Abstract
Tissue repair and regeneration are thought to involve resident cell proliferation as well as the selective recruitment of circulating stem and progenitor cell populations through complex signaling cascades. Many of these recruited cells originate from the bone marrow, and specific subpopulations of bone marrow cells have been isolated and used to augment adult tissue regeneration in preclinical models. Clinical studies of cell-based therapies have reported mixed results, however, and a variety of approaches to enhance the regenerative capacity of stem cell therapies are being developed based on emerging insights into the mechanisms of progenitor cell biology and recruitment following injury. This article discusses the function and mechanisms of recruitment of important bone marrow-derived stem and progenitor cell populations following injury, as well as the emerging therapeutic applications targeting these cells.
Keywords: endothelial progenitor cell, hematopoietic stem cell, mesenchymal stem cell, stem cell recruitment, tissue regeneration, very small embryonic-like cell
Tissue repair and regeneration following injury demand the precise orchestration of complex signaling cascades to coordinate growth of spatially proximate, but physiologically distinct structures. While this process is facilitated in many cases by proliferation, migration and differentiation of local progenitor cells, the selective recruitment of bone marrow-derived stem and progenitor cells (herein referred to as bone marrow stem cells) is also thought to play a role.
The bone marrow acts as a reservoir for multiple stem cell populations, including hematopoietic stem cells (HSCs), mesenchymal stem cells (MSCs), endothelial progenitor cells (EPCs) and very small embryonic-like cells (VSELs), which are mobilized at varying degrees into the peripheral circulation following injury [1–3]. Subsets of these cells have also demonstrated the ability to home from the circulation to a variety of experimentally injured tissues, including muscle, heart, kidney, skin, bone, liver and brain [1,3–10], where they are thought to variably contribute to tissue repair and regeneration through paracrine effects and inconsistent levels of direct differentiation [1,3,11,12].
Despite this endogenous stem cell recruitment, the inability of most adult tissue to regenerate following injury suggests that these mechanisms are easily overwhelmed. Therapies attempting to augment bone marrow stem cell involvement following insult have therefore been developed, and have shown the ability to mitigate injury and enhance the regenerative capacity of adult tissue in a variety of preclinical models [8,13–20]. Effective clinical translation of these techniques, however, has thus far lagged behind [21–23]. Poor cellular retention within the harsh injury environment, as well as the use of incompletely defined or heterogeneous cellular populations are potential limiting factors to the clinical success of stem cell therapies [21,24], which has led to ongoing studies attempting to better understand the underlying biology of stem cell recruitment, as well as to identify methods to augment stem cell survival, signaling and function.
This article discusses the role of four of the most studied bone marrow-derived stem cell populations, HSCs, MSCs, EPCs and VSELs, in endogenous and experimental tissue repair and regeneration (Figure 1). We will define these populations, explore their molecular mechanisms of mobilization and homing, identify their role within the injury microenvironement and discuss experimental methodologies to enhance their number, function and therapeutic potential.
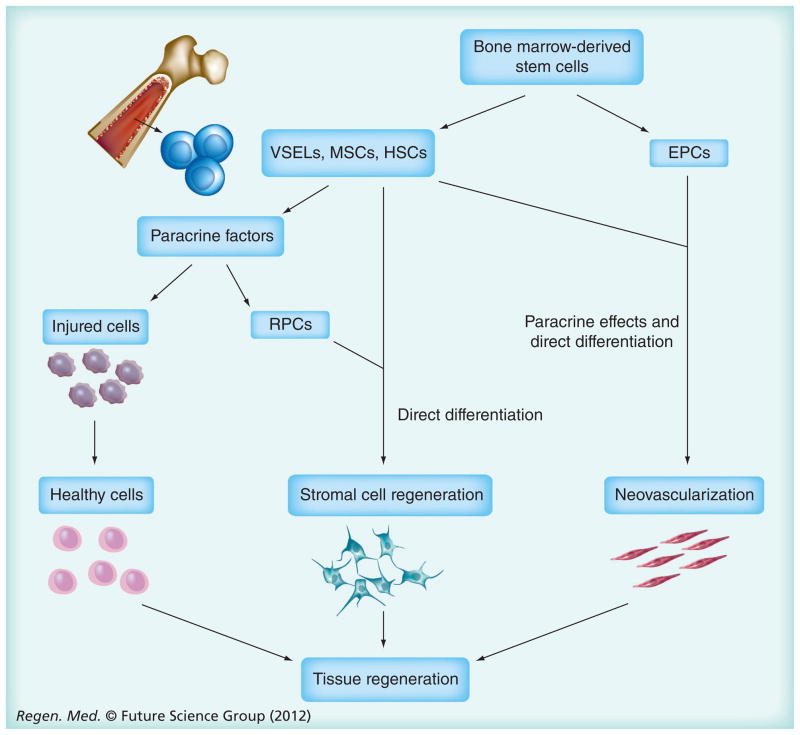
Figure 1. Proposed functions of recruited bone marrow-derived cellular subpopulations following injury.
EPCs are thought to contribute mainly to neovascularization, while VSELs, MSCs and HSCs variably support neovascularization and tissue regeneration through paracrine effects on native cell survival and RPC proliferation, as well as infrequent direct cellular differentiation.
EPC: Endothelial progenitor cell; HSC: Hematopoietic stem cell; MSC: Mesenchymal stem cell; RPC: Resident progenitor cell; VSEL: Very small embryonic-like cell.
Hematopoietic stem cells
HSCs are self-renewing, multipotent bone marrow cells that are responsible for replenishing all cellular components of the blood, including leukocytes, erythrocytes and platelets. HSCs are relatively rare, comprising approximately 0.01–0.15% of nucleated bone marrow cells [1,25], and can be further characterized based on their capacity for sustained bone marrow reconstitution (long- versus short-term HSCs). HSCs are typically isolated based on surface antigen expression, and although these profiles are constantly evolving, commonly used definitions include lack of lineage-specific markers and positivity for CD45, c-kit and/or Sca-1 (murine), or CD34 and CD133 (human) [4,26]. Combinations of cell surface receptors from the SLAM family, including CD150, CD244 and CD48, have also been used for simplified murine HSC isolation and identification within tissue sections [27], but are not equally expressed in humans [28].
Clinically, HSCs have been shown to mobilize from the bone marrow into the circulation following a variety of injuries, including myocardial infarction [29], stroke [30], liver injury [31] and skin burns [32], although their contribution to tissue repair and regeneration is uncertain. It was initially thought, based on early preclinical studies, that HSCs could help repopulate injured tissue through direct differentiation [33,34]; however, the strongest current evidence for HSC plasticity is limited to rare differentiation events within the mesodermal lineage [4]. In fact, work from our laboratory showed that HSC recruitment and engraftment within murine ischemic tissue was minor compared to changes in bone marrow-derived MSCs (BM-MSCs) [1], casting doubt on the importance of endogenous HSCs within the wound environment.
Nonetheless, delivery of exogenous HSCs may still be therapeutic, as both systemic and local injection of HSCs has been shown to ameliorate experimentally induced injuries via hematopoietic lineage (myeloid) restricted differentiation and cytokine effects [12,35].
Endothelial progenitor cells
EPCs are rare circulating cells that have the ability to incorporate into foci of neovascularization. The mechanistic contribution of these cells to de novo postnatal neovascular formation is termed vasculogenesis, and represents a paradigm shift in adult vascular biology, as neovascularization was previously thought to occur through a strictly angiogenic mechanism, (whereby pre-existing endothelial cells undergo in situ proliferation and migration to form new blood vessels) [36]. First described in 1997[37], the definition of EPCs has evolved alongside new discoveries of their lineage, resulting in two proposed subpopulations (hematopoietic and non-hematopoietic EPCs) with distinct surface marker and functional characteristics [36].
Hematopoietic EPCs (including the alternatively described early EPC and circulating angiogenic cell populations) [38,39] may represent a vasculogenic subpopulation of bone marrow-derived HSCs [36]. While a unifying cell surface antigen profile does not exist, these cells are often described as CD34 (human) or c-kit/Sca-1 (mouse) positive, with co-expression of endothelial cell markers (CD31, vWF, VEGFR2), hematopoietic lineage markers (CD45) and inconsistent expression of monocyte markers (CD14 and CD163) [39–42]. Hematopoietic EPCs secrete high levels of cytokines, including VEGF, IL-8, HGF and G-CSF, and are thought to contribute to vascular repair mainly through paracrine mechanisms [39,41], but subsets of these cells have shown the ability to directly incorporate into the endothelium [43,44].
By contrast, non-hematopoietic EPCs (including late outgrowth cells and outgrowth endothelial cells, or EOCs) do not express CD45 or monocyte markers, and show a surface marker profile more closely resembling mature endothelial cells [39–41]. Non-hematopoetic EPCs exhibit low levels of cytokine production and are thought to contribute to vascular repair mainly through the direct formation of vessels [41]. The origin of non-hematopoetic EPCs remains unclear, but it is speculated that they derive from organ blood vessels or non-hematopoietic bone marrow cells [36].
While subpopulation delineations are often not made, it is assumed that EPCs are mobilized in response to ischemic injury [29,45], and contribute to neovascularization in small animal models through a combination of direct cellular differentiation and indirect production of cytokines and growth factors (VEGF, SDF-1, and IGF-1) to promote the migration of mature endothelial cells and resident progenitor cells [3,46]. The critical role of EPCs is suggested by their dysfunction and reduced levels in clinical disease states associated with poor wound healing, such as diabetes [47,48], and the observation that EPC transplantation can ameliorate injury and improve functional outcomes in models of stroke [13], myocardial infarction [14] and acute liver and lung injury [15,16].
Mesenchymal stem cells
MSCs are multipotent, non-hematopoietic stromal cells that can be isolated from various adult organs and tissues, including bone marrow [49], adipose tissue [50], peripheral blood [51], lung [52], brain [52] and skeletal muscle [53]. MSCs are thought to reside in a perivascular niche in vivo [52,54], and are capable of differentiating into various mesenchymal lineages in vitro, including bone, muscle, cartilage and fat [49], as well as forming cells from other germ layers, such as keratinocytes and neuron-like cells [55,56]. While there is no universally accepted definition, and surface antigen expression can vary by source tissue, a list of potential criteria for human BM-MSCs includes: plastic adherence under standard culture conditions; positive expression of CD105, CD73 and CD90, with absence of lineage-specific markers and CD34; and in vitro differentiation capacity to form osteoblasts, adipocytes and chondroblasts [57]. Murine BM-MSCs share these functional characteristics, but are often isolated based on positive expression of Sca-1 and/or PDGFRα, with negative expression of hematopoietic or mature cellular markers [1,58].
BM-MSCs comprise approximately 0.001–0.08% of cells within the bone marrow [1,49], and have been shown to mobilize to the peripheral circulation following experimental injury [1,11]. Mobilized BM-MSCs home to sites of injury [1,11], where they are thought to contribute to tissue repair and regeneration mainly through paracrine support of injured cells (HGF, EGF, VEGF, sFRP-4) [59,60] and regulation of extracellular matrix remodeling [59,61,62], immune response (IL-1 antagonism, IL-10) [63,64] and local progenitor cell proliferation and differentiation [65]. Like EPCs, BM-MSCs are also thought to contribute to the restoration of vascular integrity and neovascularization following injury, as seen by their incorporation into almost 25% of new blood vessel endothelium in ischemic murine skin [1], as well as their ability to upregulate expression of pro-angiogenic factors, such as FGF, in response to environmental cues [66]. BM-MSCs have also been reported to undergo direct cellular differentiation and/or fusion to form a variety of other cell types following in vivo experimental injury, including myocardiocytes [67], kidney mesangial cells [68], osteoblasts [7], skeletal muscle cells [69] and neuron-like cells [5]; however, these events are rare, and likely less important than the aforementioned mechanisms of action.
The likely multifactorial role of BM-MSCs within the injury environment makes them especially appealing for cell-based therapies, as illustrated by their ability to support neovascularization, increase efficiency of cardiomyocyte mitochondrial oxidative phosphorylation and improve overall cardiac function in models of cardiac ischemia [67,70]. Further highlighting their therapeutic potential, transplantation of BM-MSCs has been shown to ameliorate experimental injury in almost all major organs, including the brain [17], liver [8], kidney [6] and lungs [19], and can even promote immune tolerance in tissue transplant models via cytokine activation of Tregs [71,72]. Given these diverse beneficial effects in preclinical models, an explosion of clinical trials involving BM-MSCs is currently underway to further evaluate these cells.
Very small embryonic-like cells
VSELs are a population of developmentally primitive pluripotent stem cells found in bone marrow and other adult organs [73–75]. These cells share several features typical for embryonic stem cells, including small size, a large nucleus surrounded by a narrow cytoplasmic rim, open-type chromatin and the ability to differentiate into all three germ layers [73]. VSELs comprise approximately 0.006% of all murine bone marrow cells [74], and are typically identified as being lineage- and CD45-negative, and CXCR4, Sca-1 (mouse), CD133 (human) and CD34 (human) positive [74,75]. Additionally, VSELs exhibit positive expression of pluripotency (Oct-4, SSEA-1) [73] and epiblast/germ line stem cell markers [76].
VSELs are hypothesized to be deposited in developing tissues and organs during early gastrulation, and play a role in the repopulation of more tissue specific stem cells under homeostatic conditions [77]. VSELs are also likely involved in tissue regeneration following injury, as they are mobilized into the peripheral circulation following both experimental insult and clinical cases of cardiac ischemia and stroke [2,30,78], and can improve cardiac function when delivered locally following induced myocardial infarction [20]. While a small proportion of VSELs may undergo direct cellular differentiation within the injury environment [20], their low long-term engraftment rate indicates the main beneficial effect of these cells is more likely due to paracrine mechanisms.
Mechanisms of bone marrow stem cell recruitment following injury
A complex signaling network likely underlies the selective recruitment of the aforementioned bone marrow stem cell populations following injury, which is best described for HSCs [79], but may be similar in other cell types [80,81]. Important steps in this process include cellular mobilization from the bone marrow into the circulation, homing to the injury site, vascular rolling and adhesion, endothelial transmigration and, finally, movement within the extracellular space to the injury site. Interactions of the cytokine SDF-1 with its receptor (CXCR-4) on bone marrow cells is one of the more well-described mechanisms underlying cellular mobilization and homing [82,83]; however, a variety of other molecules have been shown to affect each step of the recruitment process [12,84–87].
Cellular mobilization & homing
Under physiologic conditions, bone marrow stem cells are thought to be maintained within their niche through tightly controlled interactions of chemokines, cytokines and growth factors with cellular receptors, as well as through the presence of specific adhesion and extracellular matrix molecules [80,88]. Following injury, there is evidence that cytokine release by vascular endothelium and activated platelets, combined with local upregulation of growth factors, alters this homeostasis by providing a signal gradient for bone marrow stem cell mobilization and homing [89–91]. SDF-1 and other molecules implicated in this process are discussed below.
SDF-1
The cytokine SDF-1 is thought to play an important role in stem cell maintenance within the bone marrow, as well as cell mobilization and release following injury. SDF-1 is regulated in part by the transcription factor HIF-1α [89], and during homeostasis, SDF-1 is upregulated within discrete regions of hypoxia in the bone marrow, promoting stem cell tropism through interactions with its cellular receptor CXCR4 [83], and likely downstream modulation of adhesion molecule expression, cell proliferation and cell survival [92–94]. Following insult, SDF-1 is released by hypoxic endothelium and activated platelets at the injury site, creating a chemokine gradient that is thought to promote CXCR4-mediated bone marrow stem cell mobilization and recruitment [83,89,90]. Demonstrating the importance of this pathway, antibody blockade of SDF-1 in ischemic tissue, or CXCR4 on circulating cells, severely limits EPC recruitment to sites of experimental injury [83], and augmentation of SDF-1 expression in ischemic tissue models enhances HSC and EPC recruitment [84,95]. While the SDF-1/CXCR4 pathway is best described for HSCs and EPCs, it is also likely involved in the mobilization and recruitment BM-MSCs and VSELs, as both of these populations express CXCR4 [73,94].
Despite its demonstrated importance, the exact mechanism by which SDF-1 causes both tropism and mobilization of bone marrow stem cells is incompletely understood. There is evidence, however, that circulating SDF-1, as seen following injury, promotes cell mobilization from the bone marrow through CXCR4 receptor desensitization [83], as well as stromal cell upregulation of the protease MMP-9 [96]. Following cell mobilization, the increased binding capacity of immobilized SDF-1 found on or around ischemic blood vessels may then overcome CXCR4 desensitization to promote tissue specific adhesion and localization [83,97].
Nitric oxide
Nitric oxide (NO) is a gaseous signaling molecule that plays an important role in homeostatic vascular health. Interestingly, NO may also be involved in SDF-1/CXCR4-mediated bone marrow stem cell recruitment following injury, as endothelial nitric oxide synthase (eNOS) has been shown to increase SDF-1 expression through a cGMP-dependent mechanism in ischemic murine tissue [98], and experimental blockage of eNOS inhibits SDF-1-mediated EPC homing [84]. Additionally, eNOS has been shown to play a crucial role in progenitor cell adhesion to the vascular endothelium through an ICAM-1- and CXCR4-dependent mechanism [99].
Jagged/Notch interactions
The Notch signaling pathway plays an integral role in embryonic development, but is also active in many adult processes, including regulation of stem cell self-renewal, expansion, survival and differentiation [100–102]. Notch1 interactions with its ligand Jagged have also demonstrated importance for murine BM-MSC and EPC recruitment and therapeutic effect following ischemic injury [85,86], with knockout models having particularly deleterious effects on neovascularization. While incompletely understood, the mechanism of this effect is likely due in part to modulation of CXCR4, as Notch knockout decreases CXCR4 expression in murine BM-MSCs [86], and Notch-mediated upregulation of CXCR4 has been reported in other bone marrow-derived cells [103].
MCP-1/CCR2 interactions
MCP-1 is a chemokine that is best know for its ability to recruit monocytes following injury. However, there is also evidence that MCP-1 contributes to bone marrow stem cell recruitment, as MCP-1 binding to its receptor CCR2 is required for efficient BM-MSC homing and engraftment in a murine model of cardiac ischemia [87], and CCR2 expression is important for mobilized murine HSC trafficking to sites of inflammation [12]. This pathway is thought to act in part by stimulating chemotaxis through promotion of asymmetric lamellipodia protrusions [87], but may not be as ubiquitous as the SDF-1/CXCR4 axis, since CCR2 expression was found to be low in human EPCs [104].
Growth factors
Growth factors, such as VEGF and G-CSF, may also contribute to bone marrow stem cell mobilization and recruitment following injury, as exogenous administration of G-CSF and VEGF has been shown to enhance the mobilization of specific stem cell populations, and promote neovascularization and tissue regeneration within ischemic or traumatic injury models [105–108]. Mechanistically, G-CSF administration has been shown to promote murine HSC and EPC mobilization by reducing SDF-1 expression in the bone marrow, as well as CXCR4 expression on HSCs [106,109]. VEGF, meanwhile, has been shown to cause divergent effects on murine bone marrow populations based on receptor profiles, inhibiting HSC mobilization through VEGF receptor 1 (VEGFR1), while stimulating EPC migration and survival through VEGFR2 [106]. Further supporting an endogenous cell recruitment role for these factors, VEGF and G-CSF are upregulated following specific types of human ischemic injuries [91,110], and VEGF is known to play a crucial role in HIF-1α-induced murine adult neovascularization [111].
Cellular adhesion, endothelial transmigration & extracellular migration
Once mobilized and homed to an area of injury, a variety of molecules have been implicated in stem cell vascular rolling and adhesion, endothelial transmigration and movement within the extracellular space. These include selectins (P-selectin, E-selectin) for cell rolling [112,113], protein/integrin interactions (VCAM-1/VLA-4, ICAM-1/β2 integrin) for adhesion [112–114], chemokines (CXCL9, CXCL16, CCL20, CCL25) for transendothelial migration [115] and matrix degrading enzymes/inhibitors (MMP-2, MMP-9, tissue inhibitor of metalloproteinase-2) for cellular migration within injured tissue [116,117]. Working together, it is thought that the coordinated expression of this complex molecular network enables bone marrow stem cells to mobilize and congregate at the original site of injury, facilitating the cell-specific cytokine and direct contributions previously described.
Strategies for enhancing stem & progenitor cell involvement following injury
Despite our growing mechanistic understanding of bone marrow stem cell recruitment, the reasons behind the relatively limited endogenous cell response following major injury remain unclear. Regardless of the efficacy seen in small animal models [6,8,12–17,19,20,35], therapies to enhance stem cell involvement following injury have only had muted clinical success thus far [21]. While this discrepancy may be partially due to variations in clinical study design [118], the effects of low cellular retention seen even in small animal models [119–121] may also be exacerbated by differences in physiology and stem cell phenotype between largely divergent species [122,123]. In support of this theory, a meta-analysis of stem cell therapies in large animal models of cardiac ischemia replicated the modest therapeutic efficacy of clinical trials [124]. This same work, however, provides potential insights for the improvement of cell-based therapies, as efficacy was increased in those studies using higher cell doses and more defined populations [124]. In fact, cellular heterogeneity is becoming increasingly recognized amongst even putatively homogenous stem cell populations [125,126], making further refinements in cell characterization and purification important areas of ongoing study.
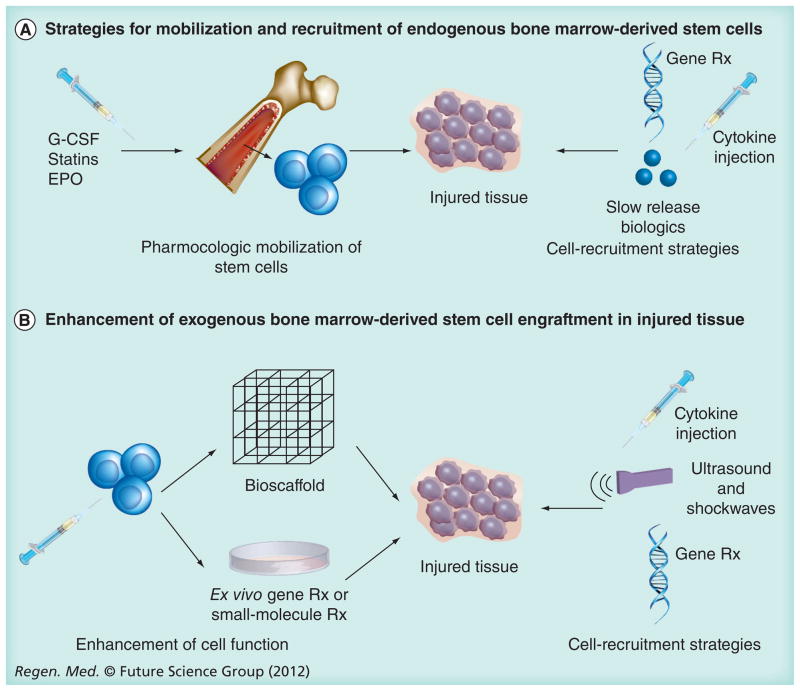
The limited clinical efficacy of this field has also led to the development of a wide range of promising preclinical techniques to enhance stem cell function following injury. Mechanistically, these approaches can be divided into two main categories: enhancement of the endogenous stem cell response and augmentation of cell-based therapies (Figure 2).
Figure 2. Stem cell enhancement strategies following injury.
Clinical trials on stem cell therapies have shown mixed efficacy, but experimental approaches targeting the endogenous cellular response (A) or enhancement of cell delivery (B) can improve stem cell function, survival and/or homing, leading to improved outcomes following injury.
EPO: Erythropoietin.
Enhancement of the endogenous stem cell response
Enhancing a patient’s endogenous stem cell response following injury is clinically appealing due to the elimination of time and costs associated with cell harvest, ex vivo processing and transplantation. A variety of experimental techniques have shown efficacy in this setting (Table 1).
Table 1.
Preclinical methods for enhancing endogenous bone marrow stem and progenitor cell response after injury.
| Molecules | Cell type | Injured tissue | Ref. |
|---|---|---|---|
| Increased cell mobilization | |||
| Modulation of SDF-1/CXCR4 axis | |||
| G-CSF | MSC, HSC, EPC, BMC | Brain, liver, artery | [5,108,129,130] |
| Plerixafor (CXCR4 antagonist) | HSC | Liver | [131] |
| Cilostazol (PDE-3 inhibitor) | EPC | Artery | [132] |
| Modulation of PI3K/Akt pathway | |||
| Statins | EPC | Heart, kidney | [133,197] |
| EPO | EPC | Artery | [134] |
| Pioglitazone (PPARγ agonist) | EPC | Subcutaneous implant | [135] |
| Increased cell homing | |||
| Local gene therapy | |||
| HIF-1α | BMC | Heart | [140] |
| SDF-1 | EPC, HSC | Heart, skeletal muscle | [84,95] |
| IGF-1 | c-kit+/CD34+ cells | Heart | [141] |
| Local injection | |||
| SDF-1 | BMC | Lung, heart | [137,138] |
| E-selectin | EPC | Skeletal muscle | [136] |
| Slow release biologics | |||
| SDF-1 | MSC, sca1+/c-kit+ cells | Heart, in vitro | [139,142,143] |
| Combined mobilization and homing | |||
| G-CSF with local SDF-1 | BMC, c-kit+ cells | Lung, heart | [137,147] |
| Substance P (mobilization) with local SDF-1 | CD29+/CD45− cells, c-kit+ cells | Skeletal muscle implant | [145] |
| G-CSF with CXCR4 antagonist with local SDF-1 | BMC | Brain | [146] |
BMC: Bone marrow-derived cell; EPC: Endothelial progenitor cell; EPO: Erythropoietin; HSC: Hematopoietic stem cell; MSC: Mesenchymal stem cell; PDE-3: Phosphodiesterase-3.
Promoting bone marrow stem cell mobilization is a common strategy to augment the cellular yield of peripheral blood apheresis for clinical stem cell transplants [127], and a similar approach has been suggested to increase the number of circulating cells available for homing following injury. In fact, a variety of compounds have shown the ability to mobilize bone marrow-derived HSCs, MSCs, EPCs and VSELs [2,38,106,128], with differential mobilization of cellular populations seen depending on the agent [106].
Selected mobilizing agents have been tested for in vivo beneficial effects following experimental injury, with modulation of the SDF-1/CXCR4 axis being the most common strategy. As discussed, G-CSF decreases SDF-1 levels in the bone marrow [109], and systemic administration of G-CSF has been shown to mobilize HSCs, EPCs and BM-MSCs, and improve outcomes in models of brain, liver and blood vessel injury [5,108,129,130]. Similarly, plerixafor (a CXCR4 antagonist) can act alone or synergistically with G-CSF to mobilize HSCs and decrease hepatic injury in a rat model of acute liver failure [131]. The dual role of the SDF-1/CXCR4 axis in bone marrow retention and peripheral recruitment creates a potential logistical problem with this approach, however, as CXCR4 blockade presumably forces mobilized cells to rely on alternative homing mechanisms to reach injured tissue.
Targeting the other side of the SDF-1/CXCR4 axis avoids this problem, as seen with oral administration of the phosphodiesterase 3 inhibitor cilostazol causing mobilization of EPCs partly through increased SDF-1 expression at the injury site [132]. Interestingly, cilostazol also upregulates the expression of CXCR4, integrin αvβ3 and VEGF in EPCs, and significantly enhances EPC-mediated inhibition of neointimal formation and acceleration of re-endothelialization following experimental arterial injury [132]. Similarly, systemic administration of agents targeting the PI3K–Akt pathway, an important mediator of cell survival and upstream modifier of eNOS, has been shown to mobilize EPCs and enhance their in vivo regenerative role [133–135], although the exact mechanism of action requires further study.
Direct amplification of the cytokine signal within injured tissue is also possible, as local injection of molecules known to be involved in stem cell homing (SDF-1, E-selectin), has been shown to enhance bone marrow cell recruitment and beneficial effects following experimental ischemic and traumatic injuries of the heart, lungs and soft tissue [136–138]. However, the short-term nature of cytokine release following injury is thought to partially limit the endogenous stem cell response [139], and local injection of quickly degraded molecules does not address this concern.
Direct- or cell-based gene therapies have therefore been used to provide more sustained transgene expression at sites of injury, and localized amplification of HIF-1α and SDF-1 gene expression has been shown to enhance bone marrow cell recruitment and improve neovascularization in ischemic injury models [84,95,140,141]. Safety concerns regarding viral vector use and regulation of transgene expression at the end of the therapeutic window may limit the translational potential of in vivo gene therapies, but SDF-1 containing slow release biologics may provide a more regulated cytokine release at the injury site [139,142,143], increasing their clinical appeal.
Despite these experimental findings, selective modulation of only one aspect of endogenous stem cell signaling may not translate to a therapeutic effect in less controlled settings, as suggested by the disappointing results of clinical trials using stem cell mobilizing agents for cardiac repair [144]. While experimental models combining local cytokine delivery with systemic mobilization have shown synergistic effects of combined treatments [137,145–147], the intrinsic constraints in endogenous stem cell number may limit the efficacy of any therapy relying solely on native cells.
Enhancement of exogenous stem cell function
The other main experimental approach to augment stem cell involvement following injury is to bolster cellular engraftment and/or function following transplantation, and a variety of cellular or injury environment modifications have shown beneficial effects (Table 2).
Table 2.
Preclinical methods for enhancing exogenous bone marrow stem and progenitor cell engraftment and function following injury.
| Molecules/methods | Cell type | Injured tissue | Ref. |
|---|---|---|---|
| Increased cell homing/engraftment | |||
| Enhancement of injured tissue homing/engraftment signal | |||
| SDF-1 local injection | EPC | Skeletal muscle | [148] |
| SDF-1 local gene therapy | EPC | Skeletal muscle | [149] |
| U/S to upregulate local SDF-1, VEGF, ICAM-1, VCAM-1 | MSC, BM-MNC | Heart | [198–201] |
| Low-energy shockwave to upregulate SDF-1 | EPC | Skeletal muscle | [150] |
| Systemic coadministration of growth factors/cytokines | |||
| G-CSF | BM-MNC | Brain, liver | [202,203] |
| HGF | BM-MNC | Liver | [204] |
| SDF-1 | BM-MNC | Liver | [205] |
| Ex vivo modulation of cell function: gene therapies | |||
| Enhancement of cell homing/function | |||
| eNOS, CXCR4 | MSC, EPC | Artery, heart | [157,158,166–169,206] |
| Enhancement of cell survival | |||
| TERT | EPC | Skeletal muscle | [207] |
| HSP-70, Bcl-2, Akt, GSK, ILK | MSC | Heart | [152–156] |
| Enhancement of cell survival/paracrine signaling | |||
| HGF | EPC | Artery | [164] |
| VEGF | EPC | Skeletal muscle | [163] |
| SDF-1 | MSC | Heart | [165] |
| IGF-1 | EPC | Heart | [208] |
| Ex vivo modulation of cell function: small molecules | |||
| Enhancement of cell survival/function | |||
| AVE9488 (eNOS enhancer) | EPC | Heart | [159] |
| PPARγ agonist | MSC | Heart | [209] |
| Inhibition of apoptosis | |||
| p38 kinase inhibitor | EPC | Skeletal muscle | [210] |
| Activation of selectins/integrins | |||
| Ephrin-B2-Fc, activating β2-integrin antibody | EPC | Skeletal muscle | [170,171] |
| Enhancement of paracrine signaling | |||
| TGF-α, estradiol | MSC | Heart | [172,173] |
| Enhancement of differentiation capacity | |||
| Angiotensin receptor blocker | MSC | Heart | [211] |
| Altered cellular microenvironment | |||
| Bioscaffolds | MSC | Heart, skin, bone | [180–184,187–189] |
| Combined approaches | |||
| Ex vivo VEGF gene therapy with local SDF-1 delivery | EPC | Heart | [174] |
| Bioscaffold with SDF-1 pretreatment | EPC | Heart | [212] |
| Bioscaffold with IL-10 gene therapy | MSC | Heart | [213] |
BM-MNC: Bone marrow-derived mononuclear cell; EPC: Endothelial progenitor cell; Ephrin-B2-Fc: Ligand for erythropoietin-producing human hepatocellular carcinoma receptor B4; MSC: Mesenchymal stem cell; U/S: Ultrasound.
Similar to studies focusing on endogenous recruitment, enhancement of SDF-1 signaling within injured tissue can also be used to augment cellular transplantation, as gene therapies, direct cytokine injection and low-energy shockwave treatments to increase SDF-1 concentration in ischemic injury models have been shown to improve the recruitment and neovascularization potential of intravenously infused EPCs [148–150].
The ex vivo modulation of cells prior to transplantation is another popular mechanism to enhance their therapeutic effect, with gene transfer and small-molecule modulation being commonly used techniques [151]. For example, the ex vivo transduction of BM-MSCs with genes encoding various kinases and anti-apoptotic proteins (e.g., Akt, Bcl-2, HSP-70, ILK and GSK-3β) has been found to improve vascularization and functional outcomes following induced myocardial infarction, likely due to enhanced BM-MSC survival [152–156]. Interestingly, GSK-3β transduction also promoted cardiomyocyte-specific BM-MSC differentiation and VEGF-independent improvement of cardiac function [153], suggesting that it may be possible to coordinate overexpression of specific genes with the promotion of organ-specific tissue regeneration.
Shifting targets, the genetic or pharmacologic (AVE9488) enhancement of eNOS signaling in EPCs has also been shown to improve transplanted cell survival and function within intimal or ischemic injury models [157–159]. While it is unclear if this effect is mediated by the previously discussed mechanisms, eNOS signaling in both damaged endothelium and EPCs is clearly important for EPC homing [84,160], making this approach particularly appealing for use in clinical disease states associated with reduced NO bioavailability, such as diabetes and coronary artery disease [161,162]. Similarly, the ex vivo transduction or small-molecule activation of growth factors, cytokines, integrins and cell receptors important for stem cell recruitment and function, such as of CXCR4, SDF-1, VEGF and HGF, has been shown to enhance transplanted BM-MSC and EPC homing and paracrine effects in ischemic or intimal injury models [163–173].
Perhaps not surprisingly, a combination of the aforementioned approaches may be even more efficacious than singularly focused therapies, as illustrated by the synergistic beneficial effects of VEGF transduction of EPCs delivered in combination with local SDF-1 injection in a murine model of peripheral ischemia [174]. Tempering the obvious potential of ex vivo manipulation for enhancing cell-based therapies, however, is the use of clinically unappealing viral vectors in many of these studies, as well as the presumably short modulatory effect of small molecules, which would need to be addressed prior to translational work.
Providing the appropriate environmental cues to delivered cells within the injury site is also thought to be a crucial aspect of tissue regeneration [175,176], and there has recently been an increased focus on alterations of the cellular microenvironment to not only enhance stem cell survival and engraftment, but also modulate cellular proliferation, paracrine activity and differentiation [177–179].
Bioscaffolds, in particular, are commonly used to control the microenvironment of exogenously delivered cells. Building upon earlier work suggesting that local delivery of BM-MSCs within a simple collagen matrix could support cellular engraftment following experimentally induced cardiac ischemia [180], more sophisticated methodologies have since utilized external BM-MSC seeding and directed collagen hydrogel contraction to form 3D cell-based constructs capable of augmenting contractile skin wound healing [181]. Additionally, BM-MSC seeding of a variety of scaffolds designed to mimic the microcomposition of native extracellular matrix has been used for the directive regeneration of a variety of tissues in vitro and in vivo, including bone [182–184], cartilage [179,185,186] and myocardium [187].
Similarly, our laboratory has shown that BM-MSC-seeded pullulan-collagen hydrogels not only improve BM-MSC survival and engraftment within the high-oxidative-stress environment of ischemic murine skin wounds, but also create a ‘stem cell niche’ that enhances cytokine secretion (VEGF, MCP-1, FGF-1 and MMPs), improves angiogenesis and accelerates wound healing [188,189].
Composite tissue & organ regeneration: an extension of stem cell therapies
The regeneration of composite tissues and organs is an obvious extension of stem cell-based therapies, but the complex cellularity and growth volume limitations in the absence of a functional perfusion system are significant barriers to the large-scale fabrication of engineered tissue. While advances in bioscaffold design have shown that spatial variance of mechanical and biochemical properties can be used to stimulate multilayer complex tissue from a single stem cell population [179,185], and the use of multiple stem cell populations can synergistically promote vascularization within engineered tissue [190,191], these constructs may still require complex vascular ingrowth when placed in vivo.
Explantable microvascular beds (EMBs) bypass these limitations by creating functional microcirculatory systems through the isolation and ex vivo manipulation of host tissue [192]. EMBs can be seeded with cells and subsequently re-planted with immediate circulatory integrity (direct vessel-to-vessel connections) [192]. Our laboratory has shown that EMBs can be maintained ex vivo for up to 24 h using a bioreactor, and intravascularly seeded with BM-MSCs, which remain viable following in vivo reimplantation [193]. Further illustrating the potential of this approach, ongoing work in our laboratory has found that EMBs seeded with BM-MSCs are also capable of directed differentiation in vivo [Gurtner GC, Unpublished Data].
Conclusion & future perspective
The evidence for endogenous bone marrow-derived stem cell contribution following injury varies by population, yet all four cell types discussed in this article have shown beneficial effects when applied to preclinical injury models. Our mechanistic understanding of this cellular behavior is rapidly evolving, and despite early clinical setbacks using cell-based therapies, advances in tissue engineering and cell manipulation have already begun to leverage our knowledge of stem cell–microenvironment interactions to enhance the regenerative potential of these cells following injury, while simultaneously laying the groundwork for neo-organ fabrication.
Looking towards the future, we expect that further characterization of bone marrow cellular mobilization, recruitment and function will continue to provide valuable insights for unlocking our innate regenerative potential, while providing additional targets for therapeutic modulation. Based on the synergism observed with the parallel use of multiple experimental manipulations [137,145–147,174], we believe that a combination of strategies, such as enhancing cell purity, intrinsic function and external microenvironment, will be the key to maximizing therapeutic effect and producing a clinically relevant therapy. Additionally, we anticipate that insights into the therapeutic action of exogenously delivered bone marrow-derived cells will be pertinent to more readily available sources of multipotent cells, such as those derived from adult adipose tissue [194–196]. The use of these alternative cell sources may accelerate the clinical translation of mesenchymal stem cell therapies by overcoming the limitation of obtaining adequate cell numbers without the need for in vitro expansion.
In summary, we believe that optimization of the fundamental mechanisms described herein has the potential to significantly increase the regenerative capacity of adult tissue following injury. As such, we expect to see the emergence of multiple clinically relevant cell-based therapies in the upcoming years, as the full potential of these cells is slowly realized.
Executive summary.
-
Tissue repair and regeneration involve resident cell proliferation, as well as the selective recruitment of stem and progenitor cell populations originating from the bone marrow:
Bone marrow stem and progenitor cell populations that are active following injury include hematopoietic and mesenchymal stem cells, endothelial progenitor cells and very small embryonic-like cells.
Recruited stem/progenitor cells are thought to promote tissue regeneration through some combination of cytokine release and direct cellular differentiation.
-
Bone marrow stem and progenitor cells are mobilized and recruited to injured tissue through complex signaling and cytokine cascades, including the important SDF-1/CXCR4 cytokine-receptor axis:
Nitric oxide, Jagged/Notch and MCP-1/CCR2 interactions, as well as various growth factors, are also likely to contribute to this process.
A variety of molecules have been implicated in bone marrow stem cell vascular rolling and adhesion, endothelial transmigration and movement within the extracellular space, enabling homed cells to congregate within sites of injury.
-
Cell-based therapies have shown the ability to augment tissue regeneration in animal models by increasing stem/progenitor cell involvement within the injury environment:
Clinical trials using stem cell therapies have shown mixed efficacy, partially due to poor cellular engraftment within the harsh injury environment.
-
Preclinical techniques augmenting endogenous or exogenous bone marrow stem cell function, survival and homing have been developed to increase stem cell engraftment and the overall regenerative effects of stem cell therapies:
The synergism observed with combined therapies is particularly applicable to translational applications.
Composite tissue and organ regeneration are natural extensions of stem cell therapies, with stem cell-seeded explantable microvascular beds showing promise for large-scale tissue engineering.
Ongoing research into the actions of endogenous stem cells should continue to provide clues for the improvement of stem cell-based therapies.
Footnotes
For reprint orders, please contact: reprints@futuremedicine.com
Financial & competing interests disclosure
This work was supported by funding from the following organizations: Institute of Diabetes, Digestive Disease Kidney Disease, Grant 2 RO1 DK074095-07; National Institute on Aging 2 RO1 AG025016; National Institute of Biomedical Imaging and Bioengineering 5 R01 EB005718-02. GC Gurtner is listed on the following patents assigned to Stanford University: Intelligent Biodegradable Pullulan Regenerative Matrix for Tissue Engineering; Efficient stem cell delivery into biomaterials using a novel capillary-driven encapsulation technique. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Hamou C, Callaghan MJ, Thangarajah H, et al. Mesenchymal stem cells can participate in ischemic neovascularization. Plast Reconstr Surg. 2009;123(2 Suppl):S45–S55. doi: 10.1097/PRS.0b013e318191be4a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kucia MJ, Wysoczynski M, Wu W, Zuba-Surma EK, Ratajczak J, Ratajczak MZ. Evidence that very small embryonic-like stem cells are mobilized into peripheral blood. Stem Cells. 2008;26(8):2083–2092. doi: 10.1634/stemcells.2007-0922. [DOI] [PubMed] [Google Scholar]
- 3.Tepper OM, Capla JM, Galiano RD, et al. Adult vasculogenesis occurs through in situ recruitment, proliferation, and tubulization of circulating bone marrow-derived cells. Blood. 2005;105(3):1068–1077. doi: 10.1182/blood-2004-03-1051. [DOI] [PubMed] [Google Scholar]
- 4.Xynos A, Corbella P, Belmonte N, Zini R, Manfredini R, Ferrari G. Bone marrow-derived hematopoietic cells undergo myogenic differentiation following a Pax-7 independent pathway. Stem Cells. 2010;28(5):965–973. doi: 10.1002/stem.418. [DOI] [PubMed] [Google Scholar]
- 5.Deng J, Zou ZM, Zhou TL, et al. Bone marrow mesenchymal stem cells can be mobilized into peripheral blood by G-CSF in vivo and integrate into traumatically injured cerebral tissue. Neurol Sci. 2011;32(4):641–651. doi: 10.1007/s10072-011-0608-2. [DOI] [PubMed] [Google Scholar]
- 6.Qian H, Yang H, Xu W, et al. Bone marrow mesenchymal stem cells ameliorate rat acute renal failure by differentiation into renal tubular epithelial-like cells. Int J Mol Med. 2008;22(3):325–332. [PubMed] [Google Scholar]
- 7.Park D, Spencer JA, Koh BI, et al. Endogenous bone marrow MSCs are dynamic, fate-restricted participants in bone maintenance and regeneration. Cell Stem Cell. 2012;10(3):259–272. doi: 10.1016/j.stem.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao W, Li JJ, Cao DY, et al. Intravenous injection of mesenchymal stem cells is effective in treating liver fibrosis. World J Gastroenterol. 2012;18(10):1048–1058. doi: 10.3748/wjg.v18.i10.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schenk S, Mal N, Finan A, et al. Monocyte chemotactic protein-3 is a myocardial mesenchymal stem cell homing factor. Stem Cells. 2007;25(1):245–251. doi: 10.1634/stemcells.2006-0293. [DOI] [PubMed] [Google Scholar]
- 10.Hamada H, Kim MK, Iwakura A, et al. Estrogen receptors α and β mediate contribution of bone marrow-derived endothelial progenitor cells to functional recovery after myocardial infarction. Circulation. 2006;114(21):2261–2270. doi: 10.1161/CIRCULATIONAHA.106.631465. [DOI] [PubMed] [Google Scholar]
- 11.Chen Y, Xiang LX, Shao JZ, et al. Recruitment of endogenous bone marrow mesenchymal stem cells towards injured liver. J Cell Mol Med. 2010;14(6B):1494–1508. doi: 10.1111/j.1582-4934.2009.00912.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Si Y, Tsou CL, Croft K, Charo IF. CCR2 mediates hematopoietic stem and progenitor cell trafficking to sites of inflammation in mice. J Clin Invest. 2010;120(4):1192–1203. doi: 10.1172/JCI40310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fan Y, Shen F, Frenzel T, et al. Endothelial progenitor cell transplantation improves long-term stroke outcome in mice. Ann Neurol. 2010;67(4):488–497. doi: 10.1002/ana.21919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schuh A, Liehn EA, Sasse A, et al. Transplantation of endothelial progenitor cells improves neovascularization and left ventricular function after myocardial infarction in a rat model. Basic Res Cardiol. 2008;103(1):69–77. doi: 10.1007/s00395-007-0685-9. [DOI] [PubMed] [Google Scholar]
- 15.Lam CF, Roan JN, Lee CH, et al. Transplantation of endothelial progenitor cells improves pulmonary endothelial function and gas exchange in rabbits with endotoxin-induced acute lung injury. Anesth Analg. 2011;112(3):620–627. doi: 10.1213/ANE.0b013e3182075da4. [DOI] [PubMed] [Google Scholar]
- 16.Nakamura T, Torimura T, Iwamoto H, et al. Prevention of liver fibrosis and liver reconstitution of DMN-treated rat liver by transplanted EPCs. Eur J Clin Invest. 2011 doi: 10.1111/j.1365-2362.2011.02637.x. [DOI] [PubMed] [Google Scholar]
- 17.Borlongan CV, Glover LE, Tajiri N, Kaneko Y, Freeman TB. The great migration of bone marrow-derived stem cells toward the ischemic brain: therapeutic implications for stroke and other neurological disorders. Prog Neurobiol. 2011;95(2):213–228. doi: 10.1016/j.pneurobio.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hara Y, Stolk M, Ringe J, et al. In vivo effect of bone marrow-derived mesenchymal stem cells in a rat kidney transplantation model with prolonged cold ischemia. Transpl Int. 2011;24(11):1112–1123. doi: 10.1111/j.1432-2277.2011.01328.x. [DOI] [PubMed] [Google Scholar]
- 19.Pati S, Gerber MH, Menge TD, et al. Bone marrow derived mesenchymal stem cells inhibit inflammation and preserve vascular endothelial integrity in the lungs after hemorrhagic shock. PLoS One. 2011;6(9):e25171. doi: 10.1371/journal.pone.0025171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dawn B, Tiwari S, Kucia MJ, et al. Transplantation of bone marrow-derived very small embryonic-like stem cells attenuates left ventricular dysfunction and remodeling after myocardial infarction. Stem Cells. 2008;26(6):1646–1655. doi: 10.1634/stemcells.2007-0715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21▪.Malliaras K, Kreke M, Marban E. The stuttering progress of cell therapy for heart disease. Clin Pharmacol Ther. 2011;90(4):532–541. doi: 10.1038/clpt.2011.175. Review of clinical trials using stem cell therapies for cardiac disease. [DOI] [PubMed] [Google Scholar]
- 22.Lee JW, Fang X, Krasnodembskaya A, Howard JP, Matthay MA. Concise review. Mesenchymal stem cells for acute lung injury: role of paracrine soluble factors. Stem Cells. 2011;29(6):913–919. doi: 10.1002/stem.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jablonska A, Lukomska B. Stroke induced brain changes: implications for stem cell transplantation. Acta Neurobiol Exp (Wars ) 2011;71(1):74–85. doi: 10.55782/ane-2011-1824. [DOI] [PubMed] [Google Scholar]
- 24.Phinney DD. Functional heterogeneity of mesenchymal stem cells: implications for cell therapy. J Cell Biochem. 2012;113(9):2806–2812. doi: 10.1002/jcb.24166. [DOI] [PubMed] [Google Scholar]
- 25.Challen GA, Boles N, Lin KK, Goodell MA. Mouse hematopoietic stem cell identification and analysis. Cytometry A. 2009;75(1):14–24. doi: 10.1002/cyto.a.20674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ratajczak MZ. Phenotypic and functional characterization of hematopoietic stem cells. Curr Opin Hematol. 2008;15(4):293–300. doi: 10.1097/MOH.0b013e328302c7ca. [DOI] [PubMed] [Google Scholar]
- 27.Kiel MJ, Yilmaz OH, Iwashita T, Terhorst C, Morrison SJ. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121(7):1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 28.Larochelle A, Savona M, Wiggins M, et al. Human and rhesus macaque hematopoietic stem cells cannot be purified based only on SLAM family markers. Blood. 2011;117(5):1550–1554. doi: 10.1182/blood-2009-03-212803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Massa M, Rosti V, Ferrario M, et al. Increased circulating hematopoietic and endothelial progenitor cells in the early phase of acute myocardial infarction. Blood. 2005;105(1):199–206. doi: 10.1182/blood-2004-05-1831. [DOI] [PubMed] [Google Scholar]
- 30.Paczkowska E, Kucia M, Koziarska D, et al. Clinical evidence that very small embryonic-like stem cells are mobilized into peripheral blood in patients after stroke. Stroke. 2009;40(4):1237–1244. doi: 10.1161/STROKEAHA.108.535062. [DOI] [PubMed] [Google Scholar]
- 31.Gehling UM, Willems M, Schlagner K, et al. Mobilization of hematopoietic progenitor cells in patients with liver cirrhosis. World J Gastroenterol. 2010;16(2):217–224. doi: 10.3748/wjg.v16.i2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Drukala J, Paczkowska E, Kucia M, et al. Stem cells, including a population of very small embryonic-like stem cells, are mobilized into peripheral blood in patients after skin burn injury. Stem Cell Rev. 2012;8(1):184–194. doi: 10.1007/s12015-011-9272-4. [DOI] [PubMed] [Google Scholar]
- 33.Orlic D, Kajstura J, Chimenti S, Bodine DM, Leri A, Anversa P. Transplanted adult bone marrow cells repair myocardial infarcts in mice. Ann N Y Acad Sci. 2001;938:221–229. doi: 10.1111/j.1749-6632.2001.tb03592.x. discussion 229–230. [DOI] [PubMed] [Google Scholar]
- 34.Lin F, Cordes K, Li L, et al. Hematopoietic stem cells contribute to the regeneration of renal tubules after renal ischemia-reperfusion injury in mice. J Am Soc Nephrol. 2003;14(5):1188–1199. doi: 10.1097/01.asn.0000061595.28546.a0. [DOI] [PubMed] [Google Scholar]
- 35.Balsam LB, Wagers AJ, Christensen JL, Kofidis T, Weissman IL, Robbins RC. Haematopoietic stem cells adopt mature haematopoietic fates in ischaemic myocardium. Nature. 2004;428(6983):668–673. doi: 10.1038/nature02460. [DOI] [PubMed] [Google Scholar]
- 36.Asahara T, Kawamoto A, Masuda H. Concise review: circulating endothelial progenitor cells for vascular medicine. Stem Cells. 2011;29(11):1650–1655. doi: 10.1002/stem.745. [DOI] [PubMed] [Google Scholar]
- 37▪.Asahara T, Murohara T, Sullivan A, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275(5302):964–967. doi: 10.1126/science.275.5302.964. First paper to describe endothelial progenitor cells. [DOI] [PubMed] [Google Scholar]
- 38.Shepherd RM, Capoccia BJ, Devine SM, et al. Angiogenic cells can be rapidly mobilized and efficiently harvested from the blood following treatment with AMD3100. Blood. 2006;108(12):3662–3667. doi: 10.1182/blood-2006-06-030577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yoon CH, Hur J, Park KW, et al. Synergistic neovascularization by mixed transplantation of early endothelial progenitor cells and late outgrowth endothelial cells: the role of angiogenic cytokines and matrix metalloproteinases. Circulation. 2005;112(11):1618–1627. doi: 10.1161/CIRCULATIONAHA.104.503433. [DOI] [PubMed] [Google Scholar]
- 40.Timmermans F, Van Hauwermeiren F, De Smedt M, et al. Endothelial outgrowth cells are not derived from CD133+ cells or CD45+ hematopoietic precursors. Arterioscler Thromb Vasc Biol. 2007;27(7):1572–1579. doi: 10.1161/ATVBAHA.107.144972. [DOI] [PubMed] [Google Scholar]
- 41.Yoder MC, Mead LE, Prater D, et al. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood. 2007;109(5):1801–1809. doi: 10.1182/blood-2006-08-043471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Timmermans F, Plum J, Yoder MC, Ingram DA, Vandekerckhove B, Case J. Endothelial progenitor cells: identity defined? J Cell Mol Med. 2009;13(1):87–102. doi: 10.1111/j.1582-4934.2008.00598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bailey AS, Willenbring H, Jiang S, et al. Myeloid lineage progenitors give rise to vascular endothelium. Proc Natl Acad Sci USA. 2006;103(35):13156–13161. doi: 10.1073/pnas.0604203103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Masuda H, Alev C, Akimaru H, et al. Methodological development of a clonogenic assay to determine endothelial progenitor cell potential. Circ Res. 2011;109(1):20–37. doi: 10.1161/CIRCRESAHA.110.231837. [DOI] [PubMed] [Google Scholar]
- 45.Sandri M, Beck EB, Adams V, et al. Maximal exercise, limb ischemia, and endothelial progenitor cells. Eur J Cardiovasc Prev Rehabil. 2011;18(1):55–64. doi: 10.1097/HJR.0b013e32833ba654. [DOI] [PubMed] [Google Scholar]
- 46.Urbich C, Aicher A, Heeschen C, et al. Soluble factors released by endothelial progenitor cells promote migration of endothelial cells and cardiac resident progenitor cells. J Mol Cell Cardiol. 2005;39(5):733–742. doi: 10.1016/j.yjmcc.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 47.Fadini GP, Miorin M, Facco M, et al. Circulating endothelial progenitor cells are reduced in peripheral vascular complications of type 2 diabetes mellitus. J Am Coll Cardiol. 2005;45(9):1449–1457. doi: 10.1016/j.jacc.2004.11.067. [DOI] [PubMed] [Google Scholar]
- 48.Sorrentino SA, Bahlmann FH, Besler C, et al. Oxidant stress impairs in vivo reendothelialization capacity of endothelial progenitor cells from patients with type 2 diabetes mellitus: restoration by the peroxisome proliferator-activated receptor-γ agonist rosiglitazone. Circulation. 2007;116(2):163–173. doi: 10.1161/CIRCULATIONAHA.106.684381. [DOI] [PubMed] [Google Scholar]
- 49.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 50▪.Zuk PA, Zhu M, Mizuno H, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7(2):211–228. doi: 10.1089/107632701300062859. First paper to describe isolation of mesenchymal stem cells from adipose tissue. [DOI] [PubMed] [Google Scholar]
- 51.Chong PP, Selvaratnam L, Abbas AA, Kamarul T. Human peripheral blood derived mesenchymal stem cells demonstrate similar characteristics and chondrogenic differentiation potential to bone marrow derived mesenchymal stem cells. J Orthop Res. 2012;30(4):634–642. doi: 10.1002/jor.21556. [DOI] [PubMed] [Google Scholar]
- 52.Da Silva Meirelles L, Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci. 2006;119(Pt 11):2204–2213. doi: 10.1242/jcs.02932. [DOI] [PubMed] [Google Scholar]
- 53.Dodson MV, Hausman GJ, Guan L, et al. Skeletal muscle stem cells from animals I. Basic cell biology. Int J Biol Sci. 2010;6(5):465–474. doi: 10.7150/ijbs.6.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Feng J, Mantesso A, Sharpe PT. Perivascular cells as mesenchymal stem cells. Expert Opin Biol Ther. 2010;10(10):1441–1451. doi: 10.1517/14712598.2010.517191. [DOI] [PubMed] [Google Scholar]
- 55.Sasaki M, Abe R, Fujita Y, Ando S, Inokuma D, Shimizu H. Mesenchymal stem cells are recruited into wounded skin and contribute to wound repair by transdifferentiation into multiple skin cell type. J Immunol. 2008;180(4):2581–2587. doi: 10.4049/jimmunol.180.4.2581. [DOI] [PubMed] [Google Scholar]
- 56.Bae KS, Park JB, Kim HS, Kim DS, Park DJ, Kang SJ. Neuron-like differentiation of bone marrow-derived mesenchymal stem cells. Yonsei Med J. 2011;52(3):401–412. doi: 10.3349/ymj.2011.52.3.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 58.Morikawa S, Mabuchi Y, Kubota Y, et al. Prospective identification, isolation, and systemic transplantation of multipotent mesenchymal stem cells in murine bone marrow. J Exp Med. 2009;206(11):2483–2496. doi: 10.1084/jem.20091046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nguyen BK, Maltais S, Perrault LP, et al. Improved function and myocardial repair of infarcted heart by intracoronary injection of mesenchymal stem cell-derived growth factors. J Cardiovasc Transl Res. 2010;3(5):547–558. doi: 10.1007/s12265-010-9171-0. [DOI] [PubMed] [Google Scholar]
- 60.Katsha AM, Ohkouchi S, Xin H, et al. Paracrine factors of multipotent stromal cells ameliorate lung injury in an elastase-induced emphysema model. Mol Ther. 2011;19(1):196–203. doi: 10.1038/mt.2010.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xu X, Xu Z, Xu Y, Cui G. Selective down-regulation of extracellular matrix gene expression by bone marrow derived stem cell transplantation into infarcted myocardium. Circ J. 2005;69(10):1275–1283. doi: 10.1253/circj.69.1275. [DOI] [PubMed] [Google Scholar]
- 62.Xu X, Xu Z, Xu Y, Cui G. Effects of mesenchymal stem cell transplantation on extracellular matrix after myocardial infarction in rats. Coron Artery Dis. 2005;16(4):245–255. doi: 10.1097/00019501-200506000-00006. [DOI] [PubMed] [Google Scholar]
- 63.Ortiz LA, Dutreil M, Fattman C, et al. Interleukin 1 receptor antagonist mediates the antiinflammatory and antifibrotic effect of mesenchymal stem cells during lung injury. Proc Natl Acad Sci USA. 2007;104(26):11002–11007. doi: 10.1073/pnas.0704421104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dayan V, Yannarelli G, Billia F, et al. Mesenchymal stromal cells mediate a switch to alternatively activated monocytes/macrophages after acute myocardial infarction. Basic Res Cardiol. 2011;106(6):1299–1310. doi: 10.1007/s00395-011-0221-9. [DOI] [PubMed] [Google Scholar]
- 65.Hatzistergos KE, Quevedo H, Oskouei BN, et al. Bone marrow mesenchymal stem cells stimulate cardiac stem cell proliferation and differentiation. Circ Res. 2010;107(7):913–922. doi: 10.1161/CIRCRESAHA.110.222703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kasper G, Dankert N, Tuischer J, et al. Mesenchymal stem cells regulate angiogenesis according to their mechanical environment. Stem Cells. 2007;25(4):903–910. doi: 10.1634/stemcells.2006-0432. [DOI] [PubMed] [Google Scholar]
- 67.Li Z, Guo J, Chang Q, Zhang A. Paracrine role for mesenchymal stem cells in acute myocardial infarction. Biol Pharm Bull. 2009;32(8):1343–1346. doi: 10.1248/bpb.32.1343. [DOI] [PubMed] [Google Scholar]
- 68.Wong CY, Cheong SK, Mok PL, Leong CF. Differentiation of human mesenchymal stem cells into mesangial cells in post-glomerular injury murine model. Pathology. 2008;40(1):52–57. doi: 10.1080/00313020701716367. [DOI] [PubMed] [Google Scholar]
- 69.De La Garza-Rodea AS, Van Der Velde I, Boersma H, et al. Long-term contribution of human bone marrow mesenchymal stromal cells to skeletal muscle regeneration in mice. Cell Transplant. 2011;20(2):217–231. doi: 10.3727/096368910X522117. [DOI] [PubMed] [Google Scholar]
- 70.Hughey CC, Johnsen VL, Ma L, et al. Mesenchymal stem cell transplantation for the infarcted heart: a role in minimizing abnormalities in cardiac-specific energy metabolism. Am J Physiol Endocrinol Metab. 2012;302(2):E163–E172. doi: 10.1152/ajpendo.00443.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jui HY, Lin CH, Hsu WT, et al. Autologous mesenchymal stem cells prevent transplant arteriosclerosis by enhancing local expression of interleukin-10, interferon-γ, and indoleamine 2,3-dioxygenase. Cell Transplant. 2012;21(5):971–984. doi: 10.3727/096368911X627525. [DOI] [PubMed] [Google Scholar]
- 72.Ge W, Jiang J, Arp J, Liu W, Garcia B, Wang H. Regulatory T-cell generation and kidney allograft tolerance induced by mesenchymal stem cells associated with indoleamine 2,3-dioxygenase expression. Transplantation. 2010;90(12):1312–1320. doi: 10.1097/TP.0b013e3181fed001. [DOI] [PubMed] [Google Scholar]
- 73▪.Kucia M, Reca R, Campbell FR, et al. A population of very small embryonic-like (VSEL) CXCR4+SSEA-1+Oct-4+ stem cells identified in adult bone marrow. Leukemia. 2006;20(5):857–869. doi: 10.1038/sj.leu.2404171. First paper to describe very small embryonic-like stem cells. [DOI] [PubMed] [Google Scholar]
- 74.Zuba-Surma EK, Kucia M, Wu W, et al. Very small embryonic-like stem cells are present in adult murine organs: ImageStream-based morphological analysis and distribution studies. Cytometry A. 2008;73A(12):1116–1127. doi: 10.1002/cyto.a.20667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sovalat H, Scrofani M, Eidenschenk A, Pasquet S, Rimelen V, Henon P. Identification and isolation from either adult human bone marrow or G-CSF-mobilized peripheral blood of CD34+/CD133+/CXCR4+/Lin-CD45− cells, featuring morphological, molecular, and phenotypic characteristics of very small embryonic-like (VSEL) stem cells. Exp Hematol. 2011;39(4):495–505. doi: 10.1016/j.exphem.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 76.Shin DM, Liu R, Klich I, et al. Molecular signature of adult bone marrow-purified very small embryonic-like stem cells supports their developmental epiblast/germ line origin. Leukemia. 2010;24(8):1450–1461. doi: 10.1038/leu.2010.121. [DOI] [PubMed] [Google Scholar]
- 77.Ratajczak MZ, Zuba-Surma EK, Shin DM, Ratajczak J, Kucia M. Very small embryonic-like (VSEL) stem cells in adult organs and their potential role in rejuvenation of tissues and longevity. Exp Gerontol. 2008;43(11):1009–1017. doi: 10.1016/j.exger.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wojakowski W, Tendera M, Kucia M, et al. Mobilization of bone marrow-derived Oct-4+ SSEA-4+ very small embryonic-like stem cells in patients with acute myocardial infarction. J Am Coll Cardiol. 2009;53(1):1–9. doi: 10.1016/j.jacc.2008.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kavanagh DP, Kalia N. Hematopoietic stem cell homing to injured tissues. Stem Cell Rev. 2011;7(3):672–682. doi: 10.1007/s12015-011-9240-z. [DOI] [PubMed] [Google Scholar]
- 80.Chen FM, Wu LA, Zhang M, Zhang R, Sun HH. Homing of endogenous stem/progenitor cells for in situ tissue regeneration: promises, strategies, and translational perspectives. Biomaterials. 2011;32(12):3189–3209. doi: 10.1016/j.biomaterials.2010.12.032. [DOI] [PubMed] [Google Scholar]
- 81.Chavakis E, Urbich C, Dimmeler S. Homing and engraftment of progenitor cells: a prerequisite for cell therapy. J Mol Cell Cardiol. 2008;45(4):514–522. doi: 10.1016/j.yjmcc.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 82.Abbott JD, Huang Y, Liu D, Hickey R, Krause DS, Giordano FJ. Stromal cell-derived factor-1α plays a critical role in stem cell recruitment to the heart after myocardial infarction but is not sufficient to induce homing in the absence of injury. Circulation. 2004;110(21):3300–3305. doi: 10.1161/01.CIR.0000147780.30124.CF. [DOI] [PubMed] [Google Scholar]
- 83▪▪.Ceradini DJ, Kulkarni AR, Callaghan MJ, et al. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nature Med. 2004;10(8):858–864. doi: 10.1038/nm1075. One of the first reports that recruitment of CXCR4-positive progenitor cells to regenerating tissues is mediated by hypoxic gradients via HIF-1-induced expression of SDF-1. [DOI] [PubMed] [Google Scholar]
- 84.Hiasa K, Ishibashi M, Ohtani K, et al. Gene transfer of stromal cell-derived factor-1α enhances ischemic vasculogenesis and angiogenesis via vascular endothelial growth factor/endothelial nitric oxide synthase-related pathway: next-generation chemokine therapy for therapeutic neovascularization. Circulation. 2004;109(20):2454–2461. doi: 10.1161/01.CIR.0000128213.96779.61. [DOI] [PubMed] [Google Scholar]
- 85.Kwon SM, Eguchi M, Wada M, et al. Specific Jagged-1 signal from bone marrow microenvironment is required for endothelial progenitor cell development for neovascularization. Circulation. 2008;118(2):157–165. doi: 10.1161/CIRCULATIONAHA.107.754978. [DOI] [PubMed] [Google Scholar]
- 86.Li Y, Hiroi Y, Ngoy S, et al. Notch1 in bone marrow-derived cells mediates cardiac repair after myocardial infarction. Circulation. 2011;123(8):866–876. doi: 10.1161/CIRCULATIONAHA.110.947531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Belema-Bedada F, Uchida S, Martire A, Kostin S, Braun T. Efficient homing of multipotent adult mesenchymal stem cells depends on FROUNT-mediated clustering of CCR2. Cell Stem Cell. 2008;2(6):566–575. doi: 10.1016/j.stem.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 88.Discher DE, Mooney DJ, Zandstra PW. Growth factors, matrices, and forces combine and control stem cells. Science. 2009;324(5935):1673–1677. doi: 10.1126/science.1171643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Youn SW, Lee SW, Lee J, et al. COMP-Ang1 stimulates HIF-1α-mediated SDF-1 overexpression and recovers ischemic injury through BM-derived progenitor cell recruitment. Blood. 2011;117(16):4376–4386. doi: 10.1182/blood-2010-07-295964. [DOI] [PubMed] [Google Scholar]
- 90.Massberg S, Konrad I, Schurzinger K, et al. Platelets secrete stromal cell-derived factor 1α and recruit bone marrow-derived progenitor cells to arterial thrombiin vivo. J Exp Med. 2006;203(5):1221–1233. doi: 10.1084/jem.20051772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Brandao D, Costa C, Canedo A, Vaz G, Pignatelli D. Endogenous vascular endothelial growth factor and angiopoietin-2 expression in critical limb ischemia. Int Angiol. 2011;30(1):25–34. [PubMed] [Google Scholar]
- 92.Hidalgo A, Sanz-Rodriguez F, Rodriguez-Fernandez JL, et al. Chemokine stromal cell-derived factor-1α modulates VLA-4 integrin-dependent adhesion to fibronectin and VCAM-1 on bone marrow hematopoietic progenitor cells. Exp Hematol. 2001;29(3):345–355. doi: 10.1016/s0301-472x(00)00668-8. [DOI] [PubMed] [Google Scholar]
- 93.Lataillade JJ, Clay D, Dupuy C, et al. Chemokine SDF-1 enhances circulating CD34+ cell proliferation in synergy with cytokines: possible role in progenitor survival. Blood. 2000;95(3):756–768. [PubMed] [Google Scholar]
- 94.Liu X, Duan B, Cheng Z, et al. SDF-1/CXCR4 axis modulates bone marrow mesenchymal stem cell apoptosis, migration and cytokine secretion. Protein Cell. 2011;2(10):845–854. doi: 10.1007/s13238-011-1097-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tang YL, Qian K, Zhang YC, Shen L, Phillips MI. Mobilizing of haematopoietic stem cells to ischemic myocardium by plasmid mediated stromal-cell-derived factor-1alpha (SDF-1alpha) treatment. Regul Pept. 2005;125(1–3):1–8. doi: 10.1016/j.regpep.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 96.Heissig B, Hattori K, Dias S, et al. Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9 mediated release of kit-ligand. Cell. 2002;109(5):625–637. doi: 10.1016/s0092-8674(02)00754-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Peled A, Grabovsky V, Habler L, et al. The chemokine SDF-1 stimulates integrin-mediated arrest of CD34+ cells on vascular endothelium under shear flow. J Clin Invest. 1999;104(9):1199–1211. doi: 10.1172/JCI7615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Li N, Lu X, Zhao X, et al. Endothelial nitric oxide synthase promotes bone marrow stromal cell migration to the ischemic myocardium via upregulation of stromal cell-derived factor-1alpha. Stem Cells. 2009;27(4):961–970. doi: 10.1002/stem.6. [DOI] [PubMed] [Google Scholar]
- 99.Kaminski A, Ma N, Donndorf P, et al. Endothelial NOS is required for SDF-1α/CXCR4-mediated peripheral endothelial adhesion of c-kit+ bone marrow stem cells. Lab Invest. 2008;88(1):58–69. doi: 10.1038/labinvest.3700693. [DOI] [PubMed] [Google Scholar]
- 100.Varnum-Finney B, Xu L, Brashem-Stein C, et al. Pluripotent, cytokine-dependent, hematopoietic stem cells are immortalized by constitutive Notch1 signaling. Nat Med. 2000;6(11):1278–1281. doi: 10.1038/81390. [DOI] [PubMed] [Google Scholar]
- 101.Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433(7027):760–764. doi: 10.1038/nature03260. [DOI] [PubMed] [Google Scholar]
- 102.Mizutani K, Yoon K, Dang L, Tokunaga A, Gaiano N. Differential notch signalling distinguishes neural stem cells from intermediate progenitors. Nature. 2007;449(7160):351–355. doi: 10.1038/nature06090. [DOI] [PubMed] [Google Scholar]
- 103.Wang YC, Hu XB, He F, et al. Lipopolysaccharide-induced maturation of bone marrow-derived dendritic cells is regulated by notch signaling through the up-regulation of CXCR4. J Biol Chem. 2009;284(23):15993–16003. doi: 10.1074/jbc.M901144200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Walenta KL, Bettink S, Bohm M, Friedrich EB. Differential chemokine receptor expression regulates functional specialization of endothelial progenitor cell subpopulations. Basic Res Cardiol. 2011;106(2):299–305. doi: 10.1007/s00395-010-0142-z. [DOI] [PubMed] [Google Scholar]
- 105.Hattori K, Dias S, Heissig B, et al. Vascular endothelial growth factor and angiopoietin-1 stimulate postnatal hematopoiesis by recruitment of vasculogenic and hematopoietic stem cells. J Exp Med. 2001;193(9):1005–1014. doi: 10.1084/jem.193.9.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Pitchford SC, Furze RC, Jones CP, Wengner AM, Rankin SM. Differential mobilization of subsets of progenitor cells from the bone marrow. Cell Stem Cell. 2009;4(1):62–72. doi: 10.1016/j.stem.2008.10.017. [DOI] [PubMed] [Google Scholar]
- 107.Hopkins SP, Bulgrin JP, Sims RL, Bowman B, Donovan DL, Schmidt SP. Controlled delivery of vascular endothelial growth factor promotes neovascularization and maintains limb function in a rabbit model of ischemia. J Vasc Surg. 1998;27(5):886–894. doi: 10.1016/s0741-5214(98)70269-1. discussion 895. [DOI] [PubMed] [Google Scholar]
- 108.Wu X, Wang K, Cui L, et al. Effects of granulocyte-colony stimulating factor on the repair of balloon-injured arteries. Pathology. 2008;40(5):513–519. doi: 10.1080/00313020802197947. [DOI] [PubMed] [Google Scholar]
- 109.Levesque JP, Hendy J, Takamatsu Y, Simmons PJ, Bendall LJ. Disruption of the CXCR4/CXCL12 chemotactic interaction during hematopoietic stem cell mobilization induced by GCSF or cyclophosphamide. J Clin Invest. 2003;111(2):187–196. doi: 10.1172/JCI15994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hasselblatt M, Jeibmann A, Riesmeier B, Maintz D, Schabitz WR. Granulocyte-colony stimulating factor (G-CSF) and G-CSF receptor expression in human ischemic stroke. Acta Neuropathol. 2007;113(1):45–51. doi: 10.1007/s00401-006-0152-y. [DOI] [PubMed] [Google Scholar]
- 111.Oladipupo S, Hu S, Kovalski J, et al. VEGF is essential for hypoxia-inducible factor-mediated neovascularization but dispensable for endothelial sprouting. Proc Natl Acad Sci USA. 2011;108(32):13264–13269. doi: 10.1073/pnas.1101321108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Thankamony SP, Sackstein R. Enforced hematopoietic cell E- and L-selectin ligand (HCELL) expression primes transendothelial migration of human mesenchymal stem cells. Proc Natl Acad Sci USA. 2011;108(6):2258–2263. doi: 10.1073/pnas.1018064108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ruster B, Gottig S, Ludwig RJ, et al. Mesenchymal stem cells display coordinated rolling and adhesion behavior on endothelial cells. Blood. 2006;108(12):3938–3944. doi: 10.1182/blood-2006-05-025098. [DOI] [PubMed] [Google Scholar]
- 114.Yoon CH, Hur J, Oh IY, et al. Intercellular adhesion molecule-1 is upregulated in ischemic muscle, which mediates trafficking of endothelial progenitor cells. Arterioscler Thromb Vasc Biol. 2006;26(5):1066–1072. doi: 10.1161/01.ATV.0000215001.92941.6c. [DOI] [PubMed] [Google Scholar]
- 115.Chamberlain G, Smith H, Rainger GE, Middleton J. Mesenchymal stem cells exhibit firm adhesion, crawling, spreading and transmigration across aortic endothelial cells: effects of chemokines and shear. PLoS One. 2011;6(9):e25663. doi: 10.1371/journal.pone.0025663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tondreau T, Meuleman N, Stamatopoulos B, et al. In vitro study of matrix metalloproteinase/tissue inhibitor of metalloproteinase production by mesenchymal stromal cells in response to inflammatory cytokines: the role of their migration in injured tissues. Cytotherapy. 2009;11(5):559–569. doi: 10.1080/14653240903051541. [DOI] [PubMed] [Google Scholar]
- 117.Ries C, Egea V, Karow M, Kolb H, Jochum M, Neth P. MMP-2, MT1-MMP, and TIMP-2 are essential for the invasive capacity of human mesenchymal stem cells: differential regulation by inflammatory cytokines. Blood. 2007;109(9):4055–4063. doi: 10.1182/blood-2006-10-051060. [DOI] [PubMed] [Google Scholar]
- 118.Hoover-Plow J, Gong Y. Challenges for heart disease stem cell therapy. Vasc Health Risk Manag. 2012;8:99–113. doi: 10.2147/VHRM.S25665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Teng CJ, Luo J, Chiu RC, Shum-Tim D. Massive mechanical loss of microspheres with direct intramyocardial injection in the beating heart: implications for cellular cardiomyoplasty. J Thorac Cardiovasc Surg. 2006;132(3):628–632. doi: 10.1016/j.jtcvs.2006.05.034. [DOI] [PubMed] [Google Scholar]
- 120.Iso Y, Spees JL, Serrano C, et al. Multipotent human stromal cells improve cardiac function after myocardial infarction in mice without long-term engraftment. Biochem Biophys Res Commun. 2007;354(3):700–706. doi: 10.1016/j.bbrc.2007.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Burst VR, Gillis M, Putsch F, et al. Poor cell survival limits the beneficial impact of mesenchymal stem cell transplantation on acute kidney injury. Nephron Exp Nephrol. 2010;114(3):E107–E116. doi: 10.1159/000262318. [DOI] [PubMed] [Google Scholar]
- 122.Demetrius L. Of mice and men. When it comes to studying ageing and the means to slow it down, mice are not just small humans. EMBO Rep. 2005;6(Spec No):S39–S44. doi: 10.1038/sj.embor.7400422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ginis I, Luo Y, Miura T, et al. Differences between human and mouse embryonic stem cells. Dev Biol. 2004;269(2):360–380. doi: 10.1016/j.ydbio.2003.12.034. [DOI] [PubMed] [Google Scholar]
- 124.Van Der Spoel TI, Jansen of Lorkeers SJ, Agostoni P, et al. Human relevance of pre-clinical studies in stem cell therapy: systematic review and meta-analysis of large animal models of ischaemic heart disease. Cardiovasc Res. 2011;91(4):649–658. doi: 10.1093/cvr/cvr113. [DOI] [PubMed] [Google Scholar]
- 125.Glotzbach JP, Januszyk M, Vial IN, et al. An information theoretic, microfluidic-based single cell analysis permits identification of subpopulations among putatively homogeneous stem cells. PLoS One. 2011;6(6):e21211. doi: 10.1371/journal.pone.0021211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Graf T, Stadtfeld M. Heterogeneity of embryonic and adult stem cells. Cell Stem Cell. 2008;3(5):480–483. doi: 10.1016/j.stem.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 127.Brauninger S, Bialleck H, Thorausch K, Felt T, Seifried E, Bonig H. Allogeneic donor peripheral blood “stem cell” apheresis: prospective comparison of two apheresis systems. Transfusion. 2012;52(5):1137–1145. doi: 10.1111/j.1537-2995.2011.03414.x. [DOI] [PubMed] [Google Scholar]
- 128.Broxmeyer HE, Hangoc G, Cooper S, Campbell T, Ito S, Mantel C. AMD3100 and CD26 modulate mobilization, engraftment, and survival of hematopoietic stem and progenitor cells mediated by the SDF-1/CXCL12-CXCR4 axis. Ann N Y Acad Sci. 2007;1106:1–19. doi: 10.1196/annals.1392.013. [DOI] [PubMed] [Google Scholar]
- 129.Liu F, Pan X, Chen G, et al. Hematopoietic stem cells mobilized by granulocyte colony-stimulating factor partly contribute to liver graft regeneration after partial orthotopic liver transplantation. Liver Transpl. 2006;12(7):1129–1137. doi: 10.1002/lt.20822. [DOI] [PubMed] [Google Scholar]
- 130.Takamiya M, Okigaki M, Jin D, et al. Granulocyte colony-stimulating factor-mobilized circulating c-Kit+/Flk-1+ progenitor cells regenerate endothelium and inhibit neointimal hyperplasia after vascular injury. Arterioscler Thromb Vasc Biol. 2006;26(4):751–757. doi: 10.1161/01.ATV.0000205607.98538.9a. [DOI] [PubMed] [Google Scholar]
- 131.Mark AL, Sun Z, Warren DS, et al. Stem cell mobilization is life saving in an animal model of acute liver failure. Ann Surg. 2010;252(4):591–596. doi: 10.1097/SLA.0b013e3181f4e479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kawabe-Yako R, Masaaki I, Masuo O, Asahara T, Itakura T. Cilostazol activates function of bone marrow-derived endothelial progenitor cell for re-endothelialization in a carotid balloon injury model. PLoS One. 2011;6(9):e24646. doi: 10.1371/journal.pone.0024646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Li X, Xu B. HMG-CoA reductase inhibitor regulates endothelial progenitor function through the phosphatidylinositol 3′-kinase/AKT signal transduction pathway. Appl Biochem Biotechnol. 2009;157(3):545–553. doi: 10.1007/s12010-008-8263-7. [DOI] [PubMed] [Google Scholar]
- 134.Urao N, Okigaki M, Yamada H, et al. Erythropoietin-mobilized endothelial progenitors enhance reendothelialization via Akt-endothelial nitric oxide synthase activation and prevent neointimal hyperplasia. Circ Res. 2006;98(11):1405–1413. doi: 10.1161/01.RES.0000224117.59417.f3. [DOI] [PubMed] [Google Scholar]
- 135.Gensch C, Clever YP, Werner C, Hanhoun M, Bohm M, Laufs U. The PPAR-γ agonist pioglitazone increases neoangiogenesis and prevents apoptosis of endothelial progenitor cells. Atherosclerosis. 2007;192(1):67–74. doi: 10.1016/j.atherosclerosis.2006.06.026. [DOI] [PubMed] [Google Scholar]
- 136.Oh IY, Yoon CH, Hur J, et al. Involvement of E-selectin in recruitment of endothelial progenitor cells and angiogenesis in ischemic muscle. Blood. 2007;110(12):3891–3899. doi: 10.1182/blood-2006-10-048991. [DOI] [PubMed] [Google Scholar]
- 137.Hannoush EJ, Sifri ZC, Elhassan IO, et al. Impact of enhanced mobilization of bone marrow derived cells to site of injury. J Trauma. 2011;71(2):283–289. doi: 10.1097/TA.0b013e318222f380. discussion 289–291. [DOI] [PubMed] [Google Scholar]
- 138.Sasaki T, Fukazawa R, Ogawa S, et al. Stromal cell-derived factor-1α improves infarcted heart function through angiogenesis in mice. Pediatr Int. 2007;49(6):966–971. doi: 10.1111/j.1442-200X.2007.02491.x. [DOI] [PubMed] [Google Scholar]
- 139.Cross DP, Wang C. Stromal-derived factor-1 α-loaded PLGA microspheres for stem cell recruitment. Pharm Res. 2011;28(10):2477–2489. doi: 10.1007/s11095-011-0474-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Huang M, Nguyen P, Jia F, et al. Double knockdown of prolyl hydroxylase and factor-inhibiting hypoxia-inducible factor with nonviral minicircle gene therapy enhances stem cell mobilization and angiogenesis after myocardial infarction. Circulation. 2011;124(Suppl 11):S46–S54. doi: 10.1161/CIRCULATIONAHA.110.014019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Haider H, Jiang S, Idris NM, Ashraf M. IGF-1-overexpressing mesenchymal stem cells accelerate bone marrow stem cell mobilization via paracrine activation of SDF-1α/CXCR4 signaling to promote myocardial repair. Circ Res. 2008;103(11):1300–1308. doi: 10.1161/CIRCRESAHA.108.186742. [DOI] [PubMed] [Google Scholar]
- 142.He X, Ma J, Jabbari E. Migration of marrow stromal cells in response to sustained release of stromal-derived factor-1α from poly(lactide ethylene oxide fumarate) hydrogels. Int J Pharm. 2010;390(2):107–116. doi: 10.1016/j.ijpharm.2009.12.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zhang G, Nakamura Y, Wang X, Hu Q, Suggs LJ, Zhang J. Controlled release of stromal cell-derived factor-1α in situ increases c-kit+ cell homing to the infarcted heart. Tissue Eng. 2007;13(8):2063–2071. doi: 10.1089/ten.2006.0013. [DOI] [PubMed] [Google Scholar]
- 144.Abdel-Latif A, Bolli R, Zuba-Surma EK, Tleyjeh IM, Hornung CA, Dawn B. Granulocyte colony-stimulating factor therapy for cardiac repair after acute myocardial infarction: a systematic review and meta-analysis of randomized controlled trials. Am Heart J. 2008;156(2):216–226.e9. doi: 10.1016/j.ahj.2008.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ko IK, Ju YM, Chen T, Atala A, Yoo JJ, Lee SJ. Combined systemic and local delivery of stem cell inducing/recruiting factors for in situ tissue regeneration. FASEB J. 2012;26(1):158–168. doi: 10.1096/fj.11-182998. [DOI] [PubMed] [Google Scholar]
- 146.Shin JW, Lee JK, Lee JE, et al. Combined effects of hematopoietic progenitor cell mobilization from bone marrow by granulocyte colony stimulating factor and AMD3100 and chemotaxis into the brain using stromal cell-derived factor-1α in an Alzheimer’s disease mouse model. Stem Cells. 2011;29(7):1075–1089. doi: 10.1002/stem.659. [DOI] [PubMed] [Google Scholar]
- 147.Askari AT, Unzek S, Popovic ZB, et al. Effect of stromal-cell-derived factor 1 on stem-cell homing and tissue regeneration in ischaemic cardiomyopathy. Lancet. 2003;362(9385):697–703. doi: 10.1016/S0140-6736(03)14232-8. [DOI] [PubMed] [Google Scholar]
- 148.Yamaguchi J, Kusano KF, Masuo O, et al. Stromal cell-derived factor-1 effects on ex vivo expanded endothelial progenitor cell recruitment for ischemic neovascularization. Circulation. 2003;107(9):1322–1328. doi: 10.1161/01.cir.0000055313.77510.22. [DOI] [PubMed] [Google Scholar]
- 149.Kuliszewski MA, Kobulnik J, Lindner JR, Stewart DJ, Leong-Poi H. Vascular gene transfer of SDF-1 promotes endothelial progenitor cell engraftment and enhances angiogenesis in ischemic muscle. Mol Ther. 2011;19(5):895–902. doi: 10.1038/mt.2011.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Aicher A, Heeschen C, Sasaki K, Urbich C, Zeiher AM, Dimmeler S. Low-energy shock wave for enhancing recruitment of endothelial progenitor cells: a new modality to increase efficacy of cell therapy in chronic hind limb ischemia. Circulation. 2006;114(25):2823–2830. doi: 10.1161/CIRCULATIONAHA.106.628623. [DOI] [PubMed] [Google Scholar]
- 151.Seeger FH, Zeiher AM, Dimmeler S. Cell-enhancement strategies for the treatment of ischemic heart disease. Nat Clin Pract Cardiovasc Med. 2007;4(Suppl 1):S110–S113. doi: 10.1038/ncpcardio0734. [DOI] [PubMed] [Google Scholar]
- 152.Lim SY, Kim YS, Ahn Y, et al. The effects of mesenchymal stem cells transduced with Akt in a porcine myocardial infarction model. Cardiovasc Res. 2006;70(3):530–542. doi: 10.1016/j.cardiores.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 153.Cho J, Zhai P, Maejima Y, Sadoshima J. Myocardial injection with GSK-3β-overexpressing bone marrow-derived mesenchymal stem cells attenuates cardiac dysfunction after myocardial infarction. Circ Res. 2011;108(4):478–489. doi: 10.1161/CIRCRESAHA.110.229658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Song SW, Chang W, Song BW, et al. Integrin-linked kinase is required in hypoxic mesenchymal stem cells for strengthening cell adhesion to ischemic myocardium. Stem Cells. 2009;27(6):1358–1365. doi: 10.1002/stem.47. [DOI] [PubMed] [Google Scholar]
- 155.Chang W, Song BW, Lim S, et al. Mesenchymal stem cells pretreated with delivered Hph-1-Hsp70 protein are protected from hypoxia-mediated cell death and rescue heart functions from myocardial injury. Stem Cells. 2009;27(9):2283–2292. doi: 10.1002/stem.153. [DOI] [PubMed] [Google Scholar]
- 156.Li W, Ma N, Ong LL, et al. Bcl-2 engineered MSCs inhibited apoptosis and improved heart function. Stem Cells. 2007;25(8):2118–2127. doi: 10.1634/stemcells.2006-0771. [DOI] [PubMed] [Google Scholar]
- 157.Cui B, Huang L, Fang Y, Guo R, Yin Y, Zhao X. Transplantation of endothelial progenitor cells overexpressing endothelial nitric oxide synthase enhances inhibition of neointimal hyperplasia and restores endothelium-dependent vasodilatation. Microvasc Res. 2011;81(1):143–150. doi: 10.1016/j.mvr.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 158.Kong D, Melo LG, Mangi AA, et al. Enhanced inhibition of neointimal hyperplasia by genetically engineered endothelial progenitor cells. Circulation. 2004;109(14):1769–1775. doi: 10.1161/01.CIR.0000121732.85572.6F. [DOI] [PubMed] [Google Scholar]
- 159.Sasaki K, Heeschen C, Aicher A, et al. Ex vivo pretreatment of bone marrow mononuclear cells with endothelial NO synthase enhancer AVE9488 enhances their functional activity for cell therapy. Proc Natl Acad Sci USA. 2006;103(39):14537–14541. doi: 10.1073/pnas.0604144103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Aicher A, Heeschen C, Mildner-Rihm C, et al. Essential role of endothelial nitric oxide synthase for mobilization of stem and progenitor cells. Nat Med. 2003;9(11):1370–1376. doi: 10.1038/nm948. [DOI] [PubMed] [Google Scholar]
- 161.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414(6865):813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 162.Fichtlscherer S, Breuer S, Schachinger V, Dimmeler S, Zeiher AM. C-reactive protein levels determine systemic nitric oxide bioavailability in patients with coronary artery disease. Eur Heart J. 2004;25(16):1412–1418. doi: 10.1016/j.ehj.2004.06.026. [DOI] [PubMed] [Google Scholar]
- 163.Iwaguro H, Yamaguchi J, Kalka C, et al. Endothelial progenitor cell vascular endothelial growth factor gene transfer for vascular regeneration. Circulation. 2002;105(6):732–738. doi: 10.1161/hc0602.103673. [DOI] [PubMed] [Google Scholar]
- 164.Song MB, Yu XJ, Zhu GX, Chen JF, Zhao G, Huang L. Transfection of HGF gene enhances endothelial progenitor cell (EPC) function and improves EPC transplant efficiency for balloon-induced arterial injury in hypercholesterolemic rats. Vascul Pharmacol. 2009;51(2–3):205–213. doi: 10.1016/j.vph.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 165.Tang J, Wang J, Yang J, et al. Mesenchymal stem cells over-expressing SDF-1 promote angiogenesis and improve heart function in experimental myocardial infarction in rats. Eur J Cardiothorac Surg. 2009;36(4):644–650. doi: 10.1016/j.ejcts.2009.04.052. [DOI] [PubMed] [Google Scholar]
- 166.Huang W, Wang T, Zhang D, et al. Mesenchymal stem cells overexpressing CXCR4 attenuate remodeling of postmyocardial infarction by releasing matrix metalloproteinase-9. Stem Cells Dev. 2012;21(5):778–789. doi: 10.1089/scd.2011.0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Zhang D, Fan GC, Zhou X, et al. Over-expression of CXCR4 on mesenchymal stem cells augments myoangiogenesis in the infarcted myocardium. J Mol Cell Cardiol. 2008;44(2):281–292. doi: 10.1016/j.yjmcc.2007.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Cheng Z, Ou L, Zhou X, et al. Targeted migration of mesenchymal stem cells modified with CXCR4 gene to infarcted myocardium improves cardiac performance. Mol Ther. 2008;16(3):571–579. doi: 10.1038/sj.mt.6300374. [DOI] [PubMed] [Google Scholar]
- 169.Chen L, Wu F, Xia WH, et al. CXCR4 gene transfer contributes to in vivo reendothelialization capacity of endothelial progenitor cells. Cardiovasc Res. 2010;88(3):462–470. doi: 10.1093/cvr/cvq207. [DOI] [PubMed] [Google Scholar]
- 170.Chavakis E, Aicher A, Heeschen C, et al. Role of β2-integrins for homing and neovascularization capacity of endothelial progenitor cells. J Exp Med. 2005;201(1):63–72. doi: 10.1084/jem.20041402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Foubert P, Silvestre JS, Souttou B, et al. PSGL-1-mediated activation of EphB4 increases the proangiogenic potential of endothelial progenitor cells. J Clin Invest. 2007;117(6):1527–1537. doi: 10.1172/JCI28338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Herrmann JL, Wang Y, Abarbanell AM, Weil BR, Tan J, Meldrum DR. Preconditioning mesenchymal stem cells with transforming growth factor-α improves mesenchymal stem cell-mediated cardioprotection. Shock. 2010;33(1):24–30. doi: 10.1097/SHK.0b013e3181b7d137. [DOI] [PubMed] [Google Scholar]
- 173.Erwin GS, Crisostomo PR, Wang Y, et al. Estradiol-treated mesenchymal stem cells improve myocardial recovery after ischemia. J Surg Res. 2009;152(2):319–324. doi: 10.1016/j.jss.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Yu JX, Huang XF, Lv WM, et al. Combination of stromal-derived factor-1α and vascular endothelial growth factor gene-modified endothelial progenitor cells is more effective for ischemic neovascularization. J Vasc Surg. 2009;50(3):608–616. doi: 10.1016/j.jvs.2009.05.049. [DOI] [PubMed] [Google Scholar]
- 175.Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature. 2008;453(7193):314–321. doi: 10.1038/nature07039. [DOI] [PubMed] [Google Scholar]
- 176.Mooney DJ, Vandenburgh H. Cell delivery mechanisms for tissue repair. Cell Stem Cell. 2008;2(3):205–213. doi: 10.1016/j.stem.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 177.Lund A, Yener B, Stegemann JP, Plopper GE. The natural and engineered 3D microenvironment as a regulatory cue during stem cell fate determination. Tissue Eng Part B Rev. 2009;15(3):371–380. doi: 10.1089/ten.teb.2009.0270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Schneider RK, Anraths J, Kramann R, et al. The role of biomaterials in the direction of mesenchymal stem cell properties and extracellular matrix remodelling in dermal tissue engineering. Biomaterials. 2010;31(31):7948–7959. doi: 10.1016/j.biomaterials.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 179.Nguyen LH, Kudva AK, Saxena NS, Roy K. Engineering articular cartilage with spatially-varying matrix composition and mechanical properties from a single stem cell population using a multi-layered hydrogel. Biomaterials. 2011;32(29):6946–6952. doi: 10.1016/j.biomaterials.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 180.Simpson D, Liu H, Fan TH, Nerem R, Dudley SC., Jr A tissue engineering approach to progenitor cell delivery results in significant cell engraftment and improved myocardial remodeling. Stem Cells. 2007;25(9):2350–2357. doi: 10.1634/stemcells.2007-0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Gourdie RG, Myers TA, Mcfadden A, Li YX, Potts JD. Self-organizing tissue-engineered constructs in collagen hydrogels. Microsc Microanal. 2012;18(1):99–106. doi: 10.1017/S1431927611012372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Tanaka T, Hirose M, Kotobuki N, et al. Bone augmentation by bone marrow mesenchymal stem cells cultured in three-dimensional biodegradable polymer scaffolds. J Biomed Mater Res A. 2009;91(2):428–435. doi: 10.1002/jbm.a.32253. [DOI] [PubMed] [Google Scholar]
- 183.Baba S, Inoue T, Hashimoto Y, et al. Effectiveness of scaffolds with pre-seeded mesenchymal stem cells in bone regeneration – assessment of osteogenic ability of scaffolds implanted under the periosteum of the cranial bone of rats. Dent Mater J. 2010;29(6):673–681. doi: 10.4012/dmj.2009-123. [DOI] [PubMed] [Google Scholar]
- 184.Ben-David D, Kizhner TA, Kohler T, Muller R, Livne E, Srouji S. Cell-scaffold transplant of hydrogel seeded with rat bone marrow progenitors for bone regeneration. J Craniomaxillofac Surg. 2011;39(5):364–371. doi: 10.1016/j.jcms.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 185.Nguyen LH, Kudva AK, Guckert NL, Linse KD, Roy K. Unique biomaterial compositions direct bone marrow stem cells into specific chondrocytic phenotypes corresponding to the various zones of articular cartilage. Biomaterials. 2011;32(5):1327–1338. doi: 10.1016/j.biomaterials.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 186.Liu L, Wu W, Tuo X, et al. Novel strategy to engineer trachea cartilage graft with marrow mesenchymal stem cell macroaggregate and hydrolyzable scaffold. Artif Organs. 2010;34(5):426–433. doi: 10.1111/j.1525-1594.2009.00884.x. [DOI] [PubMed] [Google Scholar]
- 187.Jin J, Jeong SI, Shin YM, et al. Transplantation of mesenchymal stem cells within a poly(lactide-co-ε-caprolactone) scaffold improves cardiac function in a rat myocardial infarction model. Eur J Heart Fail. 2009;11(2):147–153. doi: 10.1093/eurjhf/hfn017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Wong VW, Rustad KC, Glotzbach JP, et al. Pullulan hydrogels improve mesenchymal stem cell delivery into high-oxidative-stress wounds. Macromol Biosci. 2011;11(11):1458–1466. doi: 10.1002/mabi.201100180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Rustad KC, Wong VW, Sorkin M, et al. Enhancement of mesenchymal stem cell angiogenic capacity and stemness by a biomimetic hydrogel scaffold. Biomaterials. 2012;33(1):80–90. doi: 10.1016/j.biomaterials.2011.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Nukavarapu SP, Amini AR. Optimal scaffold design and effective progenitor cell identification for the regeneration of vascularized bone. Conf Proc IEEE Eng Med Biol Soc. 2011;2011:2464–2467. doi: 10.1109/IEMBS.2011.6090684. [DOI] [PubMed] [Google Scholar]
- 191.Moioli EK, Clark PA, Chen M, et al. Synergistic actions of hematopoietic and mesenchymal stem/progenitor cells in vascularizing bioengineered tissues. PLoS One. 2008;3(12):e3922. doi: 10.1371/journal.pone.0003922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Glotzbach JP, Wong VW, Gurtner GC, Longaker MT. Regenerative medicine. Curr Probl Surg. 2011;48(3):148–212. doi: 10.1067/j.cpsurg.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 193▪.Chang EI, Bonillas RG, El-Ftesi S, et al. Tissue engineering using autologous microcirculatory beds as vascularized bioscaffolds. FASEB J. 2009;23(3):906–915. doi: 10.1096/fj.08-114868. Proof-of-concept of stem cell seeding of explantable microvascular beds. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Danoviz ME, Nakamuta JS, Marques FL, et al. Rat adipose tissue-derived stem cells transplantation attenuates cardiac dysfunction post infarction and biopolymers enhance cell retention. PLoS One. 2010;5(8):e12077. doi: 10.1371/journal.pone.0012077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Sun CK, Yen CH, Lin YC, et al. Autologous transplantation of adipose-derived mesenchymal stem cells markedly reduced acute ischemia-reperfusion lung injury in a rodent model. J Transl Med. 2011;9:118. doi: 10.1186/1479-5876-9-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Chen YT, Sun CK, Lin YC, et al. Adipose-derived mesenchymal stem cell protects kidneys against ischemia-reperfusion injury through suppressing oxidative stress and inflammatory reaction. J Transl Med. 2011;9:51. doi: 10.1186/1479-5876-9-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Lavi R, Zhu XY, Chade AR, Lin J, Lerman A, Lerman LO. Simvastatin decreases endothelial progenitor cell apoptosis in the kidney of hypertensive hypercholesterolemic pigs. Arterioscler Thromb Vasc Biol. 2010;30(5):976–983. doi: 10.1161/ATVBAHA.109.201475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Zhong S, Shu S, Wang Z, et al. Enhanced homing of mesenchymal stem cells to the ischemic myocardium by ultrasound-targeted microbubble destruction. Ultrasonics. 2012;52(2):281–286. doi: 10.1016/j.ultras.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 199.Xu YL, Gao YH, Liu Z, et al. Myocardium-targeted transplantation of mesenchymal stem cells by diagnostic ultrasound-mediated microbubble destruction improves cardiac function in myocardial infarction of New Zealand rabbits. Int J Cardiol. 2010;138(2):182–195. doi: 10.1016/j.ijcard.2009.03.071. [DOI] [PubMed] [Google Scholar]
- 200.Ghanem A, Steingen C, Brenig F, et al. Focused ultrasound-induced stimulation of microbubbles augments site-targeted engraftment of mesenchymal stem cells after acute myocardial infarction. J Mol Cell Cardiol. 2009;47(3):411–418. doi: 10.1016/j.yjmcc.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 201.Zen K, Okigaki M, Hosokawa Y, et al. Myocardium-targeted delivery of endothelial progenitor cells by ultrasound-mediated microbubble destruction improves cardiac function via an angiogenic response. J Mol Cell Cardiol. 2006;40(6):799–809. doi: 10.1016/j.yjmcc.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 202.Jin SZ, Meng XW, Sun X, et al. Granulocyte colony-stimulating factor enhances bone marrow mononuclear cell homing to the liver in a mouse model of acute hepatic injury. Dig Dis Sci. 2010;55(10):2805–2813. doi: 10.1007/s10620-009-1117-5. [DOI] [PubMed] [Google Scholar]
- 203.Zhang XM, Du F, Yang D, et al. Granulocyte colony-stimulating factor increases the therapeutic efficacy of bone marrow mononuclear cell transplantation in cerebral ischemia in mice. BMC Neurosci. 2011;12:61. doi: 10.1186/1471-2202-12-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Jin SZ, Meng XW, Sun X, et al. Hepatocyte growth factor promotes liver regeneration induced by transfusion of bone marrow mononuclear cells in a murine acute liver failure model. J Hepatobiliary Pancreat Sci. 2011;18(3):397–405. doi: 10.1007/s00534-010-0343-8. [DOI] [PubMed] [Google Scholar]
- 205.Jin SZ, Meng XW, Han MZ, Sun X, Sun LY, Liu BR. Stromal cell derived factor-1 enhances bone marrow mononuclear cell migration in mice with acute liver failure. World J Gastroenterol. 2009;15(21):2657–2664. doi: 10.3748/wjg.15.2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Kanki-Horimoto S, Horimoto H, Mieno S, et al. Implantation of mesenchymal stem cells overexpressing endothelial nitric oxide synthase improves right ventricular impairments caused by pulmonary hypertension. Circulation. 2006;114(Suppl 1):I181–I185. doi: 10.1161/CIRCULATIONAHA.105.001487. [DOI] [PubMed] [Google Scholar]
- 207.Murasawa S, Llevadot J, Silver M, Isner JM, Losordo DW, Asahara T. Constitutive human telomerase reverse transcriptase expression enhances regenerative properties of endothelial progenitor cells. Circulation. 2002;106(9):1133–1139. doi: 10.1161/01.cir.0000027584.85865.b4. [DOI] [PubMed] [Google Scholar]
- 208.Sen S, Merchan J, Dean J, et al. Autologous transplantation of endothelial progenitor cells genetically modified by adeno-associated viral vector delivering insulin-like growth factor-1 gene after myocardial infarction. Hum Gene Ther. 2010;21(10):1327–1334. doi: 10.1089/hum.2010.006. [DOI] [PubMed] [Google Scholar]
- 209.Shinmura D, Togashi I, Miyoshi S, et al. Pretreatment of human mesenchymal stem cells with pioglitazone improved efficiency of cardiomyogenic transdifferentiation and cardiac function. Stem Cells. 2011;29(2):357–366. doi: 10.1002/stem.574. [DOI] [PubMed] [Google Scholar]
- 210.Seeger FH, Haendeler J, Walter DH, et al. p38 mitogen-activated protein kinase downregulates endothelial progenitor cells. Circulation. 2005;111(9):1184–1191. doi: 10.1161/01.CIR.0000157156.85397.A1. [DOI] [PubMed] [Google Scholar]
- 211.Numasawa Y, Kimura T, Miyoshi S, et al. Treatment of human mesenchymal stem cells with angiotensin receptor blocker improved efficiency of cardiomyogenic transdifferentiation and improved cardiac function via angiogenesis. Stem Cells. 2011;29(9):1405–1414. doi: 10.1002/stem.691. [DOI] [PubMed] [Google Scholar]
- 212.Frederick JR, Fitzpatrick JR, 3rd, Mccormick RC, et al. Stromal cell-derived factor-1α activation of tissue-engineered endothelial progenitor cell matrix enhances ventricular function after myocardial infarction by inducing neovasculogenesis. Circulation. 2010;122(11 Suppl):S107–S117. doi: 10.1161/CIRCULATIONAHA.109.930404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Holladay CA, Duffy AM, Chen X, Sefton MV, O’Brien TD, Pandit AS. Recovery of cardiac function mediated by MSC and interleukin-10 plasmid functionalised scaffold. Biomaterials. 2012;33(5):1303–1314. doi: 10.1016/j.biomaterials.2011.10.019. [DOI] [PubMed] [Google Scholar]