Abstract
Endothelial cell (EC) migration in response to VEGF is a critical step in both physiological and pathological angiogenesis. Although VEGF signaling has been extensively studied, the mechanisms by which VEGF-dependent reactive oxygen species (ROS) production affects EC signaling are not well-understood. The aim of this study was to elucidate the involvement of Nox2- and Nox4-dependent ROS in VEGF-mediated EC Ca2+ regulation and migration. VEGF induced migration of human aortic EC into a scratch wound over 6 hours that was inhibited by overexpression of either catalase or SOD. EC stimulation by micromolar concentrations of H2O2 was inhibited by catalase, but also unexpectedly by SOD. Both VEGF and H2O2 increased S-glutathiolation of SERCA2b and increased Ca2+ influx into EC, and these events could be blocked by overexpression of catalase or overexpression of SERCA2b in which the reactive cysteine-674 was mutated to a serine. In determining the source of VEGF-mediated ROS production, our studies show that specific knock down of either Nox2 or Nox4 inhibited VEGF-induced S-glutathiolation of SERCA, Ca2+ influx, and EC migration. Treatment with H2O2 induced S-glutathiolation of SERCA and EC Ca2+ influx, overcoming the knockdown of Nox4, but not Nox2, and Amplex Red measurements indicated that Nox4 is the source of H2O2. These results demonstrate that VEGF stimulates EC migration through increased S-glutathiolation of SERCA and Ca2+ influx in a Nox4- and H2O2-dependent manner, requiring Nox2 downstream.
Keywords: SERCA, endothelial cells, VEGF, hydrogen peroxide, calcium, Nox2, Nox4
Introduction
Vascular endothelial growth factor (VEGF) is an important mediator of endothelial cell (EC) migration and angiogenesis. Activation of several kinase cascades, including those mediated by PI3K, Src, and PLC, contribute to VEGF-mediated signaling; however, one important way in which VEGF stimulates EC migration is through production of reactive oxygen and nitrogen species (ROS/RNS). The requirement of low levels of ROS, including hydrogen peroxide (H2O2) and superoxide (O2.-), for EC migration is well documented, but the specific proteins that are targeted and the signaling that is affected are not well-defined.
In the endothelium, NADPH oxidase is an important source of both H2O2 and O2.-. Although originally studied as the source of the oxidative burst in phagocytes [1], NADPH oxidases play a role in a variety of intracellular signaling pathways, including Ca2+ homeostasis [2], activation of kinases [3], and transcriptional regulation [4]. Four NADPH oxidase isoforms are present in the endothelium at varying levels, namely Nox1, Nox2, Nox4, and Nox5, and a role for both Nox2 and Nox4 in VEGF-induced EC migration has been observed [5-7]. Nox4 is the predominant isoform expressed in EC and its activity is thought to be constitutive. The link between VEGF signaling and Nox4-dependent ROS production has not been elucidated, although Nox4-derived H2O2 increases VEGF mRNA production [8]. In addition, overexpression of Nox4 increases endothelial cell migration, which is blocked by catalase [9]. In contrast, Nox2 is activated by VEGF-dependent stimulation of the NADPH oxidase cofactor, Rac1 [10]. Nox2-dependent O2.- production is required for EC membrane ruffling and actin cytoskeletal rearrangement [11], as well as for feedback on the VEGF receptor and downstream kinase signaling [12]. In addition, NADPH oxidase-dependent ROS generation has been implicated in the regulation of Ca2+ homeostasis through interaction with the Ca2+ release protein, inositol triphosphate receptor, in EC [13] and the Ca2+ uptake protein, sarcoplasmic/endoplasmic reticulum Ca2+ ATPase (SERCA), in smooth muscle [2].
Regulation of Ca2+ signaling is critical for EC migration, and inhibition of Ca2+ influx impedes VEGF-induced EC migration and tube formation [14]. SERCA mediates uptake of Ca2+ into ER stores, acting as a critical regulator of intracellular Ca2+ concentration. SERCA2b, a ubiquitously expressed isoform that is the predominant isoform in EC [15], is susceptible to both stimulatory and inhibitory oxidative post translational modifications, particularly on reactive cysteine-674 [2, 16-18]. Earlier studies revealed that S-glutathiolation of SERCA C674 increases activity of the Ca2+ ATPase purified in phospholipid vesicles [16], smooth muscle cells [18], and cardiomyocytes. In addition, we recently reported that increased nitric oxide-dependent S-glutathiolation of SERCA cysteine-674 is required for VEGF-induced Ca2+ influx and EC migration [19].
Increased NADPH oxidase-dependent ROS production plays a role in the pathological oxidation of SERCA in diabetic rat smooth muscle cells [2] and in leptin-deficient mouse heart [20], but the role of physiological levels of ROS in regulating redox changes in EC SERCA is unknown. Therefore, to further elucidate the mechanism of VEGF-induced redox regulation of SERCA, this study focuses on the regulation of SERCA and EC Ca2+ influx by ROS. Our findings show that either overexpression of antioxidant enzymes or knockdown of Nox4 or Nox2 inhibits VEGF-induced EC migration and Ca2+ influx, and that Nox4-dependent H2O2 is upstream of Nox2. Additionally, we show that the mechanism by which Ca2+ influx is regulated is through ROS-dependent S-glutathiolation of SERCA cysteine-674, providing a novel redox mechanism for NADPH oxidase-dependent regulation of EC migration.
Materials and Methods
Materials
TaqMan primers and RT-PCR reagents were purchased from Applied Biosystems (Carlsbad, CA, USA). Human aortic endothelial cells (HAEC) and endothelial growth medium were purchased from Lonza (Walkersville, MD, USA). SERCA2 IID8 antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Rabbit polyclonal SERCA antibody was generated by Bethyl Laboratory (Montgomery, TX, USA). Glutathione antibody was from Virogen (Watertown, MA, USA). Recombinant human VEGF was purchased from R&D Biosciences (Minneapolis, MN, USA). Nox4 antibody was from Abcam (Cambridge, MA, USA). H2O2 solution was purchased from Sigma (St. Louis, MO, USA).
Adenoviral Constructs and Transduction
Adenoviral wild type and mutant SERCA2b (C674S) were designed as previously described [16, 18]. SERCA adenoviruses were screened for proper constructs by extraction of viral DNA using the RedExtract-N-Amp Tissue PCR kit (Sigma). Extracted DNA was subjected to PCR using a forward primer specific to the viral promoter and a reverse primer located beyond the C674 codon: 5′-ACCGTCAGATCCGCTAGAGA-3′and 5′-GCCACAATGGTGGAGAAGTT-3′. PCR products were cleaned using a QIAGEN QIAQuick PCR Purification kit (Valencia, CA, USA) then sequenced using the following sequencing primer: 5′-GATCACTGGGGACAACAAGG-3′. SERCA adenovirus was purified using the double cesium chloride purification technique and tissue culture infectious dose TCID50 and total viral particle units (VPU) were determined. Purified adenovirus was tested for the presence of viral E1A contamination as described previously [21, 22]. Endothelial cells were transduced to achieve equal expression of wild type SERCA and SERCA C674S protein at a multiplicity of infection (MOI) ≈10. Adenoviral overexpression of catalase and CuZnSOD were previously reported by our laboratory in EC [23] . Cells were transduced in media containing no FBS and 10 μg/mL Polybrene (Sigma) for 48 hours. Cells were made quiescent for 24 hrs in medium with 0.1% serum before treatments.
Specific knockdown
To knock down Nox2, HAECs were cultured for 48 hours with the Nox2-specific siRNA constructs 5′-CCGGGUUUAUGAUAUUCCAtt-3′ from Ambion (Naugatuck, CT, USA) or a scrambled siRNA control from Invitrogen (Frederick, MD, USA) with Lipofectamine (Invitrogen) reagent in serum-free, antibiotic-free EBM-2. Nox4 knockdown was achieved as previously reported [24] by adenoviral transduction with Nox4-specific short hairpin RNA encoding the siRNA sequence 5′-AGACCTGGCCAGTATATTA-3′ or scrambled shRNA. Adenoviral transduction was performed as above for 72 hours. For experiments in which SERCA was overexpressed in cells in which Nox2 or Nox4 had been knocked down, Nox knockdown was performed as described above. 24 hours after the initial knockdown, SERCA adenovirus was applied to HAEC as described above. The total knockdown time was 72 hours and the time of SERCA overexpression was 48 hours.
Migration Assay
HAEC were grown to 80% confluency and made quiescent overnight. Scratch wounds were applied to EC monolayers in low serum media as previously described [19, 25]. VEGF (50 ng/mL) or H2O2 (1 μmol/L) was given at the time of the scratch in serum-free medium. Images were taken at 3 different positions along the scratch at zero and six hours. Migration was assessed in blinded images using NIH ImageJ software and evaluated as the change in individual cell centroid location between 0 and 6 hours, averaged over the 3 images per condition.
Amplex Red Assay
Following Nox2 and Nox4 knockdown, HAEC were seeded in 96-well plates at a density of 20,000 cells/well and made quiescent overnight. Cells were then treated in triplicate with either serum-free medium alone or serum-free medium with VEGF (50 ng/mL) for 6 hours. Extracellular H2O2 was detected using the Amplex® UltraRed reagent (Invitrogen). Cells were washed once with PBS then incubated with the Amplex® UltraRed reagent (50 μM) and horseradish peroxidase (2 U/mL, Sigma) in HBSS at 37°C for one hour. Fluorescence intensity was measured on a Tecan Infinite M1000 (Morrisville, NC, USA) plate reader using i-control™ 1.5 software (Tecan) at 540/580 excitation/emission. A background reading (reagents in HBSS in wells containing no cells) was subtracted from all sample readings prior to analysis.
qRT-PCR
Quantitative PCR was performed using pre-validated gene-specific FAM-NFQ-conjugated TaqMan hydrolysis probe primers for human Nox2 or Nox4 (Assay ID Hs00166163_m1 and Hs00418356_m1, Applied Biosystems). A VIC-NFQ-conjugated human 18S primer was used to normalize mRNA expression levels. Expression was analyzed using the comparative CT(ΔΔCT) with StepOne™ Real Time PCR Software (Applied Biosystems).
Immunoprecipitation
Nox2 or Nox4 was knocked down in HAEC followed by overexpression of WT SERCA. Cells were made quiescent in EBM-2 with 0.1% FBS overnight, then treated with VEGF (50 ng/mL) or H2O2 (1 μmol/L) for 15 minutes. Lysates were incubated with a custom polyclonal SERCA antibody and immunoblotted with monoclonal GSH antibody (Virogen). Bands were visualized using the Odyssey Infrared Imaging System (LI-COR Biosciences, Lincoln, NE, USA). Membranes were stripped and probed with monoclonal SERCA antibody (Santa Cruz). Band density was evaluated using NIH ImageJ software, and SERCA-bound glutathione was determined as the ratio of GSH to SERCA.
Intracellular Ca2+ imaging
HAEC plated on gelatin-coated glass coverslips were loaded with 2 μM Fura2-AM (Invitrogen) in the presence of 0.02% pluronic F127 (Invitrogen) in serum-free endothelial growth media at 37°C for 25 mins. Cells were washed in serum-free EGM at 37°C for 15 min then transferred to nominally Ca2+-free physiological saline solution supplemented with 2.5 mM probenecid (Alfa Aesar, Ward Hill, MA, USA). Changes in intracellular Ca2+ (F340/F380) were monitored as previously described [19, 26, 27]. Briefly, HAEC were treated with VEGF or H2O2 for 1.5 min prior to addition of CaCl2 (2 μmmol/L). Fura2-AM loading was tested by addition of ionomycin (2 mol/L) followed by quenching with MnCl2 (8 mmol/L). A dual-excitation fluorescence imaging system (Intracellular Imaging, Cincinnati, OH, USA) was used for studies of individual cells. The imaging system was calibrated such that Rmax = 1.582 at 602 nM Ca2+, Rmin = 0.493 at 0 nM Ca2+. The changes in intracellular Ca2+ were expressed as ΔRatio, which was calculated as the difference between the peak F340/F380 ratio after extracellular Ca2+ was added, and its level immediately before Ca2+ addition. Data were summarized from 3-5 identical experiments done in at least 3 cell preparations.
Western Blotting
Samples in Laemmli buffer were run on 10% electrophoresis gels. Proteins were transferred onto supported nitrocellulose membranes and blocked with 5% milk. Primary antibodies were incubated overnight at 4°C in milk. IR dye-conjugated secondary antibodies were used. Blots were imaged using film or the LI-COR system.
Statistical Analysis
Statistical analysis was performed using a Student’s t-test or a 1-way analysis of variance with a Bonferroni multiple comparisons post-test. Results are expressed as means ± s.e.m. p<0.05 was considered significant.
Results
VEGF-stimulated Ca2+ influx and EC migration require both H2O2 and O2.-
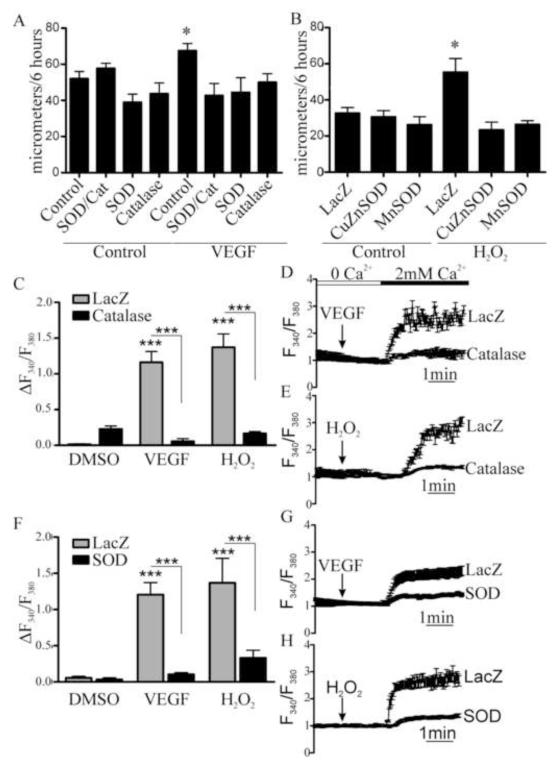
To assess the contribution of ROS to VEGF-induced EC migration, cellular antioxidants were overexpressed. LacZ, CuZnSOD, to specifically scavenge O2.-, and/or catalase, to specifically scavenge H2O2, were overexpressed in HAEC 48 hours prior to assessing migration in response to VEGF (50 ng/mL). Over 6 hours, VEGF significantly increased migration in LacZ-infected cells, but this was prevented by overexpression of either SOD or catalase alone, or both SOD and catalase together (Fig. 1A), indicating an important role for both O2.- and H2O2 in VEGF-induced EC migration. Preliminary studies showed that HAEC migration was exquisitely sensitive to exogenous H2O2, with 0.1 and 1 μM significantly increasing migration over 6 hours, while higher concentrations failed to do so (Supplemental Fig. 1). Unexpectedly, EC migration stimulated by H2O2 (1μM), was inhibited by overexpression of SOD (Fig. 1B), indicating a role of O2.- production which is downstream of H2O2. Because VEGF stimulates EC Ca2+ entry[19], the role of H2O2 in VEGF-induced Ca2+ influx was evaluated using Fura2 in HAEC overexpressing either LacZ, SOD, or catalase. Either VEGF or H2O2 stimulated Ca2+ influx in LacZ-infected EC, and this was inhibited by overexpression of SOD or catalase (Fig. 1C, 1D). These results indicate that VEGF-induced EC Ca2+ influx and migration are mediated by both H2O2 and O2-.
Figure 1.
Oxidants are required for VEGF-induced EC migration and Ca2t influx. A. HAEC monolayers overexpressing CuZnSOD and/or catalase were treated with VEGF (50 ng/mL) and migration into a scratch wound was measured over 6 hours (n = 3, *p< 0.05). B. HAEC monolayers overexpressing either MnSOD or CuZnSOD were treated with H2O2 (1μM) and migration was measured over 6 hours (n = 3, *p< 0.05). C - E. Catalase was overexpressed in HAEC and VEGF or H2O2 was added in the absence of extracellular Ca2+. The maximal increase in intracellular Ca2+ associated with Ca2+ influx upon Ca2+ readdition (2 mmol/L) was measured. Data shown are representative VEGF response trace (D) or H2O2 response trace (E) with mean ± s.e.m. of Ca2+ for measured cells and quantification of change in Fura2 fluorescence ratio between baseline and maximal Ca2+ in VEGF and H2O2 stimulated cells (C, n = at least 4, ***p< 0.001). G and H. Representative Ca2+ influx trace in HAEC overexpressing CuZnSOD and treated with VEGF or H2O2 and quantification of maximal Ca2+ associated with Ca2+ influx (F, n = at least 4, ***p< 0.001).
Nox2 and Nox4 are required for VEGF-induced EC migration and Ca2+ influx
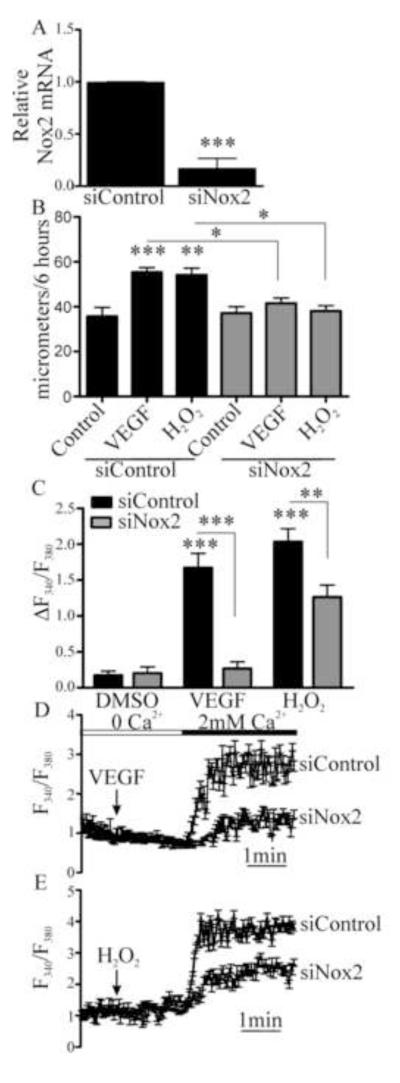
To determine the enzymatic sources of oxidants required for VEGF-induced migration and Ca2+ influx, first Nox2 was selectively knocked down with siRNA (Fig. 2A and Fig. S2). Migration in response to VEGF or H2O2 was inhibited in HAEC treated with Nox2 siRNA, but not in those treated with non-targeting siRNA (Fig. 2B). Treatment with Nox2 siRNA also inhibited both VEGF and H2O2-induced Ca2+ influx (Fig. 2C-2E), indicating that Nox2 activity is downstream of H2O2 production and is required for VEGF-induced influx of Ca2+.
Figure 2.
Nox2 is required for VEGF- and H2O2-induced EC Ca2+ influx and migration. A. HAEC were treated with specific siRNA to Nox2 and knockdown was confirmed by qRT-PCR for Nox2 mRNA (n = 3, *** p< 0.001). B. Nox2 was knocked down in HAEC and then migration into a scratch wound over 6 hours was measured in response to either VEGF (50 ng/mL) or H2O2 (1 μM, n = 3, *p < 0.05, **p < 0.01, *** p< 0.001). C. HAEC were treated with siRNA for Nox2 and the maximal increase in intracellular Ca2+ associated with Ca2+ entry upon Ca2+ readdition was measured (n = at least 4, ***p< 0.001). D and E. Representative Ca2+ influx trace in response to VEGF or H2O2 in HAEC in which Nox2 was knocked down.
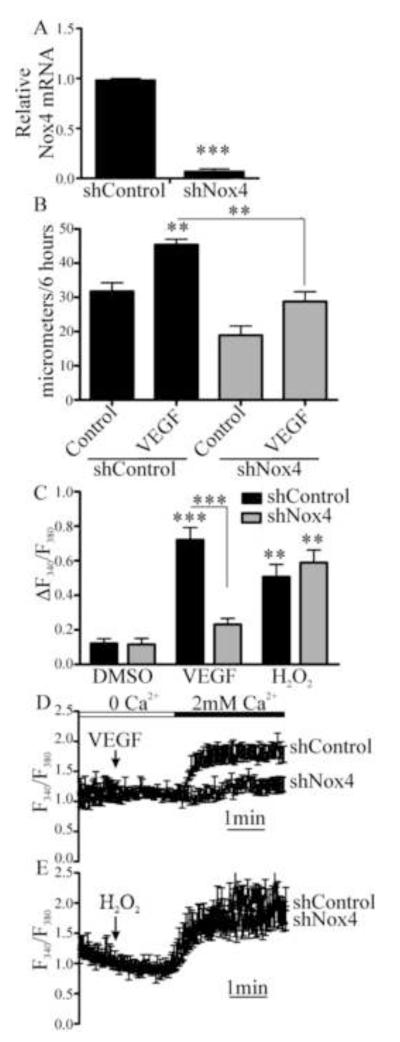
To assess the role of Nox4 in VEGF-induced EC migration, HAEC were transfected with adenoviral shRNA specifically targeting Nox4 or non-targeting control shRNA (Fig. 3A and Fig. S2) and migration over 6 hours in response to VEGF (50 ng/mL) measured. Migration induced by VEGF was inhibited in EC in which Nox4 was knocked down (Fig. 3B). H2O2 production in response to VEGF was confirmed by Amplex Red, and this was significantly inhibited following knock down of Nox4 but not Nox2 (Fig. S3). Similarly, knockdown of Nox4 inhibited VEGF-induced Ca2+ influx but, in contrast to knockdown of Nox2, knockdown of Nox4 did not inhibit Ca2+ influx in response to H2O2 (Fig. 3C-3E). Because H2O2 was able to overcome the knockdown of Nox4, but not Nox2, these data suggest that VEGF signaling requires Nox4-dependent H2O2 production, which is upstream of Nox2 activation.
Figure 3.
Nox4 is required for VEGF-induced EC Ca2+ influx and migration. A. HAEC were treated with adenoviral shRNA specific to Nox4 and knockdown was confirmed by qRT-PCR for Nox4 mRNA (n = 3, *** p< 0.001). B. Nox4 was knocked down in HAEC and then migration into a scratch wound over 6 hours was measured in response to VEGF (50 ng/mL, n = 3, **p < 0.01). C. HAEC were treated with shRNA for Nox4 and the maximal increase in intracellular Ca2+ associated with Ca2+ entry upon Ca2+ readdition was measured (n = at least 4, **p< 0.01, ***p< 0.001). D and E. Representative Ca2+ influx trace in response to VEGF or H2O2 (1 μM) in HAEC in which Nox4 was knocked down.
Nox4-derived H2O2 production is required for S-glutathiolation of SERCA C674 and EC migration
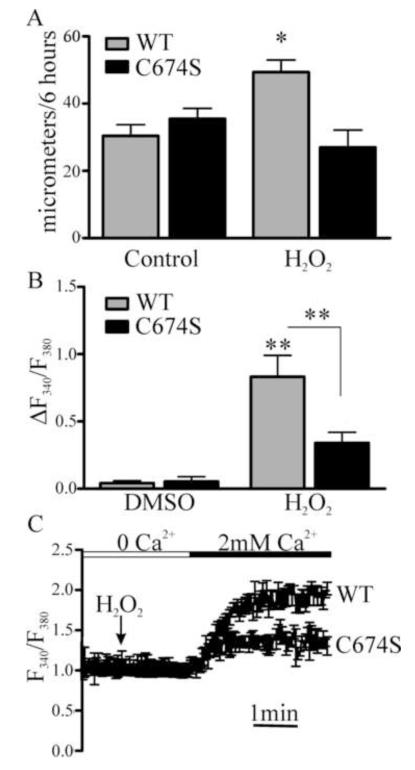
Previous studies demonstrated a role for SERCA2b C674 in VEGF- and nitric oxide-induced EC migration[19]. To assess the importance of this cysteine in H2O2-mediated EC migration, HAEC were transduced with either WT SERCA2b or SERCA2b C674S and migration into a scratch wound was measured over 6 hours. H2O2 stimulated migration in EC overexpressing WT SERCA2b similar to control HAEC, but the response was prevented by overexpression of SERCA2b C674S (Fig. 4A). Similarly, overexpression of SERCA2b C674S inhibited H2O2-induced Ca2+ influx (Fig. 4B-C), indicating an essential role for SERCA C674 in H2O2-mediated EC migration and Ca2+ influx.
Figure 4.
Mutation of the SERCA2b reactive cysteine-674 prevents VEGF- or H2O2-induced EC migration. A. Either SERCA2b WT or SERCA2b in which cysteine-674 was mutated to a serine was overexpressed in HAEC and migration into a scratch wound over six hours was measured in response to H2O2 (1 μM, n = 3, *p< 0.05). Ca2+ levels associated with Ca2+ influx were measured in HAEC overexpressing either WT SERCA2b or SERCA2b C674S. B. Summary data of change in Fura2 fluorescence ratio between baseline and maximal Ca2+ (n = at least 4, **p< 0.01). C. Representative trace of Fura2 fluorescence ratio in HAEC displayed as mean +/− s.e.m.
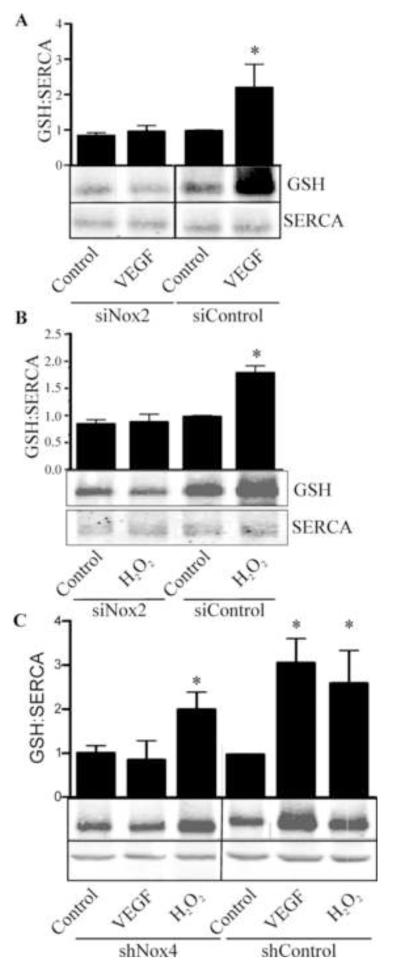
To investigate the mechanism of altered Ca2+ influx regulated by NADPH oxidase-derived ROS, glutathiolation of SERCA which occurs abundantly on cysteine-674 in VEGF-stimulated EC was assessed. WT SERCA was overexpressed in HAEC treated with control siRNA or siRNA targeting Nox2, and SERCA-bound glutathione adducts were assessed by immunoprecipitation of SERCA and western blot for glutathione protein adducts following treatment with H2O2 for 15 minutes. H2O2 increased glutathiolation of SERCA, but in HAEC in which Nox2 was knocked down, H2O2-induced glutathiolation of SERCA was prevented (Fig. 5A). In contrast, while knockdown of Nox4 inhibited VEGF-induced SERCA S-glutathiolation, H2O2-induced S-glutathiolation of SERCA was unaffected by knockdown of Nox4 (Fig.5B). Taken together, these data indicate that H2O2 is required for VEGF-induced SERCA S-glutathiolation and that the mechanism of H2O2-dependent, VEGF-stimulation requires Nox2.
Figure 5.
Nox2 and Nox4 are required for VEGF-induced S-glutathiolation of SERCA2b. A. HAEC were treated with siRNA for Nox2 and VEGF-induced (50 ng/mL) S-glutathiolation of SERCA2b was detected immunochemically following SERCA2b immunoprecipitation (n = 3, *p< 0.05). B. S-glutathiolation of SERCA2b in response to H2O2 treatment (1 μM) was assayed in HAEC in which Nox2 was knocked down (n = 3, *p< 0.05). C. Nox4 was knocked down by shRNA in HAEC and S-glutathiolation of SERCA2b in response to either VEGF or H2O2 was assessed (n = 3, *p< 0.05).
Discussion
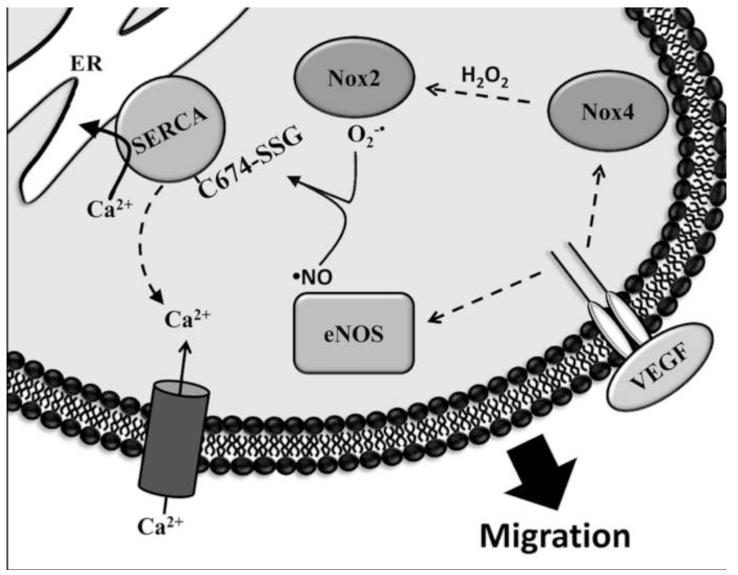
The contribution of ROS to VEGF signaling has long been recognized, but the specific proteins targeted by ROS are poorly understood. The data presented here introduce the novel concept that VEGF-induced ROS increase EC migration through S-glutathiolation of SERCA and alteration of Ca2+ homeostasis, and that this signaling depends on both Nox4 and Nox2. As demonstrated previously [19] , nearly all of the increase in S-glutathione adducts on SERCA caused by VEGF in EC expressing wild type SERCA is eliminated in cells transduced with a C674S mutant, indicating that the accumulation of the single glutathione adduct on the most reactive cysteine thiol on the protein [16], is responsible for the VEGF-mediated responses. The novel results presented here suggest that Nox4-dependent H2O2 is required for VEGF-induced SERCA glutathiolation, Ca2+ influx, and EC migration and that this requires downstream Nox2-dependent ROS production. A scheme is proposed in Figure 6.
Figure 6.
Proposed scheme of Nox2 and Nox4-dependent VEGF-induced EC migration. Upon stimulation with VEGF, Nox4-derived H2O2 works upstream of Nox2 to stimulate production of superoxide (O2-•). VEGF also stimulates activation of eNOS to produce ·NO. Together, O2-• and ·NO cause S-glutathiolation of SERCA2b cysteine-674, increasing Ca2+ uptake into the endoplasmic reticulum and Ca2+ influx into the cytosol, stimulating EC migration.
The role of H2O2 in VEGF signaling is complex. Nox4-dependent H2O2 production is required for VEGF mRNA production and VEGF receptor autophosphorylation [8]. Additionally, the regulation of Nox4 by VEGF is currently unclear. Unlike Nox2, Nox4 does not require the cofactors Rac1, p47phox or p67phox. Association with p22phox appears to stabilize Nox4 rather than activate it, indicating that activity of Nox4 may play a permissive rather than active role following VEGF stimulation. Interestingly, H2O2 has been shown to increase p22phox protein levels [28]. Through this mechanism, Nox4-derived ROS may not only promote Nox4 stability but, by increasing an essential cofactor, also increase the ability of VEGF to activate Nox2. Co-dependence of basal EC ROS production on Nox4 and Nox2 has been proposed [29], however, no direct effect of H2O2 on Nox2 activity has been found [1, 30]. Here, we demonstrate that Nox4-derived ROS are required for VEGF-induced EC migration confirmed by the fact that prevention of VEGF-induced Ca2+ influx and EC migration by knockdown of Nox4 was overcome with H2O2 supplementation.
VEGF signaling has both ROS-dependent and -independent pathways. Activation of PI3K, Akt and MAPK all require Nox2 while ERK and JNK signaling are independent of Nox2-derived ROS [12]. However, both in vivo and in vitro studies have shown that Nox2 is required for VEGF-induced angiogenesis [5, 31]. We confirmed a role of Nox2 in VEGF-mediated EC migration and further demonstrated that Nox2 is required for VEGF-mediated S-glutathiolation of SERCA and Ca2+ influx, suggesting a new mechanism by which Nox2 mediates EC migration. Interestingly, Nox2 activity is increased by Ca2+ influx in inflammatory cells, suggesting that this mechanism might represent a positive ROS feedback loop in EC [32].
Nox2-derived superoxide (O2.-) may signal directly or can be dismutated to H2O2 spontaneously or specifically by SOD. However, in contrast to knockdown of Nox4, inhibition of SERCA S-glutathiolation and Ca2+ influx by knockdown of Nox2 could not be overcome by H2O2 treatment, indicating that the Nox2-derived ROS, O2.-, is required in a final common pathway mediated by Nox4 and Nox2. In these studies, Nox4 and H2O2 appear to act upstream of Nox2-derived O2.-. H2O2-mediated Ca2+ influx and EC migration were prevented by overexpression of SOD, supporting the suggestion that O2.-, the product of Nox2, functions downstream of H2O2 signaling. Although no direct link between Nox4 and Nox2 signaling has previously been shown in EC, there is evidence showing a role of H2O2-mediated ERK signaling and activation of Nox2. Chatterjee et al. demonstrated the requirement of ERK-dependent phosphorylation of peroxiredoxin-6 for the translocation of p47phox and Rac1, and subsequent Nox2 activation in response to agonist stimulation [33].
Ca2+ signaling is required for VEGF-induced EC migration, and a role for S-glutathiolation of SERCA, and subsequent changes in Ca2+ influx, have been demonstrated previously in EC. In vitro studies using purified SERCA2 or SERCA2b expressed in HEK293 cells demonstrated that treatment with peroxynitrite in the presence of glutathione increased SERCA S-glutathiolation specifically on cysteine-674 and Ca2+ uptake activity [16]. Indeed, VEGF-induced S-glutathiolation of SERCA cysteine-674, Ca2+ influx, and EC migration also require nitric oxide [19], and we speculate that our data support a role for a nitric oxide and O2.- derived RNS, possibly peroxynitrite, in the S-glutathiolation of EC SERCA cysteine-674 (Figure 6). Peroxynitrite has previously been proposed to mediate VEGF-induced EC migration [34]. Interestingly, increased Nox4 expression and activity in aortic smooth muscle cells of Zucker obese rats increased irreversible oxidation of SERCA [2], indicating that the mechanism of SERCA S-glutathiolation occurs in a window of physiological ROS levels, and suggesting an important mechanism by which increased ROS in disease might contribute to EC dysfunction.
Supplementary Material
Figure S1. H2O2 has a concentration dependent effect on EC migration. HAEC were treated with H2O2 and migration of the EC monolayer into a scratch wound was assessed over 6 hours (n = 3, *p< 0.05).
Figure S2. Specificity of Nox2 and Nox4 knockdown. A. HAEC were treated with siRNA against Nox2 (siNox2) or an siRNA control (siCon) and assayed for non-specific knockdown of Nox4 mRNA (n = 7). B and C. HAEC were treated with siCon or siNox2, or with shRNA specific for Nox4 (shNox4) or control shRNA (shCon) and specificity of knockdown was assayed by western blot against Nox2 (B, n = 3, *p< 0.05) and Nox4 (C, n=4, *p< 0.05). Nox2 and Nox4 images are from a single western blot and share a loading control.
Figure S3. VEGF stimulates H2O2 production via Nox4 but not Nox2. HAEC in which either Nox2 or Nox4 was knocked down were treated with serum-free media or VEGF (50 ng/mL) for 6 hours. H2O2 production was measured by Amplex Red (n=3, *p<0.05 compared to serum-free siControl or shControl).
Research Highlights.
VEGF- or H2O2-induced EC migration was inhibited by overexpression of catalase or SOD.
H2O2-induced S-glutathiolation of SERCA2b cysteine-674 and EC Ca2+ influx required Nox2.
VEGF-induced S-glutathiolation of SERCA2b and EC Ca2+ influx required both Nox4 and Nox2.
Nox4-derived H2O2 mediates VEGF-induced SERCA S-glutathiolation, Ca2+ influx, and EC migration.
Non-standard Abbreviations
- C674S
cysteine-674 to serine mutation
- EC
endothelial cells
- SERCA
Sarcoplasmic/Endoplasmic Reticulum Ca2+ ATPase
- VEGF
Vascular endothelial growth factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Babior BM. NADPH oxidase: an update. Blood. 1999;93:1464–76. [PubMed] [Google Scholar]
- [2].Tong X, Hou X, Jourd’heuil D, Weisbrod RM, Cohen RA. Upregulation of Nox4 by TGF{beta}1 oxidizes SERCA and inhibits NO in arterial smooth muscle of the prediabetic Zucker rat. Circ Res. 2010;107:975–83. doi: 10.1161/CIRCRESAHA.110.221242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Mahadev K, Motoshima H, Wu X, Ruddy JM, Arnold RS, Cheng G, et al. The NAD(P)H oxidase homolog Nox4 modulates insulin-stimulated generation of H2O2 and plays an integral role in insulin signal transduction. Mol Cell Biol. 2004;24:1844–54. doi: 10.1128/MCB.24.5.1844-1854.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Clempus RE, Sorescu D, Dikalova AE, Pounkova L, Jo P, Sorescu GP, et al. Nox4 is required for maintenance of the differentiated vascular smooth muscle cell phenotype. Arterioscler Thromb Vasc Biol. 2007;27:42–8. doi: 10.1161/01.ATV.0000251500.94478.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ushio-Fukai M, Tang Y, Fukai T, Dikalov SI, Ma Y, Fujimoto M, et al. Novel role of gp91(phox)-containing NAD(P)H oxidase in vascular endothelial growth factor-induced signaling and angiogenesis. Circ Res. 2002;91:1160–7. doi: 10.1161/01.res.0000046227.65158.f8. [DOI] [PubMed] [Google Scholar]
- [6].Yamaoka-Tojo M, Ushio-Fukai M, Hilenski L, Dikalov SI, Chen YE, Tojo T, et al. IQGAP1, a novel vascular endothelial growth factor receptor binding protein, is involved in reactive oxygen species--dependent endothelial migration and proliferation. Circ Res. 2004;95:276–83. doi: 10.1161/01.RES.0000136522.58649.60. [DOI] [PubMed] [Google Scholar]
- [7].Datla SR, Peshavariya H, Dusting GJ, Mahadev K, Goldstein BJ, Jiang F. Important role of Nox4 type NADPH oxidase in angiogenic responses in human microvascular endothelial cells in vitro. Arterioscler Thromb Vasc Biol. 2007;27:2319–24. doi: 10.1161/ATVBAHA.107.149450. [DOI] [PubMed] [Google Scholar]
- [8].Gonzalez-Pacheco FR, Deudero JJ, Castellanos MC, Castilla MA, varez-Arroyo MV, Yague S, et al. Mechanisms of endothelial response to oxidative aggression: protective role of autologous VEGF and induction of VEGFR2 by H2O2. Am J Physiol Heart Circ Physiol. 2006;291:H1395–H1401. doi: 10.1152/ajpheart.01277.2005. [DOI] [PubMed] [Google Scholar]
- [9].Craige SM, Chen K, Pei Y, Li C, Huang X, Chen C, et al. NADPH oxidase 4 promotes endothelial angiogenesis through endothelial nitric oxide synthase activation. Circulation. 2011;124:731–40. doi: 10.1161/CIRCULATIONAHA.111.030775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Li SM, Zeng LW, Feng L, Chen DB. Rac1-dependent intracellular superoxide formation mediates vascular endothelial growth factor-induced placental angiogenesis in vitro. Endocrinology. 2010;151:5315–25. doi: 10.1210/en.2010-0178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Moldovan L, Moldovan NI, Sohn RH, Parikh SA, Goldschmidt-Clermont PJ. Redox changes of cultured endothelial cells and actin dynamics. Circ Res. 2000;86:549–57. doi: 10.1161/01.res.86.5.549. [DOI] [PubMed] [Google Scholar]
- [12].Abid MR, Spokes KC, Shih SC, Aird WC. NADPH oxidase activity selectively modulates vascular endothelial growth factor signaling pathways. J Biol Chem. 2007;282:35373–85. doi: 10.1074/jbc.M702175200. [DOI] [PubMed] [Google Scholar]
- [13].Hu Q, Zheng G, Zweier JL, Deshpande S, Irani K, Ziegelstein RC. NADPH oxidase activation increases the sensitivity of intracellular Ca2+ stores to inositol 1,4,5-trisphosphate in human endothelial cells. J Biol Chem. 2000;275:15749–57. doi: 10.1074/jbc.M000381200. [DOI] [PubMed] [Google Scholar]
- [14].Li J, Cubbon RM, Wilson LA, Amer MS, McKeown L, Hou B, et al. Orai1 and CRAC Channel Dependence of VEGF-Activated Ca2+ Entry and Endothelial Tube Formation. Circ Res. 2011;108:1190–8. doi: 10.1161/CIRCRESAHA.111.243352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wu KD, Lee WS, Wey J, Bungard D, Lytton J. Localization and quantification of endoplasmic reticulum Ca(2+)-ATPase isoform transcripts. Am J Physiol. 1995;269:C775–C784. doi: 10.1152/ajpcell.1995.269.3.C775. [DOI] [PubMed] [Google Scholar]
- [16].Adachi T, Weisbrod RM, Pimentel DR, Ying J, Sharov VS, Schoneich C, et al. S-Glutathiolation by peroxynitrite activates SERCA during arterial relaxation by nitric oxide. Nat Med. 2004;10:1200–7. doi: 10.1038/nm1119. [DOI] [PubMed] [Google Scholar]
- [17].Tong X, Ying J, Pimentel DR, Trucillo M, Adachi T, Cohen RA. High glucose oxidizes SERCA cysteine-674 and prevents inhibition by nitric oxide of smooth muscle cell migration. J Mol Cell Cardiol. 2008;44:361–9. doi: 10.1016/j.yjmcc.2007.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ying J, Tong X, Pimentel DR, Weisbrod RM, Trucillo MP, Adachi T, et al. Cysteine-674 of the sarco/endoplasmic reticulum calcium ATPase is required for the inhibition of cell migration by nitric oxide. Arterioscler Thromb Vasc Biol. 2007;27:783–90. doi: 10.1161/01.ATV.0000258413.72747.23. [DOI] [PubMed] [Google Scholar]
- [19].Evangelista AM, Thompson MD, Weisbrod RM, Pimental DR, Tong X, Bolotina VM, et al. Redox regulation of SERCA2 is required for VEGF-induced signaling and endothelial cell migration. Antioxid Redox Signal. 2012 doi: 10.1089/ars.2011.4022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Li SY, Yang X, Ceylan-Isik AF, Du M, Sreejayan N, Ren J. Cardiac contractile dysfunction in Lep/Lep obesity is accompanied by NADPH oxidase activation, oxidative modification of sarco(endo)plasmic reticulum Ca2+-ATPase and myosin heavy chain isozyme switch. Diabetologia. 2006;49:1434–46. doi: 10.1007/s00125-006-0229-0. [DOI] [PubMed] [Google Scholar]
- [21].Lavine JA, Raess PW, Davis DB, Rabaglia ME, Presley BK, Keller MP, et al. Contamination with E1A-positive wild-type adenovirus accounts for species-specific stimulation of islet cell proliferation by CCK: a cautionary note. Mol Endocrinol. 2010;24:464–7. doi: 10.1210/me.2009-0384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Haeussler DJ, Evangelista AM, Burgoyne JR, Cohen RA, Bachschmid MM, Pimental DR. Checkpoints in adenoviral production: cross-contamination and E1A. PLoS One. 2011;6:e23160. doi: 10.1371/journal.pone.0023160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Cayatte AJ, Rupin A, Oliver-Krasinski J, Maitland K, Sansilvestri-Morel P, Boussard MF, et al. S17834, a new inhibitor of cell adhesion and atherosclerosis that targets nadph oxidase. Arterioscler Thromb Vasc Biol. 2001;21:1577–84. doi: 10.1161/hq1001.096723. [DOI] [PubMed] [Google Scholar]
- [24].Chen K, Kirber MT, Xiao H, Yang Y, Keaney JF., Jr Regulation of ROS signal transduction by NADPH oxidase 4 localization. J Cell Biol. 2008;181:1129–39. doi: 10.1083/jcb.200709049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Tong X, Ying J, Pimentel DR, Trucillo M, Adachi T, Cohen RA. High glucose oxidizes SERCA cysteine-674 and prevents inhibition by nitric oxide of smooth muscle cell migration. J Mol Cell Cardiol. 2008;44:361–9. doi: 10.1016/j.yjmcc.2007.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Csutora P, Zarayskiy V, Peter K, Monje F, Smani T, Zakharov SI, et al. Activation mechanism for CRAC current and store-operated Ca2+ entry: calcium influx factor and Ca2+-independent phospholipase A2beta-mediated pathway. J Biol Chem. 2006;281:34926–35. doi: 10.1074/jbc.M606504200. [DOI] [PubMed] [Google Scholar]
- [27].Smani T, Zakharov SI, Csutora P, Leno E, Trepakova ES, Bolotina VM. A novel mechanism for the store-operated calcium influx pathway. Nat Cell Biol. 2004;6:113–20. doi: 10.1038/ncb1089. [DOI] [PubMed] [Google Scholar]
- [28].Djordjevic T, Pogrebniak A, BelAiba RS, Bonello S, Wotzlaw C, Acker H, et al. The expression of the NADPH oxidase subunit p22phox is regulated by a redox-sensitive pathway in endothelial cells. Free Radic Biol Med. 2005;38:616–30. doi: 10.1016/j.freeradbiomed.2004.09.036. [DOI] [PubMed] [Google Scholar]
- [29].Petry A, Djordjevic T, Weitnauer M, Kietzmann T, Hess J, Gorlach A. NOX2 and NOX4 mediate proliferative response in endothelial cells. Antioxid Redox Signal. 2006;8:1473–84. doi: 10.1089/ars.2006.8.1473. [DOI] [PubMed] [Google Scholar]
- [30].Tauber AI, Gabig TG, Babior BM. Evidence for production of oxidizing radicals by the particulate O-2-forming system from human neutrophils. Blood. 1979;53:666–76. [PubMed] [Google Scholar]
- [31].Tojo T, Ushio-Fukai M, Yamaoka-Tojo M, Ikeda S, Patrushev N, Alexander RW. Role of gp91phox (Nox2)-containing NAD(P)H oxidase in angiogenesis in response to hindlimb ischemia. Circulation. 2005;111:2347–55. doi: 10.1161/01.CIR.0000164261.62586.14. [DOI] [PubMed] [Google Scholar]
- [32].El JA, Valente AJ, Clark RA. Regulation of phagocyte NADPH oxidase by hydrogen peroxide through a Ca(2+)/c-Abl signaling pathway. Free Radic Biol Med. 2010;48:798–810. doi: 10.1016/j.freeradbiomed.2009.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Chatterjee S, Feinstein SI, Dodia C, Sorokina E, Lien YC, Nguyen S, et al. Peroxiredoxin 6 phosphorylation and subsequent phospholipase A2 activity are required for agonist-mediated activation of NADPH oxidase in mouse pulmonary microvascular endothelium and alveolar macrophages. J Biol Chem. 2011;286:11696–706. doi: 10.1074/jbc.M110.206623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].El-Remessy AB, Al-Shabrawey M, Platt DH, Bartoli M, Behzadian MA, Ghaly N, et al. Peroxynitrite mediates VEGF’s angiogenic signal and function via a nitration-independent mechanism in endothelial cells. FASEB J. 2007;21:2528–39. doi: 10.1096/fj.06-7854com. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. H2O2 has a concentration dependent effect on EC migration. HAEC were treated with H2O2 and migration of the EC monolayer into a scratch wound was assessed over 6 hours (n = 3, *p< 0.05).
Figure S2. Specificity of Nox2 and Nox4 knockdown. A. HAEC were treated with siRNA against Nox2 (siNox2) or an siRNA control (siCon) and assayed for non-specific knockdown of Nox4 mRNA (n = 7). B and C. HAEC were treated with siCon or siNox2, or with shRNA specific for Nox4 (shNox4) or control shRNA (shCon) and specificity of knockdown was assayed by western blot against Nox2 (B, n = 3, *p< 0.05) and Nox4 (C, n=4, *p< 0.05). Nox2 and Nox4 images are from a single western blot and share a loading control.
Figure S3. VEGF stimulates H2O2 production via Nox4 but not Nox2. HAEC in which either Nox2 or Nox4 was knocked down were treated with serum-free media or VEGF (50 ng/mL) for 6 hours. H2O2 production was measured by Amplex Red (n=3, *p<0.05 compared to serum-free siControl or shControl).