Abstract
BACKGROUND
Recent reports suggest that maternal immunization against low-frequency, platelet (PLT)-specific glycoprotein (GP) polymorphisms is a more common cause of neonatal alloimmune thrombocytopenia (NATP) than previously thought.
STUDY DESIGN AND METHODS
Serologic and molecular studies were performed on PLTs and DNA from three families in which an infant was born with apparent NATP not attributable to maternal immunization against known PLT-specific alloantigens.
RESULTS
Antibodies reactive only with paternal PLTs were identified in each mother. In Cases 2 (Kno) and 3 (Nos), but not Case 1 (Sta), antibody recognized paternal GPIIb/IIIa in solid-phase assays. Unique mutations encoding amino acid substitutions in GPIIb (Case 2) or GPIIIa (Cases 1 and 3) were identified in paternal DNA and in DNA from two of the affected infants. Antibody from all three cases recognized recombinant GPIIIa (Case 1 [Sta] and Case 3 [Nos]) and GPIIb (Case 2, Kno) mutated to contain the polymorphisms identified in the respective fathers. None of 100 unselected normal subjects possessed the paternal mutations. Enzyme-linked immunosorbent assay and flow cytometric studies suggested that failure of maternal serum from Case 1 (Sta) to react with paternal GPIIIa in solid-phase assays resulted from use of a monoclonal antibody AP2, for antigen immobilization that competed with the maternal antibody for binding to the Sta epitope.
CONCLUSION
NATP in the three cases was caused by maternal immunization against previously unreported, low-frequency GP polymorphisms. Maternal immunization against low-frequency PLT-specific alloantigens should be considered in cases of apparent NATP not resolved by conventional serologic and molecular testing.
Neonatal alloimmune thrombocytopenia (NATP), caused by transplacentally acquired maternal antibodies reactive with fetal platelet (PLT) antigens, occurs about once in every 1000 live births.1,2 Although many cases are mild and require no specific treatment, a subset of affected infants is at risk for intracranial hemorrhage, sometimes leading to death or permanent disability.3,4 Even in mild cases, it is important that a specific serologic diagnosis be established whenever possible because children born subsequently to the same mother can be severely affected and effective prenatal therapy is available.4–6
Early studies of NATP identified the PLT-specific antigen HPA-1a (PlA1, Zwa) as the most common stimulus for antibodies capable of causing NATP.7 Subsequently, its allele, HPA-1b (PlA2, Zwb)8 and other antigens belonging to systems designated HPA-2 carried on glycoprotein (GP)Ibα, HPA-3 on GPIIb, and HPA-5 on GPIa (reviewed by Metcalfe and coworkers9) were shown to be capable of causing maternal immunization during pregnancy, leading to NATP. The HPA-1, -2, -3, and -5 systems consist of two major alloantigens, each of which is relatively common in the general population and can be immunogenic. In recent years, other PLT-specific alloantigens have been identified but, with the exception of HPA-15a/b (Govb/a) carried on CD109,10 each of the new systems consists of one common allele and a second quite rare allele having a gene frequency less than 0.01 in the general population. Twelve antigen systems containing one rare and one common allele have been designated HPA-4, HPA-6 to -14, HPA-16, and HPA-17.9,11,12 To date, only one of the high-frequency alleles (HPA-4a) has been shown to cause maternal immunization,13 owing presumably to the fact that very few women are homozygous for a “private” antigen and are thus susceptible to immunization by its high-frequency counterpart. Each of the rare alleles referenced has, however, been implicated as an immunizing antigen in at least one case of NATP.11,12,14–24 Recent reports suggest that maternal immunization against private PLT-specific antigens, especially HPA-9b (Max), is a more important cause of NATP than has been thought.18,19,25 Here, we describe three previously unidentified, low-frequency alloantigens that appear to have caused maternal immunization leading to NATP.
CASE REPORTS
Case 1 (Sta)
The first child born to a 29-year-old woman was delivered by cesarean section at 36 weeks’ gestation because of fetal distress during labor. Apgar score was 6 at birth, but improved to 8 in 5 minutes and 10 several hours later. Scattered petechial hemorrhages were noted at approximately 4 hours. Blood count revealed a PLT count of 26 × 109/L, white blood cells (WBCs) 12 × 109/L, and hematocrit (Hct) 42%. Blood culture and studies to detect viral infection were negative. A transfusion of random-donor PLTs was given. On the next day (Day 2), the PLT count 61 × 109/L and a cranial ultrasound study provided evidence of a small intraparenchymal hemorrhage in the right thalamus. However, there were no localizing neurologic findings. Subsequent PLT counts were 30 × 109/L on Day 3, 157 × 109/L on Day 4, and 225 × 109/L on Day 7. A CT scan performed 1 week later demonstrated a small lesion in the right thalamus that was thought to be a resolving porencephalic cyst. The child was discharged and was developing normally at 3 years of age.
Case 2 (Kno)
The first child born to a 27-year-old woman with a history of two previous miscarriages was delivered by vacuum extraction at 33 weeks of gestation because of premature labor. A blood count revealed a PLT count of 61 × 109/L, WBCs 9.2 × 109/L, and Hct 37%. Later the same day, the PLT count dropped to 45 × 109/L and petechial hemorrhages were noted. A PLT transfusion from a random donor increased the PLT level to 166 × 109/L. Subsequent PLT counts were 110 × 109/L on Day 2, 120 × 109/L on Day 3, 105 × 109/L on Day 4, 89 × 109/L on Day 5, and 191 × 109/L on Day 8. The child was discharged and was developing normally at 2 years of age.
Case 3 (Nos)
The third child born to a 33-year-old woman had scattered petechial hemorrhages and a PLT count of 14 × 109/L at birth. A single PLT transfusion produced a “satisfactory” elevation of the PLT count. PLTs stabilized within the normal range at approximately 1 week and the child was developing normally at 2 years of age. Studies to identify PLT-specific antibodies were not performed and no other cause for the thrombocytopenia was identified. Two years later, a fourth child by the same father was born with normal hematologic indices, including PLT count. Serologic studies performed before delivery because of the history of thrombocytopenia in the third child demonstrated a PLT-specific antibody, presumably stimulated during the third pregnancy.
MATERIALS AND METHODS
Antibodies
Monoclonal antibodies (MoAb) AP3 specific for GPIIIa, Tab specific for GPIIb, and AP2 specific for the GPIIb-IIIa complex were described previously.26 Tab was a gift from Dr R. McEver at the University of Oklahoma. Alloantibodies specific for HPA-1a (PlA1) and HPA-4a (Pena, Yukb) were from the Platelet & Neutrophil Immunology Laboratory of the BloodCenter.
Serologic studies
Maternal samples were tested against paternal PLTs and patients from group O donors of known phenotypes by flow cytometry (FACSCalibur, Becton Dickinson, San Jose, CA) as described previously.18 Antibodies reactive with GPIIb/IIIa, GPIb/IX, GPIa/IIIa, and GPIV were detected by direct and modified antigen-capture enzyme-linked immunosorbent assay (ELISA; antigen-capture ELISA [ACE] and modified antigen-capture ELISA [MACE]) assays using MoAbs for antigen capture as previously described.18,27,28 In the ACE assay, antigen present in a lysate prepared from nonsensitized cells is captured by an immobilized monoclonal and then used as the target for a human antibody. In the MACE, intact cells expressing the antigenic protein are first sensitized by human antibody. The cells are then lysed and the target protein with human antibody attached is captured by an immobilized monoclonal. Human antibody is then detected by ELISA. Antibodies specific for Class I HLA antigens were detected with a commercial ELISA kit PAK-2MP (GTI, Inc., Waukesha, WI).
Genotyping for PLT antigens and frequency analysis of “Sta,” “Kno,” and “Nos”
Human genomic DNA from peripheral blood cells or buccal swabs was isolated as described.18 Common alloantigens HPA-1 to -5 were typed as described previously18 for Case 1 (Sta) and Case 2 (Kno). For Case 3 (Nos) human genomic DNA was typed for antigens of the HPA-1 to -6, -9, and -15 systems using assay technology (Multicode-PLx) developed by the Molecular Diagnostics Laboratory of BloodCenter in collaboration with Eragen Biosciences (Madison, WI).29 Genotyping for known single-nucleotide polymorphisms (SNPs) encoding low-frequency antigens of the HPA-6 to -14 and -16 systems was carried out after polymerase chain reaction (PCR) amplification and sequence analysis of the following exons: ITGA2 (GPIa) Exon 20 (HPA-13), GP1BB(GPIbβ) Exon 2 (HPA-12), ITGA2B (GPIIb) Exon 26 (HPA-9), and ITGB3(GPIIIa) Exons 1 to 14 (HPA-6, -7, -8, -10, -11, -14, and -16). PCR primers corresponding to intronic sequence surrounding exons of interest were designed to yield PCR products ranging from 500 to 1200 bp in length. PCR was carried out using a commercially available system (Fast-Start High Fidelity PCR system, Roche Diagnostic Corp., Indianapolis, IN). Primer sequences and amplification conditions are available upon request. Automated sequence analysis was performed in both directions as described.18 Genotyping of 100 normal donor DNA samples for the Sta and Kno mutations was performed using a Multicode-PLx assay.30 Fluorescence of PCR products was measured in a multiarray high-throughput bead analyzer (Bioplex 200, Bio-Rad, Hercules, CA). Genotyping for the Nos allele was done by PCR amplification and sequence analysis of DNA.
Preparation of cDNAs encoding truncated and mutant versions of GPIIb and GPIIIa
Throughout this report, nucleotide (Nt) 1 refers to A of the ATG translation start codon of human GPIIb or GPIIIa. All point mutations were generated using a site-directed mutagenesis kit (QuikChange II XL, Stratagene, La Jolla, CA) and were validated by direct sequencing. Mutagenic oligonucleotide sequences are available upon request. Human ITGB3 (GPIIIa) cDNA in expression vector pcDNA3.1 Zeo (Invitrogen, Carlsbad, CA) was subjected to site-directed mutagenesis to generate the Sta (Nt 487 a to c, K137Q) and Nos (Nt 1960 g to a, E628K) mutations, respectively. Similarly, human ITGA2B (GPIIb) cDNA in mammalian expression vector pCDNA3.1 Neo (Invitrogen) was subjected to site-directed mutagenesis (Nt 1949 c to t) to encode GPIIb T619M.
Stable expression of GPIIb/IIIa in Chinese hamster ovary cells and flow cytometric analysis
Chinese hamster ovary (CHO-K1) cell lines expressing wild-type and mutant GPIIb/IIIa were produced as previously described.18 In brief, CHO cells (American Type Culture Collection, Manassas, VA) were cotransfected with mutated GPIIb (T619M) or nonmutated GPIIb cDNA and with mutated GPIIIa (K137Q or E628K) or nonmutated GPIIIa cDNA. Cultured cells were sorted using a flow cytometer (FACStar Plus, Becton Dickinson) to select for high expressers18 and were maintained in Ham’s F-12K (Mediatech, Inc., Herndon, VA) supplemented with fetal bovine serum (10%), Zeocin (0.2 mg/mL), neomycin (0.6 mg/mL), and gentamicin sulfate (50 μg/mL).
Human research
All studies involving human subjects were approved by the institutional research review board of the BloodCenter of Wisconsin.
RESULTS
Serum from each mother contained an IgG antibody that reacted only with paternal PLTs
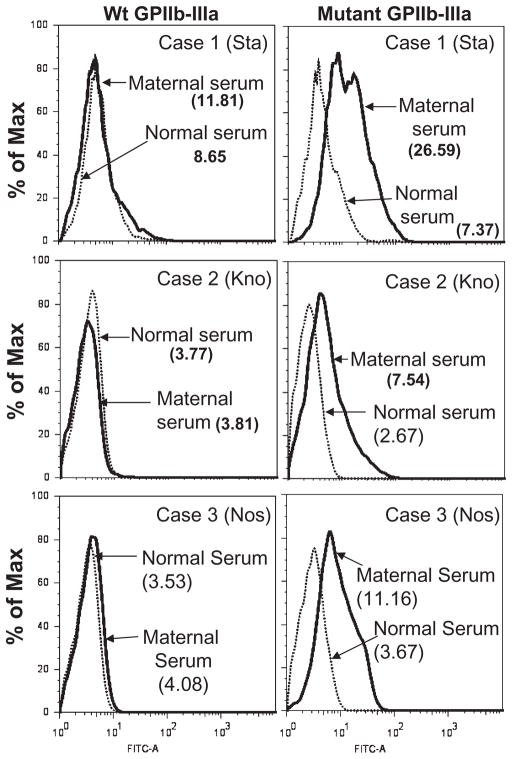
Serum from each of the three mothers contained an IgG antibody that reacted with paternal PLTs in the flow cytometric assay, but not with PLTs from group O donors carrying all antigens of the HPA-1, -2, -3, and -5 systems and the high-frequency antigen HPA-4a (Fig. 1). Class I HLA antibodies were not detected in the maternal sera with a sensitive solid-phase ELISA (data not shown). In Cases 1 (Sta) and 2 (Kno), the ACE test was used to screen maternal serum for antibodies reactive with GPIIb/IIIa, GPIb/IX, and GPIa/IIa. In Case 1 (Sta), no antibodies reactive with these GPs were detected using paternal PLTs (Fig. 1) and PLTs from normal donors positive for all antigens of the HPA-1, -2, -3, and -5 systems and for HPA-4a (not shown). In Case 2 (Kno), a strong antibody reactive with paternal GPIIb/IIIa was identified (Fig. 1). Serum from the mother of Case 3 (Nos) was screened in the MACE and reacted strongly with GPIIb/IIIa from paternal PLTs using AP2 as the capture antibody (Fig. 1). In Case 3 (Nos), the father’s blood group was A1 and mother’s was O. However, reactions of maternal serum against paternal GPIIb/IIIa were unaffected by absorption of anti-A by washed A1 RBCs. Antibodies specific for GPIb/IX, GPIV, or GPIa/IIa were not detected in any of the three maternal sera using the same solid-phase assays (not shown). A summary of these finding is shown in Table 1.
Fig. 1.
Serologic reactions of maternal serum with paternal PLTs. (Top) Each maternal serum sample contained an antibody that reacted with paternal PLTs (dark histograms) but not normal PLTs (light histograms). (Bottom) Maternal antibody from Cases 2 (Kno) (ACE test with AP2 capture) and Case 3 (Nos; MACE test) but not normal serum recognized GPIIb/IIIa from paternal PLTs. No antibody was detected in maternal serum from Case 1 (Sta) using ACE. Horizontal lines indicate mean values obtained with normal serum + 3 SD.
TABLE 1.
Summary of findings
| Molecular and serologic characterization | Case 1 (Sta) | Case 2 (Kno) | Case 3 (Nos) |
|---|---|---|---|
| NCBI dbSNP accession number | ss120032848 | ss120032852 | ss120032849 |
| Molecular characterization | |||
| Exon/nucleotide change | 3/A487C | 20/C1949T | 11/G1960A |
| Amino acid change | K137Q | T619M | E628K |
| Protein | GPIIIa | GPIIb | GPIIIa |
| Maternal serum vs. paternal GPIIb/IIIa | |||
| Flow cytometric analysis | + | + | + |
| ACE (AP2 capture of GPIIb/IIIa) | − | + | NT* |
| MACE (AP2 capture of GPIIb/IIIa) | NT | NT | + |
| Maternal serum versus recombinant GPIIb/IIIa | |||
| Flow cytometric analysis with transfected CHO cell lines | + | + | + |
| MACE with mutated GPIIb/IIIa (AP2/Tab capture) | + | + | + |
NT = not tested.
There was no paternal-maternal incompatibility for known PLT-specific alloantigens
Genotyping of parental DNA for antigens of the HPA-1, -2, -3, and -5 systems and for HPA-4a showed that all paternal antigens belonging to these systems were also present in the mother. Typing for antigens HPA-4b, HPA-6b through14bw, and HPA-16bw showed that the three fathers were negative for each of these low-frequency antigens.
Mutations predicting novel amino acid changes in GPIIIa or GPIIb were identified in DNA from the three fathers and two of the affected infants
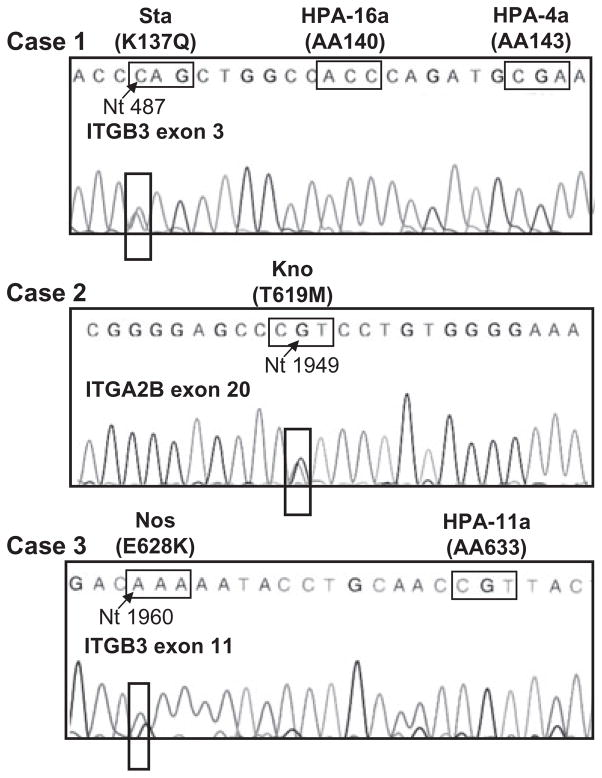
Clinical findings, the genotyping results, and the positive reactions between maternal sera and paternal PLTs but not PLTs from other donors suggested that the three mothers might be immunized against previously undescribed low-frequency PLT antigens. Since the GPIIb/IIIa complex carries most PLT-specific antigens described to date and serum from the mothers of Cases 2 (Kno) and 3 (Nos) reacted strongly with paternal GPIIb/IIIa in the ACE or MACE, extracellular domains of paternal GPIIb and GPIIIa were sequenced. In Case 1 (Sta), sequencing of ITGB3 (GPIIIa) Exon 3 revealed an A-to-C SNP at Nt 487 that predicted a lysine-to-glutamine substitution at Position 137 (Fig. 2 and Table 1). In Case 2 (Kno), sequencing of ITGA2B (GPIIb) Exon 20 revealed a C-to-T SNP at Nt 1949 that predicted a threonine to methionine switch at Position 619 (Fig. 2 and Table 1). In Case 3 (Nos), sequencing of ITGB3 Exon 11 revealed a G-to-A SNP at Nt 1960 that predicted a glutamic acid-to-lysine substitution at Position 628 (Fig. 2 and Table 1). The mutations identified in Cases 1 to 3 will be referred to as “Sta,” “Kno,” and “Nos,” respectively. The novel mutations were not present in DNA from any of the three mothers, but were identified in buccal swab DNA from the affected children in Cases 1 (Sta) and 2 (Kno). It was not possible to obtain DNA from the affected infant in Case 3 (Nos). None of the mutations was detected in any of 100 unselected normal individuals.
Fig. 2.
Previously undescribed mutations predicting amino acid substitutions in GPIIb or GPIIIa were identified in paternal DNA. The identified mutations were A to C at Nt 487 of GPIIIa Exon 3 predicting a lysine-to-glutamine substitution at Amino Acid 137 (Case 1, Sta), C to T at Nt 1949 of GPIIb Exon 20 predicting a threonine-to-methionine switch at Amino Acid 619 (Case 2, Kno), and G to A at Nt 1960 of GPIIIa Exon 11 predicting a glutamic acid-to-lysine switch at Amino Acid 628 (Case 3, Nos). Sequencing results for Case 1 (Sta) and Case 3 (Nos) are those obtained with forward primers; results shown for Case 2 (Kno) are those obtained with antisense primers. Locations of codons encoding the HPA-16 (Duv) and HPA-4 (Pen, Yuk) polymorphisms adjacent to the Sta mutation and the codon that encodes the HPA-11 polymorphism (Gro) adjacent to the Nos mutation are also shown. AA = amino acid.
Maternal antibodies from each of the mothers reacted with recombinant GPIIb/IIIa mutated to contain the identified paternal mutations
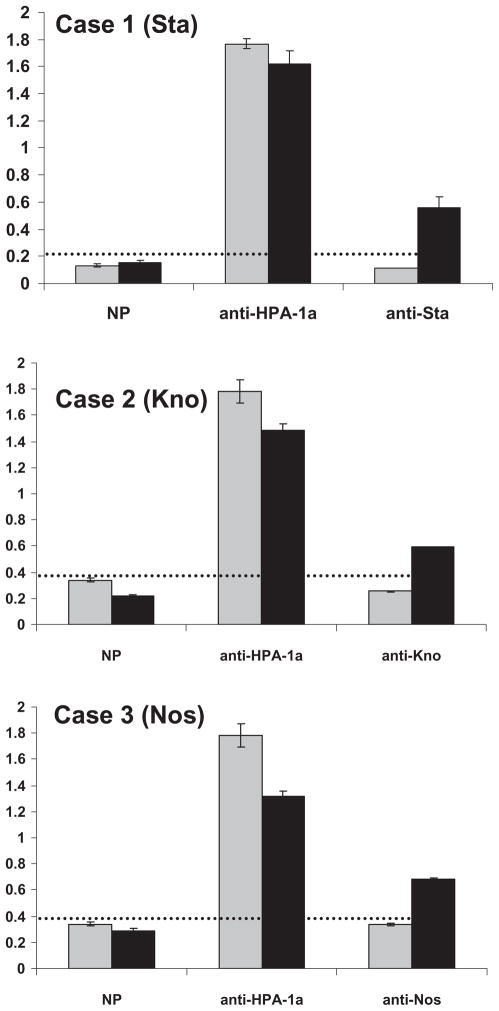
To further characterize specificity of the maternal antibodies, ITGB3(GPIIIa) containing the Sta or the Nos mutation and ITGA2B (GPIIb) containing the Kno mutation were coexpressed with wild-type GPIIb or GPIIIa in CHO cells. Using flow cytometry, it was found that maternal antibodies from Cases 1 (Sta), 2 (Kno), and 3 (Nos) recognized the appropriate mutant, but not wild-type GPIIb/IIIa (Fig. 3A, Table 1). The maternal antibodies also recognized the appropriate mutants expressed in CHO cells using MACE (Fig. 4 and Table 1).
Fig. 3.
Reactions of maternal serum with recombinant GPIIb/IIIa expressed in CHO cells (flow cytometry). Serum from Case 1 (Sta; top panel, right), Case 2 (Kno; middle panel, right), and Case 3 (Nos; bottom panel, right) recognized GPIIb/IIIa mutated to contain the amino acid change identified in paternal DNA but not wild-type (Wt) GPIIb/IIIa (left panels). An anti-HPA-1a recognized both mutated and wild-type integrin equally well (not shown), and normal serum failed to recognize either. FITC = fluorescein isothiocyanate.
Fig. 4.
Reactions of antibodies from Case 1 (Sta), Case 2 (Kno), and Case 3 (Sta) with recombinant mutated GPIIb/IIIa expressed in CHO cells using MACE. Capture of GPIIb/IIIa was achieved with a mixture of MoAbs Tab, AP2, and AP3. Maternal antibody from each case diluted 1:20 reacted with GPIIb/IIIa mutated to contain the appropriate mutation (■) but failed to recognize wild-type GPIIb/IIIa (
 ). Anti-HPA-1a recognized both constructs and normal serum (NP) recognized neither. Horizontal lines indicate mean values obtained with normal serum + 3 SD.
). Anti-HPA-1a recognized both constructs and normal serum (NP) recognized neither. Horizontal lines indicate mean values obtained with normal serum + 3 SD.
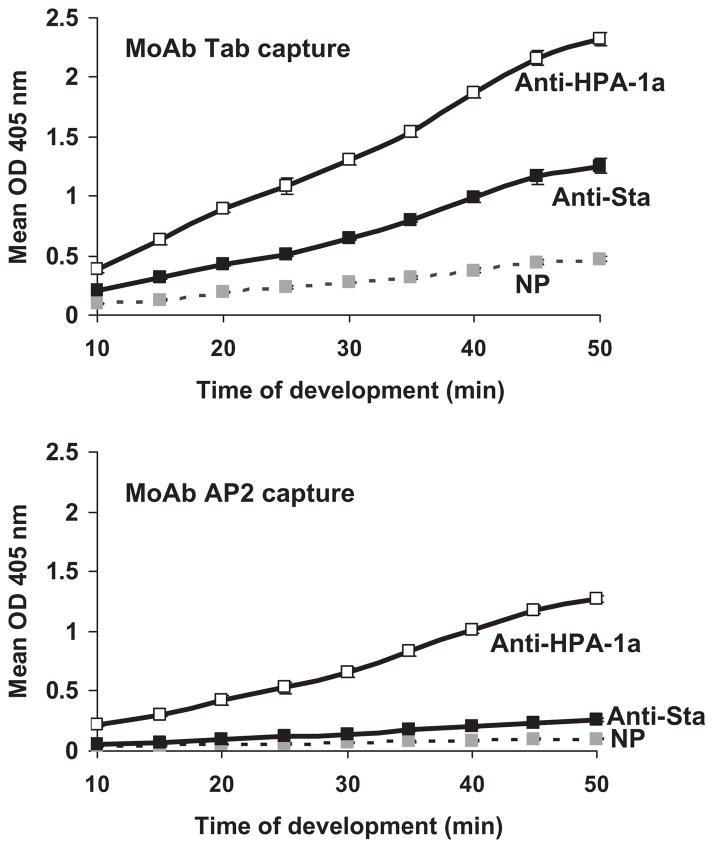
Binding of anti-Sta to recombinant GPIIb/IIIa was inhibited by monoclonal AP2, but not by Tab
Failure of the Sta antibody to react with paternal GPIIb/IIIa in the ACE using monoclonal AP2 to capture paternal GPIIb/IIIa (Fig. 1) suggested that AP2 might compete with the Sta antibody for binding to GPIIIa. Since paternal PLTs from this case were not available for further study, we used CHO cells transfected with GPIIb/IIIa mutated to contain the Sta mutation and examined possible competition between anti-Sta and AP2 using MACE. As shown in Fig. 5, the reaction of anti-Sta with the GPIIb/IIIa mutant was readily detectable when Tab (anti-GPIIb) was used for capture but was almost completely abolished when AP2 was used. This finding strongly suggests that the initial failure to detect anti-Sta in the ACE was the result of using AP2 as the capture antibody. Because the mutation encoding antigen of the HPA-4 system is located only 6 amino acid residues upstream from the Sta mutation,13 we tested whether AP2 would block the reactions of anti-HPA-4a with HPA-4a–positive PLTs using flow cytometry. These studies showed that AP2, but not AP3, also specific for GPIIIa, consistently inhibited the binding of two HPA-4a–specific antibodies by approximately 50% (data not shown).
Fig. 5.
Monoclonal AP2 (below) but not Tab (anti-GPIIb, top-) competed with anti-Sta for binding to StaGPIIb/IIIa expressed in CHO cells (MACE assay). Values shown on the ordinate are OD values obtained at various times after adding alkaline phosphatase–labeled anti-immunoglobulin. As shown below, capture with AP2 nearly abolished the reaction of anti-Sta with mutant GPIIb/IIIa. In contrast, binding of anti-HPA-1a was unaffected by the choice of MoAb.
DISCUSSION
Each of the three affected infants had severe (Cases 1 [Sta] and 3 [Nos]) or moderately severe (Case 2 [Kno]) thrombocytopenia at birth and had a satisfactory response to transfusion of PLTs from a random donor. External bleeding manifestations consisted only of petechial hemorrhages, but in Case 1, imaging studies demonstrated a small intracranial hemorrhage that may have occurred in utero. A normal PLT level was achieved after approximately 1 week in each case. Serologic studies demonstrated maternal IgG antibodies that reacted with paternal PLTs in flow cytometry (Fig. 1) but not with PLTs from normal donors positive for antigens of the HPA-1 to -6 systems and could not be attributed to Class I HLA reactivity or antibodies specific for blood groups A or B. Maternal antibody reactive with paternal GPIIb/IIIa was detected using the ACE assay in Case 2 (Kno) and the MACE assay in Case 3 (Nos) (Fig. 2) but not in Case 1 (Sta) using the ACE.
Sequencing of paternal DNA encoding ITGA2B (GPIIb) and ITGB3(GPIIIa) demonstrated previously unreported mutations predicting K137Q (Sta) and E628K (Nos) substitutions in GPIIIa (Cases 1 and 3, respectively) and a T619M (Kno) substitution in GPIIb (Case 2). Mutations identical to those found in paternal DNA were identified in the affected infants in Cases 1 (Sta) and 2 (Kno) by buccal swab testing. It was not possible to obtain DNA from the infant in Case 3 (Nos), who had been born 2 years before the maternal antibody was identified. None of the three mutations was found in DNA from 100 unrelated blood donors.
The antibodies from all three cases recognized recombinant GPIIb/IIIa mutated to contain the appropriate mutation and expressed in CHO cells (Figs. 3 and 4). The Sta antibody also reacted with biotinylated srGPIIIa mutated to contain Sta and captured with streptavidin (data not shown). However, the antibody from Case 3 (Nos) failed to recognize srGPIIIa modified to contain the Nos mutation despite its strong reactions with paternal GPIIb/IIIa and recombinant Nos GPIIb/IIIa in the MACE assay (Figs. 1 and 4 and Table 1). Together, the findings indicate that NATP in Cases 1 (Sta), 2 (Kno), and 3 (Nos) was caused by maternal antibodies specific for previously undescribed, low-frequency alloantigens carried on GPIIIa (Sta and Nos) and GPIIb (Kno). DNA sequences encoding the three newly recognized polymorphisms have been submitted to the National Center for Biotechnology Information (NCBI) and have been assigned ss numbers 120032848 (Sta), 120032852 (Kno), and 120032849 (Nos; Table 1).
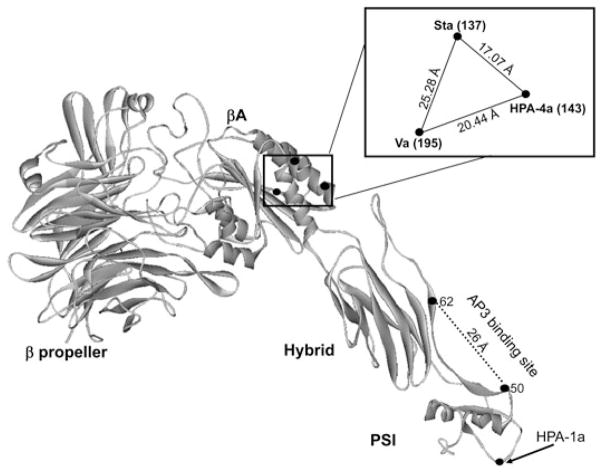
A puzzling aspect of the initial serologic evaluation of Case 1 (Sta) was the failure of maternal serum to recognize paternal GPIIb/IIIa in the ACE using monoclonal AP2 as the capture antibody. In previous experience, we had found that AP2 is satisfactory for solid-phase detection of all known HPA antibodies reactive with GPIIb/IIIa. However, in this study it was found that AP2 almost completely inhibits the reaction of anti-Sta with GPIIIa mutated to contain the Sta mutation (Fig. 5). The crystal structures of the N-terminal domains of GPIIIa and GPIIb in a complex31 and, very recently, the entire extracellular structure of GPIIb/IIIa32 have now been resolved, making it possible to assign amino acids that determine PLT-specific antigens to precise locations on the surface of the integrin. Figure 6 shows the locations of amino acids that determine the antigens HPA-4a, Sta, and a newly defined low-frequency antigen (Va) encoded by a Thr195Met substitution in GPIIIa.12 The three immunogenic amino acids are clustered in a triangle with sides about 17, 20, and 25 Å. Findings shown in Fig. 5 suggest that the binding site for monoclonal AP2 is close enough to Amino Acid 137 to inhibit the binding of anti-Sta to that site, leading to the negative result obtained in the initial ACE assay (Fig. 1). It is of interest that Kekomaki and colleagues33 found that AP2 was satisfactory for detection of anti-Va in the monoclonal antibody immobilization of platelet antigens assay but not in ACE assay, indicating that under some circumstances AP2 and anti-Va can bind to GPIIIa simultaneously, despite the proximity of the two antigens. If the “footprints” for AP2 and anti-Va are typical and occupy an area of about 1000 Å2 (radius approx. 17 Å) at the protein-solvent interface,34,35 the binding site for AP2 would need to be about 34 Å away from the Va binding site at GPIIIa residue 195 to avoid interference between the two antibodies. However, it could still be close enough to residue 137 to completely block anti-Sta and partially block anti-HPA-4a specific for Residue 143 (Fig. 6). It is likely that AP2 will also interfere with the binding of anti-HPA-16bw (Duv), which recognizes GPIIIa Residue 140,11 only three amino residues away from Sta and HPA-4a, but to our knowledge this has not been tested. The demonstration that AP2 is unsuitable as a capture antibody for detection of anti-Sta and suboptimal for detection of anti-HPA-4 emphasizes the desirability of using two or more noncompeting monoclonals to detect HPA-specific antibodies in solid-phase assays.
Fig. 6.
Location on GPIIIa of amino acids determining alloantigens HPA-4a, HPA-17bw (Va), and Sta. The three critical amino acids are clustered at the corners of a triangular area on the surface of the βA domain of GPIIIa with dimensions shown in the inset. The adjacent beta propeller domain forming the N-terminus of GPIIb and binding sites for anti-HPA-1a and AP3 are shown for reference. Not shown is the HPA-16bw (Duv) epitope located midway between the Sta and HPA-4a epitopes. The structural diagram was generated using crystal coordinates described by Zhu and colleagues.32 Intermolecular distances were calculated using WebLab Viewer Pro 3.7 (from Molecular Simulations, Inc., Princeton, NJ).
When low-frequency antigens were identified as triggers for alloantibodies capable of causing NATP, it was initially thought that each case might be unique. However, several instances of maternal immunization against HPA-4b,14,36 HPA-6bw (Ca/Tu),37,38 HPA-8bw (Sr),16,25 HPA-11bw (Gro),21,25 HPA-13bw (Sit),23,25 and at least 14 examples of immunization against HPA-9bw17–19 have now been described. The increasing number of such reports emphasizes the importance of being able to identify the corresponding antibodies. Unfortunately, alloantibodies specific for low-frequency antigens and PLTs that express them are not widely available, even to reference laboratories. Recently reports have suggested that recombinant antigens expressed in cell lines25 or as soluble proteins or protein fragments12,26,39 may provide tools to facilitate serologic evaluation of such cases.
Acknowledgments
This work was supported by Grant HL-13629 (RHA) and HL-79085 (JAP) from the National Heart, Lung, and Blood Institute.
We appreciate the technical assistance of Stephanie Balthazor. This work was supported by grant HL-13629 (RHA) and HL-79085 (JAP) from the National Heart, Lung and Blood Institute.
ABBREVIATIONS
- ACE
antigen-capture ELISA
- CHO
Chinese hamster ovary
- GP
glycoprotein
- MACE
modified antigen-capture ELISA
- NATP
neonatal alloimmune thrombocytopenia
- Nt
nucleotide (when followed by a number)
- SNP(s)
single-nucleotide polymorphism(s)
Footnotes
CONFLICT OF INTEREST
The authors have no disclaimers to make or conflicts to disclose.
References
- 1.Blanchette VS, Chen L, de Friedberg ZS, Hogan VA, Trudel E, Décary F. Alloimmunization to the PlA1 platelet antigen: results of a prospective study. Br J Haematol. 1990;74:209–15. doi: 10.1111/j.1365-2141.1990.tb02567.x. [DOI] [PubMed] [Google Scholar]
- 2.Williamson LM, Hackett G, Rennie J, Palmer CR, Maciver C, Hadfield R, Hughes D, Jobson S, Ouwehand WH. The natural history of fetomaternal alloimmunization to the platelet-specific antigen HPA-1a (PlA1, Zwa) as determined by antenatal screening. Blood. 1998;92:2280–7. [PubMed] [Google Scholar]
- 3.Bussel JB. Alloimmune thrombocytopenia in the fetus and newborn. Semin Thromb Hemost. 2001;27:245–52. doi: 10.1055/s-2001-15254. [DOI] [PubMed] [Google Scholar]
- 4.Kaplan C. Neonatal alloimmune thrombocytopenia. Haematologica. 2008;93:805–7. doi: 10.3324/haematol.13160. [DOI] [PubMed] [Google Scholar]
- 5.Bussel JB, Sola-Visner M. Current approaches to the evaluation and management of the fetus and neonate with immune thrombocytopenia. Semin Perinatol. 2009;33:35–42. doi: 10.1053/j.semperi.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 6.Kaplan C. Alloimmune thrombocytopenia of the fetus and the newborn. Blood Rev. 2002;16:69–72. doi: 10.1054/blre.2001.0187. [DOI] [PubMed] [Google Scholar]
- 7.Shulman NR, Aster RH, Pearson HA, Hiller MC. Immunoreactions involving platelet. VI. Reactions of maternal isoantibodies responsible for neonatal purpura. Differentiation of a second platelet antigen system. J Clin Invest. 1962;41:1059–69. doi: 10.1172/JCI104556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mueller-Eckhardt C, Becker T, Weisheit M, Witz C, Santoso S. Neonatal alloimmune thrombocytopenia due to fetomaternal Zwb incompatibility. Vox Sang. 1986;50:94–6. doi: 10.1111/j.1423-0410.1986.tb04853.x. [DOI] [PubMed] [Google Scholar]
- 9.Metcalfe P, Watkins NA, Ouwehand WH, Kaplan C, Newman P, Kekomaki R, De Haas M, Aster R, Shibata Y, Smith J, Kiefel V, Santoso S. Nomenclature of human platelet antigens. Vox Sang. 2003;85:240–5. doi: 10.1046/j.1423-0410.2003.00331.x. [DOI] [PubMed] [Google Scholar]
- 10.Schuh AC, Watkins NA, Nguyen Q, Harmer NJ, Lin M, Prosper JY, Campbell K, Sutherland DR, Metcalfe P, Horsfall W, Ouwehand WH. A tyrosine703serine polymorphism of CD109 defines the Gov platelet alloantigens. Blood. 2002;99:1692–8. doi: 10.1182/blood.v99.5.1692. [DOI] [PubMed] [Google Scholar]
- 11.Jallu V, Meunier M, Brement M, Kaplan C. A new platelet polymorphism Duv(a+), localized within the RGD binding domain of glycoprotein IIIa, is associated with neonatal thrombocytopenia. Blood. 2002;99:4449–56. doi: 10.1182/blood.v99.12.4449. [DOI] [PubMed] [Google Scholar]
- 12.Stafford P, Garner SF, Rankin A, Kekomaki R, Watkins NA, Ouwehand WH. A single-nucleotide polymorphism in the human ITGB3 gene is associated with the platelet-specific alloantigen Va (HPA-17bw) involved in fetal maternal alloimmune thrombocytopenia. Transfusion. 2008;48:1432–8. doi: 10.1111/j.1537-2995.2008.01737.x. [DOI] [PubMed] [Google Scholar]
- 13.Wang R, Furihata K, McFarland JG, Friedman K, Aster RH, Newman PJ. An amino acid polymorphism within the RGD binding domain of platelet membrane glycoprotein IIIa is responsible for the formation of the Pena/Penb alloantigen system. J Clin Invest. 1992;90:2038–43. doi: 10.1172/JCI116084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shibata Y, Matsuda I, Miyaji T, Ichikawa Y. Yuka, a new platelet antigen involved in two cases of neonatal alloimmune thrombocytopenia. Vox Sang. 1986;50:177–80. doi: 10.1111/j.1423-0410.1986.tb04874.x. [DOI] [PubMed] [Google Scholar]
- 15.Kuijpers RW, Simsek S, Faber NM, Goldschmeding R, van Wermerkerken RK, von dem Borne AE. Single point mutation in human glycoprotein IIIa is associated with a new platelet-specific alloantigen (Mo) involved in neonatal alloimmune thrombocytopenia. Blood. 1993;81:70–6. [PubMed] [Google Scholar]
- 16.Kroll H, Kiefel V, Santoso S, Mueller-Eckhardt C. Sra, a private platelet antigen on glycoprotein IIIa associated with neonatal alloimmune thrombocytopenia. Blood. 1990;76:2296–302. [PubMed] [Google Scholar]
- 17.Noris P, Simsek S, de Bruijne-Admiraal LG, Porcelijn L, Huiskes E, van der Vlist GL, van Leeuwen EF, van der Schoot CE, von dem Borne AE. Max(a), a new low-frequency platelet-specific antigen localized on glycoprotein IIb, is associated with neonatal alloimmune thrombocytopenia. Blood. 1995;86:1019–26. [PubMed] [Google Scholar]
- 18.Peterson JA, Balthazor SM, Curtis BR, McFarland JG, Aster RH. Maternal alloimmunization against the rare platelet-specific antigen HPA-9b (Max a) is an important cause of neonatal alloimmune thrombocytopenia. Transfusion. 2005;45:1487–95. doi: 10.1111/j.1537-2995.2005.00561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaplan C, Porcelijn L, Vanlieferinghen P, Julien E, Bianchi F, Martageix C, Bertrand G, Jallu V. Anti-HPA-9bw (Maxa) fetomaternal alloimmunization, a clinically severe neonatal thrombocytopenia: difficulties in diagnosis and therapy and report on eight families. Transfusion. 2005;45:1799–803. doi: 10.1111/j.1537-2995.2005.00606.x. [DOI] [PubMed] [Google Scholar]
- 20.Peyruchaud O, Bourre F, Morel-Kopp MC, Reviron D, Mercier P, Nurden A, Kaplan C. HPA-10w(b) (La(a)): genetic determination of a new platelet-specific alloantigen on glycoprotein IIIa and its expression in COS-7 cells. Blood. 1997;89:2422–8. [PubMed] [Google Scholar]
- 21.Simsek S, Vlekke AB, Kuijpers RW, Goldschmeding R, von dem Borne AE. A new private platelet antigen, Groa, localized on glycoprotein IIIa, involved in neonatal alloimmune thrombocytopenia. Vox Sang. 1994;67:302–6. doi: 10.1111/j.1423-0410.1994.tb01256.x. [DOI] [PubMed] [Google Scholar]
- 22.Sachs UJ, Kiefel V, Bohringer M, Afshar-Kharghan V, Kroll H, Santoso S. Single amino acid substitution in human platelet glycoprotein Ibbeta is responsible for the formation of the platelet-specific alloantigen Iy(a) Blood. 2000;95:1849–55. [PubMed] [Google Scholar]
- 23.Santoso S, Amrhein J, Hofmann HA, Sachs UJ, Walka MM, Kroll H, Kiefel V. A point mutation Thr(799)Met on the alpha(2) integrin leads to the formation of new human platelet alloantigen Sit(a) and affects collagen-induced aggregation. Blood. 1999;94:4103–11. [PubMed] [Google Scholar]
- 24.Santoso S, Kiefel V, Richter IG, Sachs UJ, Rahman A, Carl B, Kroll H. A functional platelet fibrinogen receptor with a deletion in the cysteine-rich repeat region of the beta(3) integrin: the Oe(a) alloantigen in neonatal alloimmune thrombocytopenia. Blood. 2002;99:1205–14. doi: 10.1182/blood.v99.4.1205. [DOI] [PubMed] [Google Scholar]
- 25.Kroll H, Yates J, Santoso S. Immunization against a low-frequency human platelet alloantigen in fetal alloimmune thrombocytopenia is not a single event: characterization by the combined use of reference DNA and novel allele-specific cell lines expressing recombinant antigens. Transfusion. 2005;45:353–8. doi: 10.1111/j.1537-2995.2005.04218.x. [DOI] [PubMed] [Google Scholar]
- 26.Peterson JA, Visentin GP, Newman PJ, Aster RH. A recombinant soluble form of the integrin alpha IIb beta 3 (GPIIb-IIIa) assumes an active, ligand-binding conformation and is recognized by GPIIb-IIIa-specific monoclonal, allo-, auto-, and drug-dependent platelet antibodies. Blood. 1998;92:2053–63. [PubMed] [Google Scholar]
- 27.Furihata K, Nugent DJ, Bissonette A, Aster RH, Kunicki TJ. On the association of the platelet-specific alloantigen, Pena, with glycoprotein IIIa. Evidence for heterogeneity of glycoprotein IIIa. J Clin Invest. 1987;80:1624–30. doi: 10.1172/JCI113250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Menitove JE, Pereira J, Hoffman R, Anderson T, Fried W, Aster RH. Cyclic thrombocytopenia of apparent autoimmune etiology. Blood. 1989;73:1561–9. [PubMed] [Google Scholar]
- 29.Curtis BR, Fick A, Lochowicz A, McFarland JG, Ball RH, Peterson J, Aster RH. Neonatal alloimmune thrombocytopenia associated with maternal-fetal incompatibility for blood group B. Transfusion. 2008;48:358–64. doi: 10.1111/j.1537-2995.2007.01531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Curtis BR. Genotyping for human platelet alloantigen polymorphisms: applications in the diagnosis of alloimmune platelet disorders. Semin Thromb Hemost. 2008;34:539–48. doi: 10.1055/s-0028-1103365. [DOI] [PubMed] [Google Scholar]
- 31.Xiao T, Takagi J, Coller BS, Wang JH, Springer TA. Structural basis for allostery in integrins and binding to fibrinogen-mimetic therapeutics. Nature. 2004;432:59–67. doi: 10.1038/nature02976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu J, Luo BH, Xiao T, Zhang C, Nishida N, Springer TA. Structure of a complete integrin ectodomain in a physiologic resting state and activation and deactivation by applied forces. Mol Cell. 2008;32:849–61. doi: 10.1016/j.molcel.2008.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kekomaki R, Raivio P, Kero P. A new low-frequency platelet alloantigen, Vaa, on glycoprotein IIbIIIa associated with neonatal alloimmune thrombocytopenia. Transfus Med. 1992;2:27–33. doi: 10.1111/j.1365-3148.1992.tb00131.x. [DOI] [PubMed] [Google Scholar]
- 34.Davies DR, Cohen GH. Interactions of protein antigens with antibodies. Proc Natl Acad Sci U S A. 1996;93:7–12. doi: 10.1073/pnas.93.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sundberg EJ, Mariuzza RA. Molecular recognition in antibody-antigen complexes. Adv Protein Chem. 2002;61:119–60. doi: 10.1016/s0065-3233(02)61004-6. [DOI] [PubMed] [Google Scholar]
- 36.Morel-Kopp MC, Blanchard B, Kiefel V, Joly C, Mueller-Eckhardt C, Kaplan C. Anti-HPA-4b (anti-Yuk(a)) neonatal alloimmune thrombocytopenia: first report in a Caucasian family. Transfus Med. 1992;2:273–6. doi: 10.1111/j.1365-3148.1992.tb00169.x. [DOI] [PubMed] [Google Scholar]
- 37.McFarland JG, Blanchette V, Collins J, Newman PJ, Wang R, Aster RH. Neonatal alloimmune thrombocytopenia due to a new platelet-specific alloantibody. Blood. 1993;81:3318–23. [PubMed] [Google Scholar]
- 38.Kekomaki R, Jouhikainen T, Ollikainen J, Westman P, Laes M. A new platelet alloantigen, Tua, on glycoprotein IIIa associated with neonatal alloimmune thrombocytopenia in two families. Br J Haematol. 1993;83:306–10. doi: 10.1111/j.1365-2141.1993.tb08286.x. [DOI] [PubMed] [Google Scholar]
- 39.Stafford P, Garner SF, Huiskes E, Kaplan C, Kekomaki R, Santoso S, Tsuno NH, Watkins NA, Ouwehand WH. Three novel beta3 domain-deletion peptides for the sensitive and specific detection of HPA-4 and six low frequency beta3-HPA antibodies. J Thromb Haemost. 2008;6:376–83. doi: 10.1111/j.1538-7836.2008.02843.x. [DOI] [PubMed] [Google Scholar]