Abstract
Exposure to prenatal insults such as maternal stress and pathogenic infections has been associated with an increased risk for neurodevelopmental disorders. The mechanisms by which these programing events occur likely involve complex interactions between the maternal hormonal milieu, the placenta, and the developing fetus, in addition to compounding factors such as fetal sex and gestational stage of development. Despite the diverse biological processes involved, examination of common pathways in maternal stress and immune activation offers intriguing possibilities for elucidation of mechanistic insight. Further, the endocrine and sex-specific placenta is a tissue poised to be a key mediator in fetal programing, located at the intersection of the maternal and embryonic environments. In this review, we will discuss the potential shared mechanisms of maternal stress and immune pathway activation, with a particular focus on the important contribution and role of the placenta.
Keywords: Maternal stress, Immune-activation, Fetal programming, Neurodevelopment, Epigenetic, Sex, Placenta, Glucocorticoids
Introduction
Exposure to prenatal insults such as maternal stress and pathogenic infections has been associated with an increased risk for neurodevelopmental disorders. The mechanisms by which these programing events occur likely involve complex interactions between the maternal hormonal milieu, the placenta, and the developing fetus, in addition to compounding factors such as fetal sex and gestational stage of development (Bale, 2011; Dunn et al., 2011; Mao et al., 2010). Despite the diverse biological processes involved, examination of common pathways in maternal stress and immune activation offers intriguing possibilities for elucidation of mechanistic insight. Further, the endocrine and sex-specific placenta is a tissue poised to be a key mediator in fetal programing, located at the intersection of the maternal and embryonic environments. In this review, we will discuss the potential shared mechanisms of maternal stress and immune pathway activation, with a particular focus on the important contribution and role of the placenta.
Disruptions in maternal homeostasis and fetal programing
Effects of prenatal stress
Many studies of human populations have linked maternal stressors, such as death of a family member, unwantedness of the pregnancy, military invasion, natural disaster and reported maternal depression or anxiety with an increased offspring risk for disruptions in neurodevelopment (Beversdorf et al., 2005; Buka et al., 2000; Goldstein et al., 2000; Khashan et al., 2008, 2009; Kinney et al., 2008; Li et al., 2009; Myhrman et al., 1996; van Os and Selten, 1998). Additionally, both the timing of these stressors and the sex of the offspring have been identified as key factors in the increased incidence of offspring disease development associated with maternal stress. For example, male offspring of mothers suffering from depression during pregnancy showed deficits in both motor skills and behavioral state regulation, whereas changes in behavior were not detected in female offspring (Gerardin et al., 2011). Extreme stressors during pregnancy, such as the loss of a spouse or a military invasion, produced a temporally specific effect on offspring where the gestational age was a key factor. Children born from pregnancies where the father died during mid-gestation (months 3–5) were at a higher risk for developing schizophrenia than those whose father died during late pregnancy or the first year postpartum (Huttunen and Niskanen, 1978). Similarly, children born from pregnancies that were afflicted by a military invasion were at a higher risk for the development of schizophrenia, an effect more pronounced if the invasion occurred during the first trimester (van Os and Selten, 1998).
Animal models of maternal stress across a diverse range of species, including mice, rats, guinea pigs and nonhuman primates have demonstrated that prenatal stress increases offspring hypothalamic–pituitary–adrenal (HPA) axis sensitivity, anxiety and depressive-like behaviors and cognitive deficits; endophenotypes associated with neuropsychiatric disease (Darnaudery and Maccari, 2008; Kapoor and Matthews, 2005; Kapoor et al., 2008, 2009; Lemaire et al., 2000; Mueller and Bale, 2007, 2008; Schneider et al., 2002; Weinstock, 2001). Similar to epidemiological findings, a temporal specificity of stressor exposure and fetal sex were predictive factors in animal models of maternal stress (as reviewed in Bale et al., 2010). Work in our lab and by others has demonstrated that stress during gestation results in sex-dependent HPA stress axis dysregulation and behavioral or cognitive deficits, and that there was an important temporal specificity in these outcomes (Kapoor and Matthews, 2005; Kapoor et al., 2009; Mueller and Bale, 2007, 2008). In mice, male offspring that experienced stress early in gestation showed heightened corticosterone production in response to an acute stressor, increased immobility responses in both the forced swim and tail suspension tests, and deficits in the performance and strategy used in the modified Barnes maze, a spatial learning and memory task (Mueller and Bale, 2007, 2008). Similar timing effects have also been reported in guinea pigs where gestational stress during periods of rapid brain development produced impaired learning performance, increased anxiety-like behaviors and higher basal levels of cortisol in males. Taken together, these sex and temporal specific outcomes of maternal stress offer potential mechanisms and time points to be further investigated in the search for novel targets and biomarkers predictive of disease.
Effects of maternal immune activation
Intriguingly, similar to maternal stress, maternal infection also has a strong association with increased offspring risk for neuropsychiatric and neurodevelopmental disorders. Clinical studies have found that exposure to Toxoplasma gondii, influenza or herpes simplex viruses during pregnancy increases offspring risk for schizophrenia, psychosis and autism spectrum disorders (Brown et al., 2004a, 2005; Buka et al., 2001a, 2008). Increased maternal levels of pro-inflammatory cytokines, such as TNF-α and IL-8, resultant from infection are the most likely mediators of increased offspring risk of disease development (Brown et al., 2004b; Buka et al., 2001b). Further, there is an established temporal specificity for these outcomes associated with maternal infection similar to studies on maternal stress. For instance, exposure to influenza during the first trimester or elevated levels of IL-8 during the second trimester produced an increased the risk for offspring schizophrenia development (Brown et al., 2004a, 2004b). While not directly investigated in these epidemiological datasets, there is a gender bias in the symptomatic presentation and timing of the onset of schizophrenia, likely to be recapitulated in the association with maternal immune activation (Aleman et al., 2003).
Numerous animal models of maternal infection show similar relationships where maternal immune activation produces offspring endophenotypes that are associated with neurodevelopmental disorders, especially schizophrenia (as reviewed in Meyer and Feldon, 2010). This body of research demonstrates offspring programing by several models, including maternal influenza viral infection, injection of the pathogen mimics Poly I:C and lipopolysaccharide (LPS) and the pro-inflammatory cytokine IL-6 (Borrell et al., 2002; Coyle et al., 2009; Fortier et al., 2007; Makinodan et al., 2008; Meyer et al., 2005; Romero et al., 2007; Shi et al., 2003; Smith et al., 2007). The most consistent phenotype produced in these models is a deficit in offspring pre-pulse inhibition (PPI) in response to an acoustic startle, a change that is reversed by the administration of antipsychotic drugs haloperidol or clozapine (Borrell et al., 2002; Fortier et al., 2007; Meyer et al., 2005; Romero et al., 2007; Shi et al., 2003; Smith et al., 2007). Sensorimotor gating deficiencies, a phenotype measured by PPI, is commonly associated with schizophrenia, but has also been demonstrated in numerous other psychiatric or affective disorders such as obsessive compulsive disorder, Tourette’s syndrome and post-traumatic stress disorder (Braff et al., 2001). Again, highlighting the importance of gestational timing for immune activation in producing long-term outcomes, a single administration of IL-6 at embryonic day 12.5 (E12.5) in mice was sufficient to produce deficits in both PPI and latent inhibition, while administration of an anti-IL-6 antibody following Poly I:C injection rescued this effect (Smith et al., 2007). Further, mice deficient in IL-6 failed to produce these phenotypes in response to prenatal maternal immune activation supporting an important involvement of maternal pro-inflammatory cytokines in offspring central nervous system programing (Smith et al., 2007). Similar to models of maternal stress, there is evidence to support that offspring responses to maternal immune activation are also sex-dependent. For example, male offspring of pregnant rats challenged with LPS late in gestation showed deficits in PPI, whereas females or offspring exposed earlier in pregnancy were unaffected (Fortier et al., 2007).
The intersection between immune and stress pathway activation
Beginning with classic work by famed endocrinologist Hans Selye (e.g., 1955), there has been a firm understanding that there is a suppressive role of glucocorticoids on the immune system. More recent work has highlighted a complex interplay between the HPA stress axis and immune system activation. These interactions can occur both peripherally and centrally, and depending on the context can lead to either immune activation through prevailing pro-inflammatory signaling or an immunosuppressive effect by glucocorticoid suppression of macrophages and Th1-type T cells or direct actions of CRF on peripheral CRF receptors (Goetzl et al., 2008). Likely participants in these interactions from the HPA axis include corticotropin-releasing factor (CRF), adrenocorticotropic hormone (ACTH) and glucocorticoids.
CRF, the central upstream mediator of stress pathway activation, can have a myriad of context-and concentration-dependent effects upon the immune system. For example, CRF can decrease T cell proliferation and natural killer (NK) cell cytotoxicity as a centrally mediated effect, as ICV injection of CRF antibodies prevents this suppression (Jain et al., 1991; Pawlikowski et al., 1988). Peripherally, CRF can also act as an anti-inflammatory molecule by reducing inflammatory exudate volume in various models of injury (Karalis et al., 1995; Wei et al., 1986). Despite these apparent immunosuppressive effects, CRF can also be an immune stimulant, enhancing B and T cell proliferation in response to various antigens and increasing interleukin-2 receptor numbers (Singh, 1989; Wei et al., 1986). In the context of psychological stressors, such as placement of rats in open-field settings or conditioned aversion stress, CRF triggers cytokine release and associated fever response prior to a rise in glucocorticoid levels (LeMay et al., 1990; Morrow et al., 1993). Additionally, prenatal maternal stress is positively correlated with higher circulating levels of the pro-inflammatory cytokines IL-6 and TNFα, particularly during the first trimester; potentially linking stress and maternal immune activation that could affect fetal programing (Coussons-Read et al., 2005, 2007). These effects may be mediated in part by CRF receptors found at peripheral sites of the immune system, serving to promote pro-inflammatory signaling in addition to effects of the downstream glucocorticoids (Cao et al., 2005; Karalis et al., 1991). The effects of glucocorticoids on the immune system are complex, and have been well summarized by Sapolsky et al. (2000). There is a temporal specificity in the effects glucocorticoids have on the immune system; in the acute phase glucocorticoids suppress immune responses, yet if they are present for up to a week prior to an immune challenge, glucocorticoids may serve to enhance pro-inflammatory responses. Glucocorticoids also have tissue specificity in their effects. For example, chronic elevations of glucocorticoids have suppressive effects on the peripheral immune system, yet promote a pro-inflammatory state on the immune cells in the brain (Sorrells and Sapolsky, 2007). These dynamic effects of glucocorticoids should therefore be examined in any tissue of interest.
Certainly, a reciprocal interaction of these two systems also occurs. From the immune system, pro-inflammatory cytokines can also have potent effects upon the HPA axis. For example, IL-1β promotes CRF release from the hypothalamus and ACTH from the pituitary (Berkenbosch et al., 1987; Bernton et al., 1987; Sapolsky et al., 1987). Other cytokines, including IL-2, IL-6, TNF-α, and interferon-γ (IFN-γ), are capable of stimulating the HPA axis, although none with the potency of IL-1 (as reviewed by Irwin and Miller, 2007; Wick et al., 1989). Taken as a whole, these reciprocal interactions between immune activation and HPA stress axis responses offer common mechanisms whereby the maternal environment in response to such fetal antecedents such as maternal stress or infection can affect offspring development and generate similar offspring phenotypes.
The placenta as a key mediator of offspring programing
The placenta is a conserved eutherian mammalian adaptation to facilitate in vivo gestation that has diverse functions ranging from nutrient and oxygen transport to complex endocrine and paracrine signaling. One function of critical importance is to protect the developing fetus from maternal environmental insults. For example, during either prolonged or short-term maternal nutritional deprivation, the placenta maintains fetal growth by sacrificing itself through autophagy (Alwasel et al., 2010; Broad and Keverne, 2011). Therefore, responses of the placenta to the maternal environment should be interpreted in this context of increasing offspring survival in the short-term, with the unfortunate consequence being long-term fetal developmental changes that may involve an increased risk for stress pathway dysregulation and neurodevelopmental disorders. Alternatively, the Barker hypothesis suggests that changes in offspring programing during development as a result of fetal antecedents attempts to predict the postnatal environment; this suggests that disease results when the predicted environment and the actual postnatal environment do not match (Barker and Osmond, 1986). Given that maternal cytokines and glucocorticoids are important effectors of aberrant offspring development that have been associated with neurodevelopmental disorders, the impact these molecules have on placental development and gene expression is likely important in our understanding of the etiology of disease risk. Additionally, the placenta is a tissue derived from both maternal and fetal contributions, with the majority of the tissue of fetal origin, specifically the trophoblast cell lineage, a derivative of the trophectoderm (Rossant and Cross, 2001). Therefore the placenta is sex-specific, which may in part suggest a point of convergence for effects of fetal antecedents to produce a gender bias in any effect found in the developing fetus (Bale, 2011). Prenatal stress not only has sex-specific effects on the developing fetuses, but also upon the fetal-placental unit as a whole. For example, chronic restraint stress in pregnant rats produced dramatic changes specifically in the male fetal-placental unit (Mairesse et al., 2007). Male rat fetuses exposed to this prenatal stress exhibited decreased body, pancreatic and testis weights, as well as reduced β-cell mass, plasma glucose, growth hormone and ACTH. Additionally, in the placenta associated with these fetuses there was reduced glucose transporter 1 (GLUT1) expression and 11-beta hydroxysteroid dehydrogenase 2 (11βHSD2) activity and expression. We have found similar sex-specific effects of prenatal stress on the male placenta in mice. Placentas were collected from both male and female E12.5 embryos and mRNA levels of genes related to the response to hypoxia, inflammation and nutrient transport were assessed. Compared to non-stressed controls, early prenatal stress produced male specific increases in placental gene expression of peroxisome proliferator-activated receptor α (PPARα), insulin like growth factor binding protein 1 (IGFBP-1), glucose transporter 4 (GLUT4), and hypoxia-inducible factor 3α (HIFα), all genes known to be critically important in regulating fetal growth and development (Mueller and Bale, 2008).
Glucocorticoids, members of the steroid class of hormones and thus lipophilic in nature, readily pass through the placenta. However, high levels of placental expression of the enzyme 11βHSD2, which converts active glucocorticoids to an inactive metabolite (Drake et al., 2007), protects the developing fetus from high maternal levels of this hormone (Beitins et al., 1973). Glucocorticoids are necessary for various aspects of fetal brain development including subcellular reorganization of neuron–neuron and neuron–glia interactions, as well as late gestational lung maturation. Clearly, close modulation of mechanisms involved in maintenance of appropriate hormone levels is necessary to create this differential (Matthews, 2000). Studies in humans and in animal models have linked reduced placental 11βHSD2 activity or expression with fetal exposure to increased glucocorticoids, birth complications and fetal programing of adult hypertension and hyperglycemia (Lindsay et al., 1996a, 1996b; Shams et al., 1998; Stewart et al., 1995). In rodents and humans, the activity and sensitivity of placental 11βHSD2 to maternal stimuli are sex-specific. In females, 11βHSD2 activity is reduced in response to either inflammation associated with asthma or prenatal exposure to ethanol, but in male placentas activity is actually increased (Burton and Waddell, 1994; Clifton et al., 2006; Wilcoxon and Redei, 2004). This sex specificity may therefore provide one mechanism by which sex biases in neurodevelopmental programing occurs. Finally, studies in animal models have demonstrated that placental 11βHSD2 expression and activity are also reduced by chronic maternal stress, linking this enzyme to an environment known to affect fetal programing associated with neurodevelopmental disease (Fig. 1) (Mairesse et al., 2007).
Fig. 1.
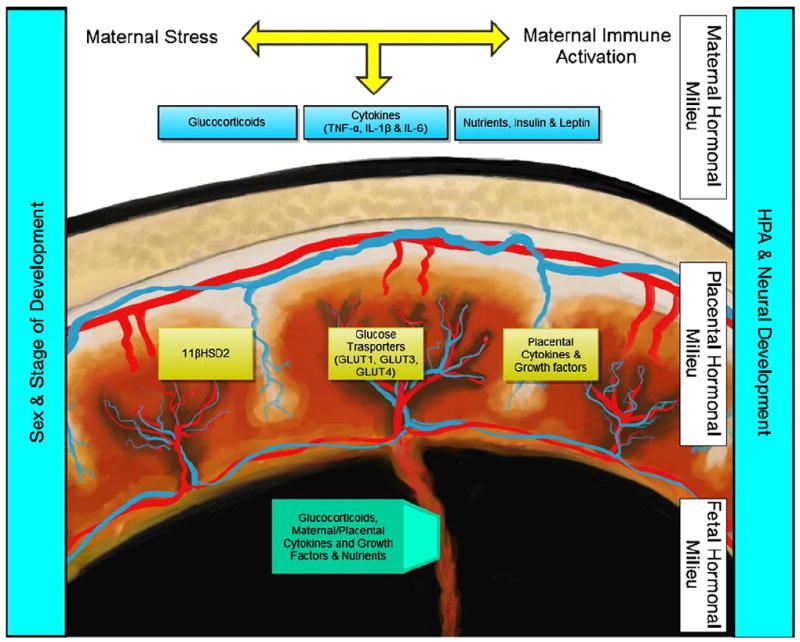
Schematic representing the proposed interaction between maternal, placental and fetal factors that may underlie aspects of programing of neurodevelopmental disease. Both maternal stress and immune activation have been associated with altered fetal programing of the developing hypothalamic–pituitary axis (HPA) and nervous system. Putative maternal contributions include increased glucocorticoids, pro-inflammatory cytokines, altered nutrient availability and dysregulated leptin and insulin signaling. In eutherian mammals, these maternal contributions are transmitted to the fetal compartment via the placenta. This transmission is achieved through passive permeability (i.e. glucocorticoids), active transport (e.g. glucose and other nutrients) and perhaps most intriguingly through endocrine and paracrine signaling within the endocrine placenta (e.g. glucocorticoid inactivation via 11-beta hydroxysteroid dehydrogenase 2 [11βHSD2] and local production of cytokines and growth factors). The resulting fetal hormonal milieu as transmitted via the placenta directly interacts with the developing embryo to shape the ontogenetic trajectory. Changes in programing are likely affected by developmental differences as well as embryo sex, which leads to chromosomal and somatic differences in both the placenta and embryo.
While changes in the activity of placental 11βHSD2 may contribute to increased glucocorticoid levels within the fetal compartment, there are also important direct effects of increased maternal glucocorticoids upon programing of the placenta itself. One example of this is the regulation of placental glucose transporters by glucocorticoids. Altered placental glucose transport from the maternal to fetal compartment has been implicated in birth complications such as intrauterine growth restriction, adult phenotypes such as diabetes and also has far reaching effects on general fetal development due to the fundamental importance of glucose as an energy substrate (Myatt, 2006). In cultured human trophoblast cells and in vivo rat placental tissues there was a significant reduction of both the mRNA and protein levels of the glucose transporters GLUT1 and GLUT3 (Hahn et al., 1999). A more recent report using spiny mice (Acomys cahirinus) reiterates these findings where the immediate response to exogenous glucocorticoid administration was a reduction in GLUT1 levels in both male and female placentas. However, observations later in gestation reveal a divergence between the sexes where males have a continued suppression of GLUT1, but females actually show an increased expression (O’Connell et al., 2011). In addition to altering the expression and activity of nutrient transporters, glucocorticoids also affect the oxidative state of both the placental and fetal compartments. Oxidative stress is exacerbated during conditions that complicate pregnancy, including inflammation and placental insufficiency, leading to developmental diseases with higher incidence in males (Auten and Davis, 2009; Myatt, 2010). Perhaps explaining the difference in disease presentation, antenatal glucocorticoids have sex-specific effects on the oxidative state of human placental tissue. In females, exogenous glucocorticoid exposure promotes antioxidant activity and reduces signs of oxidative stress postpartum, yet in males this exposure promotes a pro-oxidant state (Stark et al., 2011; Vento et al., 2009). As stated earlier, as there are temporal and tissue specific effects of glucocorticoids so careful examination of the placental specific response to these hormones should be further examined, with particular emphasis on the differences across pregnancy between the maternal and fetal components of the placenta.
The mechanism of fetal transmission of maternal cytokines is not well understood. There are several reports that support the ability for maternal cytokines to reach the fetal compartment or for the fetus to mount its own response to maternal inflammatory states. The latter would suggest an interaction at the level of the placenta that promotes fetal immune activation. As described above, maternal immune activation in pregnant mice can lead to offspring endophenotypes associated with neurodevelopmental disease, but blocking pro-inflammatory cytokine production ameliorates effects on offspring adult behaviors, therefore pro-inflammatory cytokines are likely key molecules in these programing events (Patterson, 2009). Dammann and Leviton report that maternal infection was correlated with high levels of several pro-inflammatory cytokines, including TNF-α, IL-1β and IL-6, not only within the maternal circulation, but also within the fetal circulation and CNS (1997). Another study found that maternal immune activation leads to increased levels of pro-inflammatory cytokines within the amniotic fluid and placenta (Lin et al., 2003). TNF-α and IL-1β expression was upregulated in a dose dependent manner in the fetuses of pregnant rats exposed to LPS (Gao and Cai, 2003). Additionally, maternal IL-6 was found in the fetal compartment by midgestation, whether this occurred through active transport by the placenta or passive permeability was not clear (Dahlgren et al., 2006). Activational changes at the level of the placenta were detected where knockout mouse strategies demonstrated that maternal IL-6 production was necessary for the programing effects of maternal immune activation, and that this effect occurred by placental JAK/STAT3 signaling to reduce growth hormone, insulin-like growth factor I and insulin-like growth factor binding protein 3 (Hauguel-de Mouzon and Guerre-Millo, 2006; Hsiao and Patterson, 2011). Taken together, these data suggest that maternal immune activation is a powerful effector of fetal programing, and that the signals associated with maternal inflammation may in part be transduced through direct and indirect actions at the placenta.
An important consideration is that in most placental mammals X-inactivation is a random process, yet in rodents there are at least 150 loci that escape this process, thereby leading to expression of the genes from both X-chromosomes which can account for basal sex differences of X-linked genes (Carrel and Willard, 2005). Of relevance to the above discussion on the intersection of maternal stress and immune activation, many components of the pro-inflammatory pathway are X-linked, pointing to a potentially interesting sex chromosome involvement in sex differences in immune responsivity. One such X-linked gene in NEMO (NFκB essential modulator), an X-linked regulatory protein that participated in the IKK complex that promotes nuclear translocation of NFκB dimers. Following cytokine production, NFκB binds to its target gene consensus sequence and promotes transcription of cytokine receptors and prostaglandins. Interestingly, children born with hypomorphic NEMO mutations frequently show intrauterine growth restriction, which may support a dysregulation of cytokine signaling in the placenta during development in these pregnancies (Hanson et al., 2008). Therefore the sex-specific contribution of the placenta to fetal programing may be resultant from differential expression of sex-linked traits, such as NEMO, as opposed to the traditionally held view of the influences of gonadal hormones.
Conclusion
Both maternal stress and immune activation serve as potentiating factors for alterations in offspring programing and increasing disease risk. This impressive coordination likely results from the multi-level reciprocal interactions found in these pathways where stress can serve as an immune activator and vice versa. Additionally, and perhaps even more intriguing are the seemingly temporal- and sex-specific effects of these fetal antecedents. Careful consideration of these sensitive periods of increased vulnerability that are dependent on fetal sex will likely elucidate important mechanisms by which the maternal environment invokes long-term outcomes. Finally, it is clear that the endocrine placenta is a key target tissue in need of further analyses for its important roles in fetal development including: sex-specificity of the placenta, its dynamic changes in early development, and its role in endocrine signaling for factors critical in determining somatic growth and programing. Strategies to investigate the placenta in these programing events should focus on common factors of both the stress and immune pathways, as their activation during pregnancy promote similar offspring phenotypes.
Acknowledgments
This work was supported by the National Institutes of Health Grants R01 MH-091258 and MH-087597.
References
- Aleman A, Kahn RS, Selten JP. Sex differences in the risk of schizophrenia — evidence from meta-analysis. Arch Gen Psychiatry. 2003;60:565–571. doi: 10.1001/archpsyc.60.6.565. [DOI] [PubMed] [Google Scholar]
- Alwasel SH, Abotalib Z, Aljarallah JS, Osmond C, Alkharaz SM, Alhazza IM, Badr G, Barker DJP. Changes in placental size during Ramadan. Placenta. 2010;31:607–610. doi: 10.1016/j.placenta.2010.04.010. [DOI] [PubMed] [Google Scholar]
- Auten RL, Davis JM. Oxygen toxicity and reactive oxygen species: the devil is in the details. Pediatr Res. 2009;66:121–127. doi: 10.1203/PDR.0b013e3181a9eafb. [DOI] [PubMed] [Google Scholar]
- Bale TL. Sex differences in prenatal epigenetic programing of stress pathways. Stress. 2011;14:348–356. doi: 10.3109/10253890.2011.586447. [DOI] [PubMed] [Google Scholar]
- Bale TL, Baram TZ, Brown AS, Goldstein JM, Insel TR, McCarthy MM, Nemeroff CB, Reyes TM, Simerly RB, Susser ES, Nestler EJ. Early life programming and neurodevelopmental disorders. Biol Psychiatry. 2010;68:314–319. doi: 10.1016/j.biopsych.2010.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJP, Osmond C. Infant-mortality, childhood nutrition, and ischemic-heart-disease in England and Wales. Lancet. 1986;1:1077–1081. doi: 10.1016/s0140-6736(86)91340-1. [DOI] [PubMed] [Google Scholar]
- Beitins IZ, Bayard F, Ances IG, Kowarski A, Migeon CJ. Metabolic clearance rate, blood production, interconversion and transplacental passage of cortisol and cortisone in pregnancy near term. Pediatr Res. 1973;7:509–519. doi: 10.1203/00006450-197305000-00004. [DOI] [PubMed] [Google Scholar]
- Berkenbosch F, van Oers J, del Rey A, Tilders F, Besedovsky H. Corticotropin-releasing factor-producing neurons in the rat activated by interleukin-1. Science. 1987;238:524–526. doi: 10.1126/science.2443979. [DOI] [PubMed] [Google Scholar]
- Bernton E, Beach J, Holaday J, Smallridge R, Fein H. Release of multiple hormones by a direct action of interleukin-1 on pituitary cells. Science. 1987;238:519–521. doi: 10.1126/science.2821620. [DOI] [PubMed] [Google Scholar]
- Beversdorf DQ, Manning SE, Hillier A, Anderson SL, Nordgren RE, Walters SE, Nagaraja HN, Cooley WC, Gaelic SE, Bauman ML. Timing of prenatal stressors and autism. J Autism Dev Disord. 2005;35:471–478. doi: 10.1007/s10803-005-5037-8. [DOI] [PubMed] [Google Scholar]
- Borrell J, Vela JM, Arevalo-Martin A, Molina-Holgado E, Guaza C. Prenatal immune challenge disrupts sensorimotor gating in adult rats: implications for the etiopathogenesis of schizophrenia. Neuropsychopharmacology. 2002;26:204–215. doi: 10.1016/S0893-133X(01)00360-8. [DOI] [PubMed] [Google Scholar]
- Braff DL, Geyer MA, Swerdlow NR. Human studies of prepulse inhibition of startle: normal subjects, patient groups, and pharmacological studies. Psychopharmacology. 2001;156:234–258. doi: 10.1007/s002130100810. [DOI] [PubMed] [Google Scholar]
- Broad KD, Keverne EB. Placental protection of the fetal brain during short-term food deprivation. Proc Natl Acad Sci U S A. 2011;108:15237–15241. doi: 10.1073/pnas.1106022108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AS, Begg MD, Gravenstein S, Schaefer CA, Wyatt RJ, Bresnahan M, Babulas VP, Susser ES. Serologic-evidence of prenatal influenza in the etiology of schizophrenia. Arch Gen Psychiatry. 2004a;61:774–780. doi: 10.1001/archpsyc.61.8.774. [DOI] [PubMed] [Google Scholar]
- Brown AS, Hooton J, Schaefer CA, Zhang H, Petkova E, Babulas V, Perrin M, Gorman JM, Susser ES. Elevated maternal interleukin-8 levels and risk of schizophrenia in adult offspring. Am J Psychiatry. 2004b;161:889–895. doi: 10.1176/appi.ajp.161.5.889. [DOI] [PubMed] [Google Scholar]
- Brown AS, Schaefer CA, Quesenberry CP, Liu LY, Babulas VP, Susser ES. Maternal exposure to toxoplasmosis and risk of schizophrenia in adult offspring. Am J Psychiatry. 2005;162:767–773. doi: 10.1176/appi.ajp.162.4.767. [DOI] [PubMed] [Google Scholar]
- Buka SL, Goldstein JM, Seidman LJ, Tsuang MT. Maternal recall of pregnancy history: accuracy and bias in schizophrenia research. Schizophr Bull. 2000;26:335–350. doi: 10.1093/oxfordjournals.schbul.a033457. [DOI] [PubMed] [Google Scholar]
- Buka SL, Tsuang MT, Torrey EF, Klebanoff MA, Bernstein D, Yolken RH. Maternal infections and subsequent psychosis among offspring. Arch Gen Psychiatry. 2001a;58:1032–1037. doi: 10.1001/archpsyc.58.11.1032. [DOI] [PubMed] [Google Scholar]
- Buka SL, Tsuang MT, Torrey EF, Klebanoff MA, Wagner RL, Yolken RH. Maternal cytokine levels during pregnancy and adult psychosis. Brain Behav Immun. 2001b;15:411–420. doi: 10.1006/brbi.2001.0644. [DOI] [PubMed] [Google Scholar]
- Buka SL, Cannon TD, Torrey EF, Yolken RH Collaborative Study Grp, P. Maternal exposure to herpes simplex virus and risk of psychosis among adult offspring. Biol Psychiatry. 2008;63:809–815. doi: 10.1016/j.biopsych.2007.09.022. [DOI] [PubMed] [Google Scholar]
- Burton PJ, Waddell BJ. 11-beta-hydroxysteroid dehydrogenase in the rat placenta — developmental-changes and the effects of altered glucocorticoid exposure. J Endocrinol. 1994;143:505–513. doi: 10.1677/joe.0.1430505. [DOI] [PubMed] [Google Scholar]
- Cao J, Papadopoulou N, Kempuraj D, Boucher WS, Sugimoto K, Cetrulo CL, Theoharides TC. Human mast cells express corticotropin-releasing hormone (CRH) receptors and CRH leads to selective secretion of vascular endothelial growth factor. J Immunol. 2005;174:7665–7675. doi: 10.4049/jimmunol.174.12.7665. [DOI] [PubMed] [Google Scholar]
- Carrel L, Willard HF. X-inactivation profile reveals extensive variability in X-linked gene expression in females. Nature. 2005;434:400–404. doi: 10.1038/nature03479. [DOI] [PubMed] [Google Scholar]
- Clifton VL, Rennie N, Murphy VE. Effect of inhaled glucocorticoid treatment on placental 11 beta-hydroxysteroid dehydrogenase type 2 activity and neonatal birthweight in pregnancies complicated by asthma. Aust N Z J Obstet Gynaecol. 2006;46:136–140. doi: 10.1111/j.1479-828X.2006.00543.x. [DOI] [PubMed] [Google Scholar]
- Coussons-Read ME, Okun ML, Schmitt MP, Giese S. Prenatal stress alters cytokine levels in a manner that may endanger human pregnancy. Psychosom Med. 2005;67:625–631. doi: 10.1097/01.psy.0000170331.74960.ad. [DOI] [PubMed] [Google Scholar]
- Coussons-Read ME, Okun ML, Nettles CD. Psychosocial stress increases inflammatory markers and alters cytokine production across pregnancy. Brain Behav Immun. 2007;21:343–350. doi: 10.1016/j.bbi.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Coyle P, Tran N, Fung JNT, Summers BL, Rofe AM. Maternal dietary zinc supplementation prevents aberrant behaviour in an object recognition task in mice offspring exposed to LPS in early pregnancy. Behav Brain Res. 2009;197:210–218. doi: 10.1016/j.bbr.2008.08.022. [DOI] [PubMed] [Google Scholar]
- Dahlgren J, Samuelsson AM, Jansson T, Holmang A. Interleukin-6 in the maternal circulation reaches the rat fetus in mid-gestation. Pediatr Res. 2006;60:147–151. doi: 10.1203/01.pdr.0000230026.74139.18. [DOI] [PubMed] [Google Scholar]
- Dammann O, Leviton A. Intrauterine infection, cytokines, and brain damage in the preterm newborn. Pediatr Res. 1997;42:1–8. doi: 10.1203/00006450-199707000-00001. [DOI] [PubMed] [Google Scholar]
- Darnaudery M, Maccari S. Epigenetic programming of the stress response in male and female rats by prenatal restraint stress. Brain Res Rev. 2008;57:571–585. doi: 10.1016/j.brainresrev.2007.11.004. [DOI] [PubMed] [Google Scholar]
- Drake AJ, Tang JI, Nyirenda MJ. Mechanisms underlying the role of glucocorticoids in the early life programming of adult disease. Clin Sci. 2007;113:219–232. doi: 10.1042/CS20070107. [DOI] [PubMed] [Google Scholar]
- Dunn GA, Morgan CP, Bale TL. Sex-specificity in transgenerational epigenetic programming. Horm Behav. 2011;59:290–295. doi: 10.1016/j.yhbeh.2010.05.004. [DOI] [PubMed] [Google Scholar]
- Fortier ME, Luheshi GN, Boksa P. Effects of prenatal infection on prepulse inhibition in the rat depend on the nature of the infectious agent and the stage of pregnancy. Behav Brain Res. 2007;181:270–277. doi: 10.1016/j.bbr.2007.04.016. [DOI] [PubMed] [Google Scholar]
- Gao YX, Cai FC. Experimental study on the therapeutic mechanism of high dose intravenous immunoglobulin in treatment of immune-mediated peripheral neuropathy. Zhonghua Er Ke Za Zhi. 2003;41:684–687. [PubMed] [Google Scholar]
- Gerardin P, Wendland J, Bodeau N, Galin A, Bialobos S, Tordjman S, Mazet P, Darbois Y, Nizard J, Dommergues M, Cohen D. Depression during pregnancy: is the developmental impact earlier in boys? A prospective case–control study. J Clin Psychiatry. 2011;72:378–387. doi: 10.4088/JCP.09m05724blu. [DOI] [PubMed] [Google Scholar]
- Goetzl EJ, Chan RC, Yadav M. Diverse mechanisms and consequences of immunoadoption of neuromediator systems. In: Goetzl EJ, editor. Neural Signaling: Opportunities for Novel Diagnostic Approaches and Therapies. Blackwell Publishing; Oxford: 2008. pp. 56–60. [Google Scholar]
- Goldstein JM, Seidman LJ, Buka SL, Horton NJ, Donatelli JL, Rieder RO, Tsuang MT. Impact of genetic vulnerability and hypoxia on overall intelligence by age 7 in offspring at high risk for schizophrenia compared with affective psychoses. Schizophr Bull. 2000;26:323–334. doi: 10.1093/oxfordjournals.schbul.a033456. [DOI] [PubMed] [Google Scholar]
- Hahn T, Barth S, Graf R, Engelmann M, Beslagic D, Reul J, Holsboer F, Dohr G, Desoye G. Placental glucose transporter expression is regulated by glucocorticoids. J Clin Endocrinol Metab. 1999;84:1445–1452. doi: 10.1210/jcem.84.4.5607. [DOI] [PubMed] [Google Scholar]
- Hanson EP, Monaco-Shawver L, Solt LA, Madge LA, Banerjee PP, May MJ, Orange JS. Hypomorphic nuclear factor-kappa B essential modulator mutation database and reconstitution system identifies phenotypic and immunologic diversity. J Allergy Clin Immunol. 2008;122:1169–1177. doi: 10.1016/j.jaci.2008.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauguel-de Mouzon S, Guerre-Millo M. The placenta cytokine network and inflammatory signals. Placenta. 2006;27:794–798. doi: 10.1016/j.placenta.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Hsiao EY, Patterson PH. Activation of the maternal immune system induces endocrine changes in the placenta via IL-6. Brain Behav Immun. 2011;25:604–615. doi: 10.1016/j.bbi.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttunen MO, Niskanen P. Prenatal loss of father and psychiatric-disorders. Arch Gen Psychiatry. 1978;35:429–431. doi: 10.1001/archpsyc.1978.01770280039004. [DOI] [PubMed] [Google Scholar]
- Irwin MR, Miller AH. Depressive disorders and immunity: 20 years of progress and discovery. Brain Behav Immun. 2007;21:374–383. doi: 10.1016/j.bbi.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Jain R, Zwickler D, Hollander CS, Brand H, Saperstein A, Hutchinson B, Brown C, Audhya T. Corticotropin-releasing factor modulates the immune response to stress in the rat. Endocrinology. 1991;128:1329–1336. doi: 10.1210/endo-128-3-1329. [DOI] [PubMed] [Google Scholar]
- Kapoor A, Matthews SG. Short periods of prenatal stress affect growth, behaviour and hypothalamo–pituitary–adrenal axis activity in male guinea pig offspring. J Physiol -London. 2005;566:967–977. doi: 10.1113/jphysiol.2005.090191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor A, Leen J, Matthews SG. Molecular regulation of the hypothalamic–pituitary–adrenal axis in adult male guinea pigs after prenatal stress at different stages of gestation. J Physiol -London. 2008;586:4317–4326. doi: 10.1113/jphysiol.2008.153684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor A, Kostaki A, Janus C, Matthews SG. The effects of prenatal stress on learning in adult offspring is dependent on the timing of the stressor. Behav Brain Res. 2009;197:144–149. doi: 10.1016/j.bbr.2008.08.018. [DOI] [PubMed] [Google Scholar]
- Karalis K, Sano H, Redwine J, Listwak S, Wilder RL, Chrousos GP. Autocrine or paracrine inflammatory actions of corticotropin-releasing hormone in vivo. Science. 1991;254:421–423. doi: 10.1126/science.1925600. [DOI] [PubMed] [Google Scholar]
- Karalis K, Crofford L, Wilder RL, Chrousos GP. Glucocorticoid and/or glucocorticoid antagonist effects in inflammatory disease-susceptible Lewis rats and inflammatory disease-resistant Fischer rats. Endocrinology. 1995;136:3107–3112. doi: 10.1210/endo.136.7.7789338. [DOI] [PubMed] [Google Scholar]
- Khashan AS, Abel KM, McNamee R, Pedersen MG, Webb RT, Baker PN, Kenny LC, Mortensen PB. Higher risk of offspring schizophrenia following antenatal maternal exposure to severe adverse life events. Arch Gen Psychiatry. 2008;65:146–152. doi: 10.1001/archgenpsychiatry.2007.20. [DOI] [PubMed] [Google Scholar]
- Khashan AS, McNamee R, Abel KM, Mortensen PB, Kenny LC, Pedersen MG, Webb RT, Baker PN. Rates of preterm birth following antenatal maternal exposure to severe life events: a population-based cohort study. Hum Reprod. 2009;24:429–437. doi: 10.1093/humrep/den418. [DOI] [PubMed] [Google Scholar]
- Kinney DK, Miller AM, Crowley DJ, Huang E, Gerber E. Autism prevalence following prenatal exposure to hurricanes and tropical storms in Louisiana. J Autism Dev Disord. 2008;38:481–488. doi: 10.1007/s10803-007-0414-0. [DOI] [PubMed] [Google Scholar]
- Lemaire V, Koehl M, Le Moal M, Abrous DN. Prenatal stress produces learning deficits associated with an inhibition of neurogenesis in the hippocampus. Proc Natl Acad Sci U S A. 2000;97:11032–11037. doi: 10.1073/pnas.97.20.11032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeMay LG, Vander AJ, Kluger MJ. The effects of psychological stress on plasma interleukin-6 activity in rats. Physiol Behav. 1990;47:957–961. doi: 10.1016/0031-9384(90)90024-x. [DOI] [PubMed] [Google Scholar]
- Li J, Vestergaard M, Obel C, Christensen J, Precht DH, Lu M, Olsen J. A nationwide study on the risk of autism after prenatal stress exposure to maternal bereavement. Pediatrics. 2009;123:1102–1107. doi: 10.1542/peds.2008-1734. [DOI] [PubMed] [Google Scholar]
- Lin DM, Smith MA, Eller J, Champagne C, Downey CL, Beck J, Offenbacher S. Porphyromonas gingivalis infection in pregnant mice is associated with placental dissemination, an increase in the placental Th1/Th2 cytokine ratio, and fetal growth restriction. Infect Immun. 2003;71:5163–5168. doi: 10.1128/IAI.71.9.5163-5168.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay RS, Lindsay RM, Edwards CRW, Seckl JR. Inhibition of 11 beta-hydroxysteroid dehydrogenase in pregnant rats and the programming of blood pressure in the offspring. Hypertension. 1996a;27:1200–1204. doi: 10.1161/01.hyp.27.6.1200. [DOI] [PubMed] [Google Scholar]
- Lindsay RS, Lindsay RM, Waddell BJ, Seckl JR. Prenatal glucocorticoid exposure leads to offspring hyperglycaemia in the rat: studies with the 11 beta-hydroxysteroid dehydrogenase inhibitor carbenoxolone. Diabetologia. 1996b;39:1299–1305. doi: 10.1007/s001250050573. [DOI] [PubMed] [Google Scholar]
- Mairesse J, Lesage J, Breton C, Breant B, Hahn T, Darnaudery M, Dickson SL, Seckl J, Blondeau B, Vieau D, Maccari S, Viltart O. Maternal stress alters endocrine function of the feto-placental unit in rats. Am J Physiol Endocrinol Metab. 2007;292:E1526–E1533. doi: 10.1152/ajpendo.00574.2006. [DOI] [PubMed] [Google Scholar]
- Makinodan M, Tatsumi K, Manabe T, Yamauchi T, Makinodan E, Matsuyoshi H, Shimoda S, Noriyama Y, Kishimoto T, Wanaka A. Maternal immune activation in mice delays myelination and axonal development in the hippocampus of the offspring. J Neurosci Res. 2008;86:2190–2200. doi: 10.1002/jnr.21673. [DOI] [PubMed] [Google Scholar]
- Mao J, Zhang X, Sieli PT, Falduto MT, Torres KE, Rosenfeld CS. Contrasting effects of different maternal diets on sexually dimorphic gene expression in the murine placenta. Proc Natl Acad Sci U S A. 2010;107:5557–5562. doi: 10.1073/pnas.1000440107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews SG. Antenatal glucocorticoids and programming of the developing CNS. Pediatr Res. 2000;47:291–300. doi: 10.1203/00006450-200003000-00003. [DOI] [PubMed] [Google Scholar]
- Meyer U, Feldon J. Epidemiology-driven neurodevelopmental animal models of schizophrenia. Prog Neurobiol. 2010;90:285–326. doi: 10.1016/j.pneurobio.2009.10.018. [DOI] [PubMed] [Google Scholar]
- Meyer U, Feldon J, Schedlowski M, Yee BK. Towards an immuno-precipitated neurodevelopmental animal model of schizophrenia. Neurosci Biobehav Rev. 2005;29:913–947. doi: 10.1016/j.neubiorev.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Morrow LE, McClellan JL, Conn CA, Kluger MJ. Glucocorticoids alter fever and IL-6 responses to psychological stress and to lipopolysaccharide. Am J Physiol Regul Integr Comp Physiol. 1993;264:R1010–R1016. doi: 10.1152/ajpregu.1993.264.5.R1010. [DOI] [PubMed] [Google Scholar]
- Mueller BR, Bale TL. Early prenatal stress impact on coping strategies and learning performance is sex dependent. Physiol Behav. 2007;91:55–65. doi: 10.1016/j.physbeh.2007.01.017. [DOI] [PubMed] [Google Scholar]
- Mueller BR, Bale TL. Sex-specific programming of offspring emotionality after stress early in pregnancy. J Neurosci. 2008;28:9055–9065. doi: 10.1523/JNEUROSCI.1424-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myatt L. Placental adaptive responses and fetal programming. J Physiol -London. 2006;572:25–30. doi: 10.1113/jphysiol.2006.104968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myatt L. Reactive oxygen and nitrogen species and functional adaptation of the placenta. Placenta. 2010;31:S66–S69. doi: 10.1016/j.placenta.2009.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myhrman A, Rantakallio P, Isohanni M, Jones P, Partanen U. Unwantedness of a pregnancy and schizophrenia in the child. Br J Psychiatry. 1996;169:637–640. doi: 10.1192/bjp.169.5.637. [DOI] [PubMed] [Google Scholar]
- O’Connell BA, Moritz KM, Roberts CT, Walker DW, Dickinson H. The placental response to excess maternal glucocorticoid exposure differs between the male and female conceptus in spiny mice. Biol Reprod. 2011;85:1040–1047. doi: 10.1095/biolreprod.111.093369. [DOI] [PubMed] [Google Scholar]
- Patterson PH. Immune involvement in schizophrenia and autism: etiology, pathology and animal models. Behav Brain Res. 2009;204:313–321. doi: 10.1016/j.bbr.2008.12.016. [DOI] [PubMed] [Google Scholar]
- Pawlikowski M, Zelazowski P, Dohler K, Stepien H. Effects of two neuropeptides, somatoliberin (GRF) and corticoliberin (CRF), on human lymphocyte natural killer activity. Brain Behav Immun. 1988;2:50–56. doi: 10.1016/0889-1591(88)90005-0. [DOI] [PubMed] [Google Scholar]
- Romero E, Ali C, Molina-Holgado E, Castellano B, Guaza C, Borrell J. Neuro-behavioral and immunological consequences of prenatal immune activation in rats. Influence of antipsychotics. Neuropsychopharmacology. 2007;32:1791–1804. doi: 10.1038/sj.npp.1301292. [DOI] [PubMed] [Google Scholar]
- Rossant J, Cross JC. Placental development: lessons from mouse mutants. Nat Rev Genet. 2001;2:538–548. doi: 10.1038/35080570. [DOI] [PubMed] [Google Scholar]
- Sapolsky R, Rivier C, Yamamoto G, Plotsky P, Vale W. Interleukin-1 stimulates the secretion of hypothalamic corticotropin-releasing factor. Science. 1987;238:522–524. doi: 10.1126/science.2821621. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- Schneider ML, Moore CF, Kraemer GW, Roberts AD, DeJesus OT. The impact of prenatal stress, fetal alcohol exposure, or both on development: perspectives from a primate model. Psychoneuroendocrinology. 2002;27:285–298. doi: 10.1016/s0306-4530(01)00050-6. [DOI] [PubMed] [Google Scholar]
- Selye H. Stress and disease. Science. 1955;122:625–631. doi: 10.1126/science.122.3171.625. [DOI] [PubMed] [Google Scholar]
- Shams M, Kilby MD, Somerset DA, Howie AJ, Gupta A, Wood PJ, Afnan M, Stewart PM. 11 beta-hydroxysteroid dehydrogenase type 2 in human pregnancy and reduced expression in intrauterine growth restriction. Hum Reprod. 1998;13:799–804. doi: 10.1093/humrep/13.4.799. [DOI] [PubMed] [Google Scholar]
- Shi LM, Fatemi H, Sidwell RW, Patterson PH. Maternal influenza infection causes marked behavioral and pharmacological changes in the offspring. J Neurosci. 2003;23:297–302. doi: 10.1523/JNEUROSCI.23-01-00297.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh VK. Stimulatory effect of corticotropin-releasing neurohormone on human lymphocyte proliferation and interleukin-2 receptor expression. J Neuroimmunol. 1989;23:257–262. doi: 10.1016/0165-5728(89)90058-1. [DOI] [PubMed] [Google Scholar]
- Smith SEP, Li J, Garbett K, Mirnics K, Patterson PH. Maternal immune activation alters fetal brain development through interleukin-6. J Neurosci. 2007;27:10695–10702. doi: 10.1523/JNEUROSCI.2178-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorrells SF, Sapolsky RM. An inflammatory review of glucocorticoid actions in the CNS. Brain Behav Immun. 2007;21:259–272. doi: 10.1016/j.bbi.2006.11.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark MJ, Hodyl NA, Wright IMR, Clifton VL. Influence of sex and glucocorticoid exposure on preterm placental pro-oxidant-antioxidant balance. Placenta. 2011;32:865–870. doi: 10.1016/j.placenta.2011.08.010. [DOI] [PubMed] [Google Scholar]
- Stewart PM, Rogerson FM, Mason JI. Type-2 11-beta-hydroxysteroid dehydrogenase messenger-ribonucleic-acis and activity in human placenta and fetal membranes - its relationship to birth-weight and putative role in fetal adrenal steroidgenesis. J Clin Endocrinol Metab. 1995;80:885–890. doi: 10.1210/jcem.80.3.7883847. [DOI] [PubMed] [Google Scholar]
- van Os J, Selten JP. Prenatal exposure to maternal stress and subsequent schizophrenia — the May 1940 invasion of The Netherlands. Br J Psychiatry. 1998;172:324–326. doi: 10.1192/bjp.172.4.324. [DOI] [PubMed] [Google Scholar]
- Vento M, Aguar M, Escobar J, Arduini A, Escrig R, Brugada M, Izquierdo I, Asensi MA, Sastre J, Saenz P, Gimeno A. Antenatal steroids and antioxidant enzyme activity in preterm infants: influence of gender and timing. Antioxid Redox Signal. 2009;11:2945–2955. doi: 10.1089/ars.2009.2671. [DOI] [PubMed] [Google Scholar]
- Wei ET, Kiang JG, Buchan P, Smith TW. Corticotropin-releasing factor inhibits neurogenic plasma extravasation in the rat paw. J Pharmacol Exp Ther. 1986;238:783–787. [PubMed] [Google Scholar]
- Weinstock M. Alterations induced by gestational stress in brain morphology and behaviour of the offspring. Prog Neurobiol. 2001;65:427–451. doi: 10.1016/s0301-0082(01)00018-1. [DOI] [PubMed] [Google Scholar]
- Wick G, Brezinschek HP, Hala K, Dietrich H, Wolf H, Kroemer G. The obese strain of chickens: an animal model with spontaneous autoimmune thyroiditis. Adv Immunol. 1989;47:433–500. doi: 10.1016/s0065-2776(08)60666-5. [DOI] [PubMed] [Google Scholar]
- Wilcoxon JS, Redei EE. Prenatal programming of adult thyroid function by alcohol and thyroid hormones. Am J Physiol Endocrinol Metab. 2004;287:E318–E326. doi: 10.1152/ajpendo.00022.2004. [DOI] [PubMed] [Google Scholar]


