Abstract
A 64-year-old male with Aspergillus fumigatus infection that had disseminated from the lung to the ankle and adjacent bone was treated successfully with posaconazole after therapy with itraconazole and amphotericin B lipid complex failed. Marked clinical improvement occurred within 6 weeks of initiation of posaconazole therapy; after 6 months, infection had resolved at all sites. The patient has had no recurrence of infection.
CASE REPORT
A 64-year-old man underwent a bilateral lung transplant for chronic obstructive pulmonary disease. Ten months following the transplant, the patient received antithymocyte globulin treatment for rejection. Because Aspergillus flavus and Aspergillus fumigatus were recovered from bronchoscopic lavage fluid samples obtained at the time of rejection, inhaled amphotericin B deoxycholate (AmB), 25 mg once weekly, was initiated as prophylaxis. Five months later, the patient came to the transplant clinic with complaints of increased shortness of breath, fever, and a swollen, painful right ankle (Fig. 1). He denied trauma to the ankle. His immunosuppressive regimen consisted of azathioprine, prednisone, and tacrolimus. The chest radiograph showed a loculated right-side pleural effusion. Bronchoscopy was also performed, and examination of the lavage fluid with potassium hydroxide (KOH) revealed septate hyaline hyphae. Empirical oral itraconazole solution (200 mg every 12 h) was started, and inhaled AmB therapy was continued. The culture subsequently grew A. fumigatus.
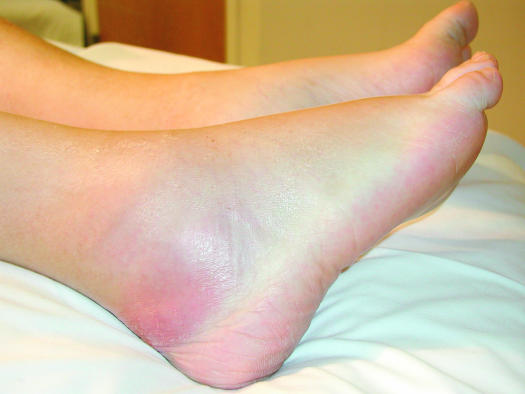
FIG. 1.
Swelling of the right ankle in a 64-year-old man who presented with invasive pulmonary aspergillosis; the infection had disseminated into the ankle and adjacent bone.
Because of persistent fever and right ankle swelling and pain, the patient was admitted to the hospital 7 days later. Relevant laboratory results on admission included a leukocyte count of 12.6 × 109/mm3, a platelet count of 370,000 × 109/mm3, a blood urea nitrogen level of 30 mg/dl, a serum creatinine level of 1.2 mg/dl, a sedimentation rate of 75 mm/h, a uric acid level of 5.5 mg/dl, and liver function test results within normal limits. Computed tomography of the chest confirmed the presence of a complex, loculated, right pleural effusion that contained numerous high-density areas; a small amount of parenchymal opacity was associated with the effusion. Thoracentesis of the effusion yielded pleural fluid with a pH of 7.02, protein level of 2.7 g/dl, and glucose level of <20 mg/dl. KOH examination of the pleural fluid revealed septate hyaline hyphae; the corresponding cultures grew A. fumigatus. Magnetic resonance imaging (MRI) of the right foot and ankle at this time revealed moderate ankle effusion and extensive signal abnormality throughout the majority of the calcaneal bone. There was sparing of the anterior process, but superior lateral calcaneal cortical destruction was noted. Arthrocentesis of the right ankle revealed septate hyaline hyphae on KOH examination, and the culture subsequently grew A. fumigatus. MRI of the brain showed no lesions.
At this time, systemic amphotericin B lipid complex (ABLC; 5 mg/kg/day) was added to his antifungal regimen. Although itraconazole treatment was continued initially, it was discontinued on hospital day 8, when the level of itraconazole in blood was determined to be undetectable. Inhaled AmB (25 mg weekly) treatment was continued.
The patient underwent exploratory thoracoscopy, thoracotomy, excision of empyema cavities, and decortication on hospital day 6. During the procedure, a number of abscess cavities were encountered; thus, chest tubes were left in place for drainage. He returned to surgery on hospital day 9 for attention to the right foot infection; on dissection into the posterior fossa, gross purulence was noted. Turbid yellow material was found within the calcaneal bone, and a partial calcanectomy was performed. Thirteen days following the initial debridement, a repeat MRI showed an inflammatory mass and fluid present in the region of the surgical bed, and repeat irrigation and debridement of the right heel wound were performed. A moderate amount of purulent, bloody drainage was encountered upon opening the wound. Necrotic tissue on all sides of the wound and the calcaneal bone were again debrided. A wound vacuum catheter was placed.
During this time, histopathology of pleuropulmonary and diaphragmatic surface tissues showed septate hyphae consistent with the diagnosis of disseminated aspergillosis. All KOH examinations showed hyphae as well, and all cultures, including those from material collected during the calcaneal debridement, grew A. fumigatus.
Despite 20 days of systemic ABLC therapy, the patient had persistent low-grade fever; moreover, there was some deterioration of renal function (the serum creatinine level increased from 1.2 mg/dl on the first day of ABLC therapy to a peak of 1.7 mg/dl). Repeat computed tomography of the chest showed an increase in basilar consolidation and atelectasis in the right lower lobe and a new 5-mm nodule in the anterior left upper lobe. Because of poor response to the therapy he had been receiving, the patient was enrolled in the limited-access posaconazole program, and posaconazole (oral suspension, 400 mg every 12 h) therapy was started. Systemic ABLC therapy was discontinued, but inhaled AmB therapy was continued.
Six weeks after posaconazole therapy was initiated, repeat computed tomography of the chest revealed marked interval improvement in the right lower lobe consolidation and complete resolution of the left upper lobe nodule. Minimal pleural thickening persisted, and a small residual loculated hydropneumothorax was noted in the right base. Repeat MRI of the foot showed two small posterior fluid collections with thick enhancing rims; ultrasound-guided aspiration attempts were unsuccessful.
By the end of his 54-day hospitalization, the patient was able to ambulate with a walker by bearing minimal weight on the right foot. His pulmonary function had improved enough that he did not require oxygen for ambulation. He was discharged home with a plan for a 1-year course of posaconazole therapy. Six months after posaconazole therapy was begun, repeat MRI of the ankle showed no evidence of osteomyelitis or soft tissue infection. The small focal fluid collections had resolved. Computed tomography of the chest revealed complete resolution of the hydropneumothorax and otherwise clear lungs. The patient was able to ambulate without the aid of a walker. The patient subsequently completed 12 months of posaconazole therapy.
Approximately 2 months after stopping posaconazole therapy, the patient suffered a probable sprain to the affected ankle. Because of persistent pain and swelling over the subsequent 3 months, an MRI was done and revealed an abnormally high signal within the talus bone and distal tibia that had not been present in posttreatment studies. Therefore, 5 months after stopping posaconazole therapy, the patient underwent core biopsies of the right distal tibia and right talus in the region of the abnormality. Histopathology showed no evidence of fungal infection by methenamine silver stain, and fungal cultures and KOH examinations were also negative. The clinical and radiographic abnormalities were thought likely to represent changes from repetitive trauma in a patient with instability of the hind foot and heel post-calcaneal resection. Twelve months after stopping posaconazole therapy, the patient is alive and well, without evidence of infection recurrence (Fig. 2).
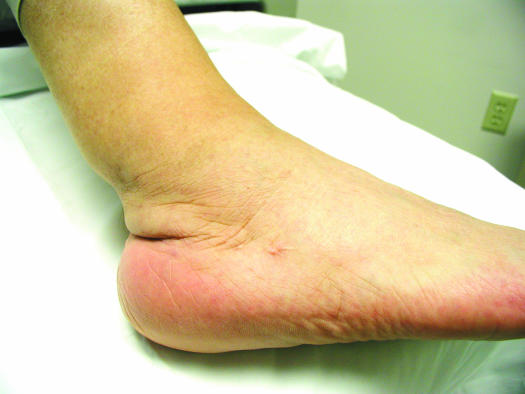
FIG. 2.
Right ankle of a 64-year-old man 2 months after completing a 1-year course of posaconazole therapy; resolution of erythema and swelling is apparent.
Aspergillus osteomyelitis is rare. For example, a 1990 review of the world literature documented a total of 26 cases of extracranial Aspergillus osteomyelitis (2). Although immunocompetent patients can acquire the disease via iatrogenic or traumatic inoculation, it more commonly results from the hematogenous spread of invasive pulmonary aspergillosis, an infection that occurs in 6 to 16% of patients after heart or lung transplantation (3). The lung transplant recipient described here presumably had primary pulmonary aspergillosis that had disseminated to the ankle and adjacent bone. Fewer than 15 cases of Aspergillus osteomyelitis after solid-organ transplantation have been reported, and only a single case in a bone marrow transplant patient has been reported (7, 9). In a majority of cases, the spine was the primary site of infection, with rare occurrences in the tibia, ribs, wrist, sternum, pelvis, and knee (3).
Because Aspergillus osteomyelitis is uncommon, the optimal treatment has yet to be defined. Historically, the mainstay of therapy has been AmB and surgical debridement. However, poor bone penetration and substantial toxicity make systemic AmB a less-than-attractive therapeutic option, particularly in patients with renal dysfunction (5, 6). Although the ABLC formulation is less nephrotoxic than the conventional formulation (6), the patient described in this report experienced worsening of renal function during treatment. In addition, prolonged therapy would have been difficult with a parenteral agent.
Itraconazole may play a role in the treatment of Aspergillus osteomyelitis, since there is some evidence that it penetrates bone, and there have been anecdotal reports of its efficacy in fungal osteomyelitis (6). Some clinicians have suggested that a polyene and an azole be used in combination therapy (1, 8). However, the patient in this case failed to achieve therapeutic concentrations of itraconazole in serum for no obvious reason. The patient was medically compliant. In addition, the patient's concomitantly administered medications are not known to interfere with the absorption or metabolism of itraconazole. It is encouraging that posaconazole treatment was successful in this patient despite previous itraconazole failure, which suggests that issues of pharmacokinetics can be important.
Compared with AmB and itraconazole, posaconazole has the highest in vitro activity against Aspergillus species (4). Moreover, clinical success has been demonstrated with posaconazole in the treatment of invasive aspergillosis in patients in whom AmB or itraconazole therapy has failed (R. Y. Hachem, I. I. Raad, C. M. Afif, R. Negroni, J. Graybill, S. Hadley, H. Kantarjian, S. Adams, and G. Mukwaya, Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 372, 2000). Since posaconazole is metabolized by the liver (P. Krieter, B. Flannery, T. Musick, R. Courtney, J. Patrick, and M. Laughlin, Abstr. 42nd Intersci. Conf. Antimicrob. Agents Chemother., abstr. A-1394, 2002), it offers an attractive treatment choice for patients with impaired renal function. The present case supports these findings in that clinical improvement and recovery of renal function were noted shortly after the initiation of posaconazole therapy. Although the length of treatment for this type of infection remains arbitrary, we were able to treat the infection for 1 year without complications and with documented cure.
In conclusion, posaconazole therapy resulted in rapid clinical improvement of Aspergillus osteomyelitis despite previous clinical failure of ABLC and itraconazole. Presumably, this success was observed because of posaconazole's potent activity against Aspergillus spp. and its penetration into the bone. Moreover, no adverse effects of treatment were noted, and the patient has had no recurrence of disease since finishing his course of therapy. Consequently, posaconazole is a potential treatment choice for patients who develop this rare infection.
Acknowledgments
This work was supported by the Schering-Plough Research Institute.
REFERENCES
- 1.Cortet, B., R. Richard, X. Deprez, L. Lucet, R. M. Flipo, X. Le Loet, B. Duquesnoy, and B. Delcambre. 1994. Aspergillus spondylodiscitis: successful conservative treatment in 9 cases. J. Rheumatol. 21:1287-1291. [PubMed] [Google Scholar]
- 2.Faure, B. T., J. X. Biondi, J. P. Flanagan, and R. Clarke. 1990. Aspergillar osteomyelitis of the acetabulum. A case report and review of the literature. Orthop. Rev. 19:58-64. [PubMed] [Google Scholar]
- 3.Hummel, M., S. Schuler, U. Weber, G. Schwertlick, S. Hempel, D. Theiss, W. Rees, J. Mueller, and R. Hetzer. 1993. Aspergillosis with aspergillus osteomyelitis and diskitis after heart transplantation: surgical and medical management. J. Heart Lung Transplant. 12:599-603. [PubMed] [Google Scholar]
- 4.Manavathu, E. K., J. L. Cutright, D. Loebenberg, and P. H. Chandrasekar. 2000. A comparative study of the in vitro susceptibilities of clinical and laboratory-selected resistant isolates of Aspergillus spp. to amphotericin B, itraconazole, voriconazole and posaconazole (SCH 56592). J. Antimicrob. Chemother. 46:229-234. [DOI] [PubMed] [Google Scholar]
- 5.Pacetti, S. A., and S. P. Gelone. 2003. Caspofungin acetate for treatment of invasive fungal infections. Ann. Pharmacother. 37:90-98. [DOI] [PubMed] [Google Scholar]
- 6.Stevens, D. A., V. L. Kan, M. A. Judson, V. A. Morrison, S. Dummer, D. W. Denning, J. E. Bennett, T. J. Walsh, T. F. Patterson, and G. A. Pankey. 2000. Practice guidelines for diseases caused by aspergillus. Clin. Infect. Dis. 30:696-709. [DOI] [PubMed] [Google Scholar]
- 7.Taillandier, J., M. Alemanni, J. Cerrina, L. F. Le Roy, and P. Dartevelle. 1997. Aspergillus osteomyelitis after heart-lung transplantation. J. Heart Lung Transplant. 16:436-438. [PubMed] [Google Scholar]
- 8.Tang, T. J., H. L. Janssen, C. H. van der Vlies, R. A. de Man, H. J. Metselaar, H. W. Tilanus, and S. de Marie. 2000. Aspergillus osteomyelitis after liver transplantation: conservative or surgical treatment? Eur. J. Gastroenterol. Hepatol. 12:123-126. [DOI] [PubMed] [Google Scholar]
- 9.Vlasveld, L. T., J. F. Delemarre, J. H. Beynen, and S. Rodenhuis. 1992. Invasive aspergillosis complicated by subclavian artery occlusion and costal osteomyelitis after autologous bone marrow transplantation. Thorax 47:136-137. [DOI] [PMC free article] [PubMed] [Google Scholar]