Abstract
Molecular and phenotyping techniques were applied to study Salmonella enterica serovar Enteritidis strains both from human cases of infection and of avian origin isolated in Uruguay from 1995 to 2002. A group of 62 isolates was subjected to random amplified polymorphic DNA (RAPD) assay and analysis of antibiotic resistance patterns. Twenty-one of these strains were further characterized by phage typing and analysis of their protein expression profiles. RAPD fingerprinting with five different primers discriminated 10 different genetic profiles. Of the 62 strains tested, 48 had a single major genetic profile, whereas the other nine profiles were evenly distributed among the other strains. The genetic diversity was greater among strains of animal origin than among isolates of human origin. Comparative examination of the results obtained by RAPD analysis and phenotypic analysis and by strain source provided evidence of the reliable discriminatory power of RAPD analysis in our study. Six avian isolates with antibiotic resistance were detected: two were nalidixic acid resistant and four had a particular β-lactam resistance pattern. The last four isolates all had the same unusual phage type (phage type 4b); however, RAPD analysis differentiated them into two groups. Two isolates with unique RAPD profiles were recovered from distinct human cases, suggesting that the technique differentiates unrelated strains. Overall, the results show the existence of a predominant genetic type that is present in poultry and that is transmitted to humans. There are also several other genotypes, but only a few of them could be recovered from human sources, suggesting the existence of different pathogenic traits among strains circulating in the country.
Salmonella enterica subsp. enterica serovar Enteritidis is a major cause of food-borne disease, and during the last two decades it has been isolated worldwide in increasing numbers (13, 42). Poultry and poultry-derived products, in particular, meat and chicken eggs, are considered important sources of human infection with this pathogen (38, 40). In Uruguay, the first outbreak of Salmonella serovar Enteritidis occurred in 1995, and since then the number of isolates has increased. Until 1994, Salmonella serovar Enteritidis had only sporadically been isolated within the country, and Salmonella serovar Typhimurium was the serotype that was isolated the most frequently from all samples (16, 37). The first outbreak of Salmonella serovar Enteritidis was associated with consumption of contaminated egg-containing food and affected an estimated 600 individuals countrywide (Uruguayan Ministry of Public Health). Since then the number of Salmonella serovar Enteritidis strains isolated annually has increased dramatically, and since 1997 it has became the most frequently identified serovar, accounting for more than 50% of all strains received each year at the National Salmonella Center (NSC) and for more than 85% of the strains from humans (our unpublished data). Although the health authorities have maintained a comprehensive policy of surveillance and reporting of every outbreak throughout this period, no detailed analysis of the strains had been performed, until now.
Epidemiological research on Salmonella spp. has traditionally been based on phenotypic approaches. Phage subtyping is a widely accepted method for the typing of Salmonella strains, and it has facilitated epidemiological tracing. However, for Salmonella serovar Enteritidis it has been found that most isolates belong to a limited number of phage types (PTs) (52) and that certain PTs prevail in different geographical locations, suggesting that the discriminatory power of phage typing might be too low for epidemiological analysis of Salmonella serovar Enteritidis. Furthermore, the requirement for specialized phage collections and bacterial strains for their propagation has resulted in a protocol that is routinely practiced in only a few reference laboratories. DNA-based methodologies have the potential for greater discriminatory power; a number of different molecular genotyping techniques such plasmid profile, ribotyping, IS200 profiling, pulsed-field gel electrophoresis (PFGE) (9, 36), and, more recently, PCR-based procedures (i.e., random amplified polymorphic DNA [RAPD] analysis, enterobacterial repetitive intergenic consensus sequence PCR, and fluorescent amplified fragment length polymorphism analysis) have all been applied to study outbreaks of salmonellosis (4, 9, 22, 25, 27, 31, 32). Although a general consensus on a standard typing method has not been achieved so far, overall these approaches have provided useful insights into the evolutionary and epidemiological relationships of strains of several Salmonella serovars, including serovar Enteritidis (3, 8, 14). Since 1995, RAPD-PCR analysis (53, 54) has been used either to differentiate Salmonella serotypes or to distinguish strains within a single serotype (3, 5, 12, 15, 18, 31, 43, 47). It offers good power for discrimination within several Salmonella serotypes, such as Dublin (20), Panama (46), Typhimurium (4), Typhi (43), and Enteritidis (7, 21, 22, 24, 25, 28). One appeal of this method is that a large number of arbitrarily selected primers can be tested to identify those that might be suitable for a particular application. Despite some concerns regarding the reproducibility of the procedure (22, 23, 49), it has been postulated that when an appropriate set of primers is chosen, RAPD analysis may provide an alternative rapid, reproducible, and powerful method for the genetic typing of Salmonella serovar Enteritidis (24, 32).
In this work, we report on the first molecular study of Salmonella serovar Enteritidis strains isolated in Uruguay. The reliable discriminatory power of RAPD analysis was demonstrated when strict criteria for interpretation of the results were used. We also compared the results of RAPD analysis with those of classical phenotyping methods and with the protein expression profiles. The results provide new insights into the genetic diversity of Salmonella serovar Enteritidis strains and suggest the existence of different pathogenic traits within strains circulating in the country.
MATERIALS AND METHODS
Bacterial strains.
A group of 62 isolates held at NSC was selected for the present study. NSC, housed in the Department of Bacteriology and Virology, Institute of Hygiene, School of Medicine, Universidad de la República, Montevideo, Uruguay, participates in the World Health Organization global Salm-Surv program (http://www.who.int/salmsurv/en/); it has catalogued and maintained Salmonella strains of medical, veterinary, and food origin isolated in Uruguay and other South American countries for the last 60 years. The strains selected for the study comprised isolates received at NSC from external laboratories and strains from food-related outbreaks isolated in our own laboratories during routine diagnostic work. Also, strains recovered during a countrywide epidemiological survey of commercially available eggs were included (Table 1).
TABLE 1.
RAPD profiles and phenotypic characteristics of the 62 strains studied
| Yr | Source | No. of strains | Strain designation | RAPD genetic profilea | Phenotypeb |
|---|---|---|---|---|---|
| 1995 | Stool | 6 | 1E, 3E, 1T, 3T, 47/95, 108/95 | AAAAA | — |
| Foodc | 1 | 33/95 | AAAAA | — | |
| Blood | 1 | 39/95 | AAAAA | — | |
| Spinal fluid | 1 | 119/95 | AAAAA | — | |
| 1996 | Stool | 2 | 33/96, 90/96 | AAAAA | — |
| Egg | 1 | 64/96 | AAAAA | — | |
| Feed | 1 | 81/96 | AAAAA | — | |
| Blood | 1 | 41/96 | AAAAA | — | |
| 1997 | Stool | 2 | 1/97, 40/97 | AAAAA | — |
| Bone | 1 | 75/97 | AAAAA | — | |
| Food | 1 | 17/97 | AAAAA | — | |
| Chicken | 1 | 56/97 | AAAAD | — | |
| 1998 | Chicken | 1 | 18/98 | AAAAA | — |
| Feed | 1 | 60/98 | AAAAA | — | |
| Bone | 1 | 80/98 | AADCA | — | |
| Food | 1 | 49/98 | BBBBB | — | |
| Stool | 1 | 14/98 | CCCAA | Dulcitol negative | |
| 1999 | Stool | 3 | 17/99, 100/99, 191/99 | AAAAA | — |
| Food | 1 | 1/99 | AAAAA | — | |
| Chicken | 1 | 116/99 | BBBBB | — | |
| 2000 | Stool | 1 | 125/00 | AAAAA | — |
| Food | 1 | 70/00 | AAAAA | — | |
| Spinal fluid | 1 | 115/00 | AAAAA | — | |
| Urine | 1 | 22/00 | AAAAA | — | |
| Hen | 1 | 32/00 | AAEDC | Nalidixic acid R | |
| 2001 | Stool | 5 | 203, 204, 251, 334, 376, | AAAAA | — |
| Food | 1 | 967 | AAAAA | Nalidixic acid R | |
| Environment | 1 | 37 | AAAAA | — | |
| GRS (2001-2002) | Stool | 11 | 4, 6, 7, 8, 68, 71, 72, 77, 82, 91, 97 | AAAAA | — |
| Eggs | 2 | F, G | AAAAA | — | |
| Stool | 2 | 65, 66 | DDFEE | — | |
| Eggs | 1 | S1 | DDFEE | — | |
| Eggs | 1 | S4 | EFGFF | — | |
| Eggs | 2 | S7, S8 | FFGGF | β-Lactam R | |
| Eggs | 2 | N11, N12 | GGHHG | β-Lactam R |
RAPD profiles other than the major one are in bold-face.
—, no distinctive phenotype; R, antibiotic resistance.
Food refers exclusively eggs or chicken-containing food.
All 62 strains had previously been identified as S. enterica serovar Enteritidis by standard bacteriological and serological methods; 61 of them were indistinguishable from each other, but 1 had a single different biochemical marker (negative for dulcitol fermentation). Of a total of about 700 strains received at NSC from 1995 to 2001, we selected 41 for the study. Since we wanted to test the discriminatory power of RAPD analysis, strain selection was designed to include strains of different origins, from patients with different clinical outcomes, and from different outbreak settings, which would maximize the chance of obtaining evidence of diversity. The other 21 strains comprised a group of isolates (the GRS group) obtained during a 3-month period from December 2001 to February 2002 that, according to previously published definitions (48), were considered epidemiologically related. Eight strains were isolated from a total of 12,400 eggs analyzed during the epidemiological survey mentioned above; and the other 13 strains were obtained from human cases of enteritis associated with egg consumption, either outbreaks or isolated cases, during the same period.
Bacterial growth.
Stock cultures were stored in Luria-Bertani (LB) broth (Sigma Chemical Co., St. Louis, Mo.) with 25% glycerol at −70°C. Cultures were grown in LB broth or were plated on LB agar.
RAPD-PCR.
Samples for RAPD analysis were prepared by boiling a suspension of bacteria. An agar plate with a pure culture was prepared for each strain; and a single colony from each plate was resuspended, washed twice in 150 μl of distilled water (Millipore, Bedford, Mass.), and boiled for 5 min. The suspension was then chilled on ice, and the supernatant collected after centrifugation at 13,000 rpm for 3 min in a microcentrifuge (Biofuge; Heraeus Instruments, Langenselbold, Germany). Five different primers (Operon Qiagen, Alameda, Calif.) previously reported (24) to provide good discriminatory power among Salmonella serovar Enteritidis strains were used, namely, (i) primer P1254 (CCGCAGCCAA), (ii) primer OPB-17 (AGGGAACGAG); (iii) primer 23L (CCGAAGCTGC); (iv) primer OPA-4 (AATCGGGCTG), and (v) primer OPB-15 (CCAGGGTGTT). The PCR mixture contained 3.5 mM MgCl, 0.2 mM deoxynucleoside triphosphates, 2.5 μM primer, 1 U of Taq polymerase, and 10 μl of DNA extract per 40 μl of PCR mixture in 1× PCR buffer (Gibco BRL, Gaithersburg, Md.). Amplification was performed in a PCR express machine (Hybaid, Ashford, United Kingdom) by using the following program: 4 cycles of 94°C for 4 min, 35°C for 4 min, and 72°C for 4 min and then 35 cycles of 94°C for 30 s, 35°C for 1 min, and 72°C for 2 min, followed by a final 10 min at 72°C. The PCR products (20 μl of each sample) were loaded on 2% agarose gels (Ultrapure; Gibco BRL, Gaithersburg, Md.) and electrophoresed in 0.5× Tris-borate buffer by using a 2-liter cube (Bio-Rad, Richmond, Calif.) and 10-cm gels at 140 V and 60 mA for 1.5 h. At least two lanes with a 100-bp ladder (Gibco) were included in each gel for reference. Gels were stained with 5 μg of ethidium bromide per ml for at least 20 min and then rinsed in distilled water for another 15 min and observed under UV light. A digital image of the gel was captured in a computer, and the amplification patterns were evaluated by visual examination of inverted gel pictures.
To confirm the reproducibility of the method, each strain was assayed four times by two different operators who used newly prepared samples and who tested all five primers each time. All other experimental conditions remained unchanged, and a result was considered valid when the same pattern was obtained at least three times.
Antibiotic susceptibility testing.
Antibiotic susceptibility testing was performed by the standard disk diffusion method in Mueller-Hinton agar (33), and the results were interpreted in accordance with the criteria of the National Committee for Clinical Laboratory Standards (34). The strains were screened for resistance to the following antibiotics (Oxoid, Basingstoke, United Kingdom): ampicillin, nalidixic acid, gentamicin, trimethoprim-sulfamethoxazole, tetracycline, and chloramphenicol. Strains showing ampicillin resistance were further tested for their susceptibilities to cephalothin, cefoxitin, cefuroxime, ceftriaxone, ceftazidime, ampicillin-sulbactam, and amoxicillin-clavulanic acid. The double-disk synergy test was used to screen for the production of extended-spectrum β-lactamases as described previously (19). Detection of inducible β-lactamases was performed by the disk approximation test by placing a 30-μg cefoxitin disk near ceftriaxone, ceftazidime, and cefuroxime disks, as described previously (26). Strains showing nalidixic acid resistance were tested for their susceptibilities to ciprofloxacin. In all cases, Escherichia coli ATCC 25922 and ATCC 35218 were used as reference strains.
Phage typing.
Phage typing was performed by using the Salmonella serovar Enteritidis phage typing scheme provided by the Laboratory of Enteric Pathogens, Public Health Laboratory Service, Central Public Health Laboratory, Colindale, United Kingdom (52).
Protein profiles.
Protein expression was evaluated with two different bacterial preparations: an outer membrane protein (OMP)-enriched fraction and a heat-extracted (HE) fraction (35), which is predominantly composed of flagella and other surface proteins. The OMP-enriched fraction was prepared as described previously (29), with modifications. Briefly, a 10-ml overnight culture of the strain in heart infusion broth (Difco, Detroit, Mich.) was harvested by centrifugation at 6,000 × g for 20 min, washed with saline solution, and suspended in 0.8 ml of 10 mM sodium phosphate buffer (pH 7). The suspension was sonicated on ice with three pulses of 1 min each and centrifuged at 16,500 × g for 1 min. The supernatant was collected and placed in a different tube, and the tube was centrifuged at 16,500 × g for 30 min. The pellet was suspended in 1% Sarkosyl, incubated at room temperature for 20 min, and washed with 1% Sarkosyl in 10 mM sodium phosphate buffer (pH 7). The pellet was recovered by centrifugation at 16,500 × g for 30 min and was suspended in 0.1 ml of Laemmli sample buffer. The samples were stored at −20°C until use. HE fractions were obtained from overnight cultures in 1% mannose LB broth, harvested by centrifugation at 1,000 × g for 25 min, suspended in 1 ml of phosphate-buffered saline, and incubated for 1 h at 65°C. The samples were centrifuged, and the supernatant was recovered and stored at −20°C until it was used.
Protein extracts from different strains were all prepared at the same time and by the exact same procedure, and the protein concentrations in all samples were determined by the bicinchoninic acid method (Sigma) according to the instructions of the manufacturer. For comparative analysis, 20 μg of each sample was resolved by standard one-dimensional sodium dodecyl sulfate-polyacrylamide gel electrophoresis on a 12% polyacrylamide gel by using a broad-range prestained protein marker (6 to 175 kDa; New England BioLabs, Hitchin, United Kingdom) in each gel. The gels were stained with silver nitrate, as described previously (44), and visually inspected.
A strain was assigned to a particular protein profile when it produced the same pattern at least three times, and two strains were considered to belong to different groups when they differed by at least three bands.
RESULTS
Reproducibility of RAPD assay.
For analysis of the RAPD amplification patterns, only bands whose sizes fell between 200 and 2,000 bp were considered, since within this range all strains produced completely reproducible patterns, whereas bands outside this range had variations between different runs. Bands were considered present or absent regardless of their intensities, and patterns were considered different when they differed by more than two bands. By use of these criteria the method produced highly reproducible results and the strains could be consistently classified into well-defined groups.
RAPD characterization.
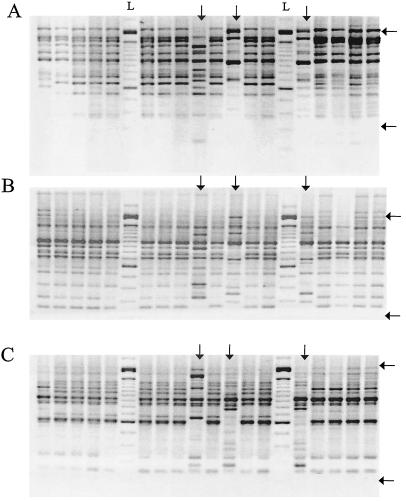
All five primers used were able to discriminate different amplification patterns among the strains. Primers 23L and OPA-4 revealed eight different patterns (identified by the letters A to H, respectively), whereas OPB-15, OPB-17, and P1254 discriminated seven patterns each (A to G, respectively) (Table 2). Figure 1 shows examples of the patterns obtained. With all primers about 80% of the strains had a single major pattern.
TABLE 2.
Distribution of strains by pattern of amplification with each primer
| Pattern | No. of strains with the indicated pattern with the following primera:
|
||||
|---|---|---|---|---|---|
| PI254 | OPB-17 | 23L | OPA-4 | OPB-15 | |
| A | 51 | 51 | 49 | 50 | 50 |
| B | 2 (49/98, 116/99) | 2 (49/98, 116/99) | 2 (49/98, 116/99) | 2 (49/98, 116/99) | 2 (49/98, 116/99) |
| C | 1 (14/98) | 1 (14/98) | 1 (14/98) | 1 (80/98) | 1 (32/00) |
| D | 3 (65, 66, S1) | 3 (65, 66, S1) | 1 (80/98) | 1 (32/00) | 1 (56/97) |
| E | 1 (S4) | 1 (S4) | 1 (32/00) | 3 (65, 66, S1) | 3 (65, 66, S1) |
| F | 2 (S7, S8) | 2 (S7, S8) | 3 (65, 66, S1) | 1 (S4) | 3 (S4, S7, S8) |
| G | 2 (N11, N12) | 2 (N11, N12) | 3 (S4, S7, S8) | 2 (S7, S8) | 2 (N11, N12) |
| H | 2 (N11, N12) | 2 (N11, N12) | |||
The strains are indicated in parentheses.
FIG. 1.
Examples of amplification patterns obtained with primer 23L (A), primer OPA-4 (B), and primer P1254 (C). Amplification patterns other than the major one are indicated with vertical arrows. Horizontal arrows mark the boundaries of the working range (200 to 2,000 bp), as described in Results. Lanes L, 100-bp ladder.
A RAPD profile identification was assigned to each strain by use of a five-letter code that refers to the pattern obtained with each primer (P1254, OPB-17, 23L, OPA-4, and OPB-15, respectively). In this way, a total of 10 different profiles were distinguished. The majority of the isolates studied (48 of 62) had a single major profile (profile AAAAA). The other 14 strains were distributed into nine minor profiles, as follows: one profile accounted for 3 strains, three profiles accounted for 2 strains each, and five profiles each accounted for a unique isolate.
The profile variation was less pronounced among strains isolated from human sources than among avian isolates. Whereas 36 of 40 (90%) strains isolated from humans had the major AAAAA profile, only 12 of 22 (54%) strains isolated from avian or food sources had the AAAAA profile; seven different minor profiles were distributed among the other 10 (46%) strains (Table 1). Isolates from every year had major profile AAAAA, albeit in different proportions.
Antibiotic susceptibility.
Of the 62 strains analyzed, 56 were susceptible to all antibiotics assayed and the other 6 showed some resistance. Two strains were resistant to nalidixic acid and susceptible to all other antibiotics tested. Both of them were isolated from nonhuman sources, the first in 2000 and the other in 2001, and they had different RAPD profiles (AAEDC and AAAAA, respectively). The other four strains showed the same pattern of resistance: resistance to ampicillin, cephalothin, cefoxitin, amoxicillin-clavulanic acid, and ampicillin-sulbactam; intermediate to cefuroxime; and susceptibility to all other tested drugs. All of them produced inducible β-lactamases active against cefuroxime but none of them seemed to produce any β-lactamase activity against extended-spectrum cephalosporins. These four strains were found among eight strains isolated from eggs in 2001 and 2002 and showed two different RAPD profiles (FFGGF for two strains and GGHHG for the other two) that were not found in human isolates.
Table 1 summarizes these results.
Analysis of GRS isolates.
Twenty-one strains isolated during 2001 and 2002 from both commercial eggs and human gastroenteritis cases were evaluated as a group of related strains. Most human isolates (11 of 13) and 2 of 8 egg isolates had the major RAPD profile (AAAAA). However, four other peculiar genotypes were recognized among the remaining strains in the GRS group. Profile DDFEE was seen in three strains (two from humans and one from eggs), whereas the other three profiles were exclusively found in strains isolated from eggs (EEGFF in one strain and FFGGF and GGHHG in two strains each). As mentioned above, four of eight egg-derived strains were resistant to β-lactam antimicrobials.
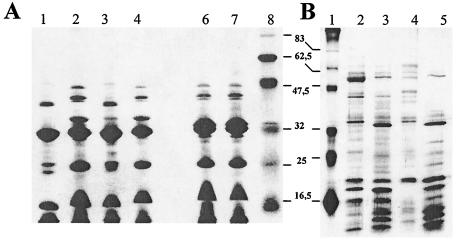
These 21 strains were also subjected to phage typing and analysis of protein expression. Phage typing classified 10 strains as PT 4 (all of them from human cases) and the other 11 strains as PT 4b (8 from eggs and 3 from human sources). The protein expression profiles obtained by the use of two different preparations discriminated six different OMP fraction profiles (profiles I to VI) and seven HE fraction profiles (profiles 1 to 7) (Table 3). Figure 2 shows examples of the profiles obtained with each preparation.
TABLE 3.
RAPD profiles, antibiotic resistance patterns, PTs, and protein expression profiles for 21 isolates (GRS group)
| Source | No. of strains | Strain designation | RAPD profile | PT | Protein profile
|
Antibiotic resistance | |
|---|---|---|---|---|---|---|---|
| OMP | HE fraction | ||||||
| Eggs | 2 | F, G | AAAAA | 4b | I | 1 | |
| 1 | S1 | DDFEE | 4b | IV | 4 | ||
| 1 | S4 | EEGFF | 4b | IV | 4 | ||
| 1 | S7 | FFGGF | 4b | V | 5 | β-Lactam | |
| 1 | S8 | FFGGF | 4b | VI | 6 | β-Lactam | |
| 1 | N11 | GGHHG | 4b | V | 7 | β-Lactam | |
| 1 | N12 | GGHHG | 4b | VI | 7 | β-Lactam | |
| Human (isolated cases) | 3 | 6, 7, 77 | AAAAA | 4b | I | 1 | |
| 1 | 82 | AAAAA | 4 | III | 3 | ||
| 2 | 4, 8 | AAAAA | 4 | I | 1 | ||
| 1 | 68 | AAAAA | 4 | II | 1 | ||
| Human (outbreak 1) | 1 | 72 | AAAAA | 4 | I | 1 | |
| 1 | 71 | AAAAA | 4 | II | 1 | ||
| Human (outbreak 2) | 2 | 65,66 | DDFEE | 4 | IV | 4 | |
| Human (outbreak 3) | 1 | 91 | AAAAA | 4 | I | 2 | |
| Human (outbreak 4) | 1 | 97 | AAAAA | 4 | III | 2 | |
FIG. 2.
Examples of protein profiles obtained with a group of strains. (A) Electrophoretic patterns of OMP extracts. Lanes 6 and 7, profile I; lanes 2 and 4, profile IV; lanes 1 and 3, profile V; lane 8, molecular size marker (the numbers between the panels are in kDa). (B) Electrophoretic patterns of HE preparations. Lane 2, profile 4; lane 3, profile 5; lane 4, profile 6; lane 5, profile 7; lane 1, molecular size marker.
DISCUSSION
We have conducted the first molecular study of S. enterica serovar Enteritidis isolates in Uruguay to look for insights into the genetic diversity of strains that have been circulating in the country since 1995, the starting date for a local shift in the epidemiological behavior of salmonellosis. We applied RAPD-PCR as a subtyping technique and also analyzed the antibiotic susceptibilities of all strains studied.
It has been argued that PCR-based typing methods may lack the sensitivity and reproducibility necessary to be used routinely in epidemiological research and that they cannot stand as reference techniques due to their inability to separate artifact variations from true polymorphisms (22, 23, 49). However, it has been postulated that RAPD analysis may have good discriminatory power for the differentiation of Salmonella strains (5, 7, 12, 14, 45, 47), and some investigators have also reported that RAPD analysis has greater discriminatory power than PFGE for the differentiation of Salmonella serovar Enteritidis strains (17, 32). In our study, we used five different primers that have previously been reported (24) to have good discriminatory power when they are used for RAPD analysis of Salmonella serovar Enteritidis strains. Working with these primers and the criteria for experimentation and analysis proposed herein, we have found that RAPD-PCR yields highly reproducible results that discriminate true polymorphisms beyond serotype and PT. Although the band patterns for a particular strain may include one or more bands that differ in intensity between assays, each strain was always classified within the same RAPD group.
We did not calculate Simpson's index of diversity for the method, since we considered that the strains used in this study could not have been defined as unrelated. The characteristic of these strains, i.e., isolation in a small country with no important geographical barriers, precluded us from making such an assumption. However, comparative analysis of the results obtained by RAPD analysis and the phenotyping tests provided strong evidence of the reliable discriminatory power of RAPD-PCR, beyond phenotypic characteristics. Four strains isolated from eggs showed the same pattern of β-lactam resistance and belonged to a single unusual PT (PT 4b) (Table 3). However, RAPD analysis divided them into two groups (strains S7 and S8 and strains N11 and N12). Interestingly, these strains have unique RAPD profiles that were not present in any other strains studied, and each of the two groups could be traced to a single farm.
Two strains exhibited resistance to nalidixic acid and had different RAPD profiles, despite their similar animal origins. One of them was classified in the major RAPD group, suggesting that mutations that produce this resistance pattern do not affect the RAPD profile. We further demonstrated this fact by subjecting several strains to antibiotic pressure in order to induce a nalidixic acid-resistant phenotype and showing that their RAPD patterns with any of the five primers were not altered by this procedure (data not shown). The most frequent mechanism of resistance to nalidixic acid involves a single mutation in the gyr gene, which codes for DNA gyrase (11), and thus would not be detected by RAPD analysis. Similar results have recently been reported in a study in which RAPD analysis of more than 400 strains with a single primer classified most of the drug-susceptible strains and the nalidixic acid-resistant strains in a single RAPD profile and the ampicillin-resistant strains in a different profile (45).
Two human isolates each had unique RAPD profiles (profiles AADCA and CCCAA, respectively), and those profiles were not found in samples of animal origin. Interestingly, these two strains were from distinct cases. One of them (strain 80/98) was isolated from a bone aspirate of a patient with osteomyelitis, and the other (strain 14/98) was isolated from a stool culture of a patient who had recently arrived from abroad, confirming that the technique differentiates unrelated or peculiar isolates.
The group of 21 strains considered epidemiologically related was also evaluated by phage typing and protein expression analysis. Phage typing revealed two different PTs: PT 4, which was only found for human isolates (10 strains), and PT 4b, which included all strains recovered from eggs and 3 human isolates. However, evaluation of the same GRS group of strains by RAPD analysis discriminated two and five different profiles among the PT 4 and PT 4b isolates, respectively (Table 3), and in many cases those differences in RAPD profiles were associated with differences in protein expression. Some protein expression profiles were exclusively observed among strains with the major RAPD profile (OMP fraction profiles I to III and HE fraction profiles 1 to 3), whereas the other profiles were observed among egg and human isolates that had other RAPD profiles (Table 3). Two human strains recovered from a single outbreak (strains 65 and 66) had the same RAPD profile (DDFEE) that was also found for an egg isolate (strain S1). These three strains were grouped together because they had the same OMP and HE fraction profiles and may be considered closely related; but whereas both human isolates belonged to PT 4, the egg isolate belonged to PT 4b. PT 4b is a very rare group (50), and to the best of our knowledge it has not been previously reported to have been isolated from eggs. Classification into PT 4b is due to the presence of an extra phage receptor (52), and PT variation during circulation in the field has been reported for Salmonella serovar Enteritidis (2, 41). The results could suggest that the absence of such a receptor may represent an advantage for transmission of the strain to humans, and in this regard, it would be interesting to conduct further genetic analyses or virulence assays with these otherwise identical strains.
Altogether, the results presented here allowed us to conclude that RAPD analysis can be a useful tool for the analysis of Salmonella serovar Enteritidis, provided that strict experimental protocols are maintained. RAPD-PCR yields reproducible results in different assays, even with different operators. Although the establishment of an international library based on RAPD analysis of the strains could prove difficult due to low intercenter reproducibility (10), the method might be used as a cost-effective tool for molecular epidemiology research in intracenter studies.
On the other hand, our results suggest that the strains circulating in Uruguay are unevenly distributed among different subtypes, with the vast majority of isolates having a single major genetic profile. The genetic diversity observed was markedly greater among strains of animal origin than among human isolates. Most of the strains of human origin (36 of 40) had the major RAPD profile, whereas almost half of the strains from animals or poultry-derived food (10 of 22) had distinctive profiles distributed among seven different minor genotypes. This provides a picture different from that presented in studies in which a balanced distribution of different genotypes has been found, as evaluated by either RAPD analysis, ribotyping, or PFGE (8, 23, 24). The high degree of homogeneity among Salmonella serovar Enteritidis isolates has also been described in studies in which all strains analyzed belonged to a single PT, particularly PT 4 (21, 27). In our study, we found that most human isolates belonged to PT 4. Also, a group of strains from the first two outbreaks in 1995 were phage typed at that time at the Central Public Health Laboratory in the United Kingdom, and all of them belonged to PT 4. This could suggest that the predominance of a single genetic profile among human isolates in Uruguay may be related to the high rate of occurrence of PT 4.
Among the seven different minor genetic types found for strains of animal origin, only one (profile DDFEE; Tables 2 and 3) was also found in human strains. This may indicate a restrictive pattern of transmission to humans due to differences in pathogenicity or to uneven survival capacities in the environment. Further research directed toward the comparative analysis of the virulence attributes of these strains could provide a basis for understanding this phenomenon.
Several strains with some antibiotic resistance were recovered from chicken eggs or chicken-containing food. Four of six antibiotic-resistant strains showed the same patterns of resistance to β-lactams. The most frequently described ampicillin resistance mechanism is inactivation of the drug by β-lactamases, encoded by bla genes, which may be located either on plasmids or on the chromosome (6). The strains from this study showed inducible β-lactamase production, suggesting that they may carry a mechanism of regulation of β-lactamase expression similar to the AmpC-AmpR mechanisms encoded by the chromosomes of some enterobacteria (26). Salmonella resistance is usually due to class A β-lactamases (Ambler classification) (30) or noninducible plasmid-encoded AmpC-like β-lactamases (39), but the transfer of AmpC-AmpR from Morganella morganii to Salmonella serovar Enteritidis has recently been reported (1, 51). Preliminary studies in our laboratories revealed that the β-lactam resistance pattern found in our strains is due to an enzyme with an isoelectric point near 8, similar to those described by Barnaud et al. (1). However, our strains remained susceptible to oximinocephalosporins. Further studies are necessary to characterize this β-lactamase activity in Salmonella.
In summary, our results suggest that an endemic strain of Salmonella serovar Enteritidis is circulating in Uruguay and that it is present in poultry-derived food and is transmitted to humans, causing enteric disease. Several other genotypes are also present in poultry, but only a few of them could be recovered from human sources. Overall, the results suggest the existence of different pathogenic traits among the strains.
These results provide information that could help define effective national control policies and stress the importance of using carefully designed epidemiological surveys that evaluate strains at the molecular level.
Acknowledgments
This work was supported by grants from the National Institute of Agricultural Research of Uruguay and C.S.I.C.
We thank María Victoria Repiso, Alicia Rigoli, Gerardo Giossa, and Patricia Barrios for technical assistance.
REFERENCES
- 1.Barnaud, G., G. Arlet, C. Verdet, O. Gaillot, P. H. Lagrange, and A. Philippon. 1998. Salmonella enteritidis: AmpC plasmid-mediated inducible β-lactamase (DHA-1) with an ampR gene from Morganella morganii. Antimicrob. Agents Chemother. 42:2352-2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown, D. J., D. L. Baggesen, D. J. Platt, and J. E. Olsen. 1999. Phage type conversion in Salmonella enterica serotype Enteritidis caused by the introduction of a resistance plasmid of incompatibility group X (IncX). Epidemiol. Infect. 122:19-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burr, M. D., K. L. Josephson, and I. L. Pepper. 1998. An evaluation of ERIC PCR and AP PCR fingerprinting for discriminating Salmonella serotypes. Lett. Appl. Microbiol. 27:24-30. [DOI] [PubMed] [Google Scholar]
- 4.Carraminana, J. J., F. Humbert, G. Ermel, and P. Colin. 1997. Molecular epidemiological investigation of Salmonella typhimurium strains related to an egg-borne outbreak. Res. Microbiol. 7:633-636. [DOI] [PubMed] [Google Scholar]
- 5.Chansiripornachai, N., P. Ramasoota, A. Bangtrakuknonth, J. Sasipreeyajan, and S. B. Svenson. 2000. Application of randomly amplified polymorphic DNA (RAPD) analysis for typing avian Salmonella enterica subsp. enterica. FEMS Immunol. Med. Microbiol. 29:221-225. [DOI] [PubMed] [Google Scholar]
- 6.Cruchaga, S., A. Echeita, A. Aladuena, J. Garcia-Pena, N. Frias, and M. A. Usera. 2001. Antimicrobial resistance in salmonellae from humans, food and animals in Spain in 1998. J. Antimicrob. Chemother. 47:315-321. [DOI] [PubMed] [Google Scholar]
- 7.De Cesare, A., G. Manfreda, T. R. Dambaugh, M. E. Guerzoni, and A. Franchini. 2001. Automated ribotyping and random amplified polymorphic DNA analysis for molecular typing of Salmonella Enteritidis and Salmonella Typhimurium strains isolated in Italy. J. Appl. Microbiol. 91:780-785. [DOI] [PubMed] [Google Scholar]
- 8.Desai, M., E. J. Threlfall, and J. Stanley. 2001. Fluorescent amplified-fragment length polymorphism subtyping of the Salmonella enterica serovar Enteritidis phage type 4 clone complex. J. Clin. Microbiol. 39:201-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dorn, C. R., R. Silapanuntakul, E. J. Angrick, and L. D. Shipman. 1993. Plasmid analysis of Salmonella enteritidis isolated from human gastroenteritis cases and from epidemiologically associated poultry flocks. Epidemiol. Infect. 111:239-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garaizar, J., N. Lopez-Molina, I. Laconcha, D. L. Baggesen, A. Rementeria, A. Vivanco, A. Audicana, and I. Perales. 2000. Suitability of PCR fingerprinting, infrequent-restriction-site PCR, and pulsed-field gel electrophoresis, combined with computerized gel analysis, in library typing of Salmonella enterica serovar Enteritidis. Appl. Environ. Microbiol. 66:5273-5281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giraud, E., A. Brisabois, J. L. Martel, and E. Chaslus-Dancla. 1999. Comparative studies of mutations in animal isolates and experimental in vitro- and in vivo-selected mutants of Salmonella spp. suggest a counterselection of highly fluoroquinolone-resistant strains in the field. Antimicrob. Agents Chemother. 43:2131-2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo, L., J. Killefer, P. B. Kenney, and J. D. Amick-Morris. 1999. Use of arbitrarily primed polymerase chain reaction to study Salmonella ecology in a turkey production environment. Poult. Sci. 78:24-31. [DOI] [PubMed] [Google Scholar]
- 13.Herikstad, H., Y. Motarjemi, and R. V. Tauxe. 2002. Salmonella surveillance: a global survey of public health serotyping. Epidemiol. Infect. 129:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hilton, A. C., J. G. Banks, and C. W. Penn. 1996. Random amplification of polymorphic DNA (RAPD) of Salmonella: strain differentiation and characterization of amplified sequences. J. Appl. Bacteriol. 81:575-584. [DOI] [PubMed] [Google Scholar]
- 15.Hilton, A. C., J. G. Banks, and C. W. Penn. 1997. Optimization of RAPD for fingerprinting Salmonella. Lett. Appl. Microbiol. 24:243-248. [DOI] [PubMed] [Google Scholar]
- 16.Hormaeche, C. E., R. De Marco, F. Schelotto, C. Alia de Montero, C. N. Rivas, D. Mutti, and N. Bello. 1977. Frecuencia de serotipos identificados en el Centro de Salmonelas de Montevideo. Rev. Urug. Patol. Clin Microbiol. 15-16:43-47. [Google Scholar]
- 17.Hudson, C. R., M. Garcia, R. K. Gast, and J. J. Maurer. 2001. Determination of close genetic relatedness of the major Salmonella Enteritidis phage types by pulse field gel electrophoresis and DNA sequence analysis of several Salmonella virulence genes. Avian Dis. 45:875-886. [PubMed] [Google Scholar]
- 18.Hudson, C. R., C. Quist, M. D. Lee, K. Keyes, S. V. Dodson, C. Morales, S. Sanchez, D. G. White, and J. J. Maurer. 2000. Genetic relatedness of Salmonella isolates from nondomestic birds in southeastern United States. J. Clin. Microbiol. 38:1860-1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jarlier, V., M. H. Nicolas, G. Fournier, and A. Philippon. 1988. Extended broad-spectrum beta-lactamases conferring transferable resistance to newer beta-lactam agents in Enterobacteriaceae: hospital prevalence and susceptibility patterns. Rev. Infect. Dis. 10:867-878. [DOI] [PubMed] [Google Scholar]
- 20.Kerouanton, A., A. Brisabois, J. Grout, and B. Picard. 1996. Molecular epidemiological tools for Salmonella Dublin typing. FEMS Immunol. Med. Microbiol. 14:25-29. [DOI] [PubMed] [Google Scholar]
- 21.Laconcha, I., N. Lopez-Molina, A. Rementeria, A. Audicana, I. Perales, and J. Garaizar. 1998. Phage typing combined with pulse-field gel electrophoresis and random amplified polymorphic DNA increases discrimination in the epidemiological analysis of Salmonella enteritidis strains. Int. J. Food Microbiol. 40:27-34. [DOI] [PubMed] [Google Scholar]
- 22.Landeras, E., M. A. Gonzalez Hevia, and M. C. Mendoza. 1998. Molecular epidemiology of Salmonella serotype Enteritidis: Relationship between food, water and pathogenic strains. Int. J. Food Microbiol. 43:81-90. [DOI] [PubMed] [Google Scholar]
- 23.Liebana, E., L. Garcia Migura, M. F. Breslin, H. R. Davies, and M. J. Woodward. 2001. Diversity of strains of Salmonella enterica serotype Enteritidis from English poultry farms assessed by multiple genetic fingerprinting. J. Clin. Microbiol. 39:154-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin, A. W., M. A. Usera, T. J. Barrett, and R. A. Godsby. 1996. Application of random amplified polymorphic DNA analysis to differentiate strains of Salmonella enteritidis. J. Clin. Microbiol. 34:870-876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ling, J. M., I. C. Koo, K. M. Kam, and A. F. Cheng. 1998. Antimicrobial susceptibilities and molecular epidemiology of Salmonella enterica serotype Enteritidis strains isolated in Hong Kong from 1986 to 1996. J. Clin. Microbiol. 36:1693-1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Livermore, D. M. 1995. β-Lactamases in laboratory and clinical resistance. Clin. Microbiol. Rev. 8:557-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lukinmaa, S., R. Schildt, T. Rinttila, and A. Siitonen. 1999. Salmonella Enteritidis phage types 1 and 4: pheno- and genotypic epidemiology of recent outbreaks in Finland. J. Clin. Microbiol. 37:2176-2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mare, L., L. M. Dick, and M. L. Van der Walt. 2001. Characterization of South African isolates of Salmonella enteritidis by phage typing, numerical analysis of RAPD-PCR banding patterns and plasmid profiles. Int. J. Food Microbiol. 64:237-245. [DOI] [PubMed] [Google Scholar]
- 29.Martinez, R. J. 1983. Plasmid-mediated and temperature-regulated surface properties of Yersinia enterocolitica. Infect. Immun. 41:921-930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Medeiros, A. 1997. Evolution and dissemination of β-lactamases accelerated by generations of β-lactam antibiotics. Clin. Infect. Dis. 24:S19-S45. [DOI] [PubMed] [Google Scholar]
- 31.Millemann, Y., M. C. Lesage-Descauses, J. P. Lanfont, and E. Chaslus-Dancla. 1996. Comparison of random amplified polymorphic DNA analysis and enterobacterial repetitive intergenic consensus-PCR for epidemiological studies of Salmonella. FEMS Immunol. Med. Microbiol. 14:129-134. [DOI] [PubMed] [Google Scholar]
- 32.Muresu, E., A. Piana, A. Azara, I. Maida, A. Nastasi, S. D. Sajid, and S. Rubino. 2001. Clonal relations among Salmonella enteritidis phage type 3 outbreaks isolates traced by DNA fingerprinting. New Microbiol. 24:371-377. [PubMed] [Google Scholar]
- 33.National Committee for Clinical Laboratory Standards. 2000. Performance standards for antimicrobial disks susceptibility tests. Approved standard, 7th ed., vol. 20, no. 1, M2-A7. NCCLS, Wayne, Pa.
- 34.National Committee for Clinical Laboratory Standards. 2001. Performance standards for antimicrobial susceptibility testing; eleventh informational supplement, vol. 21, no. 1, M100-S11. NCCLS, Wayne, Pa.
- 35.Nicholas, R. A. J., and G. A. Cullen. 1991. Development and application of an ELISA for detecting antibodies to Salmonella enteritidis in chicken flocks. Vet. Rec. 128:74-76. [DOI] [PubMed] [Google Scholar]
- 36.Olsen, J. E., M. N. Skov, E. J. Threfall, and D. J. Brown. 1994. Clonal lines of Salmonella enterica serotype Enteritidis documented by IS200-, ribo-, pulse field gel electrophoresis and RFLP typing. J. Med. Microbiol. 40:15-22. [DOI] [PubMed] [Google Scholar]
- 37.Peluffo, C. A., C. E. Hormaeche, and M. C. Coubria. 1971. Frecuencia de tipos serológicos clasificados en el Centro Nacional de Salmonelas. Rev. Urug. Patol. Clin. Microbiol. 9:143-150. [Google Scholar]
- 38.Perales, L., and A. Audicana.1988. Salmonella enteritidis and eggs. Lancet ii:1133. [DOI] [PubMed] [Google Scholar]
- 39.Philippon, A., G. Arlet, and G. A. Jacoby. 2002. Plasmid-determined AmpC-type β-lactamases. Antimicrob. Agents Chemother. 46:1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rampling, A. 1993. Salmonella enteritidis five years on. Lancet 342:317-318. [DOI] [PubMed] [Google Scholar]
- 41.Rankin, S., and D. J. Platt. 1995. Phage conversion in Salmonella enterica serotype Enteritidis: implications for epidemiology. Epidemiol. Infect. 114:227-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rodrigue, D. C., R. V. Tauxe, and B. Rowe. 1990. International increase in Salmonella enteritidis: a new pandemic? Epidemiol. Infect. 105:21-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shangkuan, Y. H., and H. C. Lin. 1998. Application of random amplified polymorphic DNA analysis to differentiate strains of Salmonella typhi and other Salmonella species. J. Appl. Microbiol. 85:693-702. [DOI] [PubMed] [Google Scholar]
- 44.Shevchenko, A., M. Wilm, O. Vorm, and M. Mann. 1996. Mass spectrometric sequencing of proteins from silver-stained polyacrylamide gels. Anal. Chem. 68:850-858. [DOI] [PubMed] [Google Scholar]
- 45.Soto, S. M., M. A. Gonzalez-Hevia, and M. C Mendoza. 2003. Antimicrobial resistance in clinical isolates of Salmonella enterica serotype Enteritidis: relationships between mutations conferring quinolone resistance, integrons, plasmids and genetic types. J. Antimicrob. Chemother. 51:1287-1291. [DOI] [PubMed] [Google Scholar]
- 46.Soto, S. M., B. Guerra, A. Del Cerro, M. A. Gonzalez-Hevia, and M. C. Mendoza. 2001. Outbreaks and sporadic cases of Salmonella serovar Panama studied by DNA fingerprinting and antimicrobial resistance. Int J. Food Microbiol. 71:35-43. [DOI] [PubMed] [Google Scholar]
- 47.Soto, S. M., B. Guerra, M. A. Gonzalez-Hevia, and M. C. Mendoza. 1999. Potential of three-way randomly amplified polymorphic DNA analysis as a typing method for twelve Salmonella serotypes. Appl. Environ. Microbiol. 65:4830-4836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tenover, F. C., R. D. Arbeit, R. V. Goering, P. A. Mickelsen, B. E. Murray, D. H. Persing, and B. Swaminathan. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis. J. Clin. Microbiol. 33:2233-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tyler, K. D., G. Wang, S. D. Tyler, and W. M. Johnson. 1997. Factors affecting reliability and reproducibility of amplification-based DNA fingerprinting of representative bacterial pathogens. J. Clin. Microbiol. 35:339-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van Duynhoven, Y. T., M. A. Widdowson, C. M de Jager, T. Fernandes, S. Neppelenbroek, W. van den Brandhof, W. J. Wannet, J. A. van Kooij, H. J. Rietveld, and W. van Pelt. 2002. Salmonella enterica serotype Enteritidis phage type 4b outbreak associated with bean sprouts. Emerg. Infect. Dis. 8:440-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Verdet, C., G. Arlet, G. Barnaud, P. H. Lagrange, and A. Philippon. 2000. A novel integron in Salmonella enteritidis serovar Enteritidis, carrying the blaDHA-1 gene and its regulator gene ampR, originated from Morganella morganii. Antimicrob. Agents Chemother. 44:222-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ward, L. R., J. D. H. de Sa, and B. Rowe. 1987. A phage typing scheme for Salmonella enteritidis. Epidemiol. Infect. 99:291-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Welsh, J., and McClelland. 1990. Fingerprinting genomes using PCR with arbitrary primers. Nucleic Acids Res. 18:7213-7218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Williams, J. G. K., A. R. Kubelik, K. J. Liviak, J. A. Rafalsky, and S. V. Tingey. 1990. DNA polymorphisms amplified by arbitrary primers are useful as genetic markers. Nucleic Acids Res. 18:6531-6535. [DOI] [PMC free article] [PubMed] [Google Scholar]