Abstract
Clostridium perfringens causes necrotic enteritis in chickens, and alpha-toxin has been suggested to be a key virulence determinant. Analysis of the alpha-toxin of 25 chicken-derived C. perfringens strains demonstrated high homology to mammal-derived strains rather than to the only avian-derived C. perfringens alpha-toxin sequence reported previously.
Clostridium perfringens is a widely distributed pathogen (6) commonly isolated from the environment and the gastrointestinal tract of birds and mammals (7, 24). C. perfringens isolates are classified into five types (A to E) according to the production of four major toxins (alpha, beta, epsilon, and iota) (11, 13). The alpha-toxin has been implicated in several diseases (18), including necrotic enteritis in chickens (3, 10). The alpha-toxin structural gene (plc or cpa) has been isolated from several strains of C. perfringens and characterized (4), and the encoded proteins were found to be highly conserved in all but one recently identified strain (8). This strain (SWCP) was isolated from a diseased swan. Justin et al. (8) found that the SWCP alpha-toxin had only 80% amino acid sequence identity to the other C. perfringens alpha-toxins and questioned if this difference in sequence was typical of all avian isolates. In this study, we examined the alpha-toxin sequences encoded by a range of isolates of C. perfringens derived from chickens to determine if the divergent SWCP alpha-toxin sequence is common in avian isolates.
The C. perfringens strains used in this study (Table 1) were isolated from chickens displaying clinical signs of necrotic enteritis (1). Genomic DNA was prepared as the template for PCR by boiling crude cells in water for 3 min. The PCR conditions and reaction concentrations were as described before (14). Two PCR products, together encompassing the complete plc gene, were amplified from each of the 25 strains and sequenced to determine the amino acid sequence of the encoded alpha-toxin (Fig. 1). In each isolate, the full-length sequence was predicted to have 398 amino acids. The toxins were all highly conserved in amino acid sequence (Fig. 2), and only five different alpha-toxin sequence types (I to V) were identified among the 25 isolates sampled from several different outbreaks of necrotic enteritis in different locations (Table 1). Each alpha-toxin gene was sequenced twice with independently generated templates to confirm that the changes were not due to sequencing or PCR errors. All the alpha-toxin sequence types from the chicken isolates closely resembled that of the toxin from the human isolate, strain 13 (20), with greater than 98% identity, but differed considerably from the swan isolate (SWCP), with only 82 to 84% identity. The SWCP isolate has between 67 and 70 amino acid differences from the chicken isolates, and of all the changes in the SWCP sequence, only two amino acid changes are found in the field isolates reported here.
TABLE 1.
Sources of C. perfringens isolates
| C. perfringens isolate | Source or geographical location | Outbreak no. | Tissue | Alpha-toxin sequence type |
|---|---|---|---|---|
| 13 | Human isolatea | |||
| R61 | South Australia | 1 | Gut sample | I |
| G45 | Queensland, site 1 | 1 | Gut sample | I |
| W1319/3 | Queensland, site 2 | 2 | Gut sample | I |
| W1319/4 | Queensland, site 2 | 2 | Gut sample | I |
| W1323/4 | Queensland, site 2 | 3 | Gut sample | I |
| W1323/5 | Queensland, site 2 | 3 | Gut sample | I |
| T3381 | Queensland, site 3 | 4 | Gut sample | II |
| T3688 | Queensland, site 3 | 5 | Gut sample | II |
| K473 | Queensland, site 4 | 6 | Gut sample | II |
| NAG-NE1 | Victoria, site 1 | 1 | Gut contents | III |
| EHE-NE3 | Victoria, site 2 | 2 | Liver sample | I |
| EHE-NE4 | Victoria, site 2 | 2 | Liver sample | I |
| EHE-NE5 | Victoria, site 2 | 2 | Kidney sample | I |
| EHE-NE6 | Victoria, site 2 | 2 | Kidney sample | IV |
| EHE-NE7 | Victoria, site 2 | 2 | Gut wall | IV |
| EHE-NE9 | Victoria, site 2 | 2 | Gut wall | IV |
| EHE-NE13 | Victoria, site 2 | 2 | Gut wall | I |
| EHE-NE14 | Victoria, site 2 | 2 | Gut contents | I |
| EUR-NE15 | Victoria, site 3 | 3 | Gut contents | I |
| EHE-NE16 | Victoria, site 2 | 4 | Gut contents | I |
| EHE-NE18 | Victoria, site 2 | 4 | Gut contents | I |
| EHE-NE19 | Victoria, site 2 | 5 | Gut contents | I |
| NAG-NE23 | Victoria, site 1 | 6 | Gut contents | V |
| NAG-NE24 | Victoria, site 1 | 6 | Gut contents | V |
| NAG-NE25 | Victoria, site 1 | 6 | Gut contents | V |
See reference 12.
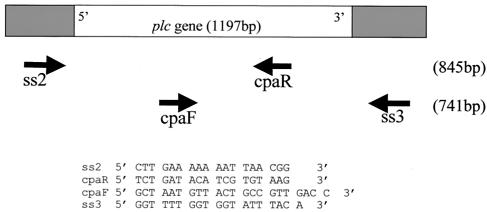
FIG. 1.
PCR primers used to amplify and sequence the plc gene. The ss2 and ss3 PCR primers were designed from the sequence of C. perfringens strain 13 (20), and primers cpaF and cpaR are the alpha-toxin typing primers designed previously (14). The predicted sizes of the PCR products are shown in parentheses.
FIG. 2.
Nonconserved amino acids in a multiple alignment of five alpha-toxin sequence types (I to V) from chicken isolates. The sequences of the alpha-toxins from the swan isolate SWCP (8) and strain 13 (20) are included for comparison. Conserved positions where the amino acid does not vary, including within the SWCP alpha-toxin, are not shown. The amino acid sequence positions of the nonconserved amino acids are shown above the alignment and refer to the full-length alpha-toxin protein (397 amino acids). Numbers are shown running vertically down, as depicted by the arrow. Amino acids are represented by the single-letter code. The number of strains with the specific alpha-toxin sequence type is shown in parentheses after each type sequence.
Sequence type I has only one amino acid difference from the strain 13 sequence and was the most common alpha-toxin found in the group sampled (Table 1). The threonine-to-alanine substitution is within the putative signal peptide sequence (21) and would not be present in the mature protein and therefore cannot affect the properties of the mature toxin (4). Alanine is also found in this position in the alpha-toxin signal sequence from strain NCTC 8237 (9). Sequence type II has two amino acid differences compared to the strain 13 sequence and includes the threonine-to-alanine change at position 13 and an isoleucine-to-valine substitution at position 373. Sequence type IV contains two amino acid changes, the threonine-to-alanine change (position 13) and a leucine-to-methionine alteration (position 54). The latter amino acid substitution is also seen in the alpha-toxin from C. perfringens strain 8-6 (19) and the phospholipase C from Clostridium novyi (22). The type V alpha-toxin sequence contains three amino acid substitutions compared to strain 13, the common threonine-to-alanine substitution at position 13, an aspartic acid-to-alanine change at position 202 (also found in the SWCP sequence), and an alanine-to-threonine substitution at position 205. The most distinct alpha-toxin sequence type seen in this study was type III. It contains six amino acid changes compared to the strain 13 alpha-toxin, including the isoleucine-to-valine substitution at position 373 and a methionine residue that replaces a lysine residue at position 54.
Overall, the amino acid differences detected in this study were minimal compared to the sequence differences observed between strains SWCP and 13. The differences that were found in the alpha-toxin sequences of the chicken isolates were all the result of single base substitutions and did not significantly alter the predicted physical properties of the encoded proteins (45.5 kDa, pI 5.58, and overall negative charge). Plating each of the strains onto egg yolk agar (2) produced a zone of precipitation around the colonies, indicating that each strain was able to produce functional alpha-toxin, although the levels of toxin activity varied. The toxin activity of some strains with identical alpha-toxin sequences was markedly different, indicating that the variable toxin levels must be due to differences in strain growth or expression rather than differences in specific activity.
None of the predicted amino acid differences occurred in the active site (5, 15, 16) or in the calcium binding pocket in the C-terminal domain (8), regions of the protein that are thought to play key roles in membrane-protein interactions. The valine-to-isoleucine change (position 373) is located in a region that is predicted to be a flexible surface-exposed loop linking two helices (4). However, this change is a conservative substitution that is unlikely to affect the tertiary structure of the protein. In regions of the protein that have been reported to be important for structural integrity, such as between residues 87 and 95 and residues 100 and 118 (17), there are no amino acid changes in the chicken isolates.
Williamson and Titball (23) showed that the protective antibody response to alpha-toxin is directed against the C-terminal domain of the protein (amino acids 247 to 370 in the mature toxin). Unlike SWCP, none of the chicken isolates have any amino acid changes in this C-terminal domain of the toxin. Therefore, it is predicted that vaccination with the C-terminal domain (23) would elicit an immune response against the alpha-toxin from these chicken-derived strains of C. perfringens.
In conclusion, the C. perfringens strains from chickens suffering necrotic enteritis have highly conserved alpha-toxin sequences that closely resemble those of the alpha-toxins found in mammalian isolates of C. perfringens but are significantly different from that of the SWCP isolate obtained from a diseased swan, the only bird-derived strain characterized previously. These results are encouraging for the development of diagnostic tests and vaccines for the control and treatment of C. perfringens infections of commercial chickens, as they signify that the vaccines and tests used for other C. perfringens infections may be able to be used in this host species.
Acknowledgments
We thank Pat Blackall (Queensland Department of Primary Industries), who supplied several strains of C. perfringens, Ambrosio Rubite and Peter Scott for facilitating access to poultry farms for sample collection, and Mark Ford (Commonwealth Scientific and Industrial Research Organisation, Livestock Industries) for animal handling and disease diagnosis.
The kind support of the Australian Rural Industries Research Development Corporation (RIRDC), through which this work was funded, is acknowledged.
REFERENCES
- 1.Al-Sheikhly, F., and R. B. Truscott. 1977. The pathology of necrotic enteritis of chickens following infusion of crude toxins of Clostridium perfringens into the duodenum. Avian Dis. 21:241-255. [PubMed] [Google Scholar]
- 2.Awad, M. M., A. E. Bryant, D. L. Stevens, and J. I. Rood. 1995. Virulence studies on chromosomal alpha-toxin and theta-toxin mutants constructed by allelic exchange provide genetic evidence for the essential role of alpha-toxin in Clostridium perfringens-mediated gas gangrene. Mol. Microbiol. 15:191-202. [DOI] [PubMed] [Google Scholar]
- 3.Baba, E., A. L. Fuller, J. M. Gilbert, S. G. Thayer, and L. R. McDougald. 1992. Effects of Eimeria brunetti infection and dietary zinc on experimental induction of necrotic enteritis in broisoleucineciner chickens. Avian Dis. 36:59-62. [PubMed] [Google Scholar]
- 4.Ginter, A., E. D. Williamson, F. Dessy, P. Coppe, H. Bullifent, A. Howells, and R. W. Titball. 1996. Molecular variation between the alpha-toxins from the type strain (NCTC 8237) and clinical isolates of Clostridium perfringens associated with disease in man and animals. Microbiology 142:191-198. [DOI] [PubMed] [Google Scholar]
- 5.Guillouard, I., T. Garnier, and S. T. Cole. 1996. Use of site-directed mutagenesis to probe structure-function relationships of alpha-toxin from Clostridium perfringens. Infect. Immun. 64:2440-2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hatheway, C. L. 1990. Toxigenic clostridia. Clin. Microbiol. Rev. 3:66-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hein, H., and L. Timms. 1972. Bacterial flora in the alimentary tract of chickens infected with Eimeria brunetti and in chickens immunized with Eimeria maxima and cross-infected with Eimeria brunetti. Exp. Parasitol. 31:188-193. [DOI] [PubMed] [Google Scholar]
- 8.Justin, N., N. Walker, H. L. Bullifent, G. Songer, D. M. Bueschel, H. Jost, C. Naylor, J. Miller, D. S. Moss, R. W. Titball, and A. K. Basak. 2002. The first strain of Clostridium perfringens isolated from an avian source has an alpha-toxin with divergent structural and kinetic properties. Biochemistry 41:6253-6262. [DOI] [PubMed] [Google Scholar]
- 9.Leslie, D., N. Fairweather, D. Pickard, G. Dougan, and M. Kehoe. 1989. Phospholipase C and haemolytic activities of Clostridium perfringens alpha-toxin cloned in Escherichia coli: sequence and homology with a Bacillus cereus phospholipase C. Mol. Microbiol. 3:383-392. [DOI] [PubMed] [Google Scholar]
- 10.Long, J. R., and R. B. Truscott. 1976. Necrotic enteritis in broisoleucineciner chickens. III. Reproduction of the disease. Can. J. Comp. Med. 40:53-59. [PMC free article] [PubMed] [Google Scholar]
- 11.MacLennan, J. D. 1962. The histotoxic clostridrial infections of man. Bacteriol. Rev. 26:177-276. [PMC free article] [PubMed] [Google Scholar]
- 12.Mahony, D. E., and T. J. Moore. 1976. Stable L-forms of Clostridium perfringens and their growth on glass surfaces. Can. J. Microbiol. 22:953-959. [DOI] [PubMed] [Google Scholar]
- 13.McDonel, J. L. 1980. Clostridium perfringens toxins (type A, B, C, D, E). Pharmacol. Ther. 10:617-655. [DOI] [PubMed] [Google Scholar]
- 14.Meer, R. R., and J. G. Songer. 1997. Multiplex polymerase chain reaction assay for genotyping Clostridium perfringens. Am. J. Vet. Res. 58:702-705. [PubMed] [Google Scholar]
- 15.Nagahama, M., T. Nakayama, K. Michiue, and J. Sakurai. 1997. Site-specific mutagenesis of Clostridium perfringens alpha-toxin: replacement of Asp-56, Asp-130, or Glu-152 causes loss of enzymatic and hemolytic activities. Infect. Immun. 65:3489-3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nagahama, M., Y. Okagawa, T. Nakayama, E. Nishioka, and J. Sakurai. 1995. Site-directed mutagenesis of histidine residues in Clostridium perfringens alpha-toxin. J. Bacteriol. 177:1179-1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Naylor, C. E., J. T. Eaton, A. Howells, N. Justin, D. S. Moss, R. W. Titball, and A. K. Basak. 1998. Structure of the key toxin in gas gangrene. Nat. Struct. Biol. 5:738-746. [DOI] [PubMed] [Google Scholar]
- 18.Rood, J. I. 1998. Virulence genes of Clostridium perfringens. Annu. Rev. Microbiol. 52:333-360. [DOI] [PubMed] [Google Scholar]
- 19.Saint-Joanis, B., T. Garnier, and S. T. Cole. 1989. Gene cloning shows the alpha-toxin of Clostridium perfringens to contain both sphingomyelinase and lecithinase activities. Mol. Gen. Genet. 219:453-460. [DOI] [PubMed] [Google Scholar]
- 20.Shimizu, T., K. Ohtani, H. Hirakawa, K. Ohshima, A. Yamashita, T. Shiba, N. Ogasawara, M. Hattori, S. Kuhara, and H. Hayashi. 2002. Complete genome sequence of Clostridium perfringens, an anaerobic flesh-eater. Proc. Natl. Acad. Sci. USA 99:996-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Titball, R. W., S. E. Hunter, K. L. Martin, B. C. Morris, A. D. Shuttleworth, T. Rubidge, D. W. Anderson, and D. C. Kelly. 1989. Molecular cloning and nucleotide sequence of the alpha-toxin (phospholipase C) of Clostridium perfringens. Infect. Immun. 57:367-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsutsui, K., J. Minami, O. Matsushita, S. Katayama, Y. Taniguchi, S. Nakamura, M. Nishioka, and A. Okabe. 1995. Phylogenetic analysis of phospholipase C genes from Clostridium perfringens types A to E and Clostridium novyi. J. Bacteriol. 177:7164-7170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Williamson, E. D., and R. W. Titball. 1993. A genetically engineered vaccine against the alpha-toxin of Clostridium perfringens protects mice against experimental gas gangrene. Vaccine 11:1253-1258. [DOI] [PubMed] [Google Scholar]
- 24.Willis, A. T. 1984. Treatment of anaerobic infections. Scand. J. Gastroenterol. Suppl. 90:53-64. [PubMed] [Google Scholar]