Abstract
BACKGROUND
From 2001 through March 2006, Planned Parenthood health centers throughout the United States provided medical abortion (abortion by means of medication) principally by a regimen of oral mifepristone followed 24 to 48 hours later by vaginal misoprostol. In response to concern about serious infections, in early 2006 Planned Parenthood changed the route of misoprostol administration from vaginal to buccal and required either routine provision of antibiotics or universal screening and treatment for chlamydia; in July 2007, Planned Parenthood began requiring routine treatment with antibiotics for all medical abortions.
METHODS
We performed a retrospective analysis assessing the rates of serious infection after medical abortion during a time when misoprostol was administered vaginally (through March 2006), as compared with rates after a change to buccal administration of misoprostol and after initiation of additional infection-reduction measures.
RESULTS
Rates of serious infection dropped significantly after the joint change to buccal misoprostol from vaginal misoprostol and to either testing for sexually transmitted infection or routine provision of antibiotics as part of the medical abortion regimen. The rate declined 73%, from 0.93 per 1000 abortions to 0.25 per 1000 (absolute reduction, 0.67 per 1000; 95% confidence interval [CI], 0.44 to 0.94; P<0.001). The subsequent change to routine provision of antibiotics led to a further significant reduction in the rate of serious infection — a 76% decline, from 0.25 per 1000 abortions to 0.06 per 1000 (absolute reduction, 0.19 per 1000; 95% CI, 0.02 to 0.34; P = 0.03).
CONCLUSIONS
The rate of serious infection after medical abortion declined by 93% after a change from vaginal to buccal administration of misoprostol combined with routine administration of antibiotics.
The Planned Parenthood Federation of America (PPFA) is a federation of 97 independent local affiliates operating 880 health centers throughout the United States; roughly 300 of those health centers provide medical abortion. In 2008, a total of 96,738 women received medical abortions (abortion by means of medication), representing 32% of first-trimester abortions in Planned Parenthood health centers. Extensive data gathering during the use of mifepristone and vaginal misoprostol indicated that efficacy (successful medical abortion without the need for surgical intervention) was 98.5%; a subsequent audit of abortions performed with the use of buccal rather than vaginal misoprostol showed virtually identical efficacy.1
Antibiotics have been routinely administered at the time of surgical abortions since the publication of a meta-analysis showing that their use resulted in a 42% reduction in postabortion infection rates.2 When medical abortion was first introduced, there was little concern about the risk of infection, because there is no use of instruments in the cervix or uterus unless the procedure fails. However, it is clear that serious infections do occur.3–6
Data are lacking to compare the rates of serious infection with antibiotic treatment and the rates without such treatment among women undergoing medical abortion. The Food and Drug Administration (FDA) states that it “does not have sufficient information to recommend the use of prophylactic antibiotics for women having a medical abortion.”7 The current American College of Obstetricians and Gynecologists Practice Bulletin on medical abortion states that no data exist to support the routine use of preventive antibiotics for medical abortion.8
By late 2005, four women in the United States and one in Canada had died from a rare bacterial infection, with Clostridium sordellii, after medical abortion with mifepristone and misoprostol.9 In contrast, no such deaths had been reported in Europe, where medical abortion had been available longer and far more women had used it. One hypothesis for the difference was that vaginal administration of misoprostol was very common in the United States but not so common in Europe.10 Another hypothesis was that periprocedural antibiotics were routinely provided in the United Kingdom but not in the United States.
Prompted by the deaths that occurred after medical abortion and internal data that show a higher-than-expected rate of serious infection, PPFA changed its medical abortion protocol at the end of March 2006. Vaginal administration of misoprostol was discontinued and replaced by buccal (or, much less commonly, oral) administration, and all health centers were required to use one of the following two regimens, with the intention of reducing the risk of infection: the routine administration of antibiotics or universal testing for chlamydia (and for gonorrhea when considered appropriate), with treatment dependent on test results. After reviewing the rates of serious infection among health centers that were using these two infection-reduction regimens, PPFA in July 2007 required all health centers to provide routine preventive antibiotic treatment. This report compares rates of serious infection before and after these changes in protocol.
METHODS
STUDY DESIGN
We obtained information about all patients who had a medical abortion from all 78 Planned Parenthood affiliates that provided this service at any time during the entire study period. A quarterly survey has been conducted by an administrator since 2001 to determine the number of patients undergoing medical abortion and the number of health centers providing it. In addition, all Planned Parenthood affiliates send a yearly report to the national office detailing the number of each clinical procedure provided (including medical and surgical abortions), and that report was used to verify the number of medical abortions in the quarterly surveys. Concordance between the two sources is high, with the annual reports containing 1.3% fewer cases than the quarterly reports; we used the caseload reported in the quarterly report, because the administrator of that report had much more frequent contact with the health professionals who reported these data than did the national office, collected data on a quarterly rather than an annual basis, and collected information only about medical abortions.
The Allendale Investigational Review Board approved the study protocol and design as a retrospective analysis of data routinely collected for quality control. The board determined that the use of these data did not require patients’ consent.
The provisions of FDA approval stipulate that any physician who orders, provides, or supervises the provision of mifepristone must sign an agreement with the sole U.S. distributor of mifepristone (Danco Laboratories) to report all serious adverse events associated with its use. Serious adverse events include all ongoing pregnancies (pregnancies that continue after the use of mifepristone or misoprostol), hemorrhage requiring emergency treatment, serious infections, hospitalizations, potentially life-threatening events, and death. Danco submits all such reports to the FDA. Staff members at Planned Parenthood health centers were trained in accurate and complete reporting of serious adverse events. Adverse-event reports are centrally tracked and monitored. Planned Parenthood health centers are audited on site for internal accreditation by the PPFA. Since 2005, concurrent with the starting date of our analysis, the accreditation process has included auditing to verify that adverse events related to the use of mifepristone for medical abortions are submitted as required.
Because the diagnosis of mild postabortion infection is clinically highly subjective and there is substantial variation in how it is defined, we focused solely on serious infections. We classified as serious infections cases in which the patient had fever accompanied by pelvic pain and was treated with intravenous antibiotics either in an emergency department or inpatient unit, or cases in which sepsis or death caused by infection was documented. Information that generates reports of serious infection may arise from the patient, from the Planned Parenthood physician providing initial care or aftercare, or from a physician providing care for the patient in a hospital emergency department or inpatient unit. Many Planned Parenthood sites provide training to obstetrics and gynecology residents, and they were often the source of reports when Planned Parenthood patients were seen in the emergency department or hospitalized.
Follow-up visits routinely scheduled 1 to 2 weeks after ingestion of mifepristone provide an additional opportunity to evaluate whether a serious adverse event has occurred. The importance of the follow-up visit is emphasized to patients, and staff members are required to make three attempts to reach patients who have not returned for follow-up by the end of 2 weeks. However, information on the proportion of women who did not return for follow-up was not available through quarterly or yearly reports. The required attempts to contact patients uncovered several reports of emergency procedures at hospitals that meet the criteria for serious adverse events. In addition, surveillance by the Centers for Disease Control and Prevention (CDC) through multiple channels to identify deaths from infection-related causes after medical abortion did not find any cases other than those already known.
After March 2006, PPFA changed the route of administration of misoprostol from vaginal to buccal (200 mg of mifepristone followed 24 to 48 hours later by 800 µg of buccal misoprostol) or, much less commonly, to oral administration. In addition, Planned Parenthood health centers that provide medical abortion were required either to screen all patients for chlamydia (and gonorrhea if endemic rates or the patient’s history or symptoms indicated the need) or to routinely provide prophylactic use of doxycycline (100 mg orally twice a day for 7 days, starting the same day as mifepristone administration) to all women. Doxycycline was chosen because it provides treatment against chlamydia, the most commonly reported sexually transmitted infection (STI) in the United States, and most gonorrhea strains11; doxycycline also has in vitro efficacy against C. sordellii.12 Theoretically, it might prevent an ascending infection and sepsis. Patients who had a positive test for an STI were treated with the standard CDC treatment recommendations, consisting of doxycycline for chlamydia (100 mg orally twice daily for 7 days), and ceftriaxone for gonorrhea (125 mg intramuscularly in a single dose).
STUDY PROCEDURES
We conducted analyses of events for the years 2005 to mid-2008 during four periods. Period 1 (January 1, 2005, through March 31, 2006) was the baseline 15-month period during which vaginal misoprostol and standard antiseptic measures were used for the abortion of fetuses through 63 days of gestation. Period 2 (April 1, 2006, through June 30, 2007) was the 15-month period during which buccal misoprostol was used through 56 days of gestation13 (or, much less commonly, oral misoprostol was used through 49 days of gestation); some Planned Parenthood clinics used the infection-reduction measure of universal screening for STI and treatment when screening was positive, whereas others routinely provided antibiotics consisting nearly uniformly of 100 mg of oral doxycycline twice a day for 7 days. Period 3 (July 1, 2007, through December 31, 2007) was the 6-month period during which buccal misoprostol was used through 56 days of gestation and all health centers routinely provided the doxycycline regimen. Period 4 (January 1, 2008, through June 30, 2008) was the 6-month period during which buccal misoprostol was used through 63 days of gestation14 and all health centers routinely provided the doxycycline regimen.
Rates of serious infection in all four periods were evaluated overall. They were also evaluated separately among two groups of health centers — health centers that switched in Period 2 to universal testing for STI (screen-and-treat) (Group 1), and health centers that switched in Period 2 to routine provision of antibiotics (Group 2).
Data were obtained from all Planned Parenthood health centers that provide medical abortion. For the present analyses, we excluded affiliates that did not provide medical abortion in all four periods and centers that in Period 2 provided routine antibiotics to only a subgroup of clients (e.g., those less than 25 years of age).
STATISTICAL ANALYSIS
We calculated 95% confidence intervals for rates as exact binomial confidence intervals. Fisher’s exact test was used to assess the significance of differences in proportions. We tested for differences in relative declines in rates between the two groups using a test for homogeneity of risk ratios. Two-sided P values of less than 0.05 were considered to indicate statistical significance. Calculations were performed with the use of Cytel Studio software, version 8 (Cytel), or Stata 10 software (StataCorp).
RESULTS
During the course of the study, 243,692 women underwent medical abortion at Planned Parenthood centers. After the exclusion of 15,869 women who did not meet eligibility criteria (<7%), the analysis population included 227,823 women, among whom 92 serious infections were reported.
During Period 1 in the analysis population, a total of 72,195 women underwent medical abortion; serious infections were reported in 67 women (0.93 per 1000 abortions). One death occurred in early 2006, from C. perfringens; this was the only death during the study periods. Rates of serious infection for each group in this and each subsequent period are shown in Table 1 and Figure 1.
Table 1.
Rates of Serious Infection in Two Groups of Patients Undergoing Medical Abortion at Planned Parenthood Health Centers during Four Periods.*
| Variable | Period 1 | Period 2 | Period 3 | Period 4 |
|---|---|---|---|---|
| Misoprostol administration | Vaginal | Buccal | Buccal | Buccal |
| Maximum no. of days of gestation | 63 | 56 | 56 | 63 |
| Analysis population | ||||
| Group 1 | ||||
| No. of abortions | 37,488 | 40,110 | 17,688 | 21,064 |
| No. of infections | 43 | 18 | 2 | 3 |
| Rate per 1000 (95% CI) | 1.15 (0.83–1.54) | 0.45 (0.27–0.71) | 0.11 (0.01–0.41) | 0.14 (0.03–0.42) |
| Group 2 | ||||
| No. of abortions | 34,707 | 38,684 | 15,780 | 22,302 |
| No. of infections | 24 | 2 | 0 | 0 |
| Rate — per 1000 (95% CI) | 0.69 (0.44–1.03) | 0.05 (0.00–0.19) | 0 (0–0.19) | 0 (0–0.13) |
| Total for Groups 1 and 2 | ||||
| No. of abortions | 72,195 | 78,794 | 33,468 | 43,366 |
| No. of infections | 67 | 20 | 2 | 3 |
| Rate per 1000 (95% CI) | 0.93 (0.72–1.18) | 0.25 (0.16–0.39) | 0.06 (0.00–0.22) | 0.07 (0.01–0.20) |
| Total for Planned Parenthood health centers† | ||||
| No. of abortions | 77,182 | 83,896 | 35,837 | 46,777 |
| No. of Infections | 69 | 20 | 2 | 3 |
| Rate per 1000 (95% CI) | 0.89 (0.70–1.13) | 0.24 (0.15–0.37) | 0.06 (0.00–0.20) | 0.06 (0.01–0.19) |
Period 1 was from January 1, 2005, through March 31, 2006, and was the baseline period. Period 2 was from April 1, 2006, through June 30, 2007; Period 3 from July 1, 2007, through December 31, 2007; and Period 4 from January 1, 2008, through June 30, 2008. Group 1 comprises health centers that switched in Period 2 to universal testing for sexually transmitted infections (STIs), and Group 2 comprises health centers that switched in Period 2 to routine provision of antibiotics. CI denotes confidence interval.
The totals for the Planned Parenthood health centers include the following two groups of affiliates that were excluded from the analysis population: those that did not provide medical abortions in all four periods, and those that in Period 2 routinely provided antibiotics to a subgroup of women (e.g., all women younger than 25 years of age) and STI screening for the remaining women.
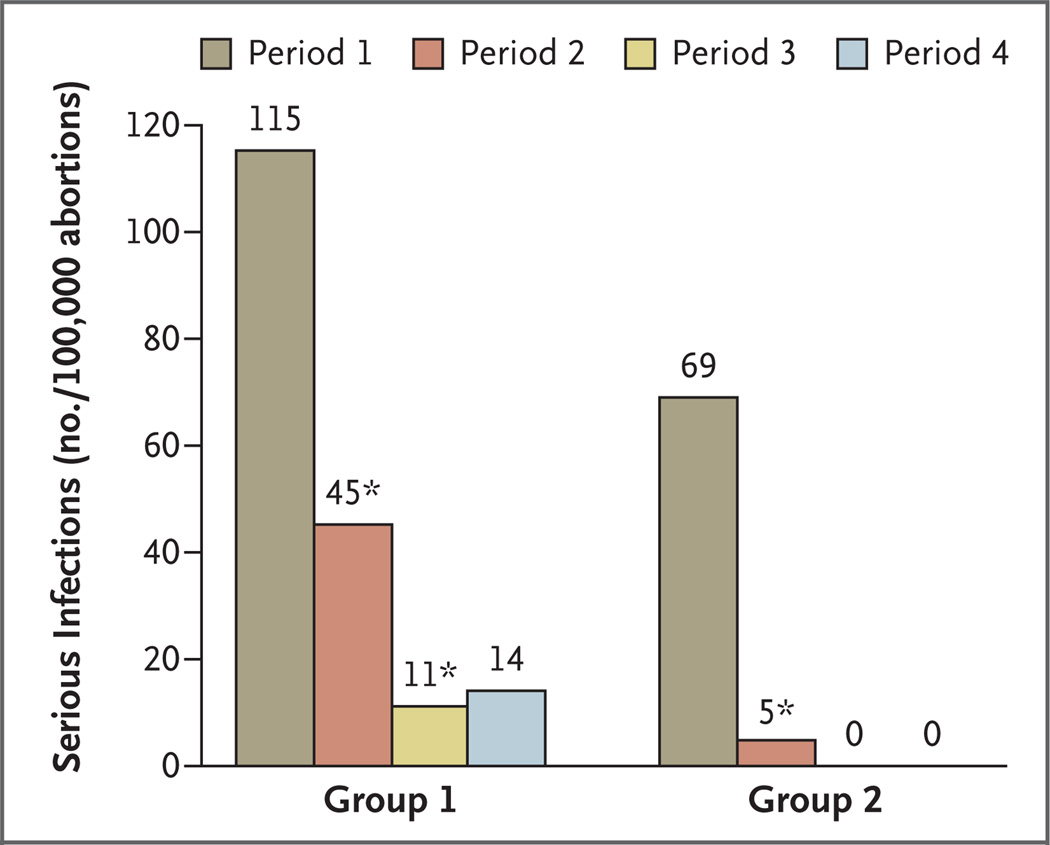
Figure 1. Rates of Serious Infection after Medical Abortion among Patients in Two Groups of Planned Parenthood Health Centers.
Period 1 ( January 1, 2005, through March 31, 2006) was the baseline period during which vaginal misoprostol and standard antiseptic measures were used for the abortion of fetuses through 63 days of gestation. During Period 2 (April 1, 2006, through June 30, 2007), buccal misoprostol was used through 56 days of gestation; some Planned Parenthood clinics used the infection-reduction measure of universal screening for sexually transmitted infections (STIs) and treatment when screening was positive, whereas others provided routine antibiotics consisting nearly uniformly of 100 mg of oral doxycycline twice a day for 7 days. During Period 3 ( July 1, 2007, through December 31, 2007), buccal misoprostol was used through 56 days of gestation and all health centers routinely provided the doxycycline regimen. During Period 4 ( January 1, 2008, through June 30, 2008) buccal misoprostol was used through 63 days of gestation, and all health centers routinely provided the doxycycline regimen. Group 1 comprises health centers that switched in Period 2 to universal testing for STI, and Group 2 comprises health centers that switched in Period 2 to routine provision of antibiotics. An asterisk denotes a significant decrease from the previous period. There were no serious infections in Periods 3 and 4 in Group 2.
The 93% relative decrease in the rate of serious infection between Period 1 and Period 4 was an absolute reduction of 0.86 per 1000 (95% confidence interval [CI], 0.64 to 1.12; P<0.001). The rate of serious infection declined significantly between Periods 1 and 2 (absolute reduction of 0.67 per 1000; 95% CI, 0.44 to 0.94; P<0.001) and between Periods 2 and 3 (absolute reduction of 0.19 per 1000; 95% CI, 0.02 to 0.34; P = 0.03). Between Periods 3 and 4, the change in the rate of serious infection was not significant (absolute increase of 0.01 per 1000; 95% CI, 0.0 to 0.15; P>0.99).
Between Periods 1 and 2, there were significant declines in the rates of serious infection in both Group 1 and Group 2, but the relative decline was significantly greater in Group 2 (93% decline) than in Group 1 (61% decline) (relative risk ratio, 0.19; 95% CI, 0.04 to 0.89; P = 0.04). Between Periods 2 and 3, there were further declines in both Group 1 (significant) and Group 2 (nonsignificant), but the relative declines did not differ significantly (75% and 100%, respectively; P = 0.07); the combined relative risk in Period 3 as compared with Period 2 was 0.24 (95% CI, 0.03 to 0.97). In Group 1, the rate of serious infection fell from 1.15 per 1000 in Period 1 to 0.11 per 1000 in Period 3. Of this absolute decline in rate of 1.04 per 1000, 33% occurred between Periods 2 and 3.
Hospital records were available for review for 45 of the 92 cases of serious infection, and for an additional 30 cases, detailed summaries of the records were provided by a Planned Parenthood clinician either after consultation with a physician who provided treatment in the hospital or emergency department or after review of the records at the hospital. In 17 cases (18%), patients reported treatment with intravenous antibiotics in the emergency department, but medical-record verification was not available. In a secondary analysis excluding these 17 cases, results were materially unchanged, although the relative decline from Period 1 to Period 2 was no longer significantly greater in Group 2 than in Group 1.
DISCUSSION
We observed significant and clinically important reductions in the risk of serious infections among patients who had undergone medical abortion after a change from vaginal to buccal administration of misoprostol and after the adoption of routine preventive treatment with antibiotics. Although the observational design of our study precludes a determination of cause and effect, it is plausible that the changes in practice patterns could explain the reductions in the rate of serious infection. Because PPFA instituted more than one measure at a time, it is difficult to estimate from our analyses the relative values of different interventions. However, the fact that Planned Parenthood health centers adopted two infection-reduction measures in Period 2 allows further exploration of this issue.
In Group 1 — the group of health centers that used the screen-and-treat strategy in Period 2 — one third of the decline in the rate of serious infection from Period 1 to Period 3 occurred between Periods 2 and 3, when the only regimen change was from screen-and-treat to routine antibiotic coverage. Moreover, the relative decline in the reported rate of serious infection from Period 1 to Period 2 was significantly greater in Group 2 than in Group 1 (in the analysis including all cases). These findings indicate that routine provision of antibiotics was associated with a greater reduction in serious infection than was the use of the screen-and-treat method. This finding could be explained by the fact that, with the screen-and-treat strategy, not all those who test positive return for treatment; also, even among those who do return for treatment, treatment is delayed for at least 2 days while they await test results.
Between Period 3 and Period 4, the only change in the regimen was an increase in the maximum gestational age at the time of medical abortion, from 56 to 63 days. Because there was no significant increase in the rate of serious infection from Period 3 to Period 4, it is unlikely that a decline in the maximum gestational age from 63 days in Period 1 to 56 days in Period 2 explains the decline over time in the rates of serious infection observed in both groups.
The rate of serious infection in Period 1 was substantially higher than the rates previously published, 3–6 even in one study that used Planned Parenthood data.6 (This finding, along with the deaths from C. sordellii after medical abortion, prompted the changes in Period 2.) It is likely that previously reported rates are underestimates and that apparent increases from previously reported data reflect improved reporting of serious adverse events to PPFA. Specifically, the official Planned Parenthood medical standards and guidelines were changed in 2004 to require that all serious adverse events be reported centrally; also, as stated earlier, since 2005, the accreditation process has included auditing to verify that adverse events related to the use of mifepristone are submitted as required.
Potential limitations of our study should be noted. We do not have data available on the rates of follow-up of women after medical abortion, and it is possible that the reporting of serious infections is incomplete. A potential concern is that serious infections might have been more likely to be underreported during Periods 2 through 4, since the intense scrutiny that occurred during Period 1 (after the reports of deaths from clostridial infections) had waned. However, we consider this unlikely, since national conference calls and meetings that were focused on the risk of infection were ongoing during the time of the study. Moreover, during Period 2 a non–Planned Parenthood patient died from C. sordellii infection after she had had a medical abortion with buccal misoprostol but was not treated with antibiotics until she presented to an emergency department in toxic shock; information about that death was widely distributed throughout the Planned Parenthood community.
Although a randomized clinical trial would be the preferred approach to determine whether the use of buccal rather than vaginal administration of misoprostol might reduce the rate of serious infection and whether a strategy of routine antibiotic coverage is superior to a strategy of screening before treating, this study design would not have been feasible. Given the low rates of serious infection, such a design would have required a prohibitively large sample. The large population that receives care at Planned Parenthood centers allowed the discerning of changes over time in the rates of serious infection after medical abortion. In summary, the current report shows that changes in PPFA policies for medical abortion that involve replacing vaginal administration of misoprostol with buccal administration and, later, providing routine antibiotics coupled with a highly monitored, systemwide surveillance network were associated with significant reductions in the rates of serious infections.
Acknowledgments
Dr. Cullins and Mrs. Fjerstad report having been employed by Planned Parenthood Federation of America (PPFA) at the time of the study. Drs. Lichtenberg and Trussell report serving on the PPFA National Medical Committee.
We thank all Planned Parenthood staff for their conscientious work and Germán Rodríguez and Kelly Cleland for help with the analysis.
Footnotes
No other conflict of interest relevant to this article was reported.
REFERENCES
- 1.Fjerstad M, Sivin I, Lichtenberg ES, Trussell J, Cleland K, Cullins V. Effectiveness of medical abortion with mifepristone and buccal misoprostol through 59 gestational days. Contraception. doi: 10.1016/j.contraception.2009.03.010. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sawaya GF, Grady D, Kerlikowske K, Grimes DA. Antibiotics at the time of induced abortion: the case for universal prophylaxis based on a meta-analysis. Obstet Gynecol. 1996;87:884–890. [PubMed] [Google Scholar]
- 3.Spitz IM, Bardin CW, Benton L, Robbins A. Early pregnancy termination with mifepristone and misoprostol in the United States. N Engl J Med. 1998;338:1241–1227. doi: 10.1056/NEJM199804303381801. [DOI] [PubMed] [Google Scholar]
- 4.Hausknecht R. Mifepristone and misoprostol for early medical abortion: 18 months experience in the United States. Contraception. 2003;67:463–465. doi: 10.1016/s0010-7824(03)00049-0. [DOI] [PubMed] [Google Scholar]
- 5.Shannon C, Brothers LP, Philip NM, Winikoff B. Infection after medical abortion: a review of the literature. Contraception. 2004;70:183–190. doi: 10.1016/j.contraception.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 6.Henderson JT, Hwang AC, Harper CC, Stewart FH. Safety of mifepristone abortions in clinical use. Contraception. 2005;72:175–178. doi: 10.1016/j.contraception.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 7.Mifeprex (mifepristone) information. Silver Spring, MD: Food and Drug Administration; 2007. [Accessed June 12, 2009]. (at http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm111323.htm.) [Google Scholar]
- 8.ACOG practice bulletin: clinical management guidelines of obstetrician-gynecologists. Number 67, October 2005. Medical management of abortion. Obstet Gynecol. 2005;106:871–882. doi: 10.1097/00006250-200510000-00051. [DOI] [PubMed] [Google Scholar]
- 9.Fischer M, Bhatnagar J, Guarner J, et al. Fatal toxic shock syndrome associated with Clostridium sordellii after medical abortion. N Engl J Med. 2005;353:2352–2360. doi: 10.1056/NEJMoa051620. [DOI] [PubMed] [Google Scholar]
- 10.Darney PD. Deaths associated with medication abortion. Contraception. 2005;72:319. doi: 10.1016/j.contraception.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 11.Sexually transmitted disease surveillance 2006 supplement: Gonococcal Isolate Surveillance Project (GISP) annual report 2006. Atlanta: Centers for Disease Control and Prevention; 2008. Apr, [Google Scholar]
- 12.Aldape MJ, Bryant AE, Stevens DL. Clostridium sordellii infection: epidemiology, clinical findings, and current perspectives on diagnosis and treatment. Clin Infect Dis. 2006;43:1436–1446. doi: 10.1086/508866. [DOI] [PubMed] [Google Scholar]
- 13.Middleton T, Schaff E, Fielding SL, et al. Randomized trial of mifepristone and buccal or vaginal misoprostol for abortion through 56 days of last menstrual period. Contraception. 2005;72:328–332. doi: 10.1016/j.contraception.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 14.Winikoff B, Dzuba IG, Creinin MD, et al. Two distinct oral routes of misoprostol in mifepristone medical abortion: a randomized controlled trial. Obstet Gynecol. 2008;112:1303–1310. doi: 10.1097/AOG.0b013e31818d8eb4. [DOI] [PubMed] [Google Scholar]