Abstract
Cells die by a variety of mechanisms, only some of which have been elucidated in detail. A number of `active' forms of cell death exist in which the cell participates in its own death, including apoptosis, programmed necrosis, mitotic catastrophe, and the recently described ferroptosis, among other processes. Here, we attempt to explain why there are so many different forms of cell death, and propose a distinction between active death that is `suicide' versus `sabotage.'
The history of life might be viewed as a collection of makeshift `fixes' against all of the things that might go wrong (and often do) with any complex mechanism, giving us life when there are so many ways to die. A recent report describes anexample of a novel, active form of cell death, `ferroptosis,' seen in Ras-transformed cells in response to a potentially therapeutic agent (1). Our goal here is not so much to place ferroptosis in the assemblage of death types (`ptosis,' often affixed to names for cell death types, means `fall,' hence our title), but to explore why this variety of modes of cellular catharsis exists at all.
The nomenclature of cell death lists three major forms (`apoptosis,' `autophagic cell death,' and `necrosis,') and notes many minor forms, based on morphology (2) and what is known of the mechanism (3). One sometimes useful distinction we may make is between modes of cell death that are passive (the cell is irreparably damaged, and thus `killed') and those that are active (the cell actively participates in its death, essentially a `suicide, see Figure 1). The former includes massive damage to, and/or disruption of, processes that are essential for the maintenance of life, such as loss of energy required to sustain water and solute distributions across membranes. Whereas passive cell death can be blocked only by eliminating the source of the damage or repairing it, active cell death can potentially be blocked by intervention of the cell's molecular participation, despite the persistence of the death signal. This, however, can be a source of confusion, depending on our experimental criteria for a cell being `alive.'
Figure 1.
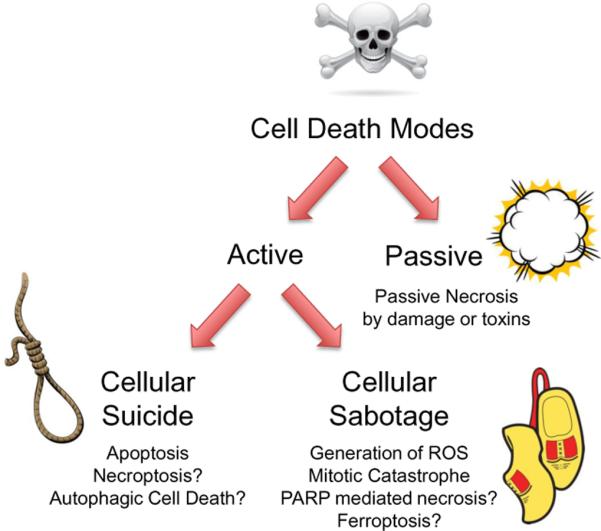
Active and passive cell deaths. Passive cell death occurs when a vital cellular function is directly damaged or inhibited, thus killing the cell. Active cell death can be of two forms. One can be classified as cellular `suicide,' in which a pathway that is specialized for cell death signaling is engaged. The best example of this is apoptosis, although there may be others. Alternatively, cell death can be active -- that is, a cell participates in its own demise -- if cellular processes are `sabotaged' such that the continuation of the process is lethal. This distinction may prove useful in understanding why there appear to be so many ways that cells can actively die (images from iStockphoto.com).
Undoubtedly, the best described form of active cell death is apoptosis, involving the activation and consequences of caspase proteases (4). The most common apoptotic death is via the mitochondrial pathway, characterized by mitochondrial outer membrane permeabilization (MOMP). However, even if caspase activation does not occur, a cell that has undergone MOMP will usually die as a consequence of the organellar catastrophe, although the subsequent death has an appearance distinct from apoptosis (5).
There are other types of active cell death as well. `Pyroptosis,' involving so-called inflammatory caspases, occurs in response to pathogens or other `danger' signals (6). `Necroptosis' involves activation and function of a kinase, RIPK3, leading (somehow) to a distinct necrotic death (7). `Autophagic cell death,' which is accompanied by autophagy (and may or may not (8) require autophagy components for the death to occur) is again a distinct form. As we have noted, there are many ways to die.
What do we mean, though, when we say that a cell `participates' in its suicide? In each case of active cell death we have so far discussed, the death appears to occur as a consequence of specialized molecular events that have presumably evolved to mediate the sacrifice of the cell for the altruistic benefit of the organism.
We can, however, envision another scenario—one that may be far more common: we can imagine a process of cellular sabotage. Intuitively, we know that any complex mechanism can be prone to system `crashes,' wherein a relatively minor disturbance is amplified by running of the machine to result in catastrophic damage. Indeed `sabotage' has its origins in the idea of wooden shoes (sabots) tossed into 15th century looms by hand weavers objecting to the automation of their craft (the appearance of the word in English only in the 20th century has been ascribed to the removal of wooden `shoes' securing rails in a train strike around 1912). In a sense, when such sabotage occurs, the machine participates in its destruction.
Perhaps the best known (if only somewhat understood) example of cell death by sabotage is via the generation of reactive oxygen species when mechanisms to restrict them are impaired, or when oxidases are engaged. Another may be mitotic catastrophe, at least as it is often suggested to occur (9). The process of chromosome segregation of damaged and cross-linked chromosomes leads to cellular destruction. The death is active in the sense that inhibition of mitosis keeps the cell alive. This can be for many years, such as described for lymphocytes in the circulation of survivors of nuclear cataclysms; the cells persist for decades despite the readily discernable chromosomal damage precluding effective cell division (10). Yet another example may be the proposed role of poly-ADP ribose polymerase (PARP) in some forms of necrosis, where it has been proposed that activation of the enzyme so rapidly depletes NADH that the cell dies (11).
Recently, Dixon et al. (1) characterized ferroptosis, which they observed in Ras-transformed cells treated with the drug erastin. The cell death, distinct from apoptosis, requires both iron and new protein synthesis, and erastin appears to target the mitochondrial outer membrane ATP channel, VDAC2 (12). In their recent report, Dixon et al. (1) have now implicated -- in addition to molecules involved in iron metabolism -- enzymes that function in fatty acid synthesis, and inhibition of a plasma membrane glutamate/cysteine antiporter (important for anti-oxidant defense). This leads to the idea that specific (albeit unspecified) oxidized lipids are responsible for the toxic effects of erastin.
While cells actively participate in the process of ferroptosis in response to erastin, it may be worth asking whether this represents a situation relating to cell suicide or that of cell sabotage. It seems unlikely that fatty acid synthesis, iron metabolism, and cysteine uptake were selected in evolution for this form of cell death for the benefit of the individual. To those who seek to understand the mechanism of action of erastin to potentially promote cancer therapy (or, perhaps, as a way to block pathologic excitotoxic death in neurons, which may have a related mechanism (1)), this distinction between sabotage and suicide may well not matter. But as a physiological mechanism, this may be no more a pathway of cell death than is the mitotic machinery.
This brings a different light to our original question: given that there are likely to be many ways in which the machinery of a living cell can be sabotaged to effectively crash the system, why is it that so few mechanisms of active cell death appear to have been selected to be bona fide suicide pathways? What is it about apoptosis (and possibly a few other types of cell death) that has made it overwhelmingly the favored mode of cell death in development and homeostasis throughout the animal kingdom?
The answer to this riddle may lie not in how the cells die, but in the consequences of cell death for the remaining living cells of the organism. Recent ideas about the functions of caspases in the control of inflammation (13), the immunologic consequences of dying cells (14), and cell death as a defense against infection (8) may point the way to resolving this conundrum. Similarly, the signals generated by dying cells that trigger regeneration and repair (15) may ultimately prove fundamental to understanding why cells tend to die as they do. While there are many ways that cells can die -- a pantheon of the fallen -- only a few may have evolved as general mechanisms to benefit the cosmos of the organism. Sometime in the evolution of animals, some forms of sabotage were co-opted to become suicides. Our challenge may be to find out how this event relates to the emergence of multicellularity, and, ultimately, us.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dixon SJ, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–1072. doi: 10.1016/j.cell.2012.03.042. 22632970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kroemer G, et al. Classification of cell death: recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death Differ. 2009;16:3–11. doi: 10.1038/cdd.2008.150. 18846107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Galluzzi L, et al. Molecular definitions of cell death subroutines: recommendations of the Nomenclature Committee on Cell Death 2012. Cell Death Differ. 2012;19:107–120. doi: 10.1038/cdd.2011.96. 21760595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Green DR. Means to an End: Apoptosis and Other Cell Death Mechanisms. Cold Spring Harbor Press; Cold Spring Harbor: 2011. [Google Scholar]
- 5.Tait SW, Green DR. Mitochondria and cell death: outer membrane permeabilization and beyond. Nat Rev Mol Cell Biol. 2010;11:621–632. doi: 10.1038/nrm2952. 20683470. [DOI] [PubMed] [Google Scholar]
- 6.Strowig T, et al. Inflammasomes in health and disease. Nature. 2012;481:278–286. doi: 10.1038/nature10759. 22258606. [DOI] [PubMed] [Google Scholar]
- 7.Green DR, et al. RIPK-dependent necrosis and its regulation by caspases: a mystery in five acts. Mol Cell. 2011;44:9–16. doi: 10.1016/j.molcel.2011.09.003. 21981915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Das G, et al. Regulation and function of autophagy during cell survival and cell death. Cold Spring Harb Perspect Biol. 2012;4 doi: 10.1101/cshperspect.a008813. 22661635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vakifahmetoglu H, et al. Death through a tragedy: mitotic catastrophe. Cell Death Differ. 2008;15:1153–1162. doi: 10.1038/cdd.2008.47. 18404154. [DOI] [PubMed] [Google Scholar]
- 10.Awa AA. Review of thirty years study of Hiroshima and Nagasaki atomic bomb survivors. II. Biological effects. G. Chromosome aberrations in somatic cells. J Radiat Res. 1975;16(Suppl):122–131. doi: 10.1269/jrr.16.supplement_122. 1195195. [DOI] [PubMed] [Google Scholar]
- 11.Bouchard VJ, et al. PARP-1, a determinant of cell survival in response to DNA damage. Exp Hematol. 2003;31:446–454. doi: 10.1016/s0301-472x(03)00083-3. 12829019. [DOI] [PubMed] [Google Scholar]
- 12.Yagoda N, et al. RAS-RAF-MEK-dependent oxidative cell death involving voltage-dependent anion channels. Nature. 2007;447:864–868. doi: 10.1038/nature05859. 17568748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martin SJ, et al. A perspective on mammalian caspases as positive and negative regulators of inflammation. Mol Cell. 2012;46:387–397. doi: 10.1016/j.molcel.2012.04.026. 22633487. [DOI] [PubMed] [Google Scholar]
- 14.Green DR, et al. Immunogenic and tolerogenic cell death. Nat Rev Immunol. 2009;9:353–363. doi: 10.1038/nri2545. 19365408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bergmann A, Steller H. Apoptosis, stem cells, and tissue regeneration. Sci Signal. 2010;3:re8. doi: 10.1126/scisignal.3145re8. 20978240. [DOI] [PMC free article] [PubMed] [Google Scholar]


