Abstract
Objective
To quantify the role patellofemoral and tibiofemoral kinematics may play in development of anterior knee pain (AKP) in individuals with cerebral palsy (CP).
Design
Case-Control
Setting
Clinical Research Center
Participants
Twenty knees from individuals diagnosed with CP and 40 control knees were evaluated. Controls were matched for sex and age based on the group average. Matching by height and weight was a secondary priority. Subjects in the control cohort were asymptomatic with no history of lower leg abnormality, surgery, or major injury. Only individuals who were physically capable of sustaining slow cyclic knee flexion-extension for 2.5 minutes and had no contraindications to MR imaging were enrolled. Both groups were samples of convenience.
Interventions
Not applicable.
Main Outcome Measure
The 3D patellofemoral and tibiofemoral joint kinematics, acquired during active leg extension, under volitional control.
Results
Participants with CP and AKP (n=8) demonstrated significantly greater patellofemoral extension, valgus rotation, superior, and posterior displacement relative to controls and to the subgroup of participants with CP and no AKP (n=12). Patellofemoral extension discriminated AKP in individuals with CP with 100% accuracy.
Conclusions
In quantifying the 3D in vivo knee joint kinematics during a volitional extension task, kinematic markers that discriminate AKP in individuals with CP were identified. This provides an ability to predict which individuals with CP are most likely to advance into AKP and could enable aggressive conservative treatment, aimed at reducing patella alta and excessive PF extension to be prescribed prior to considering surgical options. The current findings will likely lead to improved clinical diagnostics and interventions for individuals with CP, with the ultimate goal of helping maintain, if not improve functional mobility throughout the lifespan.
Keywords: MRI, patellofemoral, tibiofemoral, dynamics
Chronic pain in individuals with cerebral palsy (CP) affects quality of life,1 limits participation in activities, and reduces function.2 The overall prevalence of pain in individuals with CP (up to 79%)1, 3–5 is greater than in the general population and it increases with age.4 In addition, pain has been identified as a factor in the loss of previously obtained functional mobility skills.6 For example, the presence of anterior knee pain (AKP) can severely limit ambulation. Idiopathic AKP in the general, active population (often referred to as patellofemoral pain syndrome, PFPS) is common, constituting 14–17% of all injuries presenting to sports injury clinics7, 8 and having a prevalence of 23.7% among midshipman at the U.S. Naval Academy.9 For individuals with CP, this prevalence is much larger (39%).4
Despite its prevalence, the causes of AKP remains poorly characterized in individuals with CP.4, 10–13 Most studies evaluating AKP in individuals with CP have focused on static radiographic findings of patellar fragmentation and patella alta,10, 12, 13 as well as knee flexion contractures.10, 12 Using incidence rates, such studies have assumed an association between these variables and AKP, but direct correlations have not been documented. Rosenthal and Levine10 presented some of the earliest research in this area, but found no direct correlation between AKP and knee flexion contractures or patellar pole fragmentation. A recent study12 categorized three groups of children with CP and AKP, with all groups sharing the common traits of patella alta and rectus spasticity. Although this study highlighted potential causes of AKP among individuals with CP, it did not provide an ability to predict AKP. Accurately describing the origins of joint dysfunction14–16 and having the ability to predict AKP will likely allow more directed treatments, which may help preserve functional mobility in individuals with CP.
While the etiology of AKP remains poorly characterized in individuals with CP, PFPS has been well studied. The advent of new imaging-based techniques that can accurately quantify 3D in vivo patellofemoral (PF) kinematics has rapidly advanced the understanding of PFPS over the last few years.17–20 It is interesting to note that patella alta has been documented in both the CP 2, 10, 13, 21, 22 and PFPS23, 24 populations, with subsets from both populations experiencing patellar dislocation.12, 25 Surprisingly, no study to date has quantified PF kinematics or alignment in individuals with CP and AKP, outside the sagittal plane. An understanding of 3D PF and tibiofemoral (TF) kinematics in individuals with CP and AKP will likely help elucidate the source of this pain and provide a better understanding of knee joint dysfunction within the CP population.
Therefore, the purpose of this study was to quantify the role that knee joint kinematics may play in the development of AKP in individuals with CP and to determine if these kinematics could discriminate the presence of AKP in a population of individuals with CP. To accomplish this, a novel dynamic MR imaging technique (cine-phase contrast or CPC) was used to quantify the in vivo 3D knee joint kinematics during a volitional leg extension task in a group of individuals diagnosed with CP, but varying in the reporting of AKP. Three specific hypotheses were tested: 1) PF superior position is greater in individuals with CP that report AKP, in comparison to those that do not. 2) This difference is large enough to enable the prediction of AKP in CP based on a discriminate analysis and 3) The presence of patella alta in individuals with CP leads to increased lateral shift and tilt, in a manner similar to that documented in PFPS.24
METHODS
In this case-control study, 18 individuals with CP were recruited from local physical medicine/rehabilitation clinics and ongoing National Institutes of Health (NIH) studies. Upon entering this study, all participants gave written informed consent or, if the participant was a minor, written assent with a legal guardian providing written consent. This study was approved by the internal review board of the National Institutes of Child Health and Human Development at the NIH. Only individuals who were physically capable of sustaining slow cyclic knee flexion-extension for 2.5 minutes were accepted into the study. If both lower limbs were involved and time permitted, both knees were scanned, for a total of 20 knees with CP (Table 1). Using a physical examination and history, an in-house physiatrist (last author) recorded diagnosis (hemiplegia, diplegia, or quadriplegia), the presence of chronic AKP (> 6 months duration), the GMFCS score26, 27, manual muscle testing score for the knee extensors (MMT)28 and a modified Ashworth scale29 score for the rectus femoris and hamstrings for each participant. Each participant, was assigned to one of two subgroups (CP_Pain or CP_ noPain) based on the presence of chronic AKP.
Table 1. Demographics.
The upper portion compares the control and cerebral palsy (CP) cohorts, whereas the lower portion compares the two subgroups (CP_pain and CP_noPain) within the CP cohort. For continuous and discrete variables a two-tailed Student’s t-test or a 2-tailed chi-squares test were used to test for significance (grey values indicate, p ≥ 0.05). For categorical data (gender and the last five rows) each level is listed with the number of participants included at that level given in brackets. For continuous variables (age, height and weight) the standard deviations are given in parenthesis. All data is listed for each knee.
|
|
|||
|---|---|---|---|
| Control {n=40} | CP {n=20} | P-value | |
|
|
|||
| Gender | F{20}, M{20} | F{10}, M{10} | p=1.00 |
| Age | 22 years, 3 months (7 years, 3 months) | 21 years 8 months (10 years,1 month) | p=0.87 |
| Weight | 65.6 (12.0) kg | 55.0 (7.3) kg | p=0.01 |
| Height | 171.4 (9.4) cm | 166.3 (8.6) cm | p=0.05 |
|
|
|||
| CP w/o Pain {n=12} | CP w/pain {n=8} | P-value | |
|
|
|||
| Gender | F{5}, M{7} | F{5}, M{3} | p=0.65 |
| Age | 19 years,1 months (8 years, 6 months) | 25years, 8months (11 years, 6 months) | p=0.19 |
| Weight | 55.0 (7.3) kg | 60.9875 (11.7) kg | p=0.23 |
| Height | 167.6 (10.1) cm | 164.44 (5.7) cm | p=0.39 |
| GMFCS | I{8}, II{4} | I{1}, II{2}, III{5} | p=0.006 |
| MMT | 4-{8}, 3+{3}, 3-{1} | 3+{2}, 3-{6} | p=0.003 |
| MAS_RF | 055, 1{3}, 1+{6},2{1} | 0{1}, 1{2}, 1+{3},2{2} | p=0.77 |
| MAS_HAM | 0{1}, 1{1}, 1+{7}, 2{3}, 3{0} | 0{0}, 1{0}, 1+{2}, 2{3}, 3{3} | p=0.12 |
| Diagnosis | hemi{3}, di{9}, quad{0} | hemi{0}, di{5}, quad{3} | p=0.04 |
Abbreviations: F (M): the number of knees within the study from subjects that were women or girls (men or boys). GMFCS: gross motor function classification score with levels I, II, III, IV, and V; MMT: Manual muscle test for the rectus femoris with scores zero through five (0 = No contraction; 1 = trace contraction; 2 = active movement, with gravity eliminated; 2, 2+,3− = Active movement against gravity from 0 to 50% of the feasible range of motion; 3 = Active movement against gravity over more than 50% of the feasible range of motion (ROM); 3+,4− = Active movement against resistance over less than 50% of the feasible ROM; 4 = Active movement against resistance over more than 50% of the feasible ROM; 4+, 5− Active movement against strong resistance over the feasible ROM, but distinctly weaker than the contralateral side, 5 = Normal power); MAS_RF and MAS_HAM modified Ashworth scale for the rectus femoris and hamstrings, respectively with scores 0, 1, 1+, 2 and 3 (0 = no increase in muscle tone; 1 = slight increase in muscle tone, manifested by a catch and release or by minimal resistance at the end of the range of motion when the affected part(s) is moved in flexion or extension; 1+ = slight increase in muscle tone, manifested by a catch, followed by minimal resistance throughout the remainder (less than half) of the ROM; 2 = more marked increase in muscle tone through most of the ROM, but affected part(s) easily moved; 3 = considerable increase in muscle tone, passive movement difficult; 4 = affected part(s) rigid in flexion or extension); hemi: hemiplegia; di: diplegia; and quad: quadriplegia
The kinematics of 40 control knees, recruited from ongoing NIH studies, formed the baseline for comparison. These knees were matched for sex and age based on group averages (Table 1). Matching by both height and weight was a secondary priority, as individuals with CP tend to be lighter and of shorter stature than their peers in the general population. All subjects in the control cohort were asymptomatic with no history of lower leg abnormality, surgery, or major injury. For both cohorts, any subject with contraindications to MR imaging was excluded.
Subjects were placed supine in an MR imager with the knee bent and supported on a cushioned block (Figure 1). The subjects were then asked to cyclically flex and extend their knee while a dynamic CPC MR image set (x,y,z velocity and anatomic images frames) was acquired. Out of all the 3D in vivo techniques for measuring joint kinematics, CPC MRI is the most accurate (accuracy <0.3 mm30) and the only one that can define both muscular and skeletal kinematics. In order to establish anatomical coordinate systems, dynamic cine images (anatomic images only) were acquired in three axial planes.31, 32 Three-dimensional displacement and orientations (Figure 2) were defined for both the patella and tibia relative to the femur (PF and TF kinematics, respectively) through integration of the CPC MR velocity data.33 To account for skeletal size variations across subjects, all displacements were scaled by the ratio of the average epicondylar width from an asymptomatic population (76.9 mm31) to the epicondylar width for each individual knee. Scaling, instead of normalization was used so that the results would be directly comparable to past knee joint kinematic studies that did not use normalization.
Figure 1. Experimental Set-up.
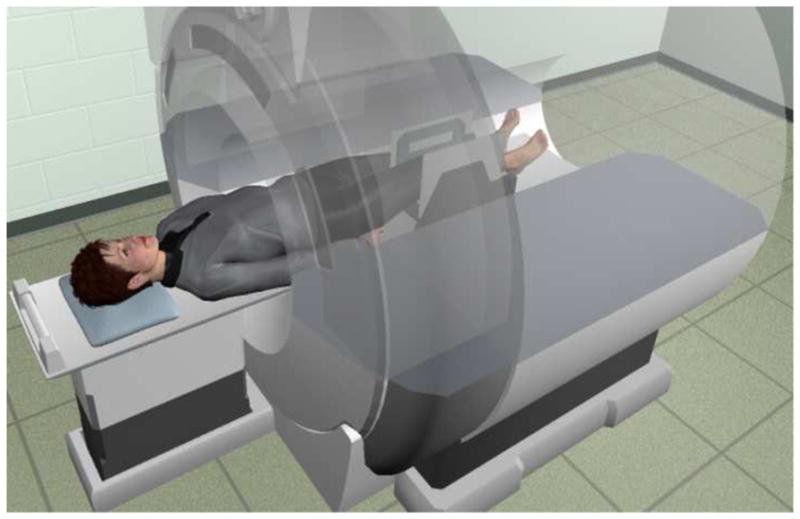
Subjects were placed supine within the MR bore. A coil holder stabilized two flexible coils medial and lateral to the knee and provided medial-lateral support for the knee. A cushion wedge was placed under the knee and adjusted so that each subject could select their own terminal extension angle, which the subject could reach repeatedly and comfortably. An auditory metronome (played through a pair of headphones) provided guidance for the repeated movement (2 beats per cycle at 35 cycles/min).
Figure 2. 3D PF Translations and Rotations.
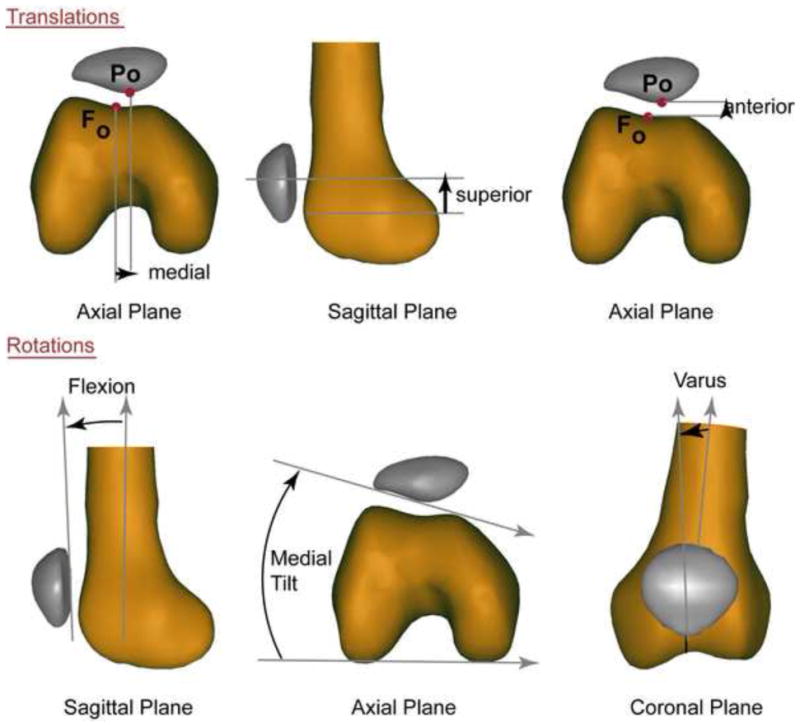
The exact directions associated with the three movement directions (positive directions = medial, superior and anterior) are based on establishing a 3-dimensional coordinate system, but can be approximated in two-dimensions using the anatomical landmarks from which the coordinate system is established.31 The patellar origin (Po) was selected to be the most posterior point on the patella its mid-section in the superior-inferior direction. The femoral origin (Fo) was selected to be the deepest point within the femoral sulcus at the level of the femoral epicondylar width. The patellofemoral displacements were derived by defining how the patellar origin moved relative to the femoral origin. The rotations are based on an xyz-body fixed Cardan rotation sequence,54 but can be approximated using anatomical lines (positive rotations = medial tilt, flexion, and varus rotation). The tibial coordinate system was defined in a similar manner, with the tibial origin being the center of the patellar tendon insertion into the tibia and tibiofemoral internal rotation being positive.
Data were collected in even temporal increments. To create population averages, each kinematic variable was interpolated to single degree knee angle increments. All data were presented for the extension portion of the movement only. An individual subject’s range of motion depended on the relationship between the leg length to the inner bore dimension, as well as the subject’s own physical ability. The experimental set-up was adapted to enable subjects to select the terminal extension angle, which they could reach comfortably and repeatedly. Therefore, not all subjects were represented in the average kinematics at all knee angles. Data representing 20% or fewer subjects were eliminated from the group average.
Patella alta was defined as a significant (p<0.05) increase in PF superior displacement. Although this is not the typical measure of patella alta (e.g., the Insall-Salvati ratio34), the current method may have more physiological relevance because the kinematic relationship is defined between the patella and the femur (not tibia) and is measured during volitional exercise with quadriceps activity (not statically). An unpublished preliminary study demonstrated that the value of PF superior displacement (as measured in the current study) in individuals with CP was moderately correlated (r=0.67, p=0.02) with the patellophyseal index, a static MR based index of patella alta which is predictive of contact area and chondral defects.35
Methods: Statistical Methods
An a priori power analysis revealed that 10 participants per subgroup (CP_pain and CP_noPain) were needed to have a detectable overall significance (α= 0.05, β= 0.8). This analysis assumed that the same differences in value and variance of PF superior position between the two subgroups (CP_pain and CP_noPain) would exist as was found in a previous study24 between a control cohort, 20.2 (SD 6.5) mm, and a cohort of individuals with PFPS,13.0 (SD 6.3) mm.
In order to simplify the interpretation of the correlations, the full range of motion for each kinematic variable was represented by a single value. The value of a kinematic variable was defined as its magnitude at 20° knee angle, this was the angle closest to full extension where all subjects were represented. Statistically significant PF and TF kinematic (12 parameters) differences (hypothesis 1) were investigated using a two-tailed Student’s t-test, assuming unequal variances between 1) the CP and control cohorts; 2) the two subgroups within the CP cohort; and 3) each individual CP subgroup and the control population. A Bonferonni-type false discovery rate procedure36 was used to adjust the p-values for the multiple comparisons. Two-tailed chi-squared and Student’s t-tests were used to evaluate differences in demographics (Table 1).
A discriminant analysis was performed to determine if the presence of pain in the CP cohort could be predicted (hypothesis 2) using one of three sets of predicting variables: (1) the value of PF superior displacement, (2) the value of PF extension, and (3) the combined values of PF superior displacement and extension. The predicting variable sets were defined based on the kinematic variables that demonstrated the largest variation from the control population.
The Pearson’s correlation coefficients (CP cohort only) for each patellofemoral degree of freedom with each of the other 5 PF degrees of freedom were calculated (hypothesis 3). Spearman’s correlations were calculated within the CP cohort between the knee joint kinematics and both the presence of AKP and the GMFCS score. In addition, the correlation between each of the PF and TF kinematic variables with each of the other kinematic variables was determined using a Pearson’s correlation. Statistical significance was set at p<0.05.
Results
Quadriceps weakness along with mild to moderate spasticity in both the hamstrings and rectus femoris existed for all participants with CP. No differences in distribution of the Modified Ashworth Score was found between the CP_pain and CP_noPain subgroups (Table 1). All participants in the CP cohort were ambulatory (GMFCS score ranging from I to III), but the CP_noPain subgroup tended to be higher functioning and stronger. The distribution of diagnosis was not equal amongst the two subgroups, likely due to the fact that AKP was correlated with functional level, GMFCS score, (ρ = 0.65, p=0.002). The CP cohort weighed less than the control cohort.
The first hypothesis (PF superior displacement was greater in individuals with CP that reported pain) was supported (Table 2, 7.9mm, p<0.001). This same result held true when comparing the entire CP cohort to controls (12.9mm, p<0.001). In addition, PF extension, varus rotation, and posterior displacement, were increased in the CP subgroup that reported AKP versus the subgroup that did not. Again, this same result held true when comparing the entire CP cohort to the control cohort. PF lateral translation and tilt was no different between the CP and control cohorts, nor was it different between the two subgroups within the CP cohort. Thus, for the entire CP cohort, patella alta was accompanied by increased PF posterior displacement, extension, and varus along with increased TF superior displacement and internal rotation. The increased extension and varus rotation caused the superior patellar pole to shift posteriorly and laterally, respectively. These same differences existed in both subgroups, but were much larger in the CP_pain subgroup (Figure 3). PF posterior shift and varus rotation were not significantly greater in the CP_noPain group than in controls. No significant differences existed between the CP_noPain and the CP_pain subgroups for TF kinematics.
Table 2. Kinematics comparisons of the values of each kinematics variable.
The value was defined as the magnitude of each variable at a knee angle of 20°. Differences are listed between the 1) CP cohort and the controls; 2) the CP_pain subgroup versus the controls; 3) the CP_noPain subgroup versus controls; and 4) the CP_pain versus the CP_noPain subgroups in the value of PF and TF kinematics (upper and lower portions, respectively).
|
|
||||||
|---|---|---|---|---|---|---|
| Displacement (mm) | Rotation (deg) | |||||
| PF | medial | superior | anterior | flexion | medial tilt | varus |
| CP vs. control | −1.4 | 12.9** | −3.5** | −10.0** | 3.5 | 2.4* |
| CP_pain vs. control | −1.0 | 20.3** | −6.4** | −17.8** | 3.1 | 4.8** |
| CP_noPain vs. control | −1.7 | 7.9 ** | −1.6 | −4.8* | 3.8 | 0.8 |
| CP_pain vs. CP_noPain | 0.7 | 12.4** | −4.8* | −13.0** | −0.7 | 3.9* |
|
| ||||||
| TF | medial | superior | anterior | flexion | internal tilt | varus |
|
| ||||||
| CP vs. control | 1.2 | 6.3** | 1.3 | −0.3 | 8.8** | 0.1 |
| CP_pain vs. control | 2.2 | 8.0** | 2.3 | −0.1 | 9.5** | 0.3 |
| CP_noPain vs. control | 0.6 | 5.2* | 0.7 | 0.5 | 8.3** | 0.0 |
| CP_pain vs. CP_noPain | 1.6 | 2.9 | 1.6 | 0.3 | 1.2 | 0.3 |
indicates p<0.001,
p<0.05m and lightened numbers indicate p ≥ 0.05
Figure 3. Comparison of patellofemoral kinematics between the cerebral palsy subgroups.
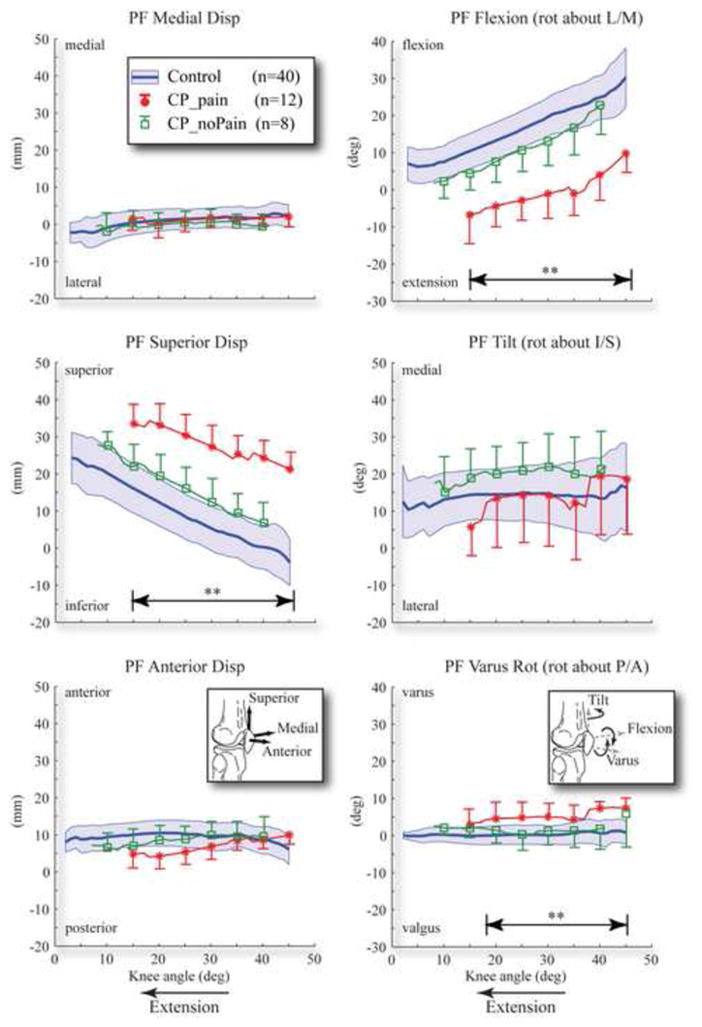
Double arrow lines with ** above denote the ranges where there are significant differences between subgroups. The control cohort is represented by a solid line with one standard deviation range shown in a shaded area. The cerebral palsy subgroups without AKP and with pain (CP_noPain and CP_pain) are represented by solid lines with a hollow square symbol and solid circular symbol, respectively. One standard deviation bar provided every 5°.
The second hypothesis was supported. PF superior displacement could discriminate the presence of AKP within the CP cohort to an accuracy of 80% (Figure 4). PF extension alone could better discriminate the presence of AKP within the CP cohort, with an accuracy of 100%. The two data points that were closest to the extension discrimination line (one from each of the subgroups) were 1.3° different from each other, well outside the accuracy and precision limits of the tracking kinematics with CPC MR and larger than the subject repeatability for PF flexion (0.9°).30
Figure 4. Discriminant Analysis.
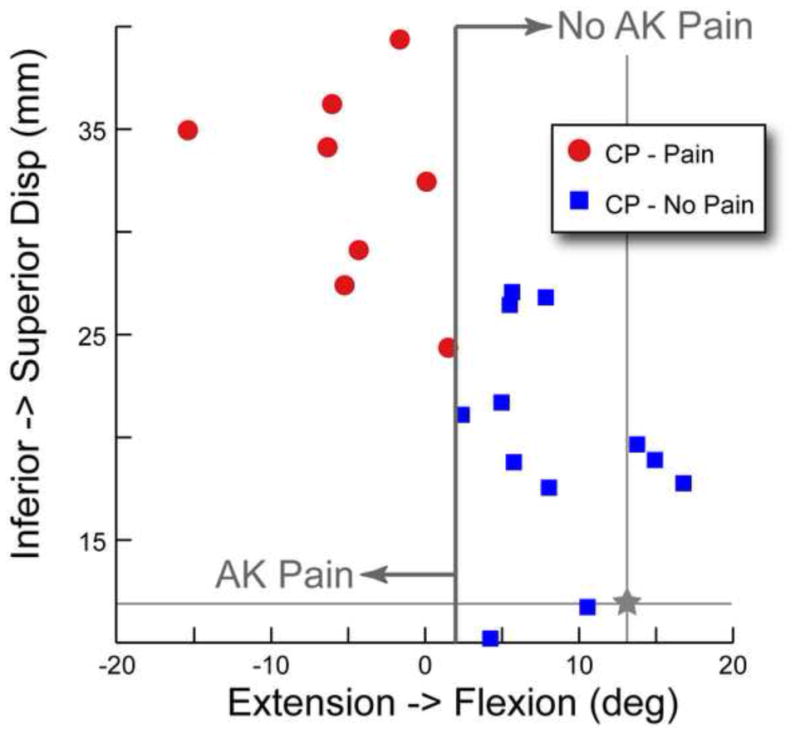
PF superior displacement versus PF extension. The participants with cerebral palsy and anterior knee pain (CP_pain) are represented by circular symbols. The participants with cerebral palsy and no anterior knee pain (CP_noPain) are represented by square symbols. The average value of patellofemoral superior displacement and extension for the control cohort is represented by a star with lines extending the width and height of the graph.
The third hypothesis (that PF superior displacement was correlated with PF lateral tilt) was not supported, yet correlations between specific PF kinematic variables with other study parameters were found. The existence of AKP was strongly correlated with the value of PF extension (ρ=0.85, p<0.001) and superior displacement (ρ=0.80, p<0.001) and moderately correlated to the GMFCS score (ρ = 0.65, p=0.002), PF posterior displacement (ρ=0.60, p=0.005), and PF valgus (ρ=0.51, p=0.02). The GMFCS score was moderately correlated with both PF extension (ρ=0.74, p<0.001) and superior displacement (ρ=0.61, p=0.004). The four PF kinematics variables that were increased in the CP cohort were all moderately correlated with each other (r=0.51 to r=0.72), likely indicating a single source for these variations. The TF kinematic variables did not correlate with any other study variable.
Discussion
In quantifying the 3D in vivo knee joint kinematics during a volitional extension task in individuals with CP, both with and without AKP, this study identified kinematic markers that clearly discriminated AKP in individuals with CP. Interestingly, few studies have even been able to establish a correlation between pain and PF kinematics in the general population24, let alone demonstrate an ability to discriminate pain based on PF kinematics. The current study provided evidence that altered PF kinematics are related both to AKP and to functional mobility (GMFCS score). This mirrors recent results that described the relationship between GMFCS score and the incidence of hip displacement as linear.37
The PF, in contrast to the TF, joint is the most affected in the individuals with CP, which agrees with the fact that clinically, individuals with CP frequently have PF joint dysfunction,16,19,25,27 limited knee extension strength, and spasticity, all of which contribute to long-term mobility impairments. Thus, treatments targeting the altered PF kinematics found within the current CP cohort may best alleviate pain and improve functional mobility. Accurately quantifying 3D joint function helps improve our clinical understanding of CP,38 by providing new information that will help guide clinicians as they explore and develop therapies/interventions for patients with CP and AKP, with the ultimate goal of reducing pain and minimizing the loss of previously obtained functional mobility skills.6
The ability to discriminate AKP in the current CP population (hypothesis 2) indicates its likely etiology. It has been reported previously that increased hamstrings spasticity, quadriceps spasticity, and/or quadriceps weakness can, over time, lengthen the patellar tendon, resulting in patella alta.10, 12, 13 Patella alta has been reported to result in increased PF contact stress.39 For the participants in the CP_pain subgroup, the combination of patella alta and femoral sulcus shape places the patella well outside the femoral sulcus in terminal extension (Figure 5). Subsequently, the excessive force within the extensor mechanism rotates (extends) the patella, until its posterior edge contacts the femoral shaft. Thus, not only is there excessive contact force, but it is exerted between cartilage and bone, rather than the normal cartilage-to-cartilage contact, likely resulting in pain. The participants in the CP_noPain subgroup appear to have less force across the extensor mechanics, evidenced by their lower PF superior displacement and extension, as compared to the CP_pain subgroup. The relatively mild PF extension in this subgroup may actually ameliorate contact stress since it brings more of the patella in contact with the trochlear cartilage. Future work focusing on 3D PF dynamic contact patterns, areas, and forces in individuals with CP is needed to more fully explore these concepts.
Figure 5. isual description of PF extension and patella alta at 20° of knee extension.
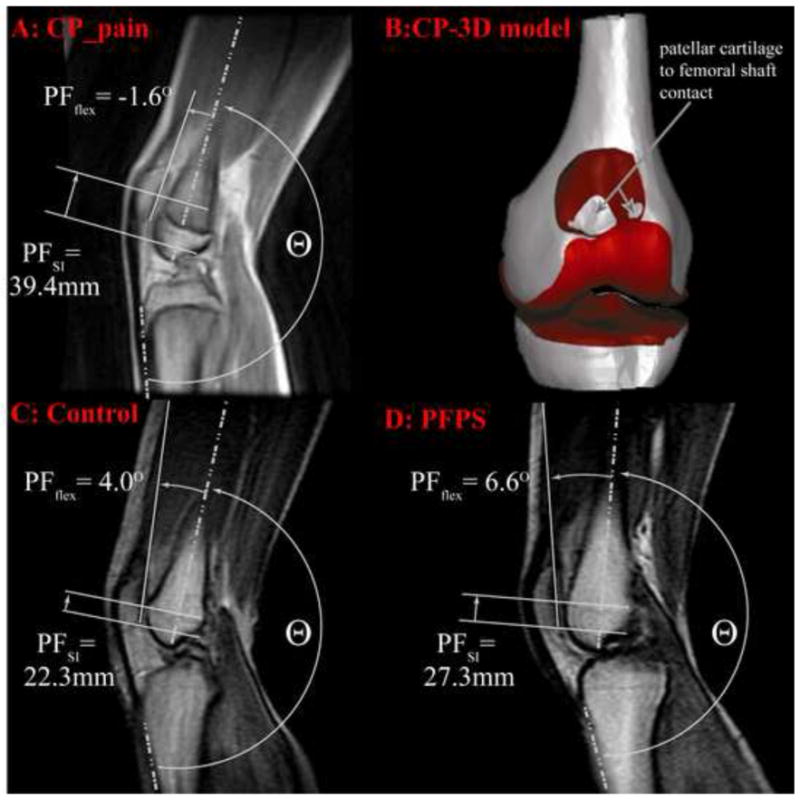
The knee angle was defined at 180°− Θ. The top row represents at single participant with CP and anterior knee pain. This participant had the most severe alta of the entire CP cohort. Image A: the cine-phase contrast (PC) magnetic resonance (MR) anatomic image. The patellofemoral superior displacement and extension is visually approximated on the image. Patellar extension can be approximated by its 2D counterpart (the angle between the vector bisecting the anterior and posterior edges of the distal femur and the vector delineating the posterior edge of the patella). The patellar superior position is the superior displacement of the patellar origin relative to the femoral origin (Figure 2) in the femoral coordinate system. Image B: A 3D model of the knee joint for this same individual at 20° knee extension. The patella is not shown, so that the contact between the patellar cartilage and femoral shaft can be seen. Image C: Cine-PC MR image for a single control subject with the 3rd highest level of patellar superior position out of all the control subjects. Image D: A cine-PC MR image from an otherwise healthy patient with PFPS from a previous study.24 Out of all the patients with PFPS in this previous study, this subject had the second highest level of patella alta.
Increased PF lateral shift and tilt did not accompany patella alta in this CP cohort (hypothesis 3), but does in the general population with PFPS,24 indicating that the etiology of kinematic alterations in these two groups is likely different (a hypo-versus a hyper-mobile patella). In the current CP cohort there is clearly patellar hypo-mobility with alta resulting from excessive force in the extensor mechanism, due to knee flexion contractures and spasticity. Although the patella is free from the constraints of the femoral groove in terminal extension (due to patella alta), the excessive force in the extensor mechanism likely inhibits lateral patellar shift and tilt, by pulling the patella posteriorly into the femur. A history of dislocation in children with CP and AKP has been documented,12 which easily fits with a hypo-mobile patella, particularly if TF valgus is present. In this situation, the extensor mechanism can be likened to a bowstring40 and a slight shift in the PF position can release the bowstring, causing an abrupt and forceful patellar dislocation. In contrast to the CP cohort, otherwise healthy individuals with patella alta and PFPS likely have a hyper-mobile patella, as patella alta has been correlated with excessive PF lateral tilt and shift24 and associated with patellar dislocation.41–43 In such individuals, patella alta is most likely due to a generalized joint laxity (evidenced by a flexed, not extended patella, Figure 5). In terminal extension the combined alta and laxity enables the patella to shift and tilt laterally, with extreme shift resulting in dislocation. Thus, unlike the CP cohort, pain in individuals with PFPS is likely due to: 1) reduced contact area 2) larger patellar movements across the femur, and 3) and higher forces required to re-engage the patella in the sulcus during early flexion. This unique etiology of pain between the CP_pain subgroup and individuals in the general population with PFPS indicates that clinical interventions aimed at eliminating AKP likely need to be tailored specifically to each group.
The current data adds more specific insights into which sub-sets of individuals with CP might benefit from both conservative and surgical intervention by delineating the severity of PF extension and patella alta that appears to lead to AKP within the CP population. Recently there has been a resurgence of interest in correcting patella alta44, 45 as part of a multi-level surgery.46–49 The strong correlations between PF extension, patella alta, and the presence of AKP indicate that when severe alta is present, reducing it while lengthening the quadriceps, will likely reduce patellar extension, restore cartilage to cartilage contact, and thereby relieve AKP. For example, traditionally the spastic/out of phase rectus is often treated with transfer surgery. Several studies50–52 have demonstrated that the mechanism by which these transfers improve knee function involves reducing the extensor torque rather than a new ability to create flexion torque. Thus, improvements in knee pain, after a rectus transfer, are likely attributable to both an increase range of motion and a normalization of PF kinematics. Having an ability to discriminate which individuals with CP are most likely to advance into AKP (either directly through quantifying PF kinematics or indirectly through functional assessments such as GMFSC score, which is correlated with PF kinematics and AKP could enable aggressive conservative treatment, aimed at reducing patella alta and excessive PF extension (e.g., stretching and mobilization), to be prescribed first. This could help maintain joint function and either delay or eliminate the need to surgically reduce the patella alta and PF extension. Studies evaluating the effects of clinical intervention on 3D joint kinematics are clearly needed to bring further clarity.
Another issue that needs further investigation is the higher levels of tibial internal rotation seen in the CP cohort. Numerous individuals within this cohort clearly demonstrated both internal femoral rotation and external tibial rotation (as measured at the ankle) during ambulation. This discrepancy likely arises from to the fact that tibial internal rotation was measured at the proximal tibia and distal femur. A twist about the long axis of the tibia could easily enable internal rotation at the knee while the distal tibia and foot remain externally rotated.
Discussion: Limitations
A limitation of the current study is that non-ambulatory individuals with CP (GMFCS scores IV or V) were excluded, as the primary focus of this study was on the movement patterns of the knee joint under volitional control. Although AKP is also reported by non-ambulatory individuals, the underlying mechanism of pain in these patients where pain is reported while sitting may be different than that in ambulatory individuals. Thus, the current results and conclusions may not be relevant when treating a more severely affected population. However, it is appropriate to have different assessment tools for different functional levels, as the clinical needs of these groups can differ. A secondary limitation is that CPC MR, as applied to the study of 3D in vivo joint kinematics, is not a standard clinical tool. Thus, the methodologies are currently not available to most clinicians treating patients. Work is ongoing to improve clinical access to this tool. The moderate correlation between the two measures of patella alta, one acquired statically and one dynamically, indicates that there is a potential for finding surrogate static MR measures that may provide a similar level of insight into joint dysfunction. Lastly, this relatively small cohort cannot represent the vast number of individuals with CP. Yet, the enrollment of individuals with a specific pathology was on par with recent work evaluating AKP in individuals with cerebral palsy.12 Work is ongoing to determine if the same relationships between knee joint kinematics, functional mobility, and AKP exist over a larger population of individuals with CP.
Conclusions
This study elucidated kinematic markers that could discriminate AKP within a cohort of individuals with CP and demonstrated clear correlations between AKP, GMFCS, and PF kinematics. In doing so, it has provided new insights into knee dysfunction with implications for clinical management of patients with CP and has opened numerous pathways for future research. Specifically, treatments that target the excessive PF superior displacement and extension may best alleviate pain and improve functional mobility. As this study focused on the knee joint in isolation, it will be important to expand future studies to incorporate the potential contributing factors at the hip and ankle as well53. These study findings will likely lead to improved clinical diagnostics and interventions for this group, with the ultimate aim of helping individuals with CP maintain, if not improve their functional mobility throughout their lifespan.
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, and the Clinical Center at the NIH. Special thanks is given to Sara Sadeghi, Abrahm Behnam, Cris Zampieri-Gallagher, Bonnie Damaska, and the Diagnostic Radiology Department at the National Institutes of Health for their support and research time.
Abbreviations
- AKP
anterior knee pain
- CP
cerebral palsy
- GMFCS
gross motor function classification system
- MMT
manual muscle testing score
- NIH
National Institutes of Health
- PF
patellofemoral
- r
Pearson’s correlation coefficient
- ρ
Spearman’s correlation coefficient
- SD
standard deviation
- TF
tibiofemoral
Footnotes
Part of this material has been previously presented at the American Academy of Cerebral Palsy in Medicine (Washington DC, September 2010) and the American Society of Biomechanics (Providence, RI, July 2010)
I certify that no party having a direct interest in the results of the research supporting this article has or will confer a benefit on me or on any organization with which I am associated AND, if applicable, I certify that all financial and material support for this research (eg, NIH or NHS grants) and work are clearly identified in the title page of the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tuzun EH, Guven DK, Eker L. Pain prevalence and its impact on the quality of life in a sample of Turkish children with cerebral palsy. Disabil Rehabil. 2010;32(9):723–8. doi: 10.3109/09638280903295433. [DOI] [PubMed] [Google Scholar]
- 2.Murphy KP. The adult with cerebral palsy. Orthop Clin North Am. 2010;41(4):595–605. doi: 10.1016/j.ocl.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 3.Andersson C, Mattsson E. Adults with cerebral palsy: a survey describing problems, needs, and resources, with special emphasis on locomotion. Dev Med Child Neurol. 2001;43(2):76–82. doi: 10.1017/s0012162201. [DOI] [PubMed] [Google Scholar]
- 4.Jahnsen R, Villien L, Aamodt G, Stanghelle JK, Holm I. Musculoskeletal pain in adults with cerebral palsy compared with the general population. J Rehabil Med. 2004;36(2):78–84. doi: 10.1080/16501970310018305. [DOI] [PubMed] [Google Scholar]
- 5.Parkinson KN, Gibson L, Dickinson HO, Colver AF. Pain in children with cerebral palsy: a cross-sectional multicentre European study. Acta Paediatr. 2010;99(3):446–51. doi: 10.1111/j.1651-2227.2009.01626.x. [DOI] [PubMed] [Google Scholar]
- 6.Bartlett DJ, Hanna SE, Avery L, Stevenson RD, Galuppi B. Correlates of decline in gross motor capacity in adolescents with cerebral palsy in Gross Motor Function Classification System levels III to V: an exploratory study. Dev Med Child Neurol. 2010;52(7):e155–60. doi: 10.1111/j.1469-8749.2010.03632.x. [DOI] [PubMed] [Google Scholar]
- 7.Taunton JE, Ryan MB, Clement DB, McKenzie DC, Lloyd-Smith DR, Zumbo BD. A retrospective case-control analysis of 2002 running injuries. Br J Sports Med. 2002;36(2):95–101. doi: 10.1136/bjsm.36.2.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iwamoto J, Takeda T, Sato Y, Matsumoto H. Retrospective case evaluation of gender differences in sports injuries in a Japanese sports medicine clinic. Gend Med. 2008;5(4):405–14. doi: 10.1016/j.genm.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 9.Boling M, Padua D, Marshall S, Guskiewicz K, Pyne S, Beutler A. Gender differences in the incidence and prevalence of patellofemoral pain syndrome. Scand J Med Sci Sports. 2009;20(8):725–30. doi: 10.1111/j.1600-0838.2009.00996.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosenthal RK, Levine DB. Fragmentation of the distal pole of the patella in spastic cerebral palsy. J Bone Joint Surg Am. 1977;59(7):934–9. [PubMed] [Google Scholar]
- 11.Samilson RL, Gill KW. Patello-femoral problems in cerebral palsy. Acta Orthop Belg. 1984;50(2):191–7. [PubMed] [Google Scholar]
- 12.Senaran H, Holden C, Dabney KW, Miller F. Anterior knee pain in children with cerebral palsy. J Pediatr Orthop. 2007;27(1):12–6. doi: 10.1097/BPO.0b013e31802b715c. [DOI] [PubMed] [Google Scholar]
- 13.Topoleski TA, Kurtz CA, Grogan DP. Radiographic abnormalities and clinical symptoms associated with patella alta in ambulatory children with cerebral palsy. J Pediatr Orthop. 2000;20(5):636–9. doi: 10.1097/00004694-200009000-00016. [DOI] [PubMed] [Google Scholar]
- 14.Campbell SK. Quantifying the effects of interventions for movement disorders resulting from cerebral palsy. J Child Neurol. 1996;11 (Suppl 1):S6–170. doi: 10.1177/0883073896011001S09. [DOI] [PubMed] [Google Scholar]
- 15.Jenkins SE, Harrington ME, Zavatsky AB, O’Connor JJ, Theologis TN. Femoral muscle attachment locations in children and adults, and their prediction from clinical measurement. Gait Posture. 2003;18(1):13–22. doi: 10.1016/s0966-6362(02)00137-6. [DOI] [PubMed] [Google Scholar]
- 16.Mackey AH, Walt SE, Lobb GA, Stott NS. Reliability of upper and lower limb three-dimensional kinematics in children with hemiplegia. Gait Posture. 2005;22(1):1–9. doi: 10.1016/j.gaitpost.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 17.MacIntyre NJ, Hill NA, Fellows RA, Ellis RE, Wilson DR. Patellofemoral joint kinematics in individuals with and without patellofemoral pain syndrome. J Bone Joint Surg Am. 2006;88(12):2596–605. doi: 10.2106/JBJS.E.00674. [DOI] [PubMed] [Google Scholar]
- 18.Sheehan FT, Derasari A, Brindle TJ, Alter KE. Understanding patellofemoral pain with maltracking in the presence of joint laxity: complete 3D in vivo patellofemoral and tibiofemoral kinematics. J Orthop Res. 2009;27(5):561–70. doi: 10.1002/jor.20783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilson NA, Sheehan FT. Dynamic in vivo quadriceps lines-of-action. J Biomech. 2010;43(11):2106–13. doi: 10.1016/j.jbiomech.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilson NA, Press JM, Koh JL, Hendrix RW, Zhang LQ. In vivo noninvasive evaluation of abnormal patellar tracking during squatting in patients with patellofemoral pain. J Bone Joint Surg Am. 2009;91(3):558–66. doi: 10.2106/JBJS.G.00572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lotman DB. Knee flexion deformity and patella alta in spastic cerebral palsy. Dev Med Child Neurol. 1976;18(3):315–9. doi: 10.1111/j.1469-8749.1976.tb03653.x. [DOI] [PubMed] [Google Scholar]
- 22.Villani C, Billi A, Morico GF, Bonsignore D. A comparative study of the extensor force of the quadriceps between subjects with a normal patella and those with patella alta of neurological pathogenesis. Ital J Orthop Traumatol. 1988;14(3):401–6. [PubMed] [Google Scholar]
- 23.Hirano A, Fukubayashi T, Ishii T, Ochiai N. Relationship between the patellar height and the disorder of the knee extensor mechanism in immature athletes. J Pediatr Orthop. 2001;21(4):541–4. [PubMed] [Google Scholar]
- 24.Sheehan FT, Derasari A, Fine KM, Brindle TJ, Alter KE. Q-angle and J-sign: indicative of maltracking subgroups in patellofemoral pain. Clin Orthop Relat Res. 2009;468(1):266–75. doi: 10.1007/s11999-009-0880-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Powers CM, Ward SR, Fredericson M, Guillet M, Shellock FG. Patellofemoral kinematics during weight-bearing and non-weight-bearing knee extension in persons with lateral subluxation of the patella: a preliminary study. J Orthop Sports Phys Ther. 2003;33(11):677–85. doi: 10.2519/jospt.2003.33.11.677. [DOI] [PubMed] [Google Scholar]
- 26.McCormick A, Brien M, Plourde J, Wood E, Rosenbaum P, McLean J. Stability of the Gross Motor Function Classification System in adults with cerebral palsy. Dev Med Child Neurol. 2007;49(4):265–9. doi: 10.1111/j.1469-8749.2007.00265.x. [DOI] [PubMed] [Google Scholar]
- 27.Rosenbaum PL, Palisano RJ, Bartlett DJ, Galuppi BE, Russell DJ. Development of the Gross Motor Function Classification System for cerebral palsy. Dev Med Child Neurol. 2008;50(4):249–53. doi: 10.1111/j.1469-8749.2008.02045.x. [DOI] [PubMed] [Google Scholar]
- 28.Paternostro-Sluga T, Grim-Stieger M, Posch M, Schuhfried O, Vacariu G, Mittermaier C, Bittner C, Fialka-Moser V. Reliability and validity of the Medical Research Council (MRC) scale and a modified scale for testing muscle strength in patients with radial palsy. J Rehabil Med. 2008;40(8):665–71. doi: 10.2340/16501977-0235. [DOI] [PubMed] [Google Scholar]
- 29.Bohannon RW, Smith MB. Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys Ther. 1987;67(2):206–7. doi: 10.1093/ptj/67.2.206. [DOI] [PubMed] [Google Scholar]
- 30.Behnam AJ, Herzka DA, Sheehan FT. Assessing the accuracy and precision of musculoskeletal motion tracking using cine-PC MRI on a 3.0T platform. J Biomech. 2011;44(1):193–7. doi: 10.1016/j.jbiomech.2010.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seisler AR, Sheehan FT. Normative three-dimensional patellofemoral and tibiofemoral kinematics: a dynamic, in vivo study. IEEE Trans Biomed Eng. 2007;54(7):1333–41. doi: 10.1109/TBME.2007.890735. [DOI] [PubMed] [Google Scholar]
- 32.Shibanuma N, Sheehan FT, Stanhope SJ. Limb positioning is critical for defining patellofemoral alignment and femoral shape. Clin Orthop Relat Res. 2005;434:198–206. doi: 10.1097/01.blo.0000155078.52475.63. [DOI] [PubMed] [Google Scholar]
- 33.Sheehan FT, Zajac FE, Drace JE. In vivo tracking of the human patella using cine phase contrast magnetic resonance imaging. J Biomech Eng. 1999;121(6):650–6. doi: 10.1115/1.2800868. [DOI] [PubMed] [Google Scholar]
- 34.Grelsamer RP, Meadows S. The modified Insall-Salvati ratio for assessment of patellar height. Clin Orthop Relat Res. 1992;282:170–6. [PubMed] [Google Scholar]
- 35.Ali SA, Helmer R, Terk MR. Patella alta: lack of correlation between patellotrochlear cartilage congruence and commonly used patellar height ratios. AJR Am J Roentgenol. 2009;193(5):136–16. doi: 10.2214/AJR.09.2729. [DOI] [PubMed] [Google Scholar]
- 36.Benjamini Y, Hochber Y. Controlling the False Discovery Rate: a Practical and Powerful Approach to Mulitple Testing. 1995;57(1):289–300. [Google Scholar]
- 37.Soo B, Howard JJ, Boyd RN, Reid SM, Lanigan A, Wolfe R, Reddihough D, Graham HK. Hip displacement in cerebral palsy. J Bone Joint Surg Am. 2006;88(1):121–9. doi: 10.2106/JBJS.E.00071. [DOI] [PubMed] [Google Scholar]
- 38.Sheehan FT, Seisler AR, Alter KE. Three-dimensional in vivo quantification of knee kinematics in cerebral palsy. Clin Orthop Relat Res. 2008;466(2):450–8. doi: 10.1007/s11999-007-0004-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Singerman R, Davy DT, Goldberg VM. Effects of patella alta and patella infera on patellofemoral contact forces. J Biomech. 1994;27(8):1059–65. doi: 10.1016/0021-9290(94)90222-4. [DOI] [PubMed] [Google Scholar]
- 40.Jackson AM. Anterior knee pain. J Bone Joint Surg Br. 2001;83(7):937–48. doi: 10.1302/0301-620x.83b7.12756. [DOI] [PubMed] [Google Scholar]
- 41.Amis AA. Current concepts on anatomy and biomechanics of patellar stability. Sports Med Arthrosc. 2007;15(2):48–56. doi: 10.1097/JSA.0b013e318053eb74. [DOI] [PubMed] [Google Scholar]
- 42.Dejour H, Walch G, Neyret P, Adeleine P. Dysplasia of the femoral trochlea. Rev Chir Orthop Reparatrice Appar Mot. 1990;76(1):45–54. [PubMed] [Google Scholar]
- 43.Escala JS, Mellado JM, Olona M, Gine J, Sauri A, Neyret P. Objective patellar instability: MR-based quantitative assessment of potentially associated anatomical features. Knee Surg Sports Traumatol Arthrosc. 2006;14(3):264–72. doi: 10.1007/s00167-005-0668-z. [DOI] [PubMed] [Google Scholar]
- 44.Roberts WM, Adams JP. The patellar-advancement operation in cerebral palsy. J Bone Joint Surg Am. 1953;35-A(4):958–66. [PubMed] [Google Scholar]
- 45.Samilson RL. Current concepts of surgical management of deformities of the lower extremities in cerebral palsy. Clin Orthop Relat Res. 1981;158:99–107. [PubMed] [Google Scholar]
- 46.Joseph B, Reddy K, Varghese RA, Shah H, Doddabasappa SN. Management of severe crouch gait in children and adolescents with cerebral palsy. J Pediatr Orthop. 2010;30(8):832–9. doi: 10.1097/BPO.0b013e3181fbfd0e. [DOI] [PubMed] [Google Scholar]
- 47.Novacheck TF, Stout JL, Gage JR, Schwartz MH. Distal femoral extension osteotomy and patellar tendon advancement to treat persistent crouch gait in cerebral palsy. Surgical technique J Bone Joint Surg Am. 2009;91 (Suppl 2):271–86. doi: 10.2106/JBJS.I.00316. [DOI] [PubMed] [Google Scholar]
- 48.Rodda JM, Graham HK, Nattrass GR, Galea MP, Baker R, Wolfe R. Correction of severe crouch gait in patients with spastic diplegia with use of multilevel orthopaedic surgery. J Bone Joint Surg Am. 2006;88(12):265–364. doi: 10.2106/JBJS.E.00993. [DOI] [PubMed] [Google Scholar]
- 49.Stout JL, Gage JR, Schwartz MH, Novacheck TF. Distal femoral extension osteotomy and patellar tendon advancement to treat persistent crouch gait in cerebral palsy. J Bone Joint Surg Am. 2008;90(11):2470–84. doi: 10.2106/JBJS.G.00327. [DOI] [PubMed] [Google Scholar]
- 50.Asakawa DS, Blemker SS, Rab GT, Bagley A, Delp SL. Three-dimensional muscle-tendon geometry after rectus femoris tendon transfer. J Bone Joint Surg Am. 2004;86-A(2):348–54. doi: 10.2106/00004623-200402000-00019. [DOI] [PubMed] [Google Scholar]
- 51.Muthusamy K, Seidl AJ, Friesen RM, Carollo JJ, Pan Z, Chang FM. Rectus femoris transfer in children with cerebral palsy: evaluation of transfer site and preoperative indicators. J Pediatr Orthop. 2008;28(6):674–8. doi: 10.1097/BPO.0b013e3181804c04. [DOI] [PubMed] [Google Scholar]
- 52.Ounpuu S, Muik E, Davis RB, 3rd, Gage JR, DeLuca PA. Rectus femoris surgery in children with cerebral palsy. Part I: The effect of rectus femoris transfer location on knee motion. J Pediatr Orthop. 1993;13(3):325–30. doi: 10.1097/01241398-199305000-00010. [DOI] [PubMed] [Google Scholar]
- 53.Davis IS, Powers CM. Patellofemoral pain syndrome: proximal, distal, and local factors, an international retreat, April 30–May 2, 2009, Fells Point, Baltimore, MD. J Orthop Sports Phys Ther. 2010;40(3):A1–16. doi: 10.2519/jospt.2010.0302. [DOI] [PubMed] [Google Scholar]
- 54.Sheehan FT, Mitiguy P. In regards to the “ISB recommendations for standardization in the reporting of kinematic data”. J Biomech. 1999;32(10):1135–6. doi: 10.1016/s0021-9290(99)00077-9. [DOI] [PubMed] [Google Scholar]


