Abstract
Ischaemic stroke represents a major health hazard in the western world, which has a severe impact on society and the health-care system. Roughly, 10% of all first ischaemic strokes can be attributed to significant atherosclerotic disease of the carotid arteries. Correct management of these lesions is essential in the prevention and treatment of carotid disease-related ischaemic events. The close relationship between diagnosis and medical and surgical management makes it necessary that all involved physicians and surgeons have profound knowledge of management strategies beyond their specific speciality. Continuous improvement in pharmacological therapy and operative techniques as well as frequently changing guidelines represent a constant challenge for the individual health-care professional. This review gives a thorough outline of the up-to-date evidence-based management of carotid artery disease and discusses its current controversies.
Keywords: Cardiovascular disease, Stroke, Carotid artery disease, Carotid endarterectomy
INTRODUCTION
Acute ischaemic stroke is the most common cause of acute neurological injury in the western world. It represents a major health hazard. Presentations are associated with a risk of death of between 20 and 30% [1]. The socioeconomic consequences are considerable and the annual estimated stroke-related costs are approximately €22 billion in Europe alone [2].
Atherosclerosis of the supra-aortic vessels accounts for up to 18% [3] of all strokes, although this includes intracranial disease. In the Northern Manhattan Study, it was found that approximately 7% of all first ischaemic strokes are associated with a corresponding carotid stenosis of >60% [4]. The careful, precise, evidence-based management of these lesions has the potential to significantly reduce the incidence of stroke (and restroke) and, therefore, stroke-related disability, death and expense. Fortunately, the management of these lesions has been the subject of good-quality medical research.
In all cases, the optimal management of carotid artery stenosis requires the use of medications to control the processes that cause atheroma as well as antiplatelet agents to reduce the risk of embolic events (so-called ‘best medical therapy’ [BMT]). In some, direct mechanical management of the carotid plaque (carotid endarterectomy [CEA] or carotid stenting [CAS]) has a supplementary role.
This review aims to outline the current recommendations concerning the management of carotid artery disease (CAD) and its evidence-base (Table 1).
Table 1:
In a nutshell
| All patients with diagnosed carotid artery atherosclerotic disease should be on an antiplatlet agent and a statin (regardless of serum cholesterol levels) |
| Only patients with a transient ischaemic attack, stroke or amaurosis fugax within the last 6 months on the isilateral side of the stenosis are considered symptomatic |
| Asymptomatic carotid disease <70% should be treated with BMT |
| Male patients with asymptomatic carotid disease of <75 years and >70% should be offered surgery |
| Female patients with asymptomatic carotid disease >70% should only be offered surgery if very young and fit |
| All patients with symptomatic carotid disease <50% should receive BMT |
| All patients with symptomatic carotid disease >50% should have surgery |
| Carotid artery endarterectomy is the treatment of choice |
| Carotid artery stenting should only be offered to symptomatic patients, which are high risk for open surgery |
| The main benefit of surgery in symptomatic patients lies within the first 14 days of symptom onset |
DEFINITIONS
Symptomatic vs asymptomatic
Patients with carotid artery stenosis may be identified after an index neurological event, or without warning symptoms. The latter group may be identified by general health screenings in the private sector, during preparation for other major surgery (for example, coronary artery bypass) or during investigation of an incidentally discovered carotid bruit.
By convention, the mainstream literature categorizes patients in the former group as ‘symptomatic’ if the stenosis is identified (and treated) within a set time of the presenting neurological event. This interval is variably stated as 6 or 3 months, or sometimes 2 weeks. Patients who have never had neurological symptoms, and those whose definitive treatment takes place later than the time frames above, are categorized as ‘asymptomatic’. Although this distinction may seem arbitrary, it is important. There is good evidence that the natural history risk of stroke and restroke is distinct for each category, and this is critical in making appropriate use of the balance of risks in deciding the place of surgical treatment strategies.
Transient ischaemic attack, stroke, amaurosis fugax
‘Transient ischaemic attacks’ (TIAs) are defined as a syndrome of acute neurological dysfunction referable to the distribution of a single brain artery and characterized by symptoms that last for <24 h [5]. The symptom duration of 24 h to 7 days is defined as ‘transient stroke’ and symptoms that persist for 7 days or more as ‘stroke’.
Amaurosis fugax is a sudden, transient monocular visual loss. It is painless and persists for <60 min (often much shorter). The clinical history is typical, with most patients describing a curtain or waterfall ascending or descending to obscure all or part of the visual field in one eye. Full visual capacity is usually recovered [6]. Occasionally, patients will suffer permanent total or partial monocular blindness. It is not uncommon for high-street optometrists to identify cholesterol emboli in the retinas of asymptomatic patients.
Retinal symptoms of all types are thought to have a less-sinister prognosis in terms of subsequent stroke than hemispheric symptoms [7] but are significantly more associated with ipsilateral carotid disease than with atrial fibrillation [8].
Best medical therapy
This review is restricted to the management of atheroma-based events and consequently does not consider the use of formal anticoagulation for non-carotid-origin cerebral emboli, or the medical management of non-atheroma carotid-origin stroke such as carotid dissection and aneurysm.
In the context of carotid artery stenosis, BMT constitutes the medical management of the major atheroma risk factors (stopping smoking and the optimal control of hypertension, hyperlipidaemia and diabetes), together with the use of antiplatelet agents.
DIAGNOSTIC TECHNIQUES
The ‘gold standard’ for diagnosing carotid disease still is two-plane angiography. Since this is an invasive method, other non-invasive modalities are gaining more importance. With the rapid technical advancements, duplex ultrasound has become the first-line method for the diagnosis of CAD. As it has a very high sensitivity and specificity, the presence of significant artheriosclerotic disease can be diagnosed on this modality alone. In fact, some surgeons are willing to act on duplex ultrasound findings alone. However, as there is strong operator dependence, a small but definite margin for error as well as a non-diagnostic rate (in tortuous or heavily calcified vessels, in the presence of stents, high bifurcations or patient intolerance), other surgeons prefer confirmatory investigations like computed tomographic angiography or magnetic resonance angiography.
There is a room for considerable confusion here: the two major randomized controlled trials (RCTs), North American Symptomatic Carotid Endarterectomy Trial (NASCET) [9] and European Carotid Surgery Trial (ECST) [10], which examined the management of symptomatic carotid stenosis used two-plane angiography as tool for the assessment of the stenosis degree. The correlation between the results of these investigations and the method used in the trials is open to interpretation. In the specific case of Duplex ultrasound, the stenosis is largely graded on the basis of flow velocity across the suspected stenosis, and these measurements are correlated with angiographic appearances [11].
To make matters worse, the major trials each used different methods in calculating stenosis based on the angiographic appearance. While the Americans use the ratio between the minimal internal carotid artery (ICA) diameter and the normal ICA diameter to calculate the percentage of stenosis, the Europeans use the ratio between the minimal ICA diameter and the diameter of the carotid bulb. Thus, a 50% NASCET stenosis is roughly equivalent to a 70% stenosis using ECST criteria.
It is essential that there is a consensus about which measurement criteria are used. To avoid confusion, the Joint Working Group of the Vascular Society of Great Britain and Ireland and the Society of Vascular Technologists [12] as well as the European Society for Vascular Surgery (ESVS) guidelines recommend the use of the NASCET criteria. In terms of ultrasound, the correlations between velocities and stenosis grades are as follows:
A 50–69% stenosis is defined as visible plaque with associated peak systolic velocities of 125–230 cm/s. Additional criteria include end-diastolic velocities of 40–100 cm/s as well as an internal to the common carotid artery peak systolic velocity ratio between 2 and 4.
Stenoses >70% have peak systolic velocities >230 cm/s, a ratio >4 and end-diastolic velocities >100 cm/s.
It should be noted that Duplex produces results in bands of stenosis, rather than specific percentage.
All stenosis grading referred to in this article therefore follow these recommendations.
BEST MEDICAL THERAPY—THE MINIMUM BASELINE
Antiplatelet therapy
The use of low-dose aspirin is well established in the prevention of stroke. The usual prescribed dose is 75–150 mg daily [13]. In fact, aspirin has been used routinely in all trials concerning the management of CAD, although in varying dosages.
The two largest clinical trials that investigated aspirin in the acute setting (Chinese Acute Stroke Trial and The International Stroke Trial) showed it to be effective in a dosage of 160–300 mg with a number to treat of 111 to prevent one stroke or death. The number needed to transform an initial ischaemic stroke into an haemorrhagic one was 500 [14].
Additional established antiplatelet strategies include the use of dipyrimadole and clopidogrel, alone or in various combinations. There is no current evidence supporting the use of dipyrimadole in primary prevention, but there is evidence for its use in combination with aspirin in the acute setting and for secondary prevention of stroke [15]. Unfortunately, dipyrimadole is frequently poorly tolerated. The value of clopidogrel as a combination drug is controversial: while the CAPRIE trial showed the superiority of treatment with clopidogrel alone by comparison with aspirin in the prevention of ischaemic events [16] (at an additional cost of circa $900 per patient per year), the MATCH study failed to show any additional benefit for the combination of both drugs in this setting [17].
Statins
The Heart Protection Study Collaborative Group completed a large RCT that showed simvastatin to be beneficial in the prevention of cardiovascular events including stroke. They investigated in excess of 20 000 individuals with known cardiac disease, diabetes and/or peripheral vascular disease [18]. Interestingly, the protective effect of statin use exists irrespective of the presence or absence of hypercholesterolemia. Recent meta-analyses have supported the case for the use of simvastatin [19] showing a relative stroke risk reduction of 19%. Data suggest that statins are even effective in patients who simply have cardiac risk factors opposed to already-established disease [20]. Other RCTs have studied the potential benefits of atrovastatin [21] and rosuvastatin [22] also with good results. A meta-analysis about the efficacy of statins from 2011 showed rosuvastatin and atrovastatin to be the most effective in lowering low-density lipoprotein-C, but failed to associate this finding with clinical superiority in cardiovascular risk reduction [23]. This suggests that the choice of statin may still be left to the treating physician.
Fibrates, although they reduce the relative risk of coronary incidents, do not play a role in the prevention of stroke [24].
Blood pressure control
Hypertension is a major risk factor for stroke and it has been postulated that the stroke risk for each 10 mmHg increase in blood pressure will increase by 30–45% [25]. Angiotensin-converting enzyme inhibitors, especially in combination with a thiazide diuretic, are the first-choice agents. They have been proven to be superior to betablockers in large RCTs [26, 27], in stroke prevention and to be particularly beneficial in diabetics [28]. Additionally, there are data that suggest an increased risk of stroke for elderly patients on betablocker therapy [29].
Diabetes control
The correlation between diabetes and microvascular disease has been well established. Although a direct effect on macrovascular disease, including strokes, has not been proven, it is widely accepted that tight glycaemic control should be achieved [30].
Life-style
Although level I evidence is lacking, large epidemiological studies show an increased risk of stroke by 25–50% in smokers [31]. There is no debate: smokers should be strongly advised to stop the habit. Regular exercise is to be encouraged as it has been suggested that individuals engaging in regular physical activity have a lower risk of stroke [32]. Metabolic syndrome and abdominal obesity are both linked to atherosclerosis and stroke [33]. Dietary advice should be offered to all patients.
THE DECISION TREE IN MANAGEMENT OF CAROTID DISEASE
In 2008, both the European Society for Vascular Surgery (ESVS) and its trans-Atlantic counterpart, the Society for Vascular Surgery (SVS) issued updated guidelines on the management of carotid disease [34, 35]. These take into consideration the current literature and their recommendations are graded according to the levels of evidence (Fig. 1).
Figure 1:
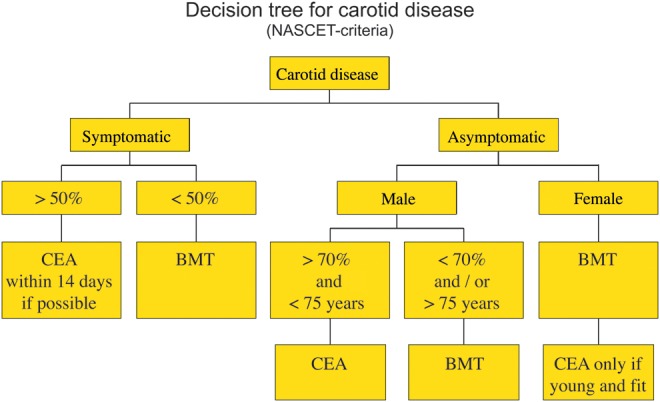
Decision tree for carotid disease (NASCET criteria).
DIRECT PLAQUE MANAGEMENT—CAROTID ENDARTERECTOMY/CAROTID STENTING
Who should be offered CEA or CAS, when and which one?
Who and when?
Symptomatic carotid disease
A large American and European trial was conducted to investigate the benefit of surgery for symptomatic carotid disease.
Between them, the ECST [10] and NASCET [9] looked at a patient group of in excess of 5800 cases. Each demonstrated a mild to moderate benefit for CEA vs medical management in patients with a 50–69% stenosis (5-year stroke risk; 15.7 vs 22.2% NASCET and marginal difference in ECST) and a high benefit with a stenosis of 70% or greater (2-year stroke risk; 9 vs 26% NASCET and 6.8 vs 20.6% ECST).
Post hoc analysis has shown that timing is essential. An analysis of the combined data shows that treatment within 14 days of the onset of ischaemic symptoms is most effective in reducing the incidence of restroke. In the subset of patients treated within this time frame, the number to treat to prevent an ipsilateral stroke was 5, but this deteriorates to 125 for patients treated >12 weeks after initial symptom onset. The reason is thought to be linked with the morphology/biology of the atheromatous plaque. Initial symptom onset is assumed to originate in plaque rupture. The ruptured plaque then acquires an initially unstable thrombotic cap, which ultimately becomes organized, stable and less prone to embolization. Consequently, both vascular societies recommend CEA for symptomatic carotid disease with a stenosis >50% using the NASCAT criteria. The aim should be to provide the service within 14 days of symptom onset, and urgent investigation and referral to the relevant specialities are crucial. To optimize stroke management, the 2008 guidelines of the National Institute for Health and Clinical Excellence recommend that all patients with suspected stroke should be admitted directly to an acute stroke unit [36]. While in 2004, only 46% of all stroke patients were admitted to specialized units, this figure improved to 74% in the 2008 UK national audit data. There is also a strong desire by the UK vascular surgeons to establish and provide a rapid access, single-stop TIA clinic service [37].
Asymptomatic carotid disease
Asymptomatic carotid disease was investigated in two major RCTs.
The Asymptomatic Carotid Surgery Trial (ACST) [38] compared CEA to the medical management of 3120 patients with asymptomatic carotid disease. It found the 5-year risk of overall stroke to be significantly lower in patients with high-grade stenosis and receiving CEA than in similar patients who did not undergo the surgery (6.4 vs 11.8%). These data include a 3% perioperative stroke risk. The benefit is pertinent in patients younger than 75 years of age with a carotid diameter reduction of 70% or more.
The North American Asymptomatic Carotid Atherosclerosis Study [39] randomized >4500 patients. It concluded that the aggregate stroke risk for patients who received BMT and also undergoing surgery was 5.1 compared with 11% in patients who were only treated medically.
Both trials showed statistically significant crossover in the incidence of stroke over time. Those patients having CEA had an initially greater risk of stroke as a consequence of the operative hazard, but the number of additional strokes over time after surgery was relatively flat in this group. By contradistinction, patients who were not offered surgery showed a greater tendency to stroke over time. The longer the time between recognition of stenosis and the end of observation, the more dramatic the difference in stroke risk becomes. Therefore, the life-benefit of surgery is greater for those with long life expectancies (the younger patients) than those with short ones.
As enlarged upon below, post hoc analysis separating the outcomes according to genders shows that these benefits only achieve statistical significance in women 3 years after surgery.
In consequence, the current ESVS recommendation is that CEA should be offered to all male patients younger than 75 years with an asymptomatic carotid stenosis of >70% (NASCET). The Americans are slightly more aggressive and offer CEA for male patients with a stenosis >60% (NASCET). CEA in asymptomatic women should be restricted to young, fit patients.
Men and women: different gender, different outcome
Naylor, a regular British author on the subject, makes a powerful case in emphasizing the importance of gender on relative stroke risk [40]. In the case of symptomatic patients with carotid stenosis, men and women both benefit from CEA within the 14 days of symptom onset. Women are at particularly high risk of stroke within 2 weeks of initial onset, which settles thereafter, while men show a much flatter risk profile. Thus, the number of strokes prevented in men is more than that in women. One thousand CEAs performed in symptomatic men, within 2 weeks of symptom onset, will prevent in excess of 400 strokes over 5 years. Even at 4 weeks, a CEA in this patient group prevents 66 strokes over 5 years.
For women, 138 strokes are prevented over 5 years if the operation was performed within 2 weeks after symptom onset. Surgery performed outside of this timeframe shows no stroke-reduction benefit [41]
CEA or CAS?
In recent years, endovascular treatment for atheromatous disease has gained attention and is evolving rapidly. CAS has been introduced and was hailed as the future in carotid intervention. However, as most recent large multicenter RCTs have ended with moderate to non-favourable results, this enthusiasm has waned considerably. The guidelines produced by the Society for Vascular Surgery (SVS) and the European Society for Vascular Surgery (ESVS) both indicate that, for symptomatic patients, open surgery remains the best option.
Numerous trials have been performed
The Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS) [42] suggested similar results in terms of stroke and mortality risks between CEA and CAS. The stent-supported percutaneous angioplasty of the carotid artery vs endarterectomy (SPACE) [43] trial showed a trend towards better results with CEA, while the endarterectomy vs angioplasty in patients with severe symptomatic carotid stenosis (EVA-3S) [44] trial was abandoned because of high stroke and death rates in the CAS group.
The stenting and angioplasty with protection in patients at high risk for endarterectomy investigators trial (SAPPHIRE) [45] specifically enrolled patients who were considered high risk for open surgery. It found the incidence for adverse effect similar for CAS and CEA in symptomatic patients. In asymptomatic patients, a lower incidence of adverse effects was reported for CAS. However, the trial has been criticized for the inclusion of a majority of asymptomatic patients. On this basis, it is thought to be vulnerable to selection bias. Another point of criticism was that asymptomatic enzyme leakage was counted as myocardial infarction.
On these grounds, the ESVS recommends CAS only in high-risk (Table 2) patients for open surgery. The American guidelines were even more conservative regarding CAS and saw it as an alternative only to open surgery in high-risk patients. They rated the level of evidence for this suggestion as having low quality.
Table 2:
High-risk criteria for open carotid surgery
| Recent myocardial infarction |
| Requirement for open heart surgery within 6 weeks |
| Unstable angina |
| Congestive heart failure (New York Heart Association III/IV) |
| Severe pulmonary disease |
| Previous CEA, contralateral laryngeal nerve pulse, radical neck dissection or cervical irradiation |
Both societies are united in the opinion that CAS is not indicated in asymptomatic carotid stenosis.
Since the publication of the guidelines in 2008, two more large carotid stenting trials have been published. The International Carotid Investigators Stenting Study (ICSS) [46] also showed CEA superior to CAS in symptomatic patients in terms of stroke and death rate in patients >70 years of age. The carotid revascularisation endarterectomy vs stenting trial (CREST) [47] found the outcomes in terms of major complications similar for CEA and CAS in symptomatic and asymptomatic patients. This made a big impact on the discussion resulting in various interest groups pushing harder for a more widespread indication for CAS. It is noteworthy that the test results are discussed controversially in the community. The main point of criticism is that myocardial infarction (which also included asymptomatic enzyme leakage) is considered an equal major complication compared with stroke, despite the unfavourable outcome in terms of rehabilitation and survival after stroke [48]. However, in the latest inter-speciality guidelines of the American colleges published in 2011, it is conceded that the CREST trial suggests CAS to be a reasonable alternative to CEA, although it is recognized that CEA still seems to be the safer treatment method, particularly in the elderly [49].
Interestingly, the American Society of Vascular Surgery only recently published an update on their guidelines in which they leave the liberal recommendations for CAS and return to their former position [50]. In view of this development, Kakisis et al. [51] reviewed the European guidelines and came to the conclusion that they still conform to the latest evidence.
TECHNICAL CONSIDERATIONS CEA and CAS
Carotid endarterectomy—the controversies
Anaesthesia
CEA can be performed under local (LA) or general anaesthesia (GA). The GALA trial [52] (GA vs LA), a large randomized trial involving 3526 patients from 95 centres in 24 countries, demonstrated that there is no significant difference in perioperative myocardial infarction, stroke or death rate between patients having either modality. The current guidelines of the European Society for Vascular Surgery (ESVS) recommend therefore that the mode of anaesthesia should be determined at the discretion of the surgeon, anaesthetist and patient.
Patch vs no patch
Traditional CEA is performed via a longitudinal arteriotomy after cross-clamping. The plaque is then endarterectomised and the incision closed by stitching in a patch. Several materials are available for this, ranging from the autologous vein to the bovine pericardium and various fabrics. The differences in outcome between all of these materials are small. The current ESVS consensus is to leave the choice of material to the surgeon's preference.
As an alternative to patch closure, the arteriotomy can be closed via direct suture repair of the arterial wall. This is a less time-consuming, but more technically demanding technique, which risks suture-line stenosis (and thus thrombosis and stroke). Additionally, there is some evidence [53] that patch angioplasty offers lesser restenosis and perioperative occlusion rates as well as a trend towards fewer non-occlusion-related strokes.
Standard vs eversion endarterectomy
Instead of the classic longitudinal incision, an eversion CEA can be performed. This employs an oblique amputation of the ICA at its origin, eversion of the adventitia, peeling the plaque out and finally reimplanting the ICA. Although a recent Cochrane review [54] suggests a lesser risk of stroke for the eversion technique, the quality of the existing studies is poor. At present, the decision on the surgical technique should depend on the expertise of the surgeon with each technique and technical considerations such at the length of the plaque and the height of the carotid bifurcation.
Shunt vs no shunt
In an attempt to reduce the risk of stroke occurring during the period of carotid cross-clamp, it is possible to maintain cerebral blood flow using a temporary shunt. These are plastic tubes placed to conduct blood from the common carotid artery into the ICA. Various styles and calibres are available, and there is some experimental research that demonstrates the relative efficiencies of each. However, there is no clinical trial evidence to support the mandatory or selective use of these devices. Many surgeons use them routinely, while others elect to do so depending on various (mainly unproven) estimates of cerebral ischaemic distress. Data from the ECST [55] showed neither advantages nor disadvantages for routine shunting. This is another technical area best left to the surgeon's discretion.
Maintaining good practice: case volume
To achieve the best possible outcomes, it has been suggested that a minimum number of procedures should be carried out by each centre. An analysis of the English Hospital Episode Statistics suggested that a minimum of 35 CEAs should be performed in each centre to maintain best surgical practice [56]. However, neither the American nor the European guidelines contain a recommendation for a minimum caseload.
THE SPECIAL CASE
Carotid artery occlusion
When the ICA is occluded, there is no risk of further distal embolization. Therefore, reconstructive surgery is unnecessary.
Carotid artery near occlusions with remaining trickle flow
Trickle flow describes the situation in which a stenosis is so severe, that the post-stenotic flow velocities actually show a significant drop instead of an increase. Subanalysis of the ECST patients has shown that near occlusions with remaining low-velocity blood flow beyond the stenosis do not benefit from surgery and should be treated medically. It is postulated that the contralateral high flow with ipsilateral low poststenotic flow acts protectively [57].
Carotid artery stenosis in patients requiring cardiac surgery
Cardiac surgery is associated with embolic stroke. While the majority of these episodes are thought to be the consequence of ascending and arch aortic disease (emboli dislodged during cannulation or other instrumentation) or pump-related particulate matter, the co-existence of high-grade carotid disease may raise this risk. However, there is a paucity of evidence and it is not possible to offer an evidence-based recommendation in the management of carotid disease in this setting [58].
In the instance of symptomatic carotid disease and an urgent need for coronary bypass, it is common for patients to be offered either precoronary carotid surgery (usually under LA), or combined carotid and cardiac surgery. The case of asymptomatic critical carotid stenosis in precardiac bypass patients is even more contentious. Level I evidence is lacking, with some studies suggesting no benefit [59], while others do report preferable outcomes for combined treatment [60]. At present, a large RCT is recruiting patients with asymptomatic carotid disease to compare synchronous CEA and open heart bypass with isolated open heart bypass alone [61]. The matter is even further complicated by the discussion whether staged or simultaneous procedures are preferable and the recent rise of CAS as an alternative to CEA.
However, the American Heart Association recommends CEA for patients with asymptomatic unilateral stenosis of greater 80% (NASCET) [62].
Carotid stump syndrome
This describes the situation when the ICA is occluded, but the patient continues to suffer from symptoms concomitant with microembolization. If other causes are excluded, surgical treatment is usually advised in the form of oversutering the distal ICA [63]. Endovascular treatment has been described, but has not gained significant acceptance in this setting yet.
Four vessel disease and cerebral global malperfusion
The impairment of all four vessels supplying the brain with consecutive generalized symptoms of cerebral malperfusion is a rare, but serious condition. Particularly in the Asian population, this scenario is mostly associated with autoimmune disease (i.e. Takayasu's arteriitis) of the arteries, but can also occur in atherosclerotic patients [64]. Here, it is often found in patients with underlying clotting disorders. Surgery is generally possible, but requires an extensive work-up and an individually tailored approach.
CAROTID ARTERY STENTING—THE CONTROVERSIES
Learning curve/maintaining good practice
One point of criticism that was used against unfavourable outcomes in the large CAS trials was the relative inexperience in CAS of recruiting centres. In fact, the learning curve in CAS seems to be extremely steep. A systematic review from 2010 came to the conclusion that centres introducing CAS may take up to 2 years before they reach acceptable stroke rates of ≤5% [65]. Similar to CEA, a minimum caseload per proceduralist to achieve the best possible outcome is discussed. Although there is consensus that high-volume operators generate better outcomes, the definition of ‘high volume’ is arbitrary and varies from study to study [66, 67].
The use of protection devices
Embolization during stent deployment is a major source of concern. To minimise this risk, various ‘embolic protection devices’ (EPDs) have been introduced to ‘catch’ embolic material during stent deployment. They function as a form of umbrella or filter, which is placed distal to the stenosis. After stent deployment, the EPD is then retrieved, capturing all potential debris. This strategy has been found to be beneficial and is associated with a reduction of the stroke/death risk from 6.2 to 2.8% [68].
Recently, the concept of ‘reversed flow’ has been introduced. The intention is that a special deployment system occludes the common and external carotid arteries, ensuring reversed (cranio-caudal) ICA flow. In this scenario, potential debris is flushed into the bulbus area and not into the cerebral circulation. However, there are no large trials examining this strategy to date.
Balloon-expandable vs self-expanding stents
Generally, self-expanding stents are used as they produce less radial force than balloon-expandable ones. The former are made of Nitinol, which has two interesting properties: it has ‘thermal memory’ (in this setting, expanding to a preferred size at body temperature) and it is relatively soft so it will adapt to the shape of the artery. It is thought that the gentler radial force results in a lower risk of dissection in fragile arteries, in addition to less debris dislodgement, even if additional ballooning of the stent is necessary. The second property allows the use of Nitinol stents in curved arterial segments.
All stents used are bare metal stents. The cell design (open vs closed) of the stent does not seem to influence the outcome [69]. There is no role for covered stents in CAS at present, neither is there experience with drug-eluting stents for this indication.
Hostile vessel anatomy
There are certain anatomical situations that should be considered when planning a patient for CAS. Steep angles between the internal and common carotid arteries and extreme vessel tortuosity risk functional stenosis due to kinking of the artery in the transition zone between the stent and native vessel.
Also, high-grade stenosis with heavily calcified plaque may be unfavourable as these lesions require high balloon pressures to achieve an acceptable post-procedural calibre. High balloon pressures carry an increased risk of unintended damage to the vessel, particularly ICA dissection. Steep or heavily calcified aortic arches make device handling difficult and carry an additional risk of embolisation.
In these cases, CEA may be the technique of choice.
Periprocedural antiplatelet therapy
Despite the findings of the MATCH study [17], which demonstrated no additional benefit, but increased bleeding complications in the prevention of stroke for the combination of aspirin and clopidogrel compared with coplidogrel alone, current opinion today is that CAS patients should be on dual antiplatelet therapy prior and post-procedural [70]. This is mainly based on the findings of the CARESS study that showed a significant reduction in microemboli [71]. There is no evidence on the required length for dual antiplatelet therapy, but general consensus is a minimum of 4 weeks, with potential benefits of prolonged treatment [72].
OUTLOOK
There are several trials currently in progress that could influence common vascular surgical practice depending on their outcomes.
Two major ongoing trials re-examining the role of CAS compared with CEA in asymptomatic patients are: the ACST-2 and the Asymptomatic Carotid Stenosis Stenting vs Endarterectomy Trial. Additionally, there have been suggestions that given recent improvements in the pharmacotherapy of atheromatous disease, the standardised BMT may be producing lower risks of stroke than was the case at the time of previous trials. The transatlantic asymptomatic carotid intervention trial will investigate current BMT in combination with CEA or CAS compared with BMT alone in patients with asymptomatic carotid disease. The SPACE 2 study will test a similar hypothesis.
Conflict of interest: none declared.
REFERENCES
- 1.Mohr JP, Caplan LR, Melski JW, Goldstein RJ, Duncan GW, Kistler JP, et al. The Harvard Cooperative Stroke Registry: a prospective registry. Neurology. 1978;28:754–62. doi: 10.1212/wnl.28.8.754. [DOI] [PubMed] [Google Scholar]
- 2.Truelsen T, Ekman M, Boysen G. Cost of stroke in Europe. Eur J Neurol. 2005;12(Suppl 1):78–84. doi: 10.1111/j.1468-1331.2005.01199.x. [DOI] [PubMed] [Google Scholar]
- 3.Petty GW, Brown RD, Jr, Whisnant JP, Sicks JD, O'Fallon WM, Wiebers DO. Ischemic stroke subtypes: a population based study of incidence and risk factors. Stroke. 1999;30:2513–6. doi: 10.1161/01.str.30.12.2513. [DOI] [PubMed] [Google Scholar]
- 4.White H, Boden-Albala B, Wang C, Elkind MS, Rundek T, Wright CB, et al. Ischemic stroke subtype incidence among whites, blacks and Hispanics: the Northern Manhattan Study. Circulation. 2005;111:1327–31. doi: 10.1161/01.CIR.0000157736.19739.D0. [DOI] [PubMed] [Google Scholar]
- 5.Goldstein LB, Bushnell CD, Adams RJ, Appel LJ, Braun LT, Chaturvedi S, et al. Guidelines for the primary prevention of stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:517–84. doi: 10.1161/STR.0b013e3181fcb238. [DOI] [PubMed] [Google Scholar]
- 6.Miller NR, Newman NJ, editors. Walsh and Hoyt's Clinical Neuro-ophthalmology. 5th edn. Baltimore: Lippencott, Williams & Wilkins; 1998. [Google Scholar]
- 7.Benavente O, Eliasziw M, Streifler JY, Fox AJ, Barnett HJ, Meldrum H North American Symptomatic Carotid Endarterectomy Trial Collaborators. Prognosis after transient monocular blindness associated with carotid-artery stenosis. N Engl J Med. 2001;345:1084–90. doi: 10.1056/NEJMoa002994. [DOI] [PubMed] [Google Scholar]
- 8.Anderson DC, Kappelle LJ, Eliasziw M, Babikian VL, Pearce LA, Barnett HJ. Occurrence of hemispheric and retinal ischemia in atrial fibrillation compared with carotid stenosis. Stroke. 2002;33:1963–7. doi: 10.1161/01.str.0000023445.20454.a8. [DOI] [PubMed] [Google Scholar]
- 9.Ferguson GG, Eliasziw M, Barr HW, Clagett GP, Barnes RW, Wallace MC, et al. The North American Symptomatic Carotid Endarterectomy Trial: surgical results in 1415 patients. Stroke. 1999;30:1751–8. doi: 10.1161/01.str.30.9.1751. [DOI] [PubMed] [Google Scholar]
- 10.Randomised trial of endarterectomy for recently symptomatic carotid stenosis: final results of the MRC European Carotid Surgery Trial (ECST) Lancet. 1998;351:1379–87. [PubMed] [Google Scholar]
- 11.Eliasziw M, Rankin RN, Fox AJ, Haynes RB, Barnett HJ. Accuracy and prognostic consequences of ultrasonography in identifying severe carotid artery stenosis. North American Symptomatic Carotid Endarterectomy Trial (NASCET) Group. Stroke. 1995;26:1747–52. doi: 10.1161/01.str.26.10.1747. [DOI] [PubMed] [Google Scholar]
- 12.Oates C, Naylor AR, Hartshorne T, Charles SM, Fail T, Humphries K, et al. Reporting carotid ultrasound investigations in the United Kingdom. Eur J Vasc Endovasc Surg. 2009;37:251–61. doi: 10.1016/j.ejvs.2008.10.015. [DOI] [PubMed] [Google Scholar]
- 13.Antithrombotic Trialists’ Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2002;324:71–86. doi: 10.1136/bmj.324.7329.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen ZM, Sandercock P, Pan HC, Counsell C, Collins R, Liu LS, et al. Indications for early aspirin use in acute ischemic stroke: a combined analysis of 40,000 randomized patients from the Chinese Acute Stroke Trial and the International Stroke Trial. Stroke. 2000;31:1240–49. doi: 10.1161/01.str.31.6.1240. [DOI] [PubMed] [Google Scholar]
- 15.De Schryver EL, Algra A, van Gijn J. Dipyridamole for preventing stroke and other vascular events in patients with vascular disease. Cochrane Database Syst Rev. 2007;3:CD001820. doi: 10.1002/14651858.CD001820.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.CAPRIE Steering Committee. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE) Lancet. 1996;348:1329–39. doi: 10.1016/s0140-6736(96)09457-3. [DOI] [PubMed] [Google Scholar]
- 17.Diener HC, Bogousslavsky J, Brass LM, Cimminiello C, Csiba L, Kaste M MATCH investigators. Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high-risk patients (MATCH): randomised, double-blind, placebo-controlled trial. Lancet. 2004;364:331–7. doi: 10.1016/S0140-6736(04)16721-4. [DOI] [PubMed] [Google Scholar]
- 18.Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360:7–22. [Google Scholar]
- 19.O'Regan C, Wu P, Arora P, Perri D, Mills EJ. Statin therapy in stroke prevention: a meta-analysis involving 121,000 patients. Am J Med. 2008;121:24–33. doi: 10.1016/j.amjmed.2007.06.033. [DOI] [PubMed] [Google Scholar]
- 20.Brugts JJ, Yetgin T, Hoeks SE, Gotto AM, Shepherd J, Westendorp RG, et al. The benefits of statins in people without established cardiovascular disease but with cardiovascular risk factors: meta-analysis of randomised controlled trials. BMJ. 2009;338:b2376. doi: 10.1136/bmj.b2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Amarenco P, Goldstein LB, Szarek M, Sillesen H, Rudolph AE, Callahan A, 3rd, et al. SPARCL Investigators. Effects of intense low-density lipoprotein cholesterol reduction in patients with stroke or transient ischemic attack: the Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) trial. Stroke. 2007;38:3198–204. doi: 10.1161/STROKEAHA.107.493106. [DOI] [PubMed] [Google Scholar]
- 22.Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM, Jr, Kastelein JJ, et al. JUPITER Study Group. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195–207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 23.Weng TC, Yang YH, Lin SJ, Tai SH. A systematic review and meta-analysis on the therapeutic equivalence of statins. J Clin Pharm Ther. 2010;35:139–51. doi: 10.1111/j.1365-2710.2009.01085.x. [DOI] [PubMed] [Google Scholar]
- 24.Jun M, Foote C, Lv J, Neal B, Patel A, Nicholls SJ, et al. Effects of fibrates on cardiovascular outcomes: a systematic review and meta-analysis. Lancet. 2010;375:1875–84. doi: 10.1016/S0140-6736(10)60656-3. [DOI] [PubMed] [Google Scholar]
- 25.Meta-analysis of hypertension treatment trials. Lancet. 1990;335:1092–4. [PubMed] [Google Scholar]
- 26.Dahlof B, Sever PS, Poulter NR, Wedel H, Beevers DG, Caulfield M, et al. Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm (ASCOT-BPLA): a multicentre randomised controlled trial. Lancet. 2005;366:895–906. doi: 10.1016/S0140-6736(05)67185-1. [DOI] [PubMed] [Google Scholar]
- 27.Dahlof B, Devereux RB, Kjeldsen SE, Julius S, Beevers G, de Faire U, et al. Cardiovascular morbidity and mortality in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet. 2002;359:995–1003. doi: 10.1016/S0140-6736(02)08089-3. [DOI] [PubMed] [Google Scholar]
- 28.Heart Outcomes Prevention Evaluation Study Investigators. Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy. Lancet. 2000;355:253–9. [PubMed] [Google Scholar]
- 29.Khan N, McAlister FA. Re-examining the efficacy of beta-blockers for the treatment of hypertension: a meta-analysis. CMAJ. 2006;174:1737–42. doi: 10.1503/cmaj.060110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kernan WN, Inzucchi SE. Type 2 diabetes mellitus and insulin resistance: stroke prevention and management. Curr Treat Options Neurol. 2004;6:443–50. doi: 10.1007/s11940-004-0002-y. [DOI] [PubMed] [Google Scholar]
- 31.Howard G, Wagenknecht LE, Cai J, Cooper L, Kraut MA, Toole JF. Cigarette smoking and other risk factors for silent cerebral infarction in the general population. Stroke. 1998;29:913–7. doi: 10.1161/01.str.29.5.913. [DOI] [PubMed] [Google Scholar]
- 32.Lee AH, Liang W. Life-long physical activity involvement and the risk of ischemic stroke in southern china. Stroke Res Treat. 2011;2010:415–41. doi: 10.4061/2010/415241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Winter Y, Rohrmann S, Linseisen J, Lanczik O, Ringleb PA, Hebebrand J, et al. Contribution of obesity and abdominal fat mass to risk of stroke and transient ischemic attacks. Stroke. 2008;39:3145–51. doi: 10.1161/STROKEAHA.108.523001. [DOI] [PubMed] [Google Scholar]
- 34.Liapis CD, Bell PR, Mikhailidis D, Sivenius J, Nicolaides A, Fernandes e Fernandes J, et al. ESVS Guidelines Collaborators. ESVS guidelines. Invasive treatment for carotid stenosis: indications, techniques. Eur J Vasc Endovasc Surg. 2009;37(Suppl 4):1–19. doi: 10.1016/j.ejvs.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 35. www.nice.org.uk .
- 36.Hobson RW, 2nd, Mackey WC, Ascher E, Murad MH, Calligaro KD, Comerota AJ, et al. Society for Vascular Surgery. Management of atherosclerotic carotid artery disease: clinical practice guidelines of the Society for Vascular Surgery. J Vasc Surg. 2008;48:480–6. doi: 10.1016/j.jvs.2008.05.036. [DOI] [PubMed] [Google Scholar]
- 37.Naylor RA. Known knowns, known unknowns and unknown unknowns: a 2010 update on carotid artery disease. Surgeon. 2010;8:79–86. doi: 10.1016/j.surge.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 38.Halliday A, Mansfield A, Marro J, Peto C, Peto R, Potter J, et al. MRC Asymptomatic Carotid Surgery Trial (ACST) Collaborative Group. Prevention of disabling and fatal strokes by successful carotid endarterectomy in patients without recent neurological symptoms: randomised controlled trial. Lancet. 2004;363:1491–502. doi: 10.1016/S0140-6736(04)16146-1. [DOI] [PubMed] [Google Scholar]
- 39.Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. Endarterectomy for asymptomatic carotid artery stenosis. JAMA. 1995;273:1421–8. [PubMed] [Google Scholar]
- 40.Naylor AR. Time is brain! Surgeon. 2007;5:23–30. doi: 10.1016/s1479-666x(07)80108-9. [DOI] [PubMed] [Google Scholar]
- 41.Rothwell PM, Eliasziw M, Gutnikov S, Warlow CP, Barnett HJ. Sex difference in the effect of time from symptoms to surgery on benefit from carotid endarterectomy for transient ischemic attack and nondisabling stroke. Stroke. 2004;35:2855–61. doi: 10.1161/01.STR.0000147040.20446.f6. [DOI] [PubMed] [Google Scholar]
- 42.Endovascular versus surgical treatment in patients with carotid stenosis in the Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS): a randomised trial. Lancet. 2001;357:1729–37. [PubMed] [Google Scholar]
- 43.Eckstein HH, Ringleb P, Allenberg JR, Berger J, Fraedrich G, Hacke W, et al. Results of the Stent-Protected Angioplasty versus Carotid Endarterectomy (SPACE) study to treat symptomatic stenoses at 2 years: a multinational, prospective, randomised trial. Lancet Neurol. 2008;7:893–902. doi: 10.1016/S1474-4422(08)70196-0. [DOI] [PubMed] [Google Scholar]
- 44.Mas JL, Chatellier G, Beyssen B, Branchereau A, Moulin T, Becquemin JP, et al. EVA-3S Investigators. Endarterectomy versus stenting in patients with symptomatic severe carotid stenosis. N Engl J Med. 2006;355:1660–71. doi: 10.1056/NEJMoa061752. [DOI] [PubMed] [Google Scholar]
- 45.Gurm HS, Yadav JS, Fayad P, Katzen BT, Mishkel GJ, Bajwa TK, et al. SAPPHIRE Investigators. Long-term results of carotid stenting versus endarterectomy in high-risk patients. N Engl J Med. 2008;358:1572–9. doi: 10.1056/NEJMoa0708028. [DOI] [PubMed] [Google Scholar]
- 46.Bonati LH, Dobson J, Algra A, Branchereau A, Chatellier G, Fraedrich G, et al. Carotid Stenting Trialists’ Collaboration. Short-term outcome after stenting versus endarterectomy for symptomatic carotid stenosis: a preplanned meta-analysis of individual patient data. Lancet. 2010;376:1062–73. doi: 10.1016/S0140-6736(10)61009-4. [DOI] [PubMed] [Google Scholar]
- 47.Brott TG, Hobson RW, II, Howard G, Roubin GS, Clark WM, Brooks W, et al. for the CREST Investigators. Stenting versus endarterectomy for treatment of carotid-artery stenosis. N Engl J Med. 2010;363:11–23. doi: 10.1056/NEJMoa0912321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Davies SM, Donnan GA. Carotid-artery stenting in stroke prevention. N Eng J Med. 2010;363:11–23. doi: 10.1056/NEJMe1005220. [DOI] [PubMed] [Google Scholar]
- 49.Brott TG, Halperin JL, Abbara S, Bacharach JM, Barr JD, Bush RL, et al. 2011 ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS Guideline on the management of patients with extracranial carotid and vertebral artery disease. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American Stroke Association, American Association of Neuroscience Nurses, American Association of Neurological Surgeons, American College of Radiology, American Society of Neuroradiology, Congress of Neurological Surgeons, Society of Atherosclerosis Imaging and Prevention, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of NeuroInterventional Surgery, Society for Vascular Medicine, and Society for Vascular Surgery Developed in Collaboration With the American Academy of Neurology and Society of Cardiovascular Computed Tomography. J Am Coll Cardiol. 2011;57:e16–94. doi: 10.1016/j.jacc.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 50.Ricotta JJ, Aburahma A, Ascher E, Eskandari M, Faries P, Lal BK. Updated society for vascular surgery guidelines for management of extracranial carotid disease. J Vasc Surg. 2011;54:e1–31. doi: 10.1016/j.jvs.2011.07.031. [DOI] [PubMed] [Google Scholar]
- 51.Kakisis JD, Avgerinos ED, Antonopoulos CN, Giannakopoulos TG, Moulakakis K, Liapis CD. The European Society for Vascular Surgery Guidelines for carotid intervention: an updated independent assessment and literature review. Eur J Vasc Endovasc Surg. 2012;44:238–43. doi: 10.1016/j.ejvs.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 52.Lewis SC, Warlow CP, Bodenham AR, Colam B, Rothwell PM, Torgerson D, et al. GALA Trial Collaborative Group. General anaesthesia versus local anaesthesia for carotid surgery (GALA): a multicentre, randomised controlled trial. Lancet. 2008;372:2132–42. doi: 10.1016/S0140-6736(08)61699-2. [DOI] [PubMed] [Google Scholar]
- 53.Rerkasem K, Rothwell PM. Patch angioplasty versus primary closure for carotid endarterectomy. Cochrane Database Syst Rev. 2009;(4):CD000160. doi: 10.1002/14651858.CD000160.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cao PG, de Rango P, Zannetti S, Giordano G, Ricci S, Celani MG. Eversion versus conventional carotid endarterectomy for preventing stroke. Cochrane Database Syst Rev. 2001;(1):CD001921. doi: 10.1002/14651858.CD001921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bond R, Warlow CP, Naylor AR, Rothwell PM European Carotid Surgery Trialists’ Collaborative Group. Variation in surgical and anaesthetic technique and associations with operative risk in the European carotid surgery trial: implications for trials of ancillary techniques. Eur J Vasc Endovasc Surg. 2002;23:117–26. doi: 10.1053/ejvs.2001.1566. [DOI] [PubMed] [Google Scholar]
- 56.Holt PJ, Poloniecki JD, Loftus IM, Thompson MM. The relationship between hospital case volume and outcome from carotid endartectomy in England from 2000 to 2005. Eur J Vasc Endovasc Surg. 2007;34:646–54. doi: 10.1016/j.ejvs.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 57.Wholey MH, Al-Mubarek N, Wholey MH. Updated review of the global carotid artery stent registry. Catheter Cardiovasc Interv. 2003;60:259–66. doi: 10.1002/ccd.10645. [DOI] [PubMed] [Google Scholar]
- 58.Naylor AR. Does the risk of post-CABG stroke merit staged or synchronous reconstruction in patients with symptomatic or asymptomatic carotid disease? J Cardiovasc Surg (Torino) 2009;50:71–81. [PubMed] [Google Scholar]
- 59.Dick AM, Brothers T, Robison JG, Elliott BM, Kratz JM, Toole JM, et al. Combined carotid endarterectomy and coronary artery bypass grafting versus coronary artery bypass grafting alone: a retrospective review of outcomes at our institution. Vasc Endovascular Surg. 2011;45:130–4. doi: 10.1177/1538574410393752. [DOI] [PubMed] [Google Scholar]
- 60.Illuminati G, Ricco JB, Caliò F, Pacilè MA, Miraldi F, Frati G, et al. Short-term results of a randomized trial examining timing of carotid endarterectomy in patients with severe asymptomatic unilateral carotid stenosis undergoing coronary artery bypass grafting. J Vasc Surg. 2011;54:993–9. doi: 10.1016/j.jvs.2011.03.284. [DOI] [PubMed] [Google Scholar]
- 61.Knipp SC, Scherag A, Beyersdorf F, Cremer J, Diener HC, Haverich JA, et al. CABACS Study Group. Randomized comparison of synchronous CABG and carotid endarterectomy vs. isolated CABG in patients with asymptomatic carotid stenosis: the CABACS trial. Int J Stroke. 2011 doi: 10.1111/j.1747-4949.2011.00687.x. Epub ahead of print doi: 10.1111/j.1747–4949.2011.00687.x. [DOI] [PubMed] [Google Scholar]
- 62.Venkatachalam S, Gray BH, Mukherjee D, Shishehbor MH. Contemporary management of concomitant carotid and coronary artery disease. Heart. 2011;97:175–80. doi: 10.1136/hrt.2010.203612. [DOI] [PubMed] [Google Scholar]
- 63.Kumar SM, Wang JC, Barry MC, Farrell L, Kelly CJ, Fitzgerald PH, et al. Carotid stump syndrome: outcome from surgical management. Eur J Vasc Endovasc Surg. 2001;21:214–9. doi: 10.1053/ejvs.2000.1292. [DOI] [PubMed] [Google Scholar]
- 64.Wang Z, Shen L, Yu J, Gu Y, Wang S, Guan H, et al. Management of cerebral ischemia due to Takayasu's arteritis. Chin Med J (Engl) 2002;115:342–6. [PubMed] [Google Scholar]
- 65.Smout J, Macdonald S, Weir G, Stansby G. Carotid artery stenting: relationship between experience and complication rate. Int J Stroke. 2010;5:477–82. doi: 10.1111/j.1747-4949.2010.00486.x. [DOI] [PubMed] [Google Scholar]
- 66.Nallamothu BK, Gurm HS, Ting HH, Goodney PP, Rogers MA, Curtis JP, et al. Operator experience and carotid stenting outcomes in Medicare beneficiaries. JAMA. 2011;306:1338–43. doi: 10.1001/jama.2011.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vogel TR, Dombrovskiy VY, Graham AM. Carotid artery stenting in the nation: the influence of hospital and physician volume on outcomes. Vasc Endovascular Surg. 2010;44:89–94. doi: 10.1177/1538574409354653. [DOI] [PubMed] [Google Scholar]
- 68.Rothwell PM, Warlow CP. Low risk of ischemic stroke in patients with reduced internal carotid artery lumen diameter distal to severe symptomatic carotid stenosis: cerebral protection due to low poststenotic flow? On behalf of the European Carotid Surgery Trialists’ Collaborative Group. Stroke. 2000;31:622–30. doi: 10.1161/01.str.31.3.622. [DOI] [PubMed] [Google Scholar]
- 69.Schillinger M, Gschwendtner M, Reimers B, Trenkler J, Stockx L, Mair J, et al. Does carotid stent cell design matter? Stroke. 2008;39:905–9. doi: 10.1161/STROKEAHA.107.499145. [DOI] [PubMed] [Google Scholar]
- 70.McKevitt FM, Randall MS, Cleveland TJ, Gaines PA, Tan KT, Venables GS. The benefits of combined anti-platelet treatment in carotid artery stenting. Eur J Vasc Endovasc Surg. 2005;29:522–27. doi: 10.1016/j.ejvs.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 71.Markus HS, Droste DW, Kaps M, Larrue V, Lees KR, Siebler M, et al. Dual antiplatelet therapy with clopidogrel and aspirin in symptomatic carotid stenosis evaluated using Doppler embolic signal detection: the Clopidogrel and Aspirin for Reduction of Emboli in Symptomatic Carotid Stenosis (CARESS) trial. Circulation. 2005;111:2233–40. doi: 10.1161/01.CIR.0000163561.90680.1C. [DOI] [PubMed] [Google Scholar]
- 72.Chaturvedi S, Yadav JS. The role of antiplatelet therapy in carotid stenting for ischemic stroke prevention. Stroke. 2006;37:1572–77. doi: 10.1161/01.STR.0000221298.43117.be. [DOI] [PubMed] [Google Scholar]


