Abstract
Primary biliary cirrhosis (PBC) has been often coined a model autoimmune disease based on the homogeneity amongst patients, the frequency and similarity of antimitochondrial antibodies, including the highly directed immune response to pyruvate dehydrogenase (PDC-E2). A significant number of patients with PBC suffer from sicca and amongst these, there are patients who also have classic Sjogren's syndrome. Indeed, both PBC and Sjogren's syndrome are characterized by inflammation of target epithelial elements. Both diseases can be considered on the basis of a number of other related clinical aspects, including proposed unique apoptotic features of the target tissue, the role of secretory IgA, and the frequency with which both diseases overlap with each other. Indeed, PBC may be considered a Sjogren's syndrome of the liver, whereas Sjogren's syndrome can be equally discussed as PBC of the salivary glands. Dissection of the genetic predispositions for both diseases and especially the molecular basis of effector mechanisms, will become critical elements in developing new therapies.
Keywords: Apoptosis, autoantibodies, epithelium, inflammation
Introduction
The current view of autoimmunity is that there will be common susceptibility backgrounds that apply to related pathologies, as supported by the frequent coexistence of more than one autoimmune disease in the same patient, the shared female predominance, and the similar genetic associations found within the MHC complex. One major example of this so-called autoimmune clustering is the coexistence of Sjögren’s syndrome (SS) and primary biliary cirrhosis (PBC). These two autoimmune diseases are characterized by the progressive immune-mediated destruction of the epithelial tissues of the salivary and lacrimal glands and the intrahepatic bile ducts, respectively.
The autoimmune destruction in SS primarily affects exocrine glands and other organs [1] and in 1979 the term “autoimmune exocrinopathy” was coined [2]. More recently, Moutsopoulos suggested the term “autoimmune epithelitis” for the condition to reflect the target of the immune mediated injury [1]. The rheumatologist or clinical immunologist following patients with SS will often be facing the possibility of encountering some degree of liver damage. This is commonly of secondary clinical relevance to the main diagnosis and the prevalence varies among series and depends on the case finding criteria.
Earlier studies based on clinical findings and serology estimated that liver abnormalities were found in 18–20% of SS cases to include autoimmune liver diseases and chronic viral hepatitis [3] while liver enlargement was reported during the examination in 2–20% of patients with SS [4–6]. This review will discuss the common themes of PBC that relate to SS and attempt to underline similarities and differences with SS; many of these are summarized in Table 1.
Table 1.
Classification criteria used for PBC and SS.
| PBC [7] | SS [14] | |
|---|---|---|
| Clinical | Fatigue Pruritus (late stages) |
Ocular and oral dry symptoms for longer than 3 months or use of tear substitute more than 3 times a day or recurrent need to drink to swallow dry food |
| Histology and functional tests | Non suppurative cholangitis with interlobular bile duct injury at liver biopsy | Abnormal Schirmer’s or Rosa Bengala test or lacrimal gland biopsy with focus score >1 and abnormal sialometry or salivary gland scintigraphy or parotid sialography or salivary gland biopsy with focus score >1 |
| Serum markers | Serum AMA > 1:40 PBC-specific ANA Serum alkaline phosphatase > 1,5-fold the normal value for more than 6 months |
Anti-SSA or anti-SSB Rheumatoid factor Speckled ANA |
Primary biliary cirrhosis (PBC)
PBC is a chronic cholestatic liver disease characterized by the immune-mediated destruction of the epithelial cells lining the small and medium-sized intra-hepatic bile ducts with resulting progressive fibrosis [7]. The diagnosis of PBC is based on the concomitant presence of two out of three internationally accepted criteria [7], i.e. (i) elevation of biochemical indices of cholestasis –e.g. alkaline phosphatase- for more than six months, (ii) the presence of serum antimitochondrial antibody (AMA) at titers exceeding 1:80, and (iii) consistent liver histology (Table 1). In some cases a nuclear magnetic resonance cholangiography is necessary to exclude the involvement of large bile ducts as in primary sclerosing cholangitis. In the past decade, the term “autoimmune cholangitis” has been used for patients with PBC but lacking AMA but positive for serum antinuclear (ANA) and anti-smooth muscle (SMA) serum antibody [8, 9]. The finding that both the salivary and lacrimal glands [10, 11] as well as the urinary tract epithelium could also be injured in PBC led the senior author of this article to suggest that PBC, similar to SS, can be considered a generalized epithelitis [12].
Diagnostic criteria
Histology remains crucial when other diagnoses of liver disease have to be ruled out, particularly in AMA-negative patients [13]. The typical histological lesion for PBC can be summarized as a chronic nonsuppurative destructive cholangitis involving small and medium interlobular bile ducts with a maximum diameter 80µ. Liver histology and staging manifests a wide sampling variability with four recognized stages ranging from portal inflammation to the appearance of fibrous septa between adjacent portal tracts and ultimately cirrhosis. At advanced stages, PBC cannot be discriminated from cirrhosis of other etiologies. Early disease stages share numerous similarities with SS in which exocrine glands are infiltrated by lymphocytes, mainly CD4+, interfering with the glandular function through the destruction of glandular elements by cell-mediated mechanisms, cytokines that activate pathways characterized by type 1 and 2 interferons, autoantibodies against muscarinic receptors, and metalloproteinases that interfere with the interaction of the epithelium with the extracellular matrix necessary for efficient glandular function [14].
Serum autoantibodies remain a crucial tool for diagnostic purposes in rheumatology and this applies to both SS and PBC. Nevertheless, there is limited overlapping in the autoantibody profiles of these two conditions, as illustrated in Table 2, and specific positivities retain their diagnostic power. It is well established that patients with SS commonly have detectable serum anti-Ro/SSA anti-La/SSB and anti-U1RNP [15], while PBC sera are characterized by serum antimitochondrial antibody (AMA), possibly the most specific autoantibody in clinical immunology [16]. AMA are highly specific for PBC and can be detected in nearly 100% of patients when sensitive diagnostic methodologies based on recombinant antigens are used [17]. In most clinical settings, however, indirect immunofluorescence techniques are used for initial screening of cases and might provide falsely positive or negative results. AMA are directed against components of the 2-oxoacid dehydrogenase (2-OADC) family of enzymes within the mitochondrial respiratory chain, most frequently the E2 and E3-binding protein (E3BP) components of the pyruvate dehydrogenase complex and the E2 components of the 2-oxo glutarate dehydrogenase and branched-chain 2-oxo acid dehydrogenase complexes. In all three antigens epitopes contain the motif DKA, with lipoic acid covalently bound to the lysine (K) residue. The role of lipoic acid in epitope recognition by AMA is unclear. The pathogenic role of AMA is debatable, since no clinical correlation can be found and animal models developing serum AMA do not develop PBC-like liver lesions.
Table 2.
Prevalence of serum autoantibodies observed in PBC and SS (highest rates are reported).
Serum antinuclear antibodies (ANA) are frequently observed in both conditions but with a higher prevalence in SS compared to PBC. In PBC, these are more frequently of the multiple nuclear dot or rim-like/membranous patterns and possibly more prevalent among AMA-negative PBC patients [9, 18–20]. The first pattern is produced by autoantibodies against gp210 and nucleoporin 62 within the nucleopore complex and the second pattern by autoantibodies against sp100, promyelocytic leukemia (PML) and small ubiquitin-like modifier (SUMO) proteins [21]. Antibodies against gp210 are associated with a more rapidly progressing disease [22–24], different from serum AMA. AMA are directed at the pyruvate dehydrogenase complex (anti-PDC) and are routinely screened with indirect immunofluorescence. In a multicenter study, AMA were present in 22% of SS, 17% of SLE, 10% of rheumatoid arthritis, and 10% of scleroderma sera [25]. Interestingly, SS per se or associated with other autoimmune diseases increases the risk of having positive serum AMA but data are burdened by the lack of more specific recombinant antigens that were recently developed [19, 26].
Clinical features and management
The major clinical features of PBC and SS are compared in Table 3. PBC at presentation is classically characterized by fatigue and pruritus while physical findings may include skin hyperpigmentation and liver and spleen enlargement [7]. End-stage symptoms are those of all types of liver cirrhosis, including ascites, jaundice, hepatic encephalopathy, and upper digestive bleeding. Fatigue is an incompletely defined, nonspecific symptom that affects up to 70% of patients with PBC and that is often overlooked, particularly in middle-aged women. Importantly, the severity of fatigue is independent of the stage of PBC or its other features (pruritus or severe cholestasis), nor does it depend on psychiatric factors. No medical treatment has been shown to be effective in alleviating this symptom, although fatigue has never been included as an endpoint in any of the large controlled clinical trials [27–32]. As many as 70% of patients with PBC and jaundice suffer from pruritus [33–36]. Longitudinal data show that the vast majority of patients will eventually experience this symptom during their lifetime; pruritus might long precede jaundice onset and typically worsens at night, following contact with wool, or in warm climates. Despite remaining a challenging symptom, the use of cholestyramine (4 g two or three times a day) ameliorates pruritus while rifampicin has been used to achieve rapid symptom relief; its prolonged use, however, should be avoided. Portal hypertension is frequently found in patients with PBC and, importantly, may precede any other sign or symptom of liver cirrhosis. Over half of untreated patients eventually develop portal hypertension over a 4-year period while medical treatment slows the development of this complication [37, 38]; once varices are found, the bleeding prevention or treatment are not different from other chronic liver diseases. An accelerated bone loss is common in long-standing cholestasis compared to sex- and age-matched healthy individuals; this is referred to as metabolic bone disease secondary to reduced bone deposition [39–41]. Current treatment of bone loss includes oral calcium supplementation, weight-bearing activity, and oral vitamin D replacement, if deficiency is found. Postmenopausal hormone replacement therapy should be considered but jaundice and other signs of liver failure should be evaluated during the first months of treatment. Hyperlipidemia is common in up to 85% of patients with PBC and both serum cholesterol and triglyceride high levels can be observed [42–45]; accordingly, statins are usually not necessary but can be well tolerated.
Table 3.
A comparison of the general features of PBC and SS.
| PBC | SS | |
|---|---|---|
| Female/male ratio | 10:1 | 9:1 |
| Mean age at diagnosis | 55 | Bimodal (20, 50) |
| Disease target | bile duct, salivary gland epithelia, and uroepithelium | salivary gland, bile duct, bronchial, alveolar, and tubular epithelia |
| Environmental factors | Bacteria, xenobiotics | Putative viral infections (EBV, retroviruses) |
| Epigenetics | Different methylation in hemidesmosome gene; disease-specific miRNAs in minor salivary glands | Different methylation at X-linked promoters |
| Proteomics | Possible serum biomarkers, no liver tissue disease-specific biomarkers | Proposed salivary biomarkers |
Autoimmune comorbidity is an important feature of PBC. Various disorders, particularly other autoimmune syndromes, are associated with PBC at various degrees [17, 46–48]. Our 2005 nationwide epidemiological study of 1032 patients with PBC reported that one-third of cases are also affected by another autoimmune disease, most commonly SS, Raynaud’s phenomenon, autoimmune thyroid disease, scleroderma, and systemic lupus erythematosus, while the prevalence of rheumatoid arthritis did not differ from controls [49]. Interestingly, recent data demonstrated that patients affected by both PBC and scleroderma manifest a less aggressive liver disease, thus suggesting an active interaction between the two conditions [50]; whether this applies also to SS remains to be determined. The association of liver involvement in SS with serum antimitochondrial antibodies (AMA) was first reported in 1970 [51] with studies on well documented SS patient populations, observing a 5–10% antibody prevalence. On one hand, about half of them have elevated liver enzymes while, on the other hand, liver enzymes may be elevated without the coexistence of AMA. According to several studies, characteristic symptoms of SS such as dry mouth or dry eyes are commonly (47–73%) found also in PBC. In addition, objective findings of dry eyes or dry mouth (such as abnormal Schirmer test, or diminished salivary flow rate) are also found in 30–50% of patients with PBC while radiological findings of sielectasia were demonstrated in 25% of PBC cases. Furthermore, these frequently (26–93%) manifest histological changes compatible with the diagnosis of SS at salivary gland biopsies [5, 52, 53]. Nevertheless, these individuals differ from the patients with primary SS. They rarely have serious sequelae and their serological and immunogenetic features are not identical to those observed in patients with primary SS. More specific, in these patients anti-Ro autoantibodies are rarely observed and the frequency of HLA-B8, -DR3 and DRW52 is lower compared to patients with primary SS. Thus, SS complicating PBC is considered similar to that occurring in RA patients (secondary SS) [54].
Epidemiology and geoepidemiology
In both PBC and SS there is a striking female predominance and female to male ratios as high as 10:1 for PBC and 9:1 for SS [55–57]. For both conditions the age at diagnosis is in the mid-50s; however, there are two age peaks of primary Sjögren's syndrome, with the first after menarche during the 20s to 30s and the second after menopause [14]. There is a significant variability in the reported prevalence of SS, ranging from 0.2 to 4.8% [58–65], based on the different diagnostic criteria adopted, as illustrated in Figure 1.
Figure 1.
The worlwide map of PBC and SS geoepidemiology [58–79, 126]. Prevalence rate (per million) are illustrated for major areas.
Data about the incidence and prevalence of PBC have generally been obtained through observational (rather than population-based) studies and might not indicate true rates in the general population. While there is general agreement on the simpler diagnostic criteria compared to SS [7], regional differences could vary on the basis of medical awareness and expertise [66]. Indeed, a population-based approach to case detection has little feasibility for PBC because of its rarity and the lack of non invasive tools for most sensitive case finding. As a result, reported prevalence ranges from 6 to 402 cases per million (Figure 1) [67–79]. By contrast, findings of serological studies with indirect immunofluorescence in large groups of unselected serum samples show that prevalence of serum AMA in the general population can be as high as 0.5%, with lower frequencies when blood donors are investigated [55]. Different from SS, the variability in the reported estimates of the incidence and prevalence of PBC is likely secondary to different case-finding methods, local physician awareness, and quality levels of health-care systems, as suggested by the socioeconomical differences [49]. On the basis of data from case-finding studies, however, a latitudinal geoepidemiological pattern of occurrence of primary biliary cirrhosis has been proposed, with the disease being more prevalent in northern Europe and North America [80, 81].
Genetics, epigenetics, and proteomics
Our knowledge of the genetics of SS and PBC remains largely incomplete and is summarized in Table 4. PBC is more frequent in relatives of affected individuals and the term ‘familial PBC’ has been coined to indicate families that have more than one case. Our data indicate 6% of cases have a first-degree relative that is also affected [49]. More importantly, the concordance rate observed among monozygotic twins for PBC is 63%, amongst the highest reported in autoimmunity, reinforcing the idea of an important role of genetics in disease susceptibility [82]. Due to the rarity of the disease several association studies have attempted to identify genes associated with PBC although no family study of genetic linkage has been performed. Associations are often not applicable to all populations but suggest that a multi-hit genetic model seems to apply to PBC, with different genetic variants conferring susceptibility (first hit) and others influencing disease progression (second hit) [81]. The study of the variants of MHC (including type I, II, and III loci) have produced associations that are often weak or limited to specific geographical areas [83]. Following the numerous association studies reported over the past decades for candidate genes in PBC, the first genome-wide case-control association studies were reported in 2009 and 2010 in patients and controls from Canada, the US, and Italy and demonstrated significant associations of PBC with IL12A, IL12RB2, and STAT4 polymorphisms [84, 85]. The importance of IL12 was most recently supported by data in a PBC animal model that IL-12p40 is crucial to autoimmunity development thus proving an ideal link between genomic studies and disease pathogenesis with potential therapeutic implications [86]. Furthermore, the growing field of microRNA effects on immune modulation has been investigated also in PBC where a specific signature was described for the first time [87]. Of note, an age-dependent enhanced monosomy X was reported in peripheral lymphocytes of women with PBC [88] and scleroderma [89], thus suggesting that PBC might ensue from a polygenic model with an X-linked major locus of susceptibility in which genes escaping inactivation are the major candidates [88, 90].
Table 4.
Proposed genetic factors involved in the etiology of PBC and SS. Data are gathered from HLA studies and genome-wide association studies (GWAS) along with the more recent epigenetic data.
| PBC | SS | |
|---|---|---|
| HLA | DR3, DR8, and DR4 in white, DR2 and DR3 in Japanese, DQA1*0102, and DQ/β1*0402 | DR2, DR3 |
| GWAS | DQB1, IL12A, IL12RB2, STAT4, IRF5, IKZF3/ORMDL3, SPIB | STAT4, IRF5 |
| Epigenetics | different methylation in hemidesmosome gene; disease-specific miRNAs in minor salivary glands | different methylation at X-linked promoters |
Beside genetics, our epidemiological data demonstrated that a high risk of developing PBC is associated with a history of urinary or vaginal infections, comorbidity with other autoimmune diseases, lifestyle factors, such as smoking, and previous pregnancies [49]. Experimental studies have focused on two main classes of agents possibly triggering PBC: infectious (bacteria and viruses) and chemical (xenobiotics) [91]. Most evidence has been reported for Escherichia coli, while contrasting data have been obtained on the role of Chlamydia pneumoniae [92]. Finally, we provided experimental evidence suggesting that Novosphingobium aromaticivorans, a ubiquitous xenobiotic-metabolizing Gram-negative bacterium, is the best candidate yet for the induction of PBC [93]. In a complementary fashion, xenobiotics (i.e. foreign compounds that may either alter or complex to defined self or nonself proteins) have been advocated to induce a change in the molecular structure of the native protein sufficient to induce an immune response. Such immune responses may then result in the cross-recognition of the self form, which could in turn perpetuate the immune response, thus leading to chronic autoimmunity. Interestingly, most xenobiotics are metabolized in the liver, thereby increasing the potential for liver-specific alteration of proteins [94].
Epigenetics is an emerging candidate link between genomics and environment in generating phenotype variability and disease susceptibility in adult life [95]. Alterations in the post-translational modification of histones and DNA methylation are the two major epigenetic mechanisms that may potentially cause a breakdown of immune tolerance and the perpetuation of autoimmune diseases. In recent years several studies both in clinical settings and experimental models proposed that the epigenome may hold the key to a better understanding of autoimmunity initiation and perpetuation. The impact of epigenetic changes has been suggested in systemic lupus erythematosus, rheumatoid arthritis, multiple sclerosis, in some cases based on observations in animal models. Data on the epigenetics of PBC are limited to one study from our group in which consistent differences were observed in the DNA methylation profile of two X-linked genes (namely CLIC2 and PIN4) in peripheral lymphocytes from discordant twins [96]. This paucity of data is reflected also in SS as only most recently there have been reports of specific microRNAs overexpressed in SS salivary glands [97]. Finally, recent efforts have been dedicated to the search through mass spectrometry (MS) for serum biomarkers of autoimmune diseases. This goal is particularly challenging in diseases such as PBC and SS in which liver and minor salivary gland biopsies are still currently used diagnostic tools. MALDI-TOF-MS combined with magnetic beads found in serum of a Chinese cohort of PBC patients found 69 presumable proteins and peptides specifically characterizing PBC [98] but an independent confirmation is awaited. More recently, an MS-based study of PBC and control liver biopsies defined a proteomic profile that characterizes PBC as well as other autoimmune liver diseases [99]. We should note that similar approaches should be encouraged in SS for which biological samples (i.e. saliva) can be easily obtained.
Immunopathogenesis
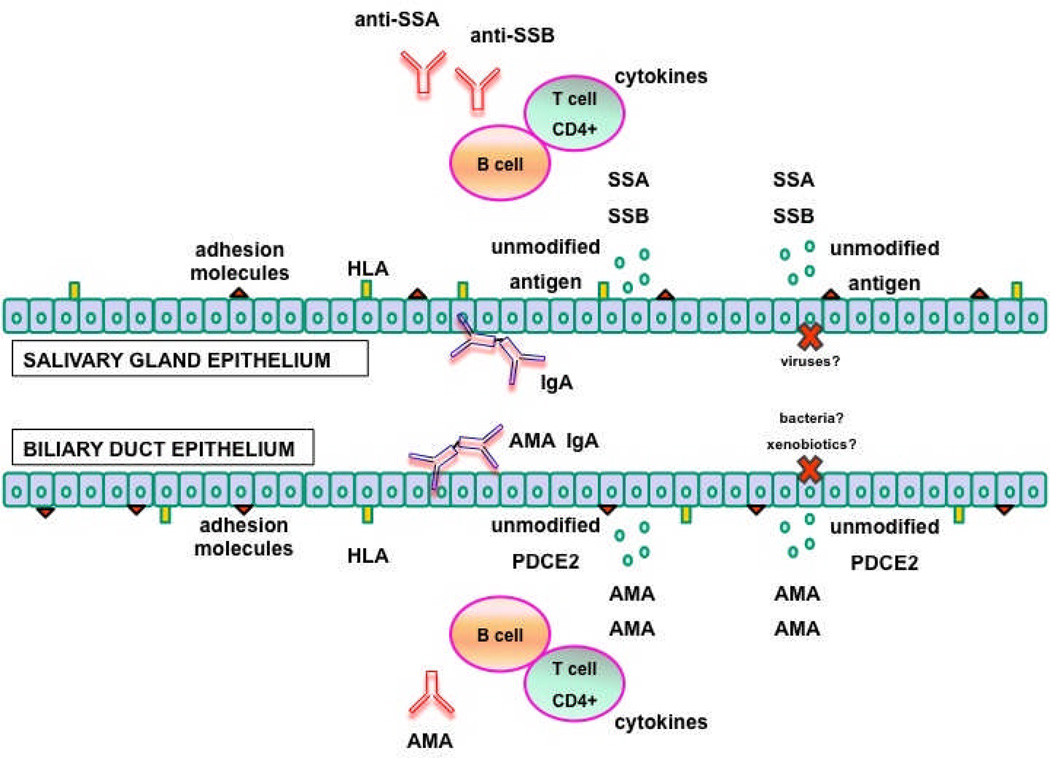
In both PBC and SS serum autoantibodies (AMA and anti-Ro, anti-La for PBC and SS respectively) target ubiquitous proteins expressed in all nucleated cells [100, 101], as illustrated in Figure 2. Nevertheless, PBC and to a lesser degree SS are organ specific diseases, indicating that epithelia (biliary epithelial cells and salivary gland epithelial cells for PBC and SS respectively) are likely active participants in the pathogenesis of the diseases [102] (Table 5). First, a hypothesis based on the unique apoptosis features in bile duct cells has been proposed to explain PBC tissue specificity [103]. It was first demonstrated that PDC-E2 remains intact and retains its immunogenicity during cholangiocyte apoptosis, secondary to a cell-specific lack of glutathionylation of biliary epithelial cells [104]. The intact PDC-E2 in apoptotic blebs (i.e. apotopes) could be then uptaken by local antigen presenting cells and transferred to regional lymph nodes for priming of cognate T cells thus initiating PBC [105, 106]. Second, biliary epithelial cells are immunologically active because they express HLA antigens, adhesion and costimulatory molecules, such as LFA3, CD40, CD80, CD86, cytokines such as IL6, IL8, chemokines, such as MCP1, and growth factors such as TGF, CTGF, PDGF, endothelin [107, 108]. Third, cholangiocytes can secrete secretory IgA in the biliary tree [12, 109] with enormous potential implications for PBC pathogenesis as secretory IgA are important effectors in mucosal immunity. IgA derived from local plasma cells are internalized into the epithelial cells as complexes with poly-Ig-receptor, transported to the apical surface of the cell, through a process called transcytosis, and secreted at the mucosal surface after poly-Ig receptor cleavage. IgA AMA have been detected not only in the bile, but also in the saliva and urine of patients [11, 110]. More importantly, it has been demonstrated that IgA AMA are locally produced supporting the hypothesis that other epithelial tissues other than cholangiocytes are involved in PBC. Whether IgA antimitochondrial producing immunocytes are primed in situ or have emigrated from other MALT is unclear but the acquired association between PBC and recurrent urinary tract infections is particularly fascinating [111]. We may hypothesize that bacterial derived mitochondrial antigens in the urinary tract may induce B lymphocyte differentiation into IgA producing plasma cells within uroepithelium. Finally, the salivary gland ducts of patients wityh PBC, independent of the presence of sicca symptoms, manifests a PBC-like immunohistochemical staining with a monoclonal antimitochondrial antibody specific for the self-antigen PDC-E2 [112], further supporting the proposed locally driven autoimmune epithelitis.
Figure 2.
A parallel comparison of the proposed immunopathogenesis of PBC and SS. In both conditions, environmental triggers (putatively infectious agents and xenobiotics) cause salivary or biliary epithelial cell apoptosis and contribute to tolerance breakdown to self antigens exposed on the apoptotic blebs (SSA and SSB) and not protected by post-translational modification (PDC-E2). Salivary and biliary epithelial cells concur to the autoimmune process also by expressing cytokines, HLA class II and adhesion molecules.
Table 5.
Immunological factors involved in the pathogenesis of PBC and SS.
| PBC | SS | |
|---|---|---|
| Breaking tolerance trigger | Proposed involvement of infections (E. coli, N aromaticivorans), xenobiotics | Proposed involvement of viral infection (EBV, CMV, retroviruses) |
| Self-antigen presentation | Lack of gluthationylation of PDC-E2 during apoptosis | Exposure of SSA/SSB on apoptotic blebs and on exosomes; lack of gluthationylation of PDC-E2 during apoptosis |
| Cellular immune response | Granuloma and predominance of CD4+ infiltrate around bile duct; | Predominance of CD4+ infiltrate around salivary duct ; |
| Humoral immune response | Epithelial transcytosis of IgA against self-antigen with locally production of AMA in bile, saliva, urine; serum reactivity against PDE2 from cholangiocyte and salivary duct epithelia; disease-specific autoantibodies (AMA) | Epithelial transcytosis of IgA against self-antigen; B cell activation with increased lymphoma risk; not disease-specific autoantibodies (anti-SSA/SSB, rheumatoid factor) |
Treatment
The only approved treatment for PBC is ursodeoxycholic acid (UDCA) at recommended doses of 13 to 15 mg/kg [13, 46]. The mechanism of action of UDCA in PBC is incompletely understood but it has been hypothesized that it is based on different factors, including modification of the bile acid pool, reduction in proinflammatory cytokines, effects on apoptosis and on vasoactive mediators [113]. A meta-analysis demonstrated that an increased survival is only obtained when a dose > 13 mg/kg is prescribed [114, 115], despite the fact that a complete biochemical response to UDCA (i.e. normalization of alkaline phosphatase) is achieved in approximately 50% of treated patients. Importantly, none of the medical treatments currently used for SS are contraindicated in patients with coexisting PBC.
Nevertheless, immunosuppressive drugs have also been used in PBC with poor efficacy, including corticosteroids, azathioprine, cyclosporine, methotrexate, penicillamine, and colchicine. Liver transplantation is the ultimate treatment for end-stage PBC but recurrence is common and its rates seem to be influenced by certain immunosuppressive†regimens, while the use of UDCA for recurrence is safe and recommended.
Conclusions
We speculate that PBC is a classic phenotype of autoimmune epithelitis and this thesis applies to salivary glands and, of course, SS. There are also major differences between SS and PBC and these include the genetic bases of disease susceptibility as well as other mechanisms well illustrated by the available animal models (Table 6). The recently completed genome-wide association studies performed reported a significant association of PBC with polymorphisms of HLA, IL-12A, IL-12RB2, and to a minor extent, STAT4 [84, 85] while data on SS can only be gathered from those in lupus [116] and manifest some degree of similarity with PBC in the minor STAT4 association. Importantly, based on the biology of the target epithelium, there likely are common effector mechanisms. Dissection of these effector mechanisms will become important in any therapeutics. Finally, we submit that PBC and SS comorbidity may identify an intriguing subgroup of patients in which common traits should be investigated.
Table 6.
Proposed animal models for PBC and SS.
Acknowledgments
Financial support provided by National Institutes of Health grant DK39588.
References
- 1.Moutsopoulos HM. Sjogren's syndrome: autoimmune epithelitis. Clin Immunol Immunopathol. 1994;72:162–165. doi: 10.1006/clin.1994.1123. [DOI] [PubMed] [Google Scholar]
- 2.Strand V, Talal N. Advances in the diagnosis and concept of Sjogren's syndrome (autoimmune exocrinopathy) Bull Rheum Dis. 1979;30:1046–1052. [PubMed] [Google Scholar]
- 3.Bloch KJ, Buchanan WW, Wohl MJ, Bunim JJ. Sjoegren's Syndrome. A Clinical, Pathological, and Serological Study of Sixty-Two Cases. Medicine (Baltimore) 1965;44:187–231. [PubMed] [Google Scholar]
- 4.Skopouli FN, Barbatis C, Moutsopoulos HM. Liver involvement in primary Sjogren's syndrome. Br J Rheumatol. 1994;33:745–748. doi: 10.1093/rheumatology/33.8.745. [DOI] [PubMed] [Google Scholar]
- 5.Kaplan MJ, Ike RW. The liver is a common non-exocrine target in primary Sjogren's syndrome: a retrospective review. BMC Gastroenterol. 2002;2:21. doi: 10.1186/1471-230X-2-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lindgren S, Manthorpe R, Eriksson S. Autoimmune liver disease in patients with primary Sjogren's syndrome. J Hepatol. 1994;20:354–358. doi: 10.1016/s0168-8278(94)80007-3. [DOI] [PubMed] [Google Scholar]
- 7.Selmi C, Bowlus CL, Gershwin ME, Coppel RL. Primary biliary cirrhosis. Lancet. 2011;377:1600–1609. doi: 10.1016/S0140-6736(10)61965-4. [DOI] [PubMed] [Google Scholar]
- 8.Invernizzi P, Crosignani A, Battezzati PM, Covini G, De Valle G, Larghi A, et al. Comparison of the clinical features and clinical course of antimitochondrial antibody-positive and -negative primary biliary cirrhosis. Hepatology. 1997;25:1090–1095. doi: 10.1002/hep.510250507. [DOI] [PubMed] [Google Scholar]
- 9.Invernizzi P, Selmi C, Ranftler C, Podda M, Wesierska-Gadek J. Antinuclear antibodies in primary biliary cirrhosis. Semin Liver Dis. 2005;25:298–310. doi: 10.1055/s-2005-916321. [DOI] [PubMed] [Google Scholar]
- 10.Epstein O, Thomas HC, Sherlock S. Primary biliary cirrhosis is a dry gland syndrome with features of chronic graft-versus-host disease. Lancet. 1980;1:1166–1168. doi: 10.1016/s0140-6736(80)91621-9. [DOI] [PubMed] [Google Scholar]
- 11.Nishio A, Van de Water J, Leung PS, Joplin R, Neuberger JM, Lake J, et al. Comparative studies of antimitochondrial autoantibodies in sera and bile in primary biliary cirrhosis. Hepatology. 1997;25:1085–1089. doi: 10.1002/hep.510250506. [DOI] [PubMed] [Google Scholar]
- 12.Tanaka A, Nalbandian G, Leung PS, Benson GD, Munoz S, Findor JA, et al. Mucosal immunity and primary biliary cirrhosis: presence of antimitochondrial antibodies in urine. Hepatology. 2000;32:910–915. doi: 10.1053/jhep.2000.19254. [DOI] [PubMed] [Google Scholar]
- 13.Lindor KD, Gershwin ME, Poupon R, Kaplan M, Bergasa NV, Heathcote EJ. Primary biliary cirrhosis. Hepatology. 2009;50:291–308. doi: 10.1002/hep.22906. [DOI] [PubMed] [Google Scholar]
- 14.Fox RI. Sjogren's syndrome. Lancet. 2005;366:321–331. doi: 10.1016/S0140-6736(05)66990-5. [DOI] [PubMed] [Google Scholar]
- 15.Hernandez-Molina G, Leal-Alegre G, Michel-Peregrina M. The meaning of anti-Ro and anti-La antibodies in primary Sjogren's syndrome. Autoimmun Rev. 2011;10:123–125. doi: 10.1016/j.autrev.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 16.Tishler M, Alosachie I, Barka N, Lin HC, Gershwin ME, Peter JB, et al. Primary Sjogren's syndrome and primary biliary cirrhosis: differences and similarities in the autoantibody profile. Clin Exp Rheumatol. 1995;13:497–500. [PubMed] [Google Scholar]
- 17.Lleo A, Battezzati PM, Selmi C, Gershwin ME, Podda M. Is autoimmunity a matter of sex? Autoimmun Rev. 2008;7:626–630. doi: 10.1016/j.autrev.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 18.Bizzaro N, Covini G, Rosina F, Muratori P, Tonutti E, Villalta D, et al. Overcoming a "Probable" Diagnosis in Antimitochondrial Antibody Negative Primary Biliary Cirrhosis: Study of 100 Sera and Review of the Literature. Clin Rev Allergy Immunol. 2010 doi: 10.1007/s12016-010-8234-y. [DOI] [PubMed] [Google Scholar]
- 19.Selmi C, Zuin M, Bowlus CL, Gershwin ME. Anti-mitochondrial antibody-negative primary biliary cirrhosis. Clin Liver Dis. 2008;12:173–185. doi: 10.1016/j.cld.2007.11.008. ix. [DOI] [PubMed] [Google Scholar]
- 20.Oertelt S, Rieger R, Selmi C, Invernizzi P, Ansari AA, Coppel RL, et al. A sensitive bead assay for antimitochondrial antibodies: Chipping away at AMA-negative primary biliary cirrhosis. Hepatology. 2007;45:659–665. doi: 10.1002/hep.21583. [DOI] [PubMed] [Google Scholar]
- 21.Janka C, Selmi C, Gershwin ME, Will H, Sternsdorf T. Small ubiquitin-related modifiers: A novel and independent class of autoantigens in primary biliary cirrhosis. Hepatology. 2005;41:609–616. doi: 10.1002/hep.20619. [DOI] [PubMed] [Google Scholar]
- 22.Bogdanos DP, Liaskos C, Pares A, Norman G, Rigopoulou EI, Caballeria L, et al. Anti-gp210 antibody mirrors disease severity in primary biliary cirrhosis. Hepatology. 2007;45:1583. doi: 10.1002/hep.21678. author reply -4. [DOI] [PubMed] [Google Scholar]
- 23.Nakamura M, Kondo H, Mori T, Komori A, Matsuyama M, Ito M, et al. Anti-gp210 and anti-centromere antibodies are different risk factors for the progression of primary biliary cirrhosis. Hepatology. 2007;45:118–127. doi: 10.1002/hep.21472. [DOI] [PubMed] [Google Scholar]
- 24.Invernizzi P, Podda M, Battezzati PM, Crosignani A, Zuin M, Hitchman E, et al. Autoantibodies against nuclear pore complexes are associated with more active and severe liver disease in primary biliary cirrhosis. J Hepatol. 2001;34:366–372. doi: 10.1016/s0168-8278(00)00040-4. [DOI] [PubMed] [Google Scholar]
- 25.Zurgil N, Bakimer R, Moutsopoulos HM, Tzioufas AG, Youinou P, Isenberg DA, et al. Antimitochondrial (pyruvate dehydrogenase) autoantibodies in autoimmune rheumatic diseases. J Clin Immunol. 1992;12:201–209. doi: 10.1007/BF00918090. [DOI] [PubMed] [Google Scholar]
- 26.Agmon-Levin N, Shapira Y, Selmi C, Barzilai O, Ram M, Szyper-Kravitz M, et al. A comprehensive evaluation of serum autoantibodies in primary biliary cirrhosis. J Autoimmun. 2010;34:55–58. doi: 10.1016/j.jaut.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 27.Bjornsson E, Simren M, Olsson R, Chapman RW. Fatigue is not a specific symptom in patients with primary biliary cirrhosis. Eur J Gastroenterol Hepatol. 2005;17:351–357. doi: 10.1097/00042737-200503000-00015. [DOI] [PubMed] [Google Scholar]
- 28.Cauch-Dudek K, Abbey S, Stewart DE, Heathcote EJ. Fatigue in primary biliary cirrhosis. Gut. 1998;43:705–710. doi: 10.1136/gut.43.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Forton DM, Patel N, Prince M, Oatridge A, Hamilton G, Goldblatt J, et al. Fatigue and primary biliary cirrhosis: association of globus pallidus magnetisation transfer ratio measurements with fatigue severity and blood manganese levels. Gut. 2004;53:587–592. doi: 10.1136/gut.2003.016766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goldblatt J, Taylor PJ, Lipman T, Prince MI, Baragiotta A, Bassendine MF, et al. The true impact of fatigue in primary biliary cirrhosis: a population study. Gastroenterology. 2002;122:1235–1241. doi: 10.1053/gast.2002.32993. [DOI] [PubMed] [Google Scholar]
- 31.Hollingsworth KG, Newton JL, Taylor R, McDonald C, Palmer JM, Blamire AM, et al. Pilot study of peripheral muscle function in primary biliary cirrhosis: potential implications for fatigue pathogenesis. Clin Gastroenterol Hepatol. 2008;6:1041–1048. doi: 10.1016/j.cgh.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 32.Newton JL, Gibson GJ, Tomlinson M, Wilton K, Jones D. Fatigue in primary biliary cirrhosis is associated with excessive daytime somnolence. Hepatology. 2006;44:91–98. doi: 10.1002/hep.21230. [DOI] [PubMed] [Google Scholar]
- 33.Bergasa NV. Pruritus in primary biliary cirrhosis: pathogenesis and therapy. Clin Liver Dis. 2008;12:385–406. doi: 10.1016/j.cld.2008.02.013. x. [DOI] [PubMed] [Google Scholar]
- 34.Bergasa NV, Schmitt JM, Talbot TL, Alling DW, Swain MG, Turner ML, et al. Open-label trial of oral nalmefene therapy for the pruritus of cholestasis. Hepatology. 1998;27:679–684. doi: 10.1002/hep.510270307. [DOI] [PubMed] [Google Scholar]
- 35.Datta DV, Sherlock S. Cholestyramine for long term relief of the pruritus complicating intrahepatic cholestasis. Gastroenterology. 1966;50:323–332. [PubMed] [Google Scholar]
- 36.Kremer AE, Martens JJ, Kulik W, Williamson C, Moolenaar WH, Kondrackiene J, et al. Increased serum autotaxin activity in cholestatic pruritus. Hepatology. 2009;50:376A. [Google Scholar]
- 37.Huet PM, Vincent C, Deslaurier J, Cote J, Matsutami S, Boileau R, et al. Portal hypertension and primary biliary cirrhosis: effect of long-term ursodeoxycholic acid treatment. Gastroenterology. 2008;135:1552–1560. doi: 10.1053/j.gastro.2008.07.019. [DOI] [PubMed] [Google Scholar]
- 38.Mayo MJ, Parkes J, Adams-Huet B, Combes B, Mills AS, Markin RS, et al. Prediction of clinical outcomes in primary biliary cirrhosis by serum enhanced liver fibrosis assay. Hepatology. 2008;48:1549–1557. doi: 10.1002/hep.22517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Benetti A, Crosignani A, Varenna M, Giussani CS, Allocca M, Zuin M, et al. Primary biliary cirrhosis is not an additional risk factor for bone loss in women receiving regular calcium and vitamin D supplementation: a controlled longitudinal study. J Clin Gastroenterol. 2008;42:306–311. doi: 10.1097/01.mcg.0000248017.31386.39. [DOI] [PubMed] [Google Scholar]
- 40.Guanabens N, Pares A, Ros I, Caballeria L, Pons F, Vidal S, et al. Severity of cholestasis and advanced histological stage but not menopausal status are the major risk factors for osteoporosis in primary biliary cirrhosis. J Hepatol. 2005;42:573–577. doi: 10.1016/j.jhep.2004.11.035. [DOI] [PubMed] [Google Scholar]
- 41.Leslie WD, Bernstein CN, Leboff MS. AGA technical review on osteoporosis in hepatic disorders. Gastroenterology. 2003;125:941–966. doi: 10.1016/s0016-5085(03)01062-x. [DOI] [PubMed] [Google Scholar]
- 42.Allocca M, Crosignani A, Gritti A, Ghilardi G, Gobatti D, Caruso D, et al. Hypercholesterolaemia is not associated with early atherosclerotic lesions in primary biliary cirrhosis. Gut. 2006;55:1795–1800. doi: 10.1136/gut.2005.079814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heathcote EJ. Management of primary biliary cirrhosis. The American Association for the Study of Liver Diseases practice guidelines. Hepatology. 2000;31:1005–1013. doi: 10.1053/he.2000.5984. [DOI] [PubMed] [Google Scholar]
- 44.Longo M, Crosignani A, Battezzati PM, Squarcia Giussani C, Invernizzi P, Zuin M, et al. Hyperlipidaemic state and cardiovascular risk in primary biliary cirrhosis. Gut. 2002;51:265–269. doi: 10.1136/gut.51.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stojakovic T, Putz-Bankuti C, Fauler G, Scharnagl H, Wagner M, Stadlbauer V, et al. Atorvastatin in patients with primary biliary cirrhosis and incomplete biochemical response to ursodeoxycholic acid. Hepatology. 2007;46:776–784. doi: 10.1002/hep.21741. [DOI] [PubMed] [Google Scholar]
- 46.EASL Clinical Practice Guidelines: management of cholestatic liver diseases. J Hepatol. 2009;51:237–267. doi: 10.1016/j.jhep.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 47.Mackay IR. Clustering and commonalities among autoimmune diseases. J Autoimmun. 2009;33:170–177. doi: 10.1016/j.jaut.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 48.Sherlock S. Immunological aspects of active chronic hepatitis and primary biliary cirrhosis. Acta Gastroenterol Belg. 1968;31:416–423. [PubMed] [Google Scholar]
- 49.Gershwin ME, Selmi C, Worman HJ, Gold EB, Watnik M, Utts J, et al. Risk factors and comorbidities in primary biliary cirrhosis: a controlled interview-based study of 1032 patients. Hepatology. 2005;42:1194–1202. doi: 10.1002/hep.20907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rigamonti C, Shand LM, Feudjo M, Bunn CC, Black CM, Denton CP, et al. Clinical features and prognosis of primary biliary cirrhosis associated with systemic sclerosis. Gut. 2006;55:388–394. doi: 10.1136/gut.2005.075002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Whaley K, Goudie RB, Williamson J, Nuki G, Dick WC, Buchanan WW. Liver disease in Sjogren's syndrome and rheumatoid arthritis. Lancet. 1970;1:861–863. doi: 10.1016/s0140-6736(70)91690-9. [DOI] [PubMed] [Google Scholar]
- 52.Tsianos EV, Hoofnagle JH, Fox PC, Alspaugh M, Jones EA, Schafer DF, et al. Sjogren's syndrome in patients with primary biliary cirrhosis. Hepatology. 1990;11:730–734. doi: 10.1002/hep.1840110504. [DOI] [PubMed] [Google Scholar]
- 53.Uddenfeldt P, Danielsson A, Forssell A, Holm M, Ostberg Y. Features of Sjogren's syndrome in patients with primary biliary cirrhosis. J Intern Med. 1991;230:443–448. doi: 10.1111/j.1365-2796.1991.tb00470.x. [DOI] [PubMed] [Google Scholar]
- 54.Andonopoulos AP, Drosos AA, Skopouli FN, Acritidis NC, Moutsopoulos HM. Secondary Sjogren's syndrome in rheumatoid arthritis. J Rheumatol. 1987;14:1098–1103. [PubMed] [Google Scholar]
- 55.Lleo A, Battezzati PM, Selmi C, Gershwin ME, Podda M. Is autoimmunity a matter of sex? Autoimmun Rev. 2008;7:626–630. doi: 10.1016/j.autrev.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 56.Lockshin MD. Sex ratio and rheumatic disease. Autoimmun Rev. 2002;1:162–167. doi: 10.1016/s1568-9972(02)00034-4. [DOI] [PubMed] [Google Scholar]
- 57.Lockshin MD. Sex ratio and rheumatic disease: excerpts from an Institute of Medicine report. Lupus. 2002;11:662–666. doi: 10.1191/0961203302lu274oa. [DOI] [PubMed] [Google Scholar]
- 58.Thomas E, Hay EM, Hajeer A, Silman AJ. Sjogren's syndrome: a community-based study of prevalence and impact. Br J Rheumatol. 1998;37:1069–1076. doi: 10.1093/rheumatology/37.10.1069. [DOI] [PubMed] [Google Scholar]
- 59.Bowman SJ, Ibrahim GH, Holmes G, Hamburger J, Ainsworth JR. Estimating the prevalence among Caucasian women of primary Sjogren's syndrome in two general practices in Birmingham, UK. Scand J Rheumatol. 2004;33:39–43. doi: 10.1080/03009740310004676. [DOI] [PubMed] [Google Scholar]
- 60.Bjerrum KB. Keratoconjunctivitis sicca and primary Sjogren's syndrome in a Danish population aged 30–60 years. Acta Ophthalmol Scand. 1997;75:281–286. doi: 10.1111/j.1600-0420.1997.tb00774.x. [DOI] [PubMed] [Google Scholar]
- 61.Alamanos Y, Tsifetaki N, Voulgari PV, Venetsanopoulou AI, Siozos C, Drosos AA. Epidemiology of primary Sjogren's syndrome in north-west Greece, 1982–2003. Rheumatology (Oxford) 2006;45:187–191. doi: 10.1093/rheumatology/kei107. [DOI] [PubMed] [Google Scholar]
- 62.Plesivcnik Novljan M, Rozman B, Hocevar A, Grmek M, Kveder T, Tomsic M. Incidence of primary Sjogren's syndrome in Slovenia. Ann Rheum Dis. 2004;63:874–876. doi: 10.1136/ard.2003.014027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Helmick CG, Felson DT, Lawrence RC, Gabriel S, Hirsch R, Kwoh CK, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part I. Arthritis Rheum. 2008;58:15–25. doi: 10.1002/art.23177. [DOI] [PubMed] [Google Scholar]
- 64.Pillemer SR, Matteson EL, Jacobsson LT, Martens PB, Melton LJ, 3rd, O'Fallon WM, et al. Incidence of physician-diagnosed primary Sjogren syndrome in residents of Olmsted County, Minnesota. Mayo Clin Proc. 2001;76:593–599. doi: 10.4065/76.6.593. [DOI] [PubMed] [Google Scholar]
- 65.Zeng QY, Chen R, Darmawan J, Xiao ZY, Chen SB, Wigley R, et al. Rheumatic diseases in China. Arthritis Res Ther. 2008;10:R17. doi: 10.1186/ar2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Invernizzi P. Geoepidemiology of autoimmune liver diseases. J Autoimmun. 2010;34:J300–J306. doi: 10.1016/j.jaut.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 67.Triger DR, Berg PA, Rodes J. Epidemiology of primary biliary cirrhosis. Liver. 1984;4:195–200. doi: 10.1111/j.1600-0676.1984.tb00927.x. [DOI] [PubMed] [Google Scholar]
- 68.Lofgren J, Jarnerot G, Danielsson D, Hemdal I. Incidence and prevalence of primary biliary cirrhosis in a defined population in Sweden. Scand J Gastroenterol. 1985;20:647–650. doi: 10.3109/00365528509089711. [DOI] [PubMed] [Google Scholar]
- 69.Myszor M, James OF. The epidemiology of primary biliary cirrhosis in north-east England: an increasingly common disease? Q J Med. 1990;75:377–385. [PubMed] [Google Scholar]
- 70.Prince MI, Chetwynd A, Diggle P, Jarner M, Metcalf JV, James OF. The geographical distribution of primary biliary cirrhosis in a well-defined cohort. Hepatology. 2001;34:1083–1088. doi: 10.1053/jhep.2001.29760. [DOI] [PubMed] [Google Scholar]
- 71.Boberg KM, Aadland E, Jahnsen J, Raknerud N, Stiris M, Bell H. Incidence and prevalence of primary biliary cirrhosis, primary sclerosing cholangitis, and autoimmune hepatitis in a Norwegian population. Scand J Gastroenterol. 1998;33:99–103. doi: 10.1080/00365529850166284. [DOI] [PubMed] [Google Scholar]
- 72.Remmel T, Remmel H, Uibo R, Salupere V. Primary biliary cirrhosis in Estonia. With special reference to incidence, prevalence, clinical features, and outcome. Scand J Gastroenterol. 1995;30:367–371. doi: 10.3109/00365529509093292. [DOI] [PubMed] [Google Scholar]
- 73.Pla X, Vergara M, Gil M, Dalmau B, Cistero B, Bella RM, et al. Incidence, prevalence and clinical course of primary biliary cirrhosis in a Spanish community. Eur J Gastroenterol Hepatol. 2007;19:859–864. doi: 10.1097/MEG.0b013e328277594a. [DOI] [PubMed] [Google Scholar]
- 74.Kim WR, Lindor KD, Locke GR, 3rd, Therneau TM, Homburger HA, Batts KP, et al. Epidemiology and natural history of primary biliary cirrhosis in a US community. Gastroenterology. 2000;119:1631–1636. doi: 10.1053/gast.2000.20197. [DOI] [PubMed] [Google Scholar]
- 75.Witt-Sullivan H, Heathcote J, Cauch K, Blendis L, Ghent C, Katz A, et al. The demography of primary biliary cirrhosis in Ontario, Canada. Hepatology. 1990;12:98–105. doi: 10.1002/hep.1840120116. [DOI] [PubMed] [Google Scholar]
- 76.Myers RP, Shaheen AA, Fong A, Burak KW, Wan A, Swain MG, et al. Epidemiology and natural history of primary biliary cirrhosis in a Canadian health region: a population-based study. Hepatology. 2009;50:1884–1892. doi: 10.1002/hep.23210. [DOI] [PubMed] [Google Scholar]
- 77.Sakauchi F, Mori M, Zeniya M, Toda G. A cross-sectional study of primary biliary cirrhosis in Japan: utilization of clinical data when patients applied to receive public financial aid. J Epidemiol. 2005;15:24–28. doi: 10.2188/jea.15.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Watson RG, Angus PW, Dewar M, Goss B, Sewell RB, Smallwood RA. Low prevalence of primary biliary cirrhosis in Victoria, Australia. Melbourne Liver Group. Gut. 1995;36:927–930. doi: 10.1136/gut.36.6.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sood S, Gow PJ, Christie JM, Angus PW. Epidemiology of primary biliary cirrhosis in Victoria, Australia: high prevalence in migrant populations. Gastroenterology. 2004;127:470–475. doi: 10.1053/j.gastro.2004.04.064. [DOI] [PubMed] [Google Scholar]
- 80.Shoenfeld Y, Selmi C, Zimlichman E, Gershwin ME. The autoimmunologist: geoepidemiology, a new center of gravity, and prime time for autoimmunity. J Autoimmun. 2008;31:325–330. doi: 10.1016/j.jaut.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 81.Selmi C, Invernizzi P, Zuin M, Podda M, Gershwin ME. Genetics and geoepidemiology of primary biliary cirrhosis: following the footprints to disease etiology. Semin Liver Dis. 2005;25:265–280. doi: 10.1055/s-2005-916319. [DOI] [PubMed] [Google Scholar]
- 82.Selmi C, Mayo MJ, Bach N, Ishibashi H, Invernizzi P, Gish RG, et al. Primary biliary cirrhosis in monozygotic and dizygotic twins: genetics, epigenetics, and environment. Gastroenterology. 2004;127:485–492. doi: 10.1053/j.gastro.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 83.Invernizzi P. Human leukocyte antigen in primary biliary cirrhosis: an old story now reviving. Hepatology. 2011;54:714–723. doi: 10.1002/hep.24414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hirschfield GM, Liu X, Xu C, Lu Y, Xie G, Gu X, et al. Primary biliary cirrhosis associated with HLA, IL12A, and IL12RB2 variants. N Engl J Med. 2009;360:2544–2555. doi: 10.1056/NEJMoa0810440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liu X, Invernizzi P, Lu Y, Kosoy R, Bianchi I, Podda M, et al. Genome-wide meta-analyses identify three loci associated with primary biliary cirrhosis. Nat Genet. 2010;42:658–660. doi: 10.1038/ng.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yoshida K, Yang GX, Zhang W, Tsuda M, Tsuneyama K, Moritoki Y, et al. Deletion of interleukin-12p40 suppresses autoimmune cholangitis in dominant negative transforming growth factor beta receptor type II mice. Hepatology. 2009;50:1494–1500. doi: 10.1002/hep.23132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Padgett KA, Lan RY, Leung PC, Lleo A, Dawson K, Pfeiff J, et al. Primary biliary cirrhosis is associated with altered hepatic microRNA expression. J Autoimmun. 2009;32:246–253. doi: 10.1016/j.jaut.2009.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Selmi C. The X in sex: how autoimmune diseases revolve around sex chromosomes. Best Pract Res Clin Rheumatol. 2008;22:913–922. doi: 10.1016/j.berh.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 89.Invernizzi P, Miozzo M, Selmi C, Persani L, Battezzati PM, Zuin M, et al. X chromosome monosomy: a common mechanism for autoimmune diseases. J Immunol. 2005;175:575–578. doi: 10.4049/jimmunol.175.1.575. [DOI] [PubMed] [Google Scholar]
- 90.Miozzo M, Selmi C, Gentilin B, Grati FR, Sirchia S, Oertelt S, et al. Preferential X chromosome loss but random inactivation characterize primary biliary cirrhosis. Hepatology. 2007;46:456–462. doi: 10.1002/hep.21696. [DOI] [PubMed] [Google Scholar]
- 91.Selmi C, Gershwin ME. The role of environmental factors in primary biliary cirrhosis. Trends Immunol. 2009;30:415–420. doi: 10.1016/j.it.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 92.Bogdanos DP, Vergani D. Bacteria and primary biliary cirrhosis. Clin Rev Allergy Immunol. 2009;36:30–39. doi: 10.1007/s12016-008-8087-9. [DOI] [PubMed] [Google Scholar]
- 93.Selmi C, Balkwill DL, Invernizzi P, Ansari AA, Coppel RL, Podda M, et al. Patients with primary biliary cirrhosis react against a ubiquitous xenobiotic-metabolizing bacterium. Hepatology. 2003;38:1250–1257. doi: 10.1053/jhep.2003.50446. [DOI] [PubMed] [Google Scholar]
- 94.Rieger R, Gershwin ME. The X and why of xenobiotics in primary biliary cirrhosis. J Autoimmun. 2007;28:76–84. doi: 10.1016/j.jaut.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Meda F, Folci M, Baccarelli A, Selmi C. The epigenetics of autoimmunity. Cell Mol Immunol. 2011;8:226–236. doi: 10.1038/cmi.2010.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mitchell MM, Lleo A, Zammataro L, Mayo MJ, Invernizzi P, Bach N, et al. Epigenetic investigation of variably X chromosome inactivated genes in monozygotic female twins discordant for primary biliary cirrhosis. Epigenetics. 2011;6:95–102. doi: 10.4161/epi.6.1.13405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lu Q, Renaudineau Y, Cha S, Ilei G, Brooks WH, Selmi C, et al. Epigenetics in autoimmune disorders: highlights of the 10th Sjogren's syndrome symposium. Autoimmun Rev. 2010;9:627–630. doi: 10.1016/j.autrev.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 98.Li YZ, Hu CJ, Leng XM, Zhao GF, Li N, Xu Y. Promising diagnostic biomarkers for primary biliary cirrhosis identified with magnetic beads and MALDI-TOF-MS. Anat Rec (Hoboken) 2009;292:455–460. doi: 10.1002/ar.20870. [DOI] [PubMed] [Google Scholar]
- 99.Bowlus CL, Seeley EH, Roder J, Grigorieva J, Roder H, Caprioli RM, et al. In situ mass spectrometry of autoimmune liver diseases. Cell Mol Immunol. 2011;8:237–242. doi: 10.1038/cmi.2010.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Selmi C, Mackay IR, Gershwin ME. The autoimmunity of primary biliary cirrhosis and the clonal selection theory. Immunol Cell Biol. 2011;89:70–80. doi: 10.1038/icb.2010.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chiorini JA, Cihakova D, Ouellette CE, Caturegli P. Sjogren syndrome: advances in the pathogenesis from animal models. J Autoimmun. 2009;33:190–196. doi: 10.1016/j.jaut.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shimoda S, Harada K, Niiro H, Yoshizumi T, Soejima Y, Taketomi A, et al. Biliary epithelial cells and primary biliary cirrhosis: the role of liver-infiltrating mononuclear cells. Hepatology. 2008;47:958–965. doi: 10.1002/hep.22102. [DOI] [PubMed] [Google Scholar]
- 103.Lleo A, Selmi C, Invernizzi P, Podda M, Gershwin ME. The consequences of apoptosis in autoimmunity. J Autoimmun. 2008;31:257–262. doi: 10.1016/j.jaut.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lleo A, Selmi C, Invernizzi P, Podda M, Coppel RL, Mackay IR, et al. Apotopes and the biliary specificity of primary biliary cirrhosis. Hepatology. 2009;49:871–879. doi: 10.1002/hep.22736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lleo A, Shimoda S, Ishibashi H, Gershwin ME. Primary biliary cirrhosis and autoimmune hepatitis: apotopes and epitopes. J Gastroenterol. 2011;46(Suppl 1):29–38. doi: 10.1007/s00535-010-0303-8. [DOI] [PubMed] [Google Scholar]
- 106.Lleo A, Bowlus CL, Yang GX, Invernizzi P, Podda M, Van de Water J, et al. Biliary apotopes and anti-mitochondrial antibodies activate innate immune responses in primary biliary cirrhosis. Hepatology. 2010;52:987–998. doi: 10.1002/hep.23783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chen XM, O'Hara SP, LaRusso NF. The immunobiology of cholangiocytes. Immunol Cell Biol. 2008;86:497–505. doi: 10.1038/icb.2008.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Reynoso-Paz S, Coppel RL, Mackay IR, Bass NM, Ansari AA, Gershwin ME. The immunobiology of bile and biliary epithelium. Hepatology. 1999;30:351–357. doi: 10.1002/hep.510300218. [DOI] [PubMed] [Google Scholar]
- 109.Tanaka A, Nezu S, Uegaki S, Mikami M, Okuyama S, Kawamura N, et al. The clinical significance of IgA antimitochondrial antibodies in sera and saliva in primary biliary cirrhosis. Ann N Y Acad Sci. 2007;1107:259–270. doi: 10.1196/annals.1381.028. [DOI] [PubMed] [Google Scholar]
- 110.Reynoso-Paz S, Leung PS, Van De Water J, Tanaka A, Munoz S, Bass N, et al. Evidence for a locally driven mucosal response and the presence of mitochondrial antigens in saliva in primary biliary cirrhosis. Hepatology. 2000;31:24–29. doi: 10.1002/hep.510310106. [DOI] [PubMed] [Google Scholar]
- 111.Selmi C, De Santis M, Cavaciocchi F, Gershwin ME. Infectious agents and xenobiotics in the etiology of primary biliary cirrhosis. Dis Markers. 2010;29:287–299. doi: 10.3233/DMA-2010-0746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kong L, Ogawa N, McGuff HS, Nakabayashi T, Sakata KM, Masago R, et al. Bcl-2 family expression in salivary glands from patients with primary Sjogren's syndrome: involvement of Bax in salivary gland destruction. Clin Immunol Immunopathol. 1998;88:133–141. doi: 10.1006/clin.1998.4556. [DOI] [PubMed] [Google Scholar]
- 113.Lazaridis KN, Gores GJ, Lindor KD. Ursodeoxycholic acid 'mechanisms of action and clinical use in hepatobiliary disorders'. J Hepatol. 2001;35:134–146. doi: 10.1016/s0168-8278(01)00092-7. [DOI] [PubMed] [Google Scholar]
- 114.Gluud C, Christensen E. Ursodeoxycholic acid for primary biliary cirrhosis. Cochrane Database Syst Rev. 2002 doi: 10.1002/14651858.CD000551. CD000551. [DOI] [PubMed] [Google Scholar]
- 115.Gong Y, Huang ZB, Christensen E, Gluud C. Ursodeoxycholic acid for primary biliary cirrhosis. Cochrane Database Syst Rev. 2008 doi: 10.1002/14651858.CD000551.pub2. CD000551. [DOI] [PubMed] [Google Scholar]
- 116.Harley JB, Alarcon-Riquelme ME, Criswell LA, Jacob CO, Kimberly RP, Moser KL, et al. Genome-wide association scan in women with systemic lupus erythematosus identifies susceptibility variants in ITGAM, PXK, KIAA1542 and other loci. Nat Genet. 2008;40:204–210. doi: 10.1038/ng.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Huo AP, Lin KC, Chou CT. Predictive and prognostic value of antinuclear antibodies and rheumatoid factor in primary Sjogren's syndrome. Int J Rheum Dis. 2010;13:39–47. doi: 10.1111/j.1756-185X.2009.01444.x. [DOI] [PubMed] [Google Scholar]
- 118.Hatzis GS, Fragoulis GE, Karatzaferis A, Delladetsima I, Barbatis C, Moutsopoulos HM. Prevalence and longterm course of primary biliary cirrhosis in primary Sjogren's syndrome. J Rheumatol. 2008;35:2012–2016. [PubMed] [Google Scholar]
- 119.Marasini B, Gagetta M, Rossi V, Ferrari P. Rheumatic disorders and primary biliary cirrhosis: an appraisal of 170 Italian patients. Ann Rheum Dis. 2001;60:1046–1049. doi: 10.1136/ard.60.11.1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bournia VK, Diamanti KD, Vlachoyiannopoulos PG, Moutsopoulos HM. Anticentromere antibody positive Sjogren's Syndrome: a retrospective descriptive analysis. Arthritis Res Ther. 2010;12:R47. doi: 10.1186/ar2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Dugar M, Cox S, Limaye V, Gordon TP, Roberts-Thomson PJ. Diagnostic utility of anti-Ro52 detection in systemic autoimmunity. Postgrad Med J. 2010;86:79–82. doi: 10.1136/pgmj.2009.089656. [DOI] [PubMed] [Google Scholar]
- 122.Mavragani CP, Tzioufas AG, Moutsopoulos HM. Sjogren's syndrome: autoantibodies to cellular antigens. Clinical and molecular aspects. Int Arch Allergy Immunol. 2000;123:46–57. doi: 10.1159/000024423. [DOI] [PubMed] [Google Scholar]
- 123.Culp KS, Fleming CR, Duffy J, Baldus WP, Dickson ER. Autoimmune associations in primary biliary cirrhosis. Mayo Clin Proc. 1982;57:365–370. [PubMed] [Google Scholar]
- 124.Oertelt S, Ridgway WM, Ansari AA, Coppel RL, Gershwin ME. Murine models of primary biliary cirrhosis: Comparisons and contrasts. Hepatol Res. 2007;37(Suppl 3):S365–S369. doi: 10.1111/j.1872-034X.2007.00226.x. [DOI] [PubMed] [Google Scholar]
- 125.Lavoie TN, Lee BH, Nguyen CQ. Current concepts: mouse models of Sjogren's syndrome. J Biomed Biotechnol. 2011;2011 doi: 10.1155/2011/549107. 549107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Liu H, Liu Y, Wang L, Xu D, Lin B, Zhong R, et al. Prevalence of primary biliary cirrhosis in adults referring hospital for annual health check-up in Southern China. BMC Gastroenterol. 2010;10:100. doi: 10.1186/1471-230X-10-100. [DOI] [PMC free article] [PubMed] [Google Scholar]