Abstract
The extracellular matrix (ECM) microenvironment consists of structural and functional molecules. The ECM relays both biochemical and biophysical cues to and from the cells to modulate cell behavior and function. The biophysical cues can be engineered and applied to cells by means of spatial patterning, matrix rigidity and matrix actuation. Tissue engineering strategies that utilize ECMs to direct stem cell organization and lineage specification show tremendous potential. This review describes the technologies for modulating ECM spatial patterning, matrix rigidity, chemical composition and matrix actuation. The role of ECMs in vascular tissue engineering is then discussed as a model of tissue engineering and regenerative medicine.
Keywords: Stem cells, extracellular matrix, mechanotransduction, nanopatterning, micropatterning, skin, spatial patterning, matrix rigidity
INTRODUCTION
Cells naturally reside within an extracellular matrix (ECM), which is a biological scaffolding material that consists of structural and functional molecules.1 Besides providing structural support to the cells, the ECM is a dynamic microenvironment that also plays a role in modulating numerous cell functions, including cell survival, migration, proliferation, and differentiation.2 Two decades ago, Langer and Vacanti pioneered the strategy of engineering tissue constructs by culturing cells on or within a matrix.3 Since then, tremendous interest has been placed into modulating matrix properties for tissue engineering applications, as well as understanding the role of matrices in the physiological environment.
Many of the desirable properties of engineered matrix materials are inspired from their physiological properties and functions. The ordered cellular geometries are, in part, associated with the alignment of ECMs upon which the cells grow, suggesting a role of ECM patterning on cellular orientation. Furthermore, solid tissues exhibit a range of matrix rigidity, with a Young’s Modulus of ~1kPa for the brain to ~100 kPa for collagenous bone, implicating the role of differential matrix rigidity in the maintenance of cell function and specification of stem cell lineage. As a result, the desirable properties of matrix materials would include being biocompatible; enabling cell survival and proliferation; biodegrading at a rate that is matched to the rate of native matrix secretion such that the neo-tissue is functionally and structurally similar to that native tissue; and having mechanical and physical properties similar to that of the native tissue.
Although the field of tissue engineering has made numerous advancements in the past few decades, including the first successful autologous tissue engineered tracheal implant, a major challenge is the fabrication of three-dimensional (3D) tissues that mimic the geometry and material properties of physiological tissues. To address this challenge, micro- and nano-scale technologies can be powerful tools for controlling matrix distribution and cellular patterning. Additionally, matrix rigidity, composition and actuation can transduce signals that modulate cellular behavior and tissue morphogenesis. This review will describe the emerging technologies to control the matrix microenvironment for stem cell engineering and regenerative medicine applications.
TYPES OF MATRIX MATERIALS
Matrix materials can generally be classified as naturally-derived or synthetic. Naturally-derived ECMs include collagen, laminin, fibrin, matrigel and many others. These ECMs can be extracted and purified from donor tissues. The advantages of these ECMs include their natural biocompatibility and the ability to preserve the intact 3D structure. However, the disadvantages of naturally-derived ECMs include the limited control of the physical and mechanical properties, along with the potential contamination of pathogens in the case of ECMs derived from non-human sources.
A unique naturally-derived ECM is decellularized matrix. Native tissues can become decellularized by detergents or enzymes to render them free of DNA and intracellular structural proteins.4 Such decellularized tissues retain tissue structural integrity as well as many aspects of the matrix chemical composition such as glycosaminoglycan content. Upon recellularization, the tissue can retain many aspects of native tissue structure and function. This approach has been successfully demonstrated in decellularized heart, lung, liver, and blood vessels.5–8
Besides naturally-derived ECMs, synthetic polymers can be used for the culture of cells for tissue engineering applications. These materials can be produced by chemical synthesis or recombinant DNA technology. For example, polyglycolic acid (PGA) and poly-L-lactic acid (PLLA) are aliphatic polyesters that are widely studied as biocompatible materials for tissue engineering. These materials can be synthesized by ring-opening polymerization and degraded by hydrolytic cleavage of ester bonds. The mechanical properties and rate of degradation can be modulated by the molecular weight of the polymers.9 Synthetic materials mimic some of the properties of naturally-derived ECMs such as biocompatibility and ability to provide structural support. However, in comparison to naturally-derived materials, the biochemical and biophysical properties of synthetic materials can be more easily controlled based on the chemistry of the material.
Synthetic materials can incorporate functional protein domains from naturally-derived ECMs or other biomolecules to improve cell binding capacity or to release angiogenic factors. For example, a family of artificial ECMs contains elastin-like domains interspersed with RGD cell-binding domains.10 The elasticity and tensile strength of these materials could be controlled by the cross-linking reactive residues interspersed within the elastin-like domains. Besides ECMs, other biomolecules can be immobilized onto polymers using polyethylene glycol (PEG), biotin, or heparin chemistries. For example, we have adhered laminin and basic fibroblast growth factor (bFGF) to heparin-functionalized nanofibrous PLLA scaffolds to simulate physical and biochemical environments that would promote neurite outgrowth and wound healing,11 and other groups have generated biodegradable poly(ester urethane)urea (PEUU) scaffolds with controlled release of bFGF.12
While many synthetic degradable materials are degraded by hydrolysis, native ECMs are generally degraded at enzyme-sensitive peptide (ESP) sequences by proteolytic enzymes. For example, GPQG↓IAGQ peptide derived from collagen renders MMP-mediated degradation,13 whereas YK↓NRD derived from fibrin enables plasmin-sensitive degradation (where ↓ indicates cleavage site).14 As a result, synthetic ECMs containing ESPs can be beneficial to tissue regeneration by mimicking the enzyme-mediated degradation properties of natural ECMs. For example, Lutolf et al. demonstrated that PEG-based hydrogels containing MMP-sensitive sequences embedded with bone morphogenetic protein-2 could enhance cell infiltration and bone regeneration in a rat calvarial bone defect model.15, 16 Furthermore, the level of bone regeneration depended on the enzymatic sensitivity of the incorporated substrate.
CONTROL OF ECM SPATIAL DISTRIBUTION AND ORGANIZATION
Cell shape regulates many aspects of cellular behavior, embryonic development, and stem cell differentiation. The ECM exerts control over cell shape in part by modulating the micro-scale and nano-scale ECM distribution and organization in two-dimensional (2D) and 3D contexts. By using various 2D or 3D geometric patterning technologies in a controlled environment, researchers have gained insights into the role of spatial patterning in cellular function and behavior, including lineage specification of stem cells. It is recognized that cells cultured on 2D or 3D environments vary in overall morphology, adhesion, migration, gene expression, and generation of stress.17 For example, one group compared MV3 melanoma cell migration on hyaluronic-acid-containing 2D or 3D substrates, and showed a direct correlation between migration rate with hyaluronic acid concentration on 2D substrates, but not in 3D substrates. This difference may be attributed to conformational changes due to substrate rigidity or the degree of polymerization. In another example, smooth muscle cells (SMCs) embedded in 3D gels underwent a marked reduction in both proliferation and phenotypic expression of smooth muscle α-actin, when compared to cells in 2D collagen gels.18 Dimensionality has also been shown to influence stem cell differentiation, as mouse embryonic stem cells (ESCs) cultured in 3D scaffolds more efficiently differentiated towards hematopoietic lineages than on 2D substrates.19 Below we briefly discuss some methods of 2D and 3D spatial patterning.
2D Spatial Patterning
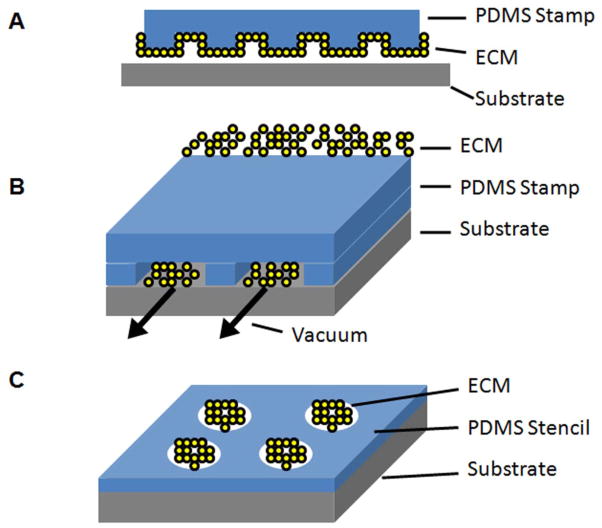
To mimic physiologically ordered tissues in vitro, spatial patterning of ECM proteins can direct cell organization, migration, proliferation, and differentiation.20 Numerous technologies have been developed to geometrically pattern ECM proteins on the micro-scale. These methods include soft lithography, dip pen nanolithography (DPN), electron beam lithography, and ECM microarrays. Soft lithography is a set of microfabrication techniques in which patterns are generated on a silicon wafer and then transferred onto a deformable material such as polydimethylsiloxane (PDMS).21, 22 The topographically patterned substrate can either be used directly for cell seeding, or serve as a template for microfluidic patterning or microcontact printing (Figure 1A–B). Microfluidic patterning is a procedure in which microchannel spaces between the PDMS template and contact surface are used to guide the flow of ECM proteins and other molecules.23 This method enables proteins to be deposited onto the contact surface in specified patterns. When microfluidic networks with multiple inlets are used, gradients of ECM proteins can be deposited.24 Such gradients enable the systematic study of haptotaxis, the migration of cells towards regions of higher ECM density. Related to microfluidic patterning is microcontact printing, in which the PDMS template is patterned with a matrix material or other molecule and then transferred to the cell culture substrate by conformal contact. The remaining non-patterned areas on the cell culture substrate can be treated with bovine serum albumin or other blocking agents to prevent non-specific cell attachment. Stencil-based techniques utilize the opposite strategy in which the PDMS containing holes are placed on top of the cell culture substrate (Figure 1C). When an ECM solution is deposited on the PDMS template, only the areas of holes will be deposited by the ECM. The advantages of soft lithography include the ability to generate micro-scale devices without the use of expensive photolithography equipment and clean room facilities.22 However, the disadvantages of this technique include the need for generating silicon wafers for each pattern of interest and the limited ability to pattern multiple types of ECMs in close proximity.
Figure 1.
Schematic of 2D micropatterning of ECM using soft lithography by A. microcontact printing, B. microfluidic printing, or C. stencils.
Using soft lithography, we and others have previously demonstrated the ability to micropattern cellular alignment, function, and cell fate.25–28 To study the role of cell shape on stem cell specification towards adipogenic or osteogenic lineages, McBeath et al. cultured human mesenchymal stem cells (MSCs) on micropatterned islands of fibronectin.28 Their results indicated that small (1024μm2) islands promoted adipogenesis rounded cellular morphology, whereas large (10,000μm2) islands preferentially induced osteogenesis and adherent morphology. The mechanism of such geometry-mediated effects appeared to be related to F-actin assembly and Rho/Rock pathways. Besides individual cells, soft lithographic tools can micropattern ESC colonies into strips or circular shapes. Lam et al. showed that micropatterned substrates restricted differentiation of the colony periphery, but supported growth and subsequent differentiation of cells in the center of colonies.27 The tendency of differentiation was consistent with the reduction of β-catenin expression. These examples demonstrate the utility of soft lithography as a tool for assessing the role of cell shape and cell-cell interactions on stem cell differentiation.
Technologies to generate geometrically defined nanoscale patterning techniques enable the cell-matrix interactions to be examined at high resolution. These methods include DPN and electron-beam lithography. DPN is a direct contact printing technique in which an atomic force microscopy (AFM) tip deposits chemical reagents onto a substrate by capillary transport.29 DPN can be used to print a variety of molecules, including proteins and antibodies, at sub-micron distance apart. The advantages of this approach include the ease of patterning without the use of silicon templates as well as the ability to print multiple types of molecules at sub-micron resolution. Another technique is electron-beam lithography, in which electron-sensitive resist is exposed to electron beams that make the exposed areas soluble or insoluble to solvents. Electron-beam lithography does not require a physical mask and is suitable for nanopatterns with 3-nm resolution.30
Recent developments now enable rapid high-throughput patterning of multiple ECMs on the same substrates. Using similar technology for printing DNA microarrays, ECM microarrays can be printed on glass slides to determine the effect of ECM on cell functions. Flaim et al. developed an ECM array to assess the optimal ECM conditions to maintain hepatic phenotype or induce hepatic differentiation of murine ESCs.31 The authors further examined the combinatorial effects of chemical signaling and ECMs on cardiac differentiation of ESCs.32 Anderson et al. probed the induction of human ESC differentiation on microarrays consisting of combinations of acrylate, diacrylate, dimethacylate, and triacrylate monomers.33 Besides probing for cell phenotype, this technology platform can also be used to deposit patterns of multiple ECMs on the same cell culture substrate for modulating cell attachment and alignment.34 These high-throughput patterning methods usually have a resolution of hundreds of microns, which make them more appropriate for the studies at multicellular level.
3D Spatial Patterning
Besides 2D patterning, micro- and nano-scale features can also be created within 3D scaffolds. In comparison to 2D constructs, 3D tissues better mimic the complexity and size of the physiological ECM environment. Furthermore, the fundamental differences in cell behavior and function between 2D and 3D environments are well-recognized.35 As a result, there is tremendous interest in the fabrication of 3D tissues. A variety of methods have been developed for the fabrication of 3D scaffolds (Figure 2). Conventional methods include salt-leaching, gas-foaming, and vacuum-drying, but these techniques suffer drawbacks in the insufficiency of interconnectivity among the pores as well as uneven dispersion of pores throughout the matrix. Furthermore, the limitations in size and the potential persistence of organic solvents in the scaffold present challenges for the clinical use of these types of scaffolds. Nevertheless, the porous scaffolds that were fabricated from these methods promoted the formation of 3D tissue-like structures. For example, Levenberg et al. demonstrated the ability for 3D PLLA/poly(lactic-co-glycolic acid) (PLGA) polymer scaffolds to support the differentiation of human ESCs and formation of complex structures such as vessel-like and rosette-like ductular structures.20
Figure 2.
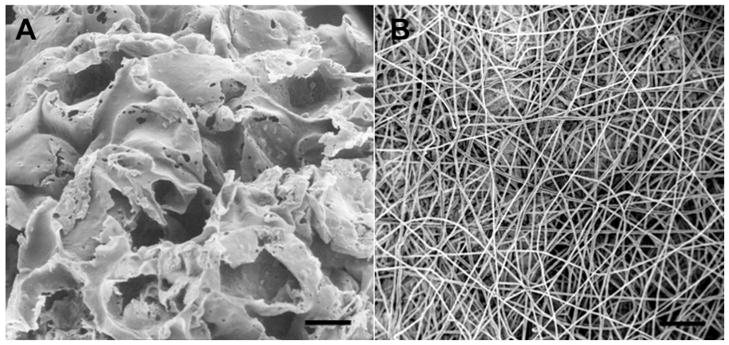
3D patterning methods. A. PLGA porous scaffold generated by salt leaching. B. PLLA nanofibrous scaffold generated by electrospinning. Scale bar, 100μm (A), 50μm (B).
3D scaffolds can also be generated with micro-scale control of physical features. One method is the stacking of microfabricated polymer films. This technique has been utilized to stack 5 to 35 layers of polymer films containing networks of vascular channels that are joined to neighboring networks in the vertical direction by machined through-holes.36 3D printing (3-DP) is another method of generating scaffolds with defined patterns using computer-aided design models. 3-DP is a fabrication process that utilizes ink-jet printing to deposit binder material into sequential powder layers.37 3-DP allows precise control of the scaffold architecture to enable the formation of interconnected pores. Numerous polymers have been used for generating 3D scaffolds using this process. The advantage of 3-DP include the flexibility of patterning desired 3D microstructures. On the other hand, the disadvantages of 3-DP include the use of organic solvents that can limit the feasibility of incorporating soluble factors or cells into the scaffold structure.
Numerous studies demonstrate the feasibility of 3-DP for tissue engineering applications. For example, copolymers of poly-lactic acid (PLA) and poly-glycolic acid (PGA) were fabricated into scaffolds by 3-DP before seeding with hepatocytes and nonparenchymal cells.38 When subjected to dynamic flow, the engineered construct produced significantly higher levels of albumin than under static conditions. To eliminate the use of organic solvents, recent studies utilize indirect methods of 3-DP, in which molds are printed and the final materials are subsequently cast into the mold.39 To examine the role of scaffold architecture on SMC growth, PLGA scaffolds were printed by indirect 3-DP technique with varying sizes of villus diameter, height, and intervillus spacing (0.5–1 mm).40 The findings suggest that small villi features (0.5 mm) supported significantly higher cell density within the scaffold than large villi features (1 mm) after 2 weeks of culture.
Another 3D matrix fabrication technology is direct-write assembly. This approach utilizes robotic deposition of nanoparticle, fugitive, and polyelectrolyte inks to generate structural features at the submicron and micron scale.41–43 Direct-write involves extrusion of the ink in a filamentous form that is patterned one layer at a time.44 This method was used to successfully fabricate 3D microvascular networks with defined connectivity.41 To test the therapeutic potential of scaffolds engineered by direct-write assembly, hydroxyapatite scaffolds generated by direct-write were implanted into trephine defects of rabbit, and the scaffolds were shown afterwards to be osteoconductive and promoted the formation of new trabecular bone.45
A recent development in the fabrication of nano- and micro-scale matrices is electrospinning. This technology enables the generation of 3D scaffolds with the 3D control of the surface topography. In the electrospinning process, a high-voltage electrostatic field causes an electrically charged polymer solution to spin to a grounded collector plate. As the nano- or micro-scale liquid stream reaches the plate, it forms a nano- or micro-scale fibrous strand after the liquid evaporates.46 A stationary collector plate can generate a matrix of randomly oriented fibers, whereas a rotating drum collector can be used to prepare aligned fibrous networks.47 Electrospinning technology has been to generate nanofibrous substrates to culture a variety of cell types, including skeletal myoblasts, MSCs, SMCs, ECs, and cardiomyocytes.25, 48–50
Nanofibrous textured substrates can promote cell viability and adhesion, guide cell migration and organization, modulate cell proliferation, and direct stem cell lineage specification.51, 52 We have previously shown that human MSCs remain viable and aligned when cultured on aligned PLLA nanofibers with diameters mimicking the physiological size of collagen fibrils (500–1000nm).49 It has been also shown that human MSCs cultured on collagen I nanofibers (~400nm diameter) have significantly faster adhesion, compared to collagen cast film.53 The spatial organization of nanofibers appears to induce phenotypic and functional changes as well. For example, myoblasts cultured on parallel-aligned PLLA nanofibers exhibited inhibited proliferation concomitantly with inducing the formation of longer multi-nucleated myotubes, when compared to randomly oriented nanofibers.25 In addition, electrospun polyurethane scaffolds were also shown to support neuronal differentiation of human ESCs, based on the expression of dopaminergic tyrosine hydroxylase (TH) and the appearance of neurite extensions that connected to adjacent cells.54 These studies demonstrate the application of 2D and 3D printing strategies for regulating cellular geometry of stem cells to direct organization and lineage specification.
Although spatial patterns are valuable for the understanding of cell-ECM interactions, they may not capture the complexity of physiological ECMs. Nevertheless, these technologies provide a useful model for studying the role of geometric patterning on cell and tissue behavior. In addition, some spatially patterned ECMs may be lacking in mechanical strength, durability, porosity, and elasticity to resemble those of physiological tissues. One challenge is to create electrospun scaffolds with sufficient porosity for cell infiltration. There has been progress in using salt leaching, fiber alignment and heparin modification to increase cell infiltration.55, 56 Many new methods that combine electrospinning with microfabricated templates and laser ablation are under development. Next generation patterning methods may also incorporate controllable degradation properties, multi-component ECM compositions, and tunable mechanical properties.
THE EFFECTS OF MATRIX RIGIDITY
Besides spatial patterning, another matrix property that regulates cellular and tissue behavior is the matrix rigidity. Matrix rigidity is a bulk property of the material that is often described by the apparent Young’s modulus. Physiologically, matrix rigidity varies over three orders of magnitude from bone to soft tissues, but it can be altered due to injury or disease.57 The mechanosensing structures within the cells include integrins and focal adhesions, which regulate the mechanotransduction process that ultimately affects cell morphology, migration, proliferation, and differentiation.
In order to study the role of matrix rigidity on cell behavior that is independent of chemical composition, Pelham and Wang developed a robust method to vary the matrix stiffness of polyacrylamide gels by changing the concentration of acrylamide and bis-acrylamide crosslinker components, while maintaining the same chemical composition.58 Since the development of polyacrylamide gels for controlling rigidity, other methods have been established using different hydrogels (e.g., PEG, alginate) and PDMS.59–61 Rigidity alone was shown to modulate fibroblast migration speed, as cells on soft substrates were associated with lower spreading area and faster migration rates, in comparison to rigid substrates.58 Furthermore, matrix rigidity also modulates the directionality of migration in a process known as durotaxis, in which cells tend to migrate from soft to hard substrates.62 The mechanism of the mechanosensing ability was associated with the αvβ3 integrin, as it was shown to colocalize at the leading edge during fibroblast migration on fibronectin substrates, and inhibiting the integrin with antibodies could impair the ability to sense fibronectin rigidity.63 In addition, fibroblasts could modulate actin cytoskeleton assembly to adapt their cellular rigidity with that of the substrate.64 By modifying matrix rigidity along with spatial patterning, Madden et al. demonstrated enhanced cardiomyocyte function.65 The authors cultured human ESC-derived cardiomyocytes in poly(2-hydroxyethyl methacrylate-co-methacrylic acid) hydrogel containing parallel channels and interconnected pores, and showed that the cardiomyocytes enhanced their survival and proliferation within these scaffolds for 2 weeks, reaching densities similar to that of adult hearts.
Rigidity not only modulates the behavior of differentiated cells, but it can also impart lineage commitment cues on stem cells. For example, Engler et al. demonstrated that when the substrate rigidity was matched to that of the physiological tissue rigidity, it could selectively induce MSC differentiation on towards osteogenic, muscular, and neuronal lineages.66 Indeed, soft matrices that resemble brain tissue stimulated neurogenesis, whereas rigid matrices resembling bone tissue induced osteogenesis. Transcriptional profiling further elucidated the upregulation of neurogenic markers on soft gels (0.1–1 kPa), myogenic markers on intermediate gels (11 kPa), and osteogenic markers on stiff gels (34 kPa). The mechanism of matrix-mediated lineage specificity appeared to be related to nonmuscle myosin II, as demonstrated by the loss of lineage specificity after treatment with blebbistatin, an inhibitor of non-muscle myosin II.
To elucidate the mechanism by which ECM cues are ultimately transformed into signaling pathways that modulate gene expression changes, a recent study demonstrated that ECM rigidity regulates the differentiation of MC3T3-E1 pre-osteoblasts through the involvement of the mitogen-activated protein kinase (MAPK) pathway.67 When pre-osteoblasts were cultured on PEG substrates of varying rigidity, it was found that alkaline phosphatase levels were significantly higher on rigid (~400 kPa) substrates when compared to soft (~14 kPa) substrates, suggesting that rigid substrates promoted osteogenesis. Similar trends were also observed for osteocalcin and sialoprotein gene expression levels. Concomitantly, MAPK activity was higher on stiffer substrates, compared to soft substrates, and pharmacological inhibition of MAPK activity resulted in lower expression levels of alkaline phosphatase, osteocalcin, and sialoprotein on both soft and rigid substrates. Taken together, these studies demonstrate that matrix rigidity plays an important role in regulating cell behavior and cell fate.
Commonly used materials for controlling matrix rigidity such as polyacrylamide or PDMS are generally synthetic materials. However, since these synthetic polymers have limited biocompatibility for in vivo applications, one of the challenges is to develop materials with controllable rigidity and improved biocompatibility. This is particularly important for 3D culture. Furthermore, with the current approach, the increase of ECM rigidity is usually coupled with the decrease of porosity, making it difficult to study the rigidity effects in 3D environment. The range of rigidity is another concern. The rigidity of hydrogels may be manipulated easily within the range of tens of kPa, but different types of tunable materials need to be developed to create well-defined rigidity between hundreds of kPa and tens of MPa for the most of soft tissues. Further research is also needed to understand the molecular mechanisms by which rigidity cues ultimately result in the changes in cell function and behavior.
CHEMICAL SIGNALING OF ECMS
Besides transducing physical and mechanical cues, ECMs also transfer chemical signals through membrane-associated signal transduction molecules like cell surface receptors, which in turn relay the signal to intracellular molecules that activate downstream pathways governing cell phenotype and function. As a result, ECM chemical composition alone has been shown to modulate stem cell renewal and cell fate. For example, hyaluronic acid (HA), a non-sulfated linear polysaccharide of (1-β-4)d-glucuronic acid and (1-β-3)N-acetyl-d-glucosamine that is abundantly expressed in human ESCs and in the developing embryo, supported ESC pluripotency by binding to CD44 and CD168 cell surface receptors.68 In particular, CD44 mediated HA-induced cell proliferation and survival pathways, whereas CD168 participated in HA-induced cell locomotion. Using high-throughput ECM microarrays, the compositions that support ESC renewal can also be quantified. Brafman et al. screened 320 unique signaling environments and reported that the optimal condition supporting long-term human ESC renewal was composed of human collagen I, collagen IV, fibronectin, and laminin.69 This ECM composition promoted cell proliferation while sustaining the expression of pluripotency markers such as Nanog and Oct3/4.
Besides promoting pluripotency, specific ECM compositions or binding domains can promote lineage specification. To explore the specificity of integrin binding by fibronectin’s central binding domains (FN III9–10) and their effect on stem cell differentiation, Martino et al. generated fibrin matrices that were functionalized with FN III9–10 variants with varying specificities to integrin α5β1.70 Their data demonstrated that variants with greater specificity for the α5β1 integrin significantly induced MSC differentiation towards osteogenic lineages in both 2D and 3D environments. To assess ligand-mediated interactions on chondrogenic differentiation, MSCs were encapsulated within the 3D RGD-functionalized PEG hydrogels containing an MMP-13 cleavage linker.71 The results show that the cells embedded in hydrogels with enzymatically cleavage RGD sequences produced 10 times more glycosaminoglycan as cells with uncleavable RGD functionalities, suggesting that temporal regulation of integrin-binding peptides regulate stem cell differentiation.
ECMs have also been shown to modulate cellular haptotaxis, the directional migration of cells along an ECM gradient. Experimental systems that regulate ligand gradients provide further information about directional cellular movements. Microfluidic systems and other microfabricated substrates have been frequently used to generate gradients of ECMs and or other chemical factors. For example, using micropatterned parallel strips of collagen I, we have previously shown that ECs develop focal adhesions and migrate towards areas of higher surface collagen density.72 To explore the contribution of surface density and concentration profiles on intestinal cell migration, Gunawan et al. generated microfluidic gradients of laminin with either similar change in local concentration (same gradient steepness) or different gradients with similar local concentration.24 Their results showed that cells migrated towards increasing laminin concentrations, independent of gradient steepness. Cell migration was also independent of gradient steepness at the same local concentration. However, directional cell migration was inhibited at high laminin densities. The ability of fibroblasts to sense the ECM gradient appears to be related to intracellular signaling proteins such as N-WASP, activated Cdc42, and FAK.73 Overexpression of N-WASP and activated Cdc42 led to increased directional migration, whereas overexpression of FAK increased the persistence of directional cell migration. Such studies provide additional clues of the signaling pathways that regulate directional cell migration.
ECM and synthetic matrix can also serve as a reservoir for the release of proteins, genes or drugs. For example, Mooney and colleagues demonstrated the release of VEGF from alginate hydrogels to stimulate neovascularization after femoral artery ligation.74 In tissue-engineered intestines, sustained VEGF release was achieved by transplanting intestinal organoids seeded on PGA scaffolds embedded with VEGF-containing PLGA microspheres.75 A hydrogel composite of oligo(poly(ethylene glycol)fumarate)-encapsulated rabbit marrow MSCs and transforming growth factor-β (TGF-β)-containing gelatin microparticles was examined for cartilage tissue engineering applications.76 The authors demonstrated that the gene expression of chondrocyte-specific type II collagen and aggrecan was only induced in groups containing TGF-β-containing gelatin microparticles in a manner dependent on TGF-β concentration. As an alternative to delivering proteins, Goldstein and colleagues examined gene delivery from plasmids embedded in polymer matrix for the repair of canine bone defects.77 They showed that the gene-activated matrices induced the gene expression for 6 weeks as well as bone formation. In addition to plasmid gene delivery, viruses can also be delivered by polymer-based approaches. Virus-encapsulated electrospun fibrous scaffolds have been shown to promote sustained and localized transduction with at least one month of transgene expression.78
Although ECMs relay chemical cues to the cells, this process is often concurrent with other dynamic mechanical cues, such as compressive loading, shear stress, or mechanical strain. As a result, cellular responses to ECM cues under static conditions may not capture the true behavior of cells under dynamic conditions. Furthermore, since the relay of ECM signaling occurs both inside-out as well as outside-in, not only are cells influenced by the ECM, but ECM may also be remodeled according to the cues from the cells, which results in a dynamic ECM environment. Since ECM is also a depot for many growth factors and cytokines, how to control the release of these bioactive factors from ECM needs further investigation.
STIMULUS-RESPONSIVE MATERIALS
“Smart materials” that can become bioactive by external stimulation have become very attractive for regenerative medicine applications. These materials can be changed or tuned by diverse stimuli, including light, temperature, and magnetic or electric fields. External actuation enables controlled stimulation, which are beneficial for regenerative medicine applications in which the release of soluble factors, cell encapsulation, or tissue remodeling may be required. Below we briefly describe several types of stimulus-responsive ECMs.
Light is a convenient and accurate stimulus to light-responsive polymers. They have potential for applications in the development of optical switches and delivery of drugs or growth factors.79 For example, spiropyran is a photo-responsive molecule that isomerizes in the presence of UV light from the hydrophobic spiro conformation to the hydrophilic conformation.80 Poly(spiropyran-co-methyl methacylate)-coated substrates were shown to stimulate UV-regulated release of fibrinogen, platelets, and mesenchymal stem cells.81 When spiropyran was incorporated into side chains of poly(N-isopropylacrylamide) (PNIPAAm), this material became a photo-responsive culture surface that enhanced cell attachment in the presence of UV light or reversibly stimulated cell detachment with visible light followed by thermal annealing.82 Azobenzene is another photo-responsive molecule that isomerizes reversibly by UV light from trans to cis form. The RGD-modified azobenzene derivatives tethered to poly(methyl methacylate) has been demonstrated to regulate cellular attachment by UV illumination.83
Thermo-responsive polymers are a commonly employed stimulus-responsive system. These polymers are characterized by a critical solution temperature in which the phase of the polymer and that of the solution are discontinuously changed based on temperature.84 When thermo-responsive polymers are heated above a critical temperature, they either become insoluble in the case of having a lower critical solution temperature (LCST), or they become more soluble in the case of having a upper critical solution temperature (UCST).85 Some thermo-responsive polymers commonly studied are PNIPAAm that have a transition temperature near body temperature, and poly(N,N′-diethylacrylamide) (PDEAAm) that have a transition temperature of 25–35°C.79 The critical temperature can also be modified by the addition of hydrophilic or hydrophobic groups. When thermo-sensitive and biodegradable poly(ethylene oxide) and poly(L-lactic acid) hydrogels loaded with bioactive molecules were injected subcutaneously, the hydrogel provided sustained release of drugs in vivo.86
Magnetic fields have also been employed for modulating polymer behavior. Tranquillo et al. demonstrated that magnetic fields can orient collagen fibrils circumferentially.87 In addition, polymers embedded with magnetic additives can respond to externally applied magnetic fields to initiate bioactivity. Commonly used magnetic particles include magnetite, maghemite, silica, iron, cobalt, and nickel.88 The magnetic particles can be incorporated to polymers such as PLGA, PLA, and chitosan by single emulsion-solvent evaporation, solvent diffusion, or nanoprecipitation methods.89, 90 Magnetic-sensitive silica nanospheres (50nm in size) loaded with ibuprofen exhibited controlled burst drug release upon stimulation with high frequency magnetic field.91 For the applications of orally administrated protein therapy, Cheng et al. co-encapsulated insulin with PLGA microparticles and then assessed their effect on hypoglycemia in mice in the presence of an external magnetic field.92 The authors reported a significantly improved hypoglycemic effect in mice that were subjected to the external magnetic field, suggesting that magnetic fields could improve the efficiency of orally administered proteins.
In addition to magnetic fields, electric fields can also influence the behavior of materials. The advantages of electric field stimulation include the control over magnitude and duration of the electric pulses.84 Electro-sensitive hydrogels can swell, shrink, or deform depending on the electric field. They are promising for the development of sensors, artificial tissues, and drug delivery systems.93 Over two decades ago, Tanaka et al. reported the effect of electrical fields in the volume change of polyacrylamide gels.94 With increasing applied voltage, the polyacrylamide gels shrank and collapsed. For artificial muscle therapeutic applications, acrylamide/acrylic acid copolymer and polypyrrole/carbon black have been used for electrical stimulation under low applied potential.95 Under electrical field stimulation, Kim et al. revealed that polycation hydrogels like chitosan/polyacrylonitrite appear to deform toward the anode, whereas polyanion hydrogels like hyaluronic acid/poly(vinyl alcohol) bend toward the cathode.96, 97
While stimulus-responsive materials are useful in the local manipulation of materials and actuation of cells, these materials also have many applications in regenerative medicine applications. For example, thermo-sensitive materials have been used to create cell sheets for tissue engineering.98 Light-sensitive gels can be used to generate gradient of surface properties by designing appropriate masks with gradient pattern.99 Although stimuli-responsive materials possess unique properties that enable local actuation, in many cases the actuation is transient and irreversible, which limits the use of these materials to tentative matrix or scaffold materials.
ENGINEERING ECMS FOR VASCULAR DIFFERENTIATION AND TISSUE REGENERATION
Blood vessels are organized in three concentric layers, namely an intimal endothelial cell (EC) monolayer, a medial layer of SMCs, and a fibroblast-rich adventitial outer layer. As a result of injury or disease, the vessels become prone to atherosclerotic lesions or other deposits that can restrict the circulation and potentially lead to myocardial infarction, stroke, atherosclerosis, or peripheral arterial disease. Despite the improvement in the quality of life by bypass surgeries, there is still a growing demand for engineered vascular grafts when donor vessels for bypass surgeries are unavailable, particularly for small-diameter grafts (<6mm diameter).
A number of polymer-based vascular grafts have been used commercially for vascular intervention. For example, one of the first FDA-approved vascular grafts is Artegraft, a tubular-shaped collagen graft prescribed as an arterial patch, an arterial bypass, and a femoropopliteal bypass. The product has mechanical strength but is also compliant and supports long-term patency. Although acellular vascular grafts have shown therapeutic benefits, they have the potential risk of thrombosis and occlusion, particularly for small diameter vascular grafts. As a result, engineering non-thrombogenic cellularized or acellular vascular grafts is a promising alternative.
Early studies demonstrated the feasibility of generating vascular conduits composed of cells embedded within a 3D scaffold. For example, Weinberg et al. generated a vascular conduit using SMCs in collagen gel that is lined with ECs and supported by a Dacron mesh.100 L’Heureux et al. reported the feasibility of generating tri-laminar vascular grafts in the absence of exogenous ECMs. To do this they cultured sheets of SMCs and wrapped them around a tubular support to mimic the medial layer, next wrapped a sheet of fibroblasts around the SMC layer to generate the adventitial layer, and finally seeded ECs in the luminal side of the vascular graft.101 A derivative of this approach is the fabrication of a bilayered vascular graft that contains concentric layers of ECs and fibroblasts.102 In addition, Niklason et al. demonstrated the feasibility of engineering vessels using a tubular PGA porous scaffold seeded with ECs and SMCs.103 The engineered vessels were mechanically strong and remained patent for up to 24 days after in vivo transplantation.
Although “smart materials” are still at early stages in the generation of vascular grafts, some studies have utilized these materials for generating vascularized tissue constructs. In vitro studies have shown that aligned matrix strips and microgrooves can guide the organization of SMCs in a way similar to that in native arteries and suppress SMC proliferation.104 The micropatterning method was also combined with thermosensitive hydrogel to engineer SMC sheet for vascular grafts construction.105 Recent approaches seek to fabricate vascular grafts with more physiologically relevant structure or function to those of native vessels by regulation of ECM spatial geometry. Using electrospinning technology to mimic the fibrous structure of physiological collagen, Hashi et al. showed that interpositional grafts composed of MSC-seeded PLA nanofibrous scaffolds supported long-term patency and the formation of EC and SMC layers in vivo.49 Furthermore, the electrospun nanofibers aligned in the circumferential direction to help organize cells in the vascular wall, and the deposition of collagen and elastin was prominent in the cell-seeded grafts. The patency of the vessels appeared to be related to the anti-thrombogenic property of MSCs, which could be attributed in part to heparin sulfate proteoglycans on the MSC cell surface. In a later study, the authors developed microfibrous PLA scaffolds conjugated with hirudin and PEG that inhibit platelet adhesion and improve in vivo patency.106 Not only did the grafts support in vivo cellular infiltration, but the grafts also showed increased mechanical strength, as assessed by elastic moduli of 3.5 to 11.1 MPa after 6 months of implantation. Bilayered electrospun vascular grafts composed of PCL and collagen were also shown to support EC organization and SMC infiltration by modulating fiber diameters.107 Nanofibers of 0.27μm fiber diameter supported ECs organization of focal adhesions and actin stress fiber assembly in the direction of the fiber direction, whereas larger fiber diameters (4.45μm) enhanced SMC infiltration. The bilayered scaffolds consisting of smaller fibers diameters in the EC layer and larger diameters in the outer layer appeared to improve vessel formation.
It appears that ECM rigidity is important for SMC differentiation in vascular grafts. When MSCs were used to construct vascular grafts, they differentiated into SMC lineage on stiffer substrates while differentiated into chondrogenic cells on soft substrates.108 These differential effects were further enhanced by TGF-β treatment. This observation suggests that appropriate stiffness of scaffold materials need to be used for vascular grafts and hydrogels are not optimal for SMC differentiation.
Chemical signaling of ECMs plays an important role in regulating stem cell specification towards vascular lineage. For example, we previously demonstrated the differential induction of vascular genes in human MSCs when cultured on substrates coated with fibrin, collagen I, laminin, or fibronectin. In the absence of growth factors, collagen I polymer substrates significantly induced the gene expression of endothelial markers FLK1 and VE-cadherin, whereas fibrin induced the expression of smooth muscle markers calponin and smooth muscle α-actin.109 Direct culture on decellularized microvascular EC matrix was also shown to promote MSC differentiation towards vascular lineage, based on induced gene expression of vascular markers PECAM1 and smooth muscle α-actin, along with enhanced tube-like formation in matrigel, when compared to MSCs cultured on tissue culture plastic.110 The inductive effect could only be partially attributed to paracrine factors, since indirect culture of MSCs with ECs led only to slight enhancement of EC markers and no effect on SMC markers, suggesting a role of direct ECM interaction on MSC differentiation.
Recently, stimulus responsive materials have also been used for vascular tissue engineering. In one study, ECs were cultured on thermo-responsive PNIPAAm micropatterned strips 20 μm-wide cell-adhesive lanes with 60-μm non-adhesive zones.111 Layers of micropatterned strips of ECs were then detached by temperature change and collected onto a gelatin-coated surface. The layers of EC strips were alternated with monolayer sheets of fibroblasts. The multi-layered tissue construct maintained the organization of ECs in the formation of vascular-like networks. In a later study in which temperature-responsive dishes were used to create stacked EC sheets sandwiched between sheets of myoblasts, the ECs formed network structures in vitro.98 Furthermore, when the multi-layered sheet constructs were implanted subcutaneously into nude rats, the sheets supported neovascularization and graft cell survival, highlighting the benefit of prevascularizing the myoblast construct prior to transplantation.
Although significant progress has been made in modulating ECMs and directing stem cell differentiation for vascular tissue engineering, there remain a number of hurdles that limit the clinical applicability of stem cell-based vascular grafts. Designing materials with adequate mechanical properties and desirable biological functions such as sustained release of bioactive molecules remains a challenge. Overcoming the potential of immune rejection caused by stem cells and xenoproteins during cell culture is another obstacle. In addition, the molecular signaling pathways that guide stem cell differentiation towards vascular lineages, as well as cell-matrix interactions, will be important to elucidate. As improved vascular grafts become available, the scalable production and storage of these engineered vessels will become another consideration. Overcoming these limitations will require multidisciplinary effort and innovative thinking.
SUMMARY AND FUTURE DIRECTIONS
In summary, the ECM microenvironment modulates cellular behavior in part by imparting cues by spatial patterning, substrate rigidity, chemical signaling, and matrix actuation (Figure 3). Although ECMs play an important role in tissue engineering and regenerative medicine, a number of concerns need to be addressed with respect to ECMs before tissue engineering can reach its full potential. Among them include the possible transmission of infectious diseases from the animals or human cadaveric donor tissue for ECM harvesting. In this regard, synthetic matrices overcome the concern of disease transmission. As the role of spatial patterning and rigidity of ECMs continues to become elucidated, the knowledge gained will facilitate the fabrication of ECMs that more accurately mimic the geometric complexity and material properties of physiological tissues. 3D ECM environments will become an increasingly important platform for evaluating cellular behavior and function. Furthermore, the roles of mechanical stimulation on engineered grafts will also be important to examine as shear stress and mechanical strain not only remodel the cells but also the ECM upon which the cells are grown. By building on the advancements of cell culture tools and engineering technologies, it is anticipated that we will improve the ability to create modulate matrix microenvironments for controlling of cell function and engineering tissues.
Figure 3.
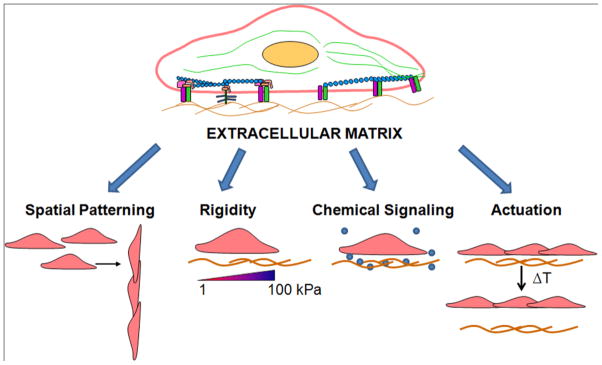
The role of the extracellular matrix (ECM) microenvironment on cell behavior and function. The ECM exerts its effects on cells by means of spatial patterning, rigidity, and chemical signaling.
Acknowledgments
This work was support in part by research grants from the American Heart Association (to N.H.) and National Institute of Health (HL098688-01A1 to N.H; HL083900 and EB12240 to S.L.)
References
- 1.Bissell MJ, Aggeler J. Dynamic reciprocity: how do extracellular matrix and hormones direct gene expression? Prog Clin Biol Res. 1987;249:251–262. [PubMed] [Google Scholar]
- 2.Daley WP, Peters SB, Larsen M. Extracellular matrix dynamics in development and regenerative medicine. J Cell Sci. 2008;121:255–264. doi: 10.1242/jcs.006064. [DOI] [PubMed] [Google Scholar]
- 3.Langer R, Vacanti JP. Tissue engineering. Science. 1993;260:920–926. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 4.Wainwright JM, Czajka CA, Patel UB, Freytes DO, Tobita K, Gilbert TW, Badylak SF. Preparation of cardiac extracellular matrix from an intact porcine heart. Tissue Eng Part C Methods. 2010;16:525–532. doi: 10.1089/ten.tec.2009.0392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ott HC, Matthiesen TS, Goh SK, Black LD, Kren SM, Netoff TI, Taylor DA. Perfusion-decellularized matrix: using nature’s platform to engineer a bioartificial heart. Nat Med. 2008;14:213–221. doi: 10.1038/nm1684. [DOI] [PubMed] [Google Scholar]
- 6.Uygun BE, Soto-Gutierrez A, Yagi H, Izamis ML, Guzzardi MA, Shulman C, Milwid J, Kobayashi N, Tilles A, Berthiaume F, Hertl M, Nahmias Y, Yarmush ML, Uygun K. Organ reengineering through development of a transplantable recellularized liver graft using decellularized liver matrix. Nat Med. 2010;16:814–820. doi: 10.1038/nm.2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ott HC, Clippinger B, Conrad C, Schuetz C, Pomerantseva I, Ikonomou L, Kotton D, Vacanti JP. Regeneration and orthotopic transplantation of a bioartificial lung. Nat Med. 2010;16:927–933. doi: 10.1038/nm.2193. [DOI] [PubMed] [Google Scholar]
- 8.Kaushal S, Amiel GE, Guleserian KJ, Shapira OM, Perry T, Sutherland FW, Rabkin E, Moran AM, Schoen FJ, Atala A, Soker S, Bischoff J, Mayer JE., Jr Functional small-diameter neovessels created using endothelial progenitor cells expanded ex vivo. Nat Med. 2001;7:1035–1040. doi: 10.1038/nm0901-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu XS, Wang N. Synthesis, characterization, biodegradation, and drug delivery application of biodegradable lactic/glycolic acid polymers. Part II: biodegradation. J Biomater Sci Polym Ed. 2001;12:21–34. doi: 10.1163/156856201744425. [DOI] [PubMed] [Google Scholar]
- 10.Liu JC, Heilshorn SC, Tirrell DA. Comparative cell response to artificial extracellular matrix proteins containing the RGD and CS5 cell-binding domains. Biomacromolecules. 2004;5:497–504. doi: 10.1021/bm034340z. [DOI] [PubMed] [Google Scholar]
- 11.Patel S, Kurpinski K, Quigley R, Gao H, Hsiao BS, Poo MM, Li S. Bioactive nanofibers: synergistic effects of nanotopography and chemical signaling on cell guidance. Nano Lett. 2007;7:2122–2128. doi: 10.1021/nl071182z. [DOI] [PubMed] [Google Scholar]
- 12.Guan J, Stankus JJ, Wagner WR. Biodegradable elastomeric scaffolds with basic fibroblast growth factor release. J Control Release. 2007;120:70–78. doi: 10.1016/j.jconrel.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DeForest CA, Polizzotti BD, Anseth KS. Sequential click reactions for synthesizing and patterning three-dimensional cell microenvironments. Nat Mater. 2009;8:659–664. doi: 10.1038/nmat2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pratt AB, Weber FE, Schmoekel HG, Muller R, Hubbell JA. Synthetic extracellular matrices for in situ tissue engineering. Biotechnol Bioeng. 2004;86:27–36. doi: 10.1002/bit.10897. [DOI] [PubMed] [Google Scholar]
- 15.Lutolf MP, Lauer-Fields JL, Schmoekel HG, Metters AT, Weber FE, Fields GB, Hubbell JA. Synthetic matrix metalloproteinase-sensitive hydrogels for the conduction of tissue regeneration: engineering cell-invasion characteristics. Proc Natl Acad Sci U S A. 2003;100:5413–5418. doi: 10.1073/pnas.0737381100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lutolf MP, Weber FE, Schmoekel HG, Schense JC, Kohler T, Muller R, Hubbell JA. Repair of bone defects using synthetic mimetics of collagenous extracellular matrices. Nat Biotechnol. 2003;21:513–518. doi: 10.1038/nbt818. [DOI] [PubMed] [Google Scholar]
- 17.Pedersen JA, Swartz MA. Mechanobiology in the third dimension. Ann Biomed Eng. 2005;33:1469–1490. doi: 10.1007/s10439-005-8159-4. [DOI] [PubMed] [Google Scholar]
- 18.Stegemann JP, Nerem RM. Altered response of vascular smooth muscle cells to exogenous biochemical stimulation in two- and three-dimensional culture. Exp Cell Res. 2003;283:146–155. doi: 10.1016/s0014-4827(02)00041-1. [DOI] [PubMed] [Google Scholar]
- 19.Liu H, Lin J, Roy K. Effect of 3D scaffold and dynamic culture condition on the global gene expression profile of mouse embryonic stem cells. Biomaterials. 2006;27:5978–5989. doi: 10.1016/j.biomaterials.2006.05.053. [DOI] [PubMed] [Google Scholar]
- 20.Levenberg S, Huang NF, Lavik E, Rogers AB, Itskovitz-Eldor J, Langer R. Differentiation of human embryonic stem cells on three-dimensional polymer scaffolds. Proc Natl Acad Sci U S A. 2003;100:12741–12746. doi: 10.1073/pnas.1735463100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singhvi R, Kumar A, Lopez GP, Stephanopoulos GN, Wang DI, Whitesides GM, Ingber DE. Engineering cell shape and function. Science. 1994;264:696–698. doi: 10.1126/science.8171320. [DOI] [PubMed] [Google Scholar]
- 22.Whitesides GM, Ostuni E, Takayama S, Jiang X, Ingber DE. Soft lithography in biology and biochemistry. Annu Rev Biomed Eng. 2001;3:335–373. doi: 10.1146/annurev.bioeng.3.1.335. [DOI] [PubMed] [Google Scholar]
- 23.Takayama S, McDonald JC, Ostuni E, Liang MN, Kenis PJ, Ismagilov RF, Whitesides GM. Patterning cells and their environments using multiple laminar fluid flows in capillary networks. Proc Natl Acad Sci U S A. 1999;96:5545–5548. doi: 10.1073/pnas.96.10.5545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gunawan RC, Silvestre J, Gaskins HR, Kenis PJ, Leckband DE. Cell migration and polarity on microfabricated gradients of extracellular matrix proteins. Langmuir. 2006;22:4250–4258. doi: 10.1021/la0531493. [DOI] [PubMed] [Google Scholar]
- 25.Huang NF, Patel S, Thakar RG, Wu J, Hsiao BS, Chu B, Lee RJ, Li S. Myotube Assembly on Nanofibrous and Micropatterned Polymers. Nano Lett. 2006;6:537–542. doi: 10.1021/nl060060o. [DOI] [PubMed] [Google Scholar]
- 26.Khademhosseini A, Eng G, Yeh J, Kucharczyk PA, Langer R, Vunjak-Novakovic G, Radisic M. Microfluidic patterning for fabrication of contractile cardiac organoids. Biomed Microdevices. 2007;9:149–157. doi: 10.1007/s10544-006-9013-7. [DOI] [PubMed] [Google Scholar]
- 27.Lam H, Patel S, Wong J, Chu J, Lau A, Li S. Localized decrease of beta-catenin contributes to the differentiation of human embryonic stem cells. Biochem Biophys Res Commun. 2008;372:601–606. doi: 10.1016/j.bbrc.2008.05.116. [DOI] [PubMed] [Google Scholar]
- 28.McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev Cell. 2004;6:483–495. doi: 10.1016/s1534-5807(04)00075-9. [DOI] [PubMed] [Google Scholar]
- 29.Piner RD, Zhu J, Xu F, Hong S, Mirkin CA. “Dip-Pen” nanolithography. Science. 1999;283:661–663. doi: 10.1126/science.283.5402.661. [DOI] [PubMed] [Google Scholar]
- 30.Vieu C, Carcenac F, Pepin A, Chen Y, Mejias M, Lebib A, Manin-Ferlazzo L, Couraud L, Launois H. Electron beam lithography: resolution limits and applications. Appl Surf Sci. 2000;164:111–117. [Google Scholar]
- 31.Flaim CJ, Chien S, Bhatia SN. An extracellular matrix microarray for probing cellular differentiation. Nat Methods. 2005;2:119–125. doi: 10.1038/nmeth736. [DOI] [PubMed] [Google Scholar]
- 32.Flaim CJ, Teng D, Chien S, Bhatia SN. Combinatorial signaling microenvironments for studying stem cell fate. Stem Cells Dev. 2008;17:29–39. doi: 10.1089/scd.2007.0085. [DOI] [PubMed] [Google Scholar]
- 33.Anderson DG, Levenberg S, Langer R. Nanoliter-scale synthesis of arrayed biomaterials and application to human embryonic stem cells. Nat Biotechnol. 2004;22:863–866. doi: 10.1038/nbt981. [DOI] [PubMed] [Google Scholar]
- 34.Mei Y, Cannizzaro C, Park H, Xu Q, Bogatyrev SR, Yi K, Goldman N, Langer R, Anderson DG. Cell-compatible, multicomponent protein arrays with subcellular feature resolution. Small. 2008;4:1600–1604. doi: 10.1002/smll. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li S, Lao J, Chen BP, Li YS, Zhao Y, Chu J, Chen KD, Tsou TC, Peck K, Chien S. Genomic analysis of smooth muscle cells in 3-dimensional collagen matrix. FASEB J. 2003;17:97–99. doi: 10.1096/fj.02-0256fje. [DOI] [PubMed] [Google Scholar]
- 36.Borenstein JT, Weinberg EJ, Orrick BK, Sundback C, Kaazempur-Mofrad MR, Vacanti JP. Microfabrication of three-dimensional engineered scaffolds. Tissue Eng. 2007;13:1837–1844. doi: 10.1089/ten.2006.0156. [DOI] [PubMed] [Google Scholar]
- 37.Hutmacher DW. Scaffold design and fabrication technologies for engineering tissues--state of the art and future perspectives. J Biomater Sci Polym Ed. 2001;12:107–124. doi: 10.1163/156856201744489. [DOI] [PubMed] [Google Scholar]
- 38.Kim SS, Utsunomiya H, Koski JA, Wu BM, Cima MJ, Sohn J, Mukai K, Griffith LG, Vacanti JP. Survival and function of hepatocytes on a novel three-dimensional synthetic biodegradable polymer scaffold with an intrinsic network of channels. Ann Surg. 1998;228:8–13. doi: 10.1097/00000658-199807000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee M, Dunn JC, Wu BM. Scaffold fabrication by indirect three-dimensional printing. Biomaterials. 2005;26:4281–4289. doi: 10.1016/j.biomaterials.2004.10.040. [DOI] [PubMed] [Google Scholar]
- 40.Lee M, Wu BM, Dunn JC. Effect of scaffold architecture and pore size on smooth muscle cell growth. J Biomed Mater Res A. 2008;87:1010–1016. doi: 10.1002/jbm.a.31816. [DOI] [PubMed] [Google Scholar]
- 41.Therriault D, White SR, Lewis JA. Chaotic mixing in three-dimensional microvascular networks fabricated by direct-write assembly. Nat Mater. 2003;2:265–271. doi: 10.1038/nmat863. [DOI] [PubMed] [Google Scholar]
- 42.Li Q, Lewis J. Nanoparticle Inks for Directed Assembly of Three-Dimensional Periodic Structures. Adv Mater. 2003;15:1639–1643. [Google Scholar]
- 43.Gratson GM, Xu M, Lewis JA. Microperiodic structures: direct writing of three-dimensional webs. Nature. 2004;428:386. doi: 10.1038/428386a. [DOI] [PubMed] [Google Scholar]
- 44.Lewis JA, Gratson GM. Direct writing in three dimensions. Materials Today. 2004;7:32–39. [Google Scholar]
- 45.Simon JL, Michna S, Lewis JA, Rekow ED, Thompson VP, Smay JE, Yampolsky A, Parsons JR, Ricci JL. In vivo bone response to 3D periodic hydroxyapatite scaffolds assembled by direct ink writing. J Biomed Mater Res A. 2007;83:747–758. doi: 10.1002/jbm.a.31329. [DOI] [PubMed] [Google Scholar]
- 46.Li WJ, Laurencin CT, Caterson EJ, Tuan RS, Ko FK. Electrospun nanofibrous structure: a novel scaffold for tissue engineering. J Biomed Mater Res. 2002;60:613–621. doi: 10.1002/jbm.10167. [DOI] [PubMed] [Google Scholar]
- 47.Xu CY, Inai R, Kotaki M, Ramakrishna S. Aligned biodegradable nanofibrous structure: a potential scaffold for blood vessel engineering. Biomaterials. 2004;25:877–886. doi: 10.1016/s0142-9612(03)00593-3. [DOI] [PubMed] [Google Scholar]
- 48.Mo XM, Xu CY, Kotaki M, Ramakrishna S. Electrospun P(LLA-CL) nanofiber: a biomimetic extracellular matrix for smooth muscle cell and endothelial cell proliferation. Biomaterials. 2004;25:1883–1890. doi: 10.1016/j.biomaterials.2003.08.042. [DOI] [PubMed] [Google Scholar]
- 49.Hashi CK, Zhu Y, Yang GY, Young WL, Hsiao BS, Wang K, Chu B, Li S. Antithrombogenic property of bone marrow mesenchymal stem cells in nanofibrous vascular grafts. Proc Natl Acad Sci U S A. 2007;104:11915–11920. doi: 10.1073/pnas.0704581104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zong X, Bien H, Chung CY, Yin L, Fang D, Hsiao BS, Chu B, Entcheva E. Electrospun fine-textured scaffolds for heart tissue constructs. Biomaterials. 2005;26:5330–5338. doi: 10.1016/j.biomaterials.2005.01.052. [DOI] [PubMed] [Google Scholar]
- 51.Li X, Xie J, Yuan X, Xia Y. Coating electrospun poly(epsilon-caprolactone) fibers with gelatin and calcium phosphate and their use as biomimetic scaffolds for bone tissue engineering. Langmuir. 2008;24:14145–14150. doi: 10.1021/la802984a. [DOI] [PubMed] [Google Scholar]
- 52.Xie J, Willerth SM, Li X, Macewan MR, Rader A, Sakiyama-Elbert SE, Xia Y. The differentiation of embryonic stem cells seeded on electrospun nanofibers into neural lineages. Biomaterials. 2009;30:354–362. doi: 10.1016/j.biomaterials.2008.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chan CK, Liao S, Li B, Lareu RR, Larrick JW, Ramakrishna S, Raghunath M. Early adhesive behavior of bone-marrow-derived mesenchymal stem cells on collagen electrospun fibers. Biomed Mater. 2009;4:35006. doi: 10.1088/1748-6041/4/3/035006. [DOI] [PubMed] [Google Scholar]
- 54.Carlberg B, Axell MZ, Nannmark U, Liu J, Kuhn HG. Electrospun polyurethane scaffolds for proliferation and neuronal differentiation of human embryonic stem cells. Biomed Mater. 2009;4:45004. doi: 10.1088/1748-6041/4/4/045004. [DOI] [PubMed] [Google Scholar]
- 55.Kurpinski KT, Stephenson JT, Janairo RR, Lee H, Li S. The effect of fiber alignment and heparin coating on cell infiltration into nanofibrous PLLA scaffolds. Biomaterials. 2010;31:3536–3542. doi: 10.1016/j.biomaterials.2010.01.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee YH, Lee JH, An IG, Kim C, Lee DS, Lee YK, Nam JD. Electrospun dual-porosity structure and biodegradation morphology of Montmorillonite reinforced PLLA nanocomposite scaffolds. Biomaterials. 2005;26:3165–3172. doi: 10.1016/j.biomaterials.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 57.Park JS, Huang NF, Kurpinski KT, Patel S, Hsu S, Li S. Mechanobiology of mesenchymal stem cells and their use in cardiovascular repair. Front Biosci. 2007;12:5098–5116. doi: 10.2741/2551. [DOI] [PubMed] [Google Scholar]
- 58.Pelham RJ, Jr, Wang Y. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc Natl Acad Sci U S A. 1997;94:13661–13665. doi: 10.1073/pnas.94.25.13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gilbert PM, Havenstrite KL, Magnusson KE, Sacco A, Leonardi NA, Kraft P, Nguyen NK, Thrun S, Lutolf MP, Blau HM. Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science. 2010;329:1078–1081. doi: 10.1126/science.1191035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ochsner M, Textor M, Vogel V, Smith ML. Dimensionality controls cytoskeleton assembly and metabolism of fibroblast cells in response to rigidity and shape. PLoS One. 2010;5:e9445. doi: 10.1371/journal.pone.0009445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kong HJ, Kaigler D, Kim K, Mooney DJ. Controlling rigidity and degradation of alginate hydrogels via molecular weight distribution. Biomacromolecules. 2004;5:1720–1727. doi: 10.1021/bm049879r. [DOI] [PubMed] [Google Scholar]
- 62.Lo CM, Wang HB, Dembo M, Wang YL. Cell movement is guided by the rigidity of the substrate. Biophys J. 2000;79:144–152. doi: 10.1016/S0006-3495(00)76279-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jiang G, Huang AH, Cai Y, Tanase M, Sheetz MP. Rigidity sensing at the leading edge through alphavbeta3 integrins and RPTPalpha. Biophys J. 2006;90:1804–1809. doi: 10.1529/biophysj.105.072462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Solon J, Levental I, Sengupta K, Georges PC, Janmey PA. Fibroblast adaptation and stiffness matching to soft elastic substrates. Biophys J. 2007;93:4453–4461. doi: 10.1529/biophysj.106.101386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Madden LR, Mortisen DJ, Sussman EM, Dupras SK, Fugate JA, Cuy JL, Hauch KD, Laflamme MA, Murry CE, Ratner BD. Proangiogenic scaffolds as functional templates for cardiac tissue engineering. Proc Natl Acad Sci U S A. 2010;107:15211–15216. doi: 10.1073/pnas.1006442107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 67.Khatiwala CB, Peyton SR, Metzke M, Putnam AJ. The regulation of osteogenesis by ECM rigidity in MC3T3-E1 cells requires MAPK activation. J Cell Physiol. 2007;211:661–672. doi: 10.1002/jcp.20974. [DOI] [PubMed] [Google Scholar]
- 68.Gerecht S, Burdick JA, Ferreira LS, Townsend SA, Langer R, Vunjak-Novakovic G. Hyaluronic acid hydrogel for controlled self-renewal and differentiation of human embryonic stem cells. Proc Natl Acad Sci U S A. 2007;104:11298–11303. doi: 10.1073/pnas.0703723104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Brafman DA, Shah KD, Fellner T, Chien S, Willert K. Defining Long-Term Maintenance Conditions of Human Embryonic Stem Cells With Arrayed Cellular Microenvironment Technology. Stem Cells Dev. 2009;18:1141–1154. doi: 10.1089/scd.2008.0410. [DOI] [PubMed] [Google Scholar]
- 70.Martino MM, Mochizuki M, Rothenfluh DA, Rempel SA, Hubbell JA, Barker TH. Controlling integrin specificity and stem cell differentiation in 2D and 3D environments through regulation of fibronectin domain stability. Biomaterials. 2009;30:1089–1097. doi: 10.1016/j.biomaterials.2008.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Salinas CN, Anseth KS. The enhancement of chondrogenic differentiation of human mesenchymal stem cells by enzymatically regulated RGD functionalities. Biomaterials. 2008;29:2370–2377. doi: 10.1016/j.biomaterials.2008.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hsu S, Thakar R, Liepmann D, Li S. Effects of shear stress on endothelial cell haptotaxis on micropatterned surfaces. Biochem Biophys Res Commun. 2005;337:401–409. doi: 10.1016/j.bbrc.2005.08.272. [DOI] [PubMed] [Google Scholar]
- 73.Rhoads DS, Guan JL. Analysis of directional cell migration on defined FN gradients: role of intracellular signaling molecules. Exp Cell Res. 2007;313:3859–3867. doi: 10.1016/j.yexcr.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lee KY, Peters MC, Anderson KW, Mooney DJ. Controlled growth factor release from synthetic extracellular matrices. Nature. 2000;408:998–1000. doi: 10.1038/35050141. [DOI] [PubMed] [Google Scholar]
- 75.Rocha FG, Sundback CA, Krebs NJ, Leach JK, Mooney DJ, Ashley SW, Vacanti JP, Whang EE. The effect of sustained delivery of vascular endothelial growth factor on angiogenesis in tissue-engineered intestine. Biomaterials. 2008;29:2884–2890. doi: 10.1016/j.biomaterials.2008.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Park H, Temenoff JS, Tabata Y, Caplan AI, Mikos AG. Injectable biodegradable hydrogel composites for rabbit marrow mesenchymal stem cell and growth factor delivery for cartilage tissue engineering. Biomaterials. 2007;28:3217–3227. doi: 10.1016/j.biomaterials.2007.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bonadio J, Smiley E, Patil P, Goldstein S. Localized, direct plasmid gene delivery in vivo: prolonged therapy results in reproducible tissue regeneration. Nat Med. 1999;5:753–759. doi: 10.1038/10473. [DOI] [PubMed] [Google Scholar]
- 78.Liao IC, Chen S, Liu JB, Leong KW. Sustained viral gene delivery through core-shell fibers. J Control Release. 2009;139:48–55. doi: 10.1016/j.jconrel.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Qiu Y, Park K. Environment-sensitive hydrogels for drug delivery. Adv Drug Deliv Rev. 2001;53:321–339. doi: 10.1016/s0169-409x(01)00203-4. [DOI] [PubMed] [Google Scholar]
- 80.Mendes PM. Stimuli-responsive surfaces for bio-applications. Chem Soc Rev. 2008;37:2512–2529. doi: 10.1039/b714635n. [DOI] [PubMed] [Google Scholar]
- 81.Higuchi A, Hamamura A, Shindo Y, Kitamura H, Yoon BO, Mori T, Uyama T, Umezawa A. Photon-modulated changes of cell attachments on poly(spiropyran-co-methyl methacrylate) membranes. Biomacromolecules. 2004;5:1770–1774. doi: 10.1021/bm049737x. [DOI] [PubMed] [Google Scholar]
- 82.Edahiro J, Sumaru K, Tada Y, Ohi K, Takagi T, Kameda M, Shinbo T, Kanamori T, Yoshimi Y. In situ control of cell adhesion using photoresponsive culture surface. Biomacromolecules. 2005;6:970–974. doi: 10.1021/bm0493382. [DOI] [PubMed] [Google Scholar]
- 83.Auernheimer J, Dahmen C, Hersel U, Bausch A, Kessler H. Photoswitched cell adhesion on surfaces with RGD peptides. J Am Chem Soc. 2005;127:16107–16110. doi: 10.1021/ja053648q. [DOI] [PubMed] [Google Scholar]
- 84.Bawa P, Pillay V, Choonara YE, du Toit LC. Stimuli-responsive polymers and their applications in drug delivery. Biomed Mater. 2009;4:022001. doi: 10.1088/1748-6041/4/2/022001. [DOI] [PubMed] [Google Scholar]
- 85.Schmaljohann D. Thermo- and pH-responsive polymers in drug delivery. Adv Drug Deliv Rev. 2006;58:1655–1670. doi: 10.1016/j.addr.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 86.Jeong B, Bae YH, Lee DS, Kim SW. Biodegradable block copolymers as injectable drug-delivery systems. Nature. 1997;388:860–862. doi: 10.1038/42218. [DOI] [PubMed] [Google Scholar]
- 87.Tranquillo RT, Girton TS, Bromberek BA, Triebes TG, Mooradian DL. Magnetically orientated tissue-equivalent tubes: application to a circumferentially orientated media-equivalent. Biomaterials. 1996;17:349–357. doi: 10.1016/0142-9612(96)85573-6. [DOI] [PubMed] [Google Scholar]
- 88.Ju XJ, Xie R, Yang L, Chu LY. Biodegradable ‘intelligent’ materials in response to physical stimuli for biomedical applications. Expert Opin Ther Pat. 2009;19:493–507. doi: 10.1517/13543770902771282. [DOI] [PubMed] [Google Scholar]
- 89.Zhou S, Sun J, Sun L, Dai Y, Liu L, Li X, Wang J, Weng J, Jia W, Zhang Z. Preparation and characterization of interferon-loaded magnetic biodegradable microspheres. J Biomed Mater Res B Appl Biomater. 2008;87:189–196. doi: 10.1002/jbm.b.31091. [DOI] [PubMed] [Google Scholar]
- 90.Gou ML, Qian ZY, Wang H, Tang YB, Huang MJ, Kan B, Wen YJ, Dai M, Li XY, Gong CY, Tu MJ. Preparation and characterization of magnetic poly(epsilon-caprolactone)-poly(ethylene glycol)-poly(epsilon-caprolactone) microspheres. J Mater Sci Mater Med. 2008;19:1033–1041. doi: 10.1007/s10856-007-3230-3. [DOI] [PubMed] [Google Scholar]
- 91.Hu SH, Liu TY, Huang HY, Liu DM, Chen SY. Magnetic-sensitive silica nanospheres for controlled drug release. Langmuir. 2008;24:239–244. doi: 10.1021/la701570z. [DOI] [PubMed] [Google Scholar]
- 92.Cheng J, Teply BA, Jeong SY, Yim CH, Ho D, Sherifi I, Jon S, Farokhzad OC, Khademhosseini A, Langer RS. Magnetically responsive polymeric microparticles for oral delivery of protein drugs. Pharm Res. 2006;23:557–564. doi: 10.1007/s11095-005-9444-5. [DOI] [PubMed] [Google Scholar]
- 93.Shang J, Shao Z, Chen X. Electrical behavior of a natural polyelectrolyte hydrogel: chitosan/carboxymethylcellulose hydrogel. Biomacromolecules. 2008;9:1208–1213. doi: 10.1021/bm701204j. [DOI] [PubMed] [Google Scholar]
- 94.Tanaka T, Nishio I, Sun ST, Ueno-Nishio S. Collapse of gels in an electric field. Science. 1982;218:467–469. doi: 10.1126/science.218.4571.467. [DOI] [PubMed] [Google Scholar]
- 95.Moschou EA, Madoub MJ, Bachasa LG, Daunert S. Voltage-switchable artificial muscles actuating at near neutral pH. Sensors and Actuators B: Chemical. 2006;115:379–383. [Google Scholar]
- 96.Kim SJ, Yoon SG, Lee YM, Kim HC, Kim SI. Electrical behavior of polymer hydrogel composed of poly(vinyl alcohol)-hyaluronic acid in solution. Biosens Bioelectron. 2004;19:531–536. doi: 10.1016/s0956-5663(03)00277-x. [DOI] [PubMed] [Google Scholar]
- 97.Kim SJ, Shin SR, Lee JH, Lee SH, Kim SI. Electrical response characterization of chitosan/polyacrylonitrile hydrogel in NaCl solutions. J Appl Polym Sci. 2003;90:91–96. [Google Scholar]
- 98.Sasagawa T, Shimizu T, Sekiya S, Haraguchi Y, Yamato M, Sawa Y, Okano T. Design of prevascularized three-dimensional cell-dense tissues using a cell sheet stacking manipulation technology. Biomaterials. 2010;31:1646–1654. doi: 10.1016/j.biomaterials.2009.11.036. [DOI] [PubMed] [Google Scholar]
- 99.Wong JY, Velasco A, Rajagopalan P, Pham Q. Directed Movement of Vascular Smooth Muscle Cells on Gradient-Compliant Hydrogels. Langmuir. 2003;19:1908–1913. [Google Scholar]
- 100.Weinberg CB, Bell E. A blood vessel model constructed from collagen and cultured vascular cells. Science. 1986;231:397–400. doi: 10.1126/science.2934816. [DOI] [PubMed] [Google Scholar]
- 101.L’Heureux N, Paquet S, Labbe R, Germain L, Auger FA. A completely biological tissue-engineered human blood vessel. Faseb J. 1998;12:47–56. doi: 10.1096/fasebj.12.1.47. [DOI] [PubMed] [Google Scholar]
- 102.L’Heureux N, Dusserre N, Konig G, Victor B, Keire P, Wight TN, Chronos NA, Kyles AE, Gregory CR, Hoyt G, Robbins RC, McAllister TN. Human tissue-engineered blood vessels for adult arterial revascularization. Nat Med. 2006;12:361–365. doi: 10.1038/nm1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Niklason LE, Gao J, Abbott WM, Hirschi KK, Houser S, Marini R, Langer R. Functional arteries grown in vitro. Science. 1999;284:489–493. doi: 10.1126/science.284.5413.489. [DOI] [PubMed] [Google Scholar]
- 104.Thakar RG, Ho F, Huang NF, Liepmann D, Li S. Regulation of vascular smooth muscle cells by micropatterning. Biochem Biophys Res Commun. 2003;307:883–890. doi: 10.1016/s0006-291x(03)01285-3. [DOI] [PubMed] [Google Scholar]
- 105.Isenberg BC, Tsuda Y, Williams C, Shimizu T, Yamato M, Okano T, Wong JY. A thermoresponsive, microtextured substrate for cell sheet engineering with defined structural organization. Biomaterials. 2008;29:2565–2572. doi: 10.1016/j.biomaterials.2008.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hashi CK, Derugin N, Janairo RR, Lee R, Schultz D, Lotz J, Li S. Antithrombogenic modification of small-diameter microfibrous vascular grafts. Arterioscler Thromb Vasc Biol. 2010;30:1621–1627. doi: 10.1161/ATVBAHA.110.208348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ju YM, Choi JS, Atala A, Yoo JJ, Lee SJ. Bilayered scaffold for engineering cellularized blood vessels. Biomaterials. 2010;31:4313–4321. doi: 10.1016/j.biomaterials.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 108.Park JS, Chu JSF, Tan E, Li S. Biomedical Engineering Society Annual Fall Meeting. Baltimore, MD: 2005. Matrix rigidity is important in transforming growth factor-β-induced changes in mesenchymal stem cells. [Google Scholar]
- 109.Huang NF, Chu J, Lee RJ, Li S. Biophysical and chemical effects of fibrin on mesenchymal stromal cell gene expression. Acta Biomater. 2010;6:3947–3956. doi: 10.1016/j.actbio.2010.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lozito TP, Kuo CK, Taboas JM, Tuan RS. Human mesenchymal stem cells express vascular cell phenotypes upon interaction with endothelial cell matrix. J Cell Biochem. 2009;107:714–722. doi: 10.1002/jcb.22167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tsuda Y, Shimizu T, Yamato M, Kikuchi A, Sasagawa T, Sekiya S, Kobayashi J, Chen G, Okano T. Cellular control of tissue architectures using a three-dimensional tissue fabrication technique. Biomaterials. 2007;28:4939–4946. doi: 10.1016/j.biomaterials.2007.08.002. [DOI] [PubMed] [Google Scholar]