. 2004;40:673–80. doi: 10.1016/j.ejca.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 27.Braithwaite D, Satariano WA, Sternfeld B, Hiatt RA, Ganz PA, Kerlikowska K, Moore DH, Slattery ML, Tammermagi M, Castillo A, Melisko M, Esserman L, Weltzien EK, Caan BJ. Long-term prognostic role of functional limitaiton among women with breast cancer. J Natl Cancer Inst. 2010;102:1–10. doi: 10.1093/jnci/djq344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patterson RE, Squib N, Natarajan L, et al. Improvement in self-reported physical health predicts longer survival among women with a history of breast cancer. Breast Cancer Res Treat. 2011;127:541–7. doi: 10.1007/s10549-010-1236-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klepin HD, Geiger AM, Tooze JA, et al. Physical performance and subsequent disability and survival in older adults with malignancy: results from the health, aging and body composition study. J Am Geriatr Soc. 2010;58:76–82. doi: 10.1111/j.1532-5415.2009.02620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Satariano WA, Ragheb NE, Buck KA, Swanson GM, Branch LG. Aging and breast cancer: a case-control comparison of instrumental functioning. J Aging Health. 1989;1:209–33. [Google Scholar]
- 31.Wenzel BL, Fairclough DL, Brady MJ, Cella D, Garrett KM, Kluhsman BC, et al. Age-related differences in the quality of life of breast carcinoma patients after treatment. Cancer. 1999;86:1768–74. [PubMed] [Google Scholar]
- 32.Ferrucci L, Harris TB, Guralnik JM, et al. Serum IL-6 level and the development of disability in older persons. J Am Geriatr Soc. 1999;47:639–46. doi: 10.1111/j.1532-5415.1999.tb01583.x. [DOI] [PubMed] [Google Scholar]
- 33.Ferrucci L, Penninx BW, Volpato S, et al. Change in muscle strength explains accelerated decline of physical function in older women with high interleukin-6 serum levels. J Am Geriatr Soc. 2002;50:1947–54. doi: 10.1046/j.1532-5415.2002.50605.x. [DOI] [PubMed] [Google Scholar]
- 34.Reuben DB, Cheh AI, Harris TB, et al. Peripheral blood markers of inflammation predict mortality and functional decline in high-functioning community-dwelling older persons. J Am Geriatr Soc. 2002;50:638–44. doi: 10.1046/j.1532-5415.2002.50157.x. [DOI] [PubMed] [Google Scholar]
- 35.Taaffe DR, Harris TB, Ferrucci L, Rowe J, Seeman TE. Cross-sectional and prospective relationships of interleukin-6 and C-reactive protein with physical performance in elderly persons: MacArthur studies of successful aging. J Gerontol A Biol Sci Med Sci. 2000;55:M709–15. doi: 10.1093/gerona/55.12.m709. [DOI] [PubMed] [Google Scholar]
- 36.Zhu S, Patel KV, Bandinelli S, Ferrucci L, Guralnik JM. Predictors of interleukin-6 elevation in older adults. J Am Geriatr Soc. 2009;57:1672–7. doi: 10.1111/j.1532-5415.2009.02426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brinkley TE, Leng X, Miller ME, et al. Chronic inflammation is associated with low physical function in older adults across multiple comorbidities. J Gerontol A Biol Sci Med Sci. 2009;64:455–61. doi: 10.1093/gerona/gln038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bower JE, Ganz PA, Tao ML, et al. Inflammatory biomarkers and fatigue during radiation therapy for breast and prostate cancer. Clin Cancer Res. 2009;15:5534–40. doi: 10.1158/1078-0432.CCR-08-2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Earle CC, Burstein HJ, Winer EP, Weeks JC. Quality of non-breast cancer health maintenance among elderly breast cancer survivors. J Clin Oncol. 2003;21:1447–51. doi: 10.1200/JCO.2003.03.060. [DOI] [PubMed] [Google Scholar]
- 40.Pahor M, Blair SN, Espeland M, et al. LIFE Study Investigators. Effects of a physical activity intervention on measures of physical performance: Results of the lifestyle interventions and independence for Elders Pilot (LIFE-P) study. J Gerontol A Biol Sci Med Sci. 2006;61:1157–65. doi: 10.1093/gerona/61.11.1157. [DOI] [PubMed] [Google Scholar]