Abstract
OBJECTIVES
Fistulas between the oesophagus and the respiratory tract can occur as a complication to anastomotic dehiscence after oesophageal resection, without any signs of local residual tumour growth. Other causes that are, by definition, benign may rarely prevail. The traditional therapeutic approach is to divert the proximal portion of the oesophagus and transpose the conduit into the abdominal cavity. With the introduction and development of self-expandable metal stents (SEMS), new therapeutic options have emerged for these severe complications. We have evaluated our stent-based strategy for managing these life-threatening situations.
METHODS
At Karolinska University Hospital, all patients admitted with an oesophago-respiratory fistula during the period 2003–2011 followed a stent-based strategy. On clinical suspicion, a prompt computed tomography scan was performed with contrast ingestion, to visualize the status of the anastomosis and the potential communications. Often an endoscopy was done to assess the oesophagus and the conduit. The respiratory tree was inspected through a concomitant bronchoscopy. The double-stent strategy presently applied meant that covered self-expandable metal stents (SEMS) were applied on the alimentary and airway sides to adequately cover the fistula orifice on both sides. The subsequent clinical course determined the ensuing therapeutic strategy.
RESULTS
During the study period, 17 cases with oesophago-respiratory fistulas were treated at our unit, of which 13 exhibited fistulation following an oesophageal resection due to cancer and 4 cases had a benign underlying disease. The cancer patients did not show any obvious demographic profile when it came to the cancer sub-location, histological type of cancer, or treatment with neoadjuvant chemo- and radiochemotherapy. There was an equal distribution between hand-sutured and stapled anastomoses. In 10 of the cases, the anastomoses were located in the upper right chest; the remainder in the neck, and all reconstructions were carried out by a tubulized stomach. The diagnosis of the fistula tract between the anastomotic area and the respiratory tract was attained on the 15th postoperative day (median), with a range from 5 to 24 days.
CONCLUSIONS
When an oesophago-respiratory fistula is diagnosed, even in a situation where no neoplastic tissue is prevailing, attempts should be made to close the fistula tract by SEMS from both directions, i.e. from the oesophageal as well as the respiratory side. By this means, a majority of these patients can be initially managed conservatively with prospects of a successful outcome, although virtually all will eventually require a single-stage resection and reconstruction.
Keywords: Oesophageal resection, Anastomotic leakage, Caustic ingestion, Postoperative complications, Postoperative morbidity, Postoperative mortality
INTRODUCTION
Fistula and direct communication between the airways and the oesophagus is a clinical condition which is followed by dramatic and life-threatening symptoms that are connected with an exceptionally high mortality, even in situations where the underlying cause is, by definition, of a benign nature [1, 2]. The most common cause behind a oesophago-respiratory fistula is advanced tumour overgrowth, either from the oesophageal or the pulmonary side [3, 4]. In these situations, the development of modern self-expandable metal stent (SEMS) technologies, with the deployment and immediate sealing of the fistula, has revolutionized the palliative management of these patients [1, 2]. Over recent years, the therapeutic, stent-based strategies have been defined and refined to a point where, whenever there is a hole in the oesophagus—basically irrespective of the genesis of the perforation—SEMS placement can be justified [5–7]. Despite some continuing controversy, it seems that the primary strategy of promptly covering the leak using a stent has gained increasing acceptance [8].
Not infrequently, an unnoticed perforation or a conservatively treated anastomotic leakage may develop and manifest itself as a fistula into the respiratory tract [9]. Another life-threatening and similar situation is penetration into the airways of the oesophageal transmural damage caused by ingestion of corrosive material. Until now, few surgeons have utilized a stent-based strategy to cope with these very demanding situations. The application and practice of a corresponding self-expandable stent-based strategy are far more complex and may, in many instances, require stenting from both sides of the fistula. We have, since 2003, tested the concept of always trying to seal an oesophago-respiratory fistula of a non-malignant nature by the deployment of expandable stents from both sides, i.e. from the alimentary as well as the respiratory tracts. We report herein our experiences with the objective of defining the ‘pros’, ‘cons’ and limitations of this therapeutic strategy.
MATERIAL AND METHODS
During the study period, between 1st January 2003 and 31st December 2011, 17 patients with a non-malignant oesophago-respiratory fistula were managed at the Department of Surgery, Karolinska University Hospital, Stockholm, Sweden. None of these patients had a communication between the alimentary and respiratory tracts due to a manifestation of a locally advanced and untreated cancer. During this time period, we explored the therapeutic concept of aiming to cover the defect from both sides. In patients with a history of oesophageal resection, we did not perform a routine investigation of the patency of the anastomosis, but instead acted promptly on clinical signs occurring during the early postoperative period. In those situations, all patients received a prompt computed tomography (CT) scan with contrast ingestion to visualize the status of the anatomosis and the potential communications. The vast majority also underwent an endoscopy in which the native oesophagus, the anastomotic area and the status of the conduit were assessed. In all situations where we had a suspicion of an airway communication, a bronchoscopy was performed, at the same time as this endoscopy, to delineate the details of the damage to the airways. The double-stent strategy presently applied meant that an SEMS was applied on the alimentary side and, during bronchoscopy, another covered stent was applied and modified to adequately cover the airway defect (Figs 1 and 2). In the cases in which we needed to put stents in the upper lobe bronchus or in the lower lobe, we used partially coated stents. The advantage of these stents is that they have a lesser tendency to migrate. In two cases, a tracheal bifurcation airway stent was deployed and, in order to apply an expanded device, a bronchial expandable stent was placed in the affected main bronchus, inside the bifurcation stent. The alimentary stents were changed, either in consequence of the development of the clinical situation or at intervals of 3–6 weeks. The airway stents were usually maintained in place for 2–3 months before being replaced.
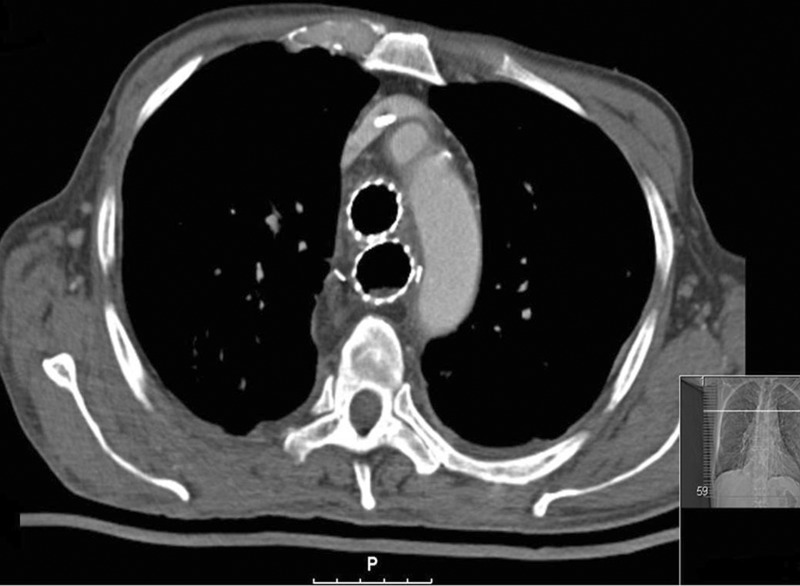
Figure 1:
Computed tomography of stents in the oesophagus and in the trachea.
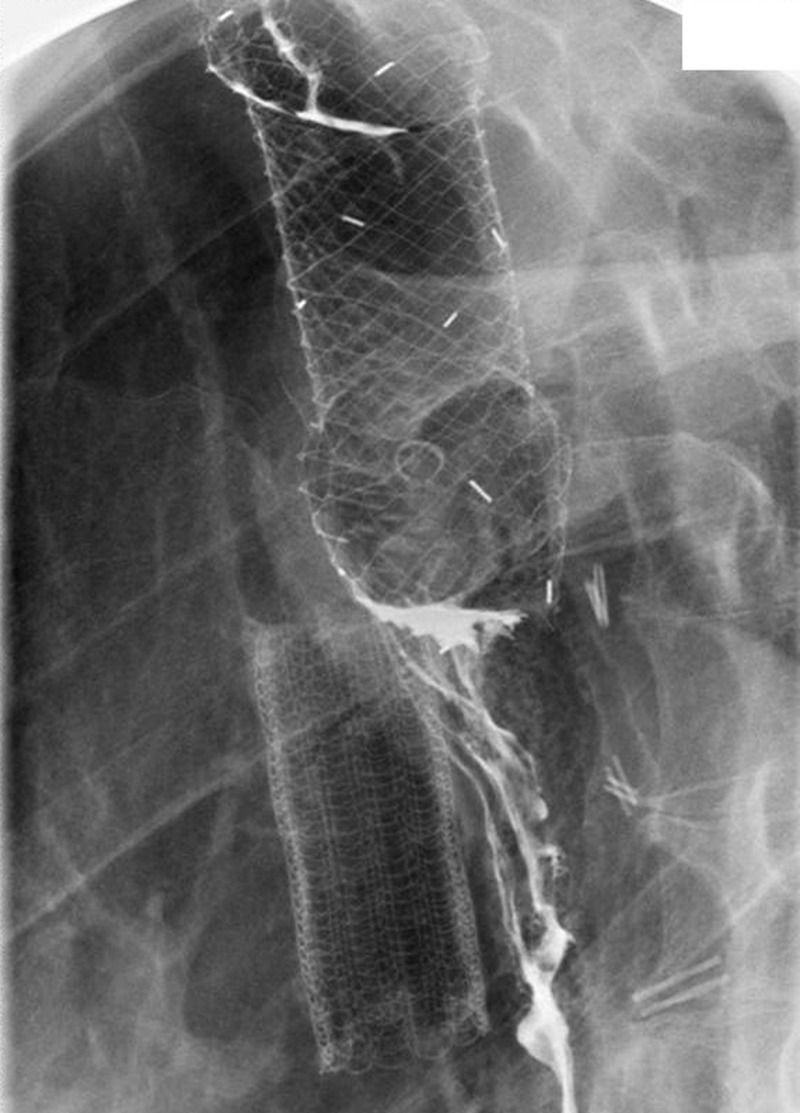
Figure 2:
Plain X-ray of double stents. The stent top right is placed in the oesophagus. Contrast passes through the stent and down into the conduit. No leakage can be seen. The stent bottom left is placed in the trachea.
RESULTS
Mixture of causes of oesophago-respiratory tract fistula
Of the four cases with a benign underlying disease, three had ingested lye, with subsequent destruction of the oesophageal wall. Of these patients, one had a tracheal fistula occurring as a consequence of endoscopic dilatation and perforation, whereupon the patient was referred after combined oesophageal and tracheal stenting had failed at the referring hospital. After resection and reconstruction with a colonic conduit, the airway fistula healed promptly (i.e. the airway stent could be removed within 1 month). One patient developed an acute tracheal fistula, connected with a complete ischaemic necrosis of a colonic conduit completed 30 days earlier. The only patient who had a fatal outcome was a 61-year old male who ingested large amounts of corrosive liquid to commit suicide. He first had a laparotomy, whereby a gastrectomy was required due to local transmural necrosis and, 10 days later, he presented with fistulation between the distal oesophagus and the left main bronchus. He had an emergency oesophagectomy with stenting of the airway defect but succumbed three days later due to multi-organ failure. Otherwise the details of these true benign cases are given in Table 1.
Table 1:
Characteristics of true benign cases treated for an oesophago-respiratory fistula
| Sex | Age | Comorbidity | Level of anastomosis | Anastom. leak | Airway leak onset (day) | No of oesophageal stents | No of airway stents | Total stent time (days) | Fistula sealed by stent | In-hospital death | In-hospital stay | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | 78 | Yes | Neck | No | 17 | 6 | 3 | 143 | No | No | 123 | Reconstructiona |
| M | 50 | No | n.a. | No | n.a. | 6 | 3 | unknown | No | No | 10 | Reconstructionb |
| M | 61 | Yes | n.a. | n.a. | 10 | 0 | 1 | 19 | No | Yes | 53 | Diedc |
| F | 35 | No | Neck | Yes | 36 | 0 | 3 | 30 | No | No | 91 | Reconstructiond |
aLye injury since childhood. Developed chronic strictures and was operated with oesophageal resection. After operation developed fistulas. Reconstructed 118 days postoperatively.
bReferred to Karolinska University Hospital 1025 days after accidental lye injury. Initially operated at home hospital but developed fistulas. Reconstructed 1075 days after lye injury.
cDied from lye injury.
dFistulation after oesophageal resection. Reconstructed 329 days postoperatively.
n.a.: not applicable.
Oesophago-respiratory tract fistula in the postoperative period
In 13 cases, the communication appeared between the anastomotic area, after an oesophageal resection, and the respiratory tree. As seen in Table 2 there seemed to be no obvious demographic profile of these patients when it came to the location or type of resected cancer, treatment with neoadjuvant chemo- and radiochemotherapy. There was an equal distribution between hand-sutured and stapled anastomoses, which basically reflected the preferences of the operating surgeons. The location of the anastomoses was in the upper right chest in 10 of the cases, the remainder in the neck and all reconstructions were carried out by a tubulized stomach. The diagnosis of the fistula tract between the anastomotic area and the respiratory tract was established on the 15th postoperative day (median) with a range from 5 to 24 days. Of particular interest was the fact that no clinical suspicion of or documented anastomotic dehiscence was reported in six of these patients, whereas the preceding anastomotic leakage was documented at a median of 5 days postoperatively (range 2–12 days) in the remaining seven patients. The average elapsed time from the appearance of an anastomotic leak and the documentation of an airway fistula was 10.4 days (range 6–20) in these seven patients.
Table 2:
Characteristics of patients treated for post resectional oesophago-respiratory fistulas
| Sex | Age | Comorbidity | Tumour histology | T-stage | Level of anastomosis | Neo-adjuvant therapy | Anast. leak | Airway leak | No of oesophageal stent | No of airway stent | Total stent time (days) | Fistula sealed by stent | Salvage oesophag- ectomy | In-hospital death | In-hospital stay | Outcome (days after primary operation) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | 58 | Yes | AC | 1 | Thx | No | Day 4 | Day 10 | 1 | 1 | 7 | No | Yes | – | 93 days | Reconstruction (568 days) |
| M | 58 | Yes | SCC | 2 | Thx | No | No | 21 | 6 | 4 | 198 | No | – | Yes | 221 | Died (219) |
| M | 56 | Yes | AC | 2 | Thx | Yes | No | 12 | 9 | 9 | 521 | No | – | – | 60 | Reconstruction (549) |
| M | 76 | Yes | SCC | 3 | Thx | No | No | 15 | 1 | 2 | 5 | No | – | – | 8 | Died (33) |
| M | 57 | No | SCC | 3 | Neck | No | No | 16 | 2 | 7 | 77 | Noa | – | – | 34 | Died (716) |
| F | 57 | Yes | SCC | 3 | Thx | Yes | Day 10 | 17 | 7 | 0 | 203 | No | – | – | 37 | Reconstruction (519) |
| M | 68 | No | AC | 1 | Thx | No | Day 2 | 23 | 7 | 1 | 308 | No | – | – | 59 | Reconstruction (975) |
| M | 68 | Yes | SCC | 3 | Neck | No | Day 12 | 24 | 2 | 0 | 92 | No | – | – | 61 | Died (114) |
| M | 64 | No | AC | 1 | Thx | No | No | 15 | 5 | 1 | 105 | Yes | – | – | 79 | No complications after stents |
| M | 71 | Yes | SCC | 2 | Neck | Yes | Day 4 | 8 | 0 | 3 | 46 | No | Yes | – | 94 | Reconstruction (252) |
| F | 57 | Yes | AC | 3 | Thx | No | Day 5 | 6 | 1 | 2 | 1 | No | Yes | – | 25 | Has so far declined reconstruction |
| M | 49 | Yes | SCC | 2 | Neck | No | No | 24 | 1 | 1 | 32 | Yes | – | – | 61 | No complications after stents |
| M | 60 | Yes | AC | 2 | Thx | No | Day 5 | 30 | 6 | 0 | unknown | No | – | – | 34 | Referred to home hospital |
aIn this patient stenting did seal the fistula for more than 2 months but, due to local recurrence of tumour, the fistula recurred.
Thx: Thorax.
In one case, a gastric tube necrosis was documented at the diagnosis of the tracheal fistula: otherwise, typical appearances and location of an anastomotic dehiscence were documented. Nine of the fistulas entered mainly into the trachea and the rest were equally distributed between engagement of the right and left bronchi. The two fistula tracts on the right side emptied into the upper lobe bronchus. Only two patients healed completely by stent treatment and thus needed no reconstruction. One of these patients had a fistula with a long tract from the anastomotic area in the thoracic aperture to the left main bronchus. The other patient had a fistula to the trachea. A chronic fistula, seen through a gastroscope, is shown in Fig. 3.
Figure 3:
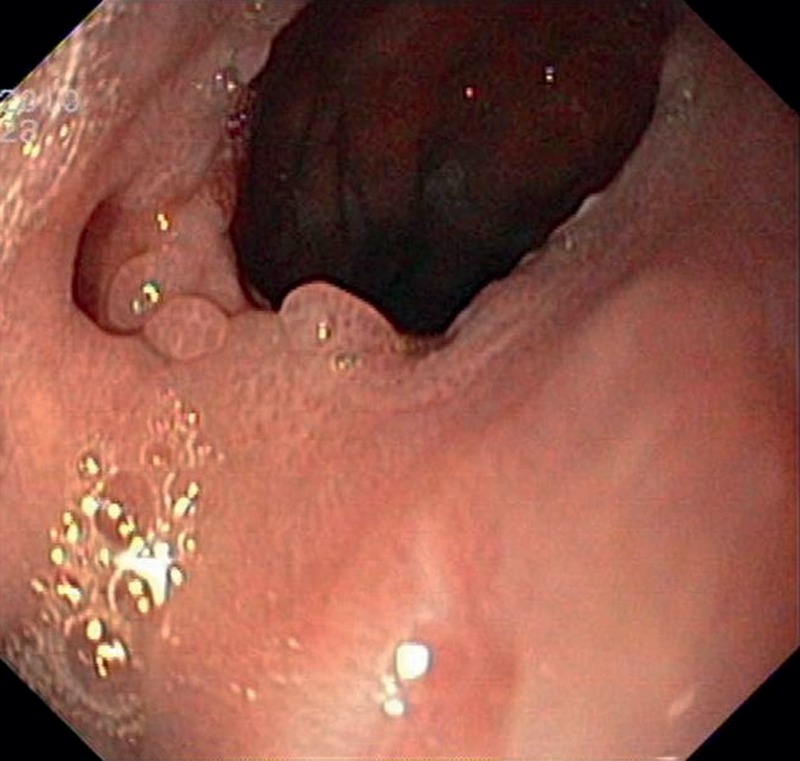
Endoscopic view of a chronic fistula in the anastomosis.
Three patients underwent a salvage oesophagectomy/conduit resection and proximal-end oesophagostomy, due to insufficient damage control. In two of these cases, double stenting had been applied for a limited time period but failed to control the situation. All but one of these had a large fistula into the mid-portion of the trachea. At the time of oesophagectomy, all patients received a repositioning of the airway stent(s). During the subsequent clinical course, most patients had to have the oesophageal SEMS replaced (Table 2), whereas three patients required more than two replacements of the airway stents. The duration of hospital stay was extensive, at a mean of 58 days (range 10–123). At the time of writing, four patients have undergone a reconstruction under elective circumstances, with resection of the fistula tract and restoration of the alimentary continuity. In two cases a simultaneous pulmonary lobectomy was completed. After reconstruction, it has been possible to remove all airway stents within the predicted time span. During follow-up, no airway strictures have been observed. Three patients underwent an emergency oesophagectomy: two have been successfully reconstructed but one patient has so far declined further surgery. Overall procedure-related mortality was 2/17 (12%) but another patient died due to fistula-related problems that were found to be due to recurrent local tumour growth.
DISCUSSION
Our series of patients with oesophago-respiratory fistulas, clearly demonstrates that an expandable stent-based management strategy can be a temporary option to control such a life-threatening situation. During the last eight years we have recorded a series of 17 patients where we noted a procedure-related mortality of only 12%. This figure should be compared to the results reported in the very scarce surgical literature covering the traditional therapeutic approaches to these situations, where surgical treatment of these fistulas has been associated with significant morbidity and an exceedingly high mortality rate [10–13]. The main features and outcomes of the present case series can be summarized as follows:
No particular demographic characteristics are attached to those who develop this devastating complication.
Most fistulas develop from an anastomotic leak into the membranous part of the trachea.
A substantial proportion of these fistulas manifest themselves without obvious clinical and radiological signs of anastomotic dehiscence, and are diagnosed rather late in the postoperative course following oesophagectomy (median 15 days).
Moreover, a significant number of these patients can be successfully managed by a double SEMS-based stent strategy, i.e. a stent applied on both sides of the fistula.
In total only five of the cases required an emergency oesophagectomy, among whom four patients had a shorter period of unsuccessful stenting with incomplete sealing of the leakage. In fact, among the 13 patients with a leaking post-oesophagectomy anastomotic leak, only two had a salvage oesophagectomy. This experience again illustrates the vital importance of continuously re-evaluating these patients on a daily basis, being prepared to switch strategy if damage control is insufficient [8, 14]. The patient's clinical condition has to determine—as assessed from day to day—whether the therapeutic strategy initially embarked upon should be abandoned. Despite the fact that the majority of our cases could be brought past the acute life-threatening phase by the stent-based therapeutic strategy, all but two patients required a subsequent resection and reconstruction with a viable conduit. The only patients whose fistulas healed achieved this within a few months. In addition, one of the patients had a fistula which emptied into the distal left segmental bronchus, an observation which may harbour an important piece of therapeutic information.
It is, however, crucial to carefully balance the ‘pros’ and ‘cons’ of a SEMS-based therapeutic strategy. The first issue relates the early recognition of this devastating complication. Most of the fistulas that we have seen so far seemed to manifest themselves rather late [14]. In fact, this complication arose even at a time when the patient had been referred to either a general ward or discharged to a rehabilitation unit. When we carefully scrutinized the clinical picture preceding the establishment of the complication, it was however noticed that all patients presented with some diffuse respiratory distress symptoms, combined with elevated CRP levels [7, 15]. Through the early recognition and interpretation of corresponding signs and symptoms, options become available for proactive, preventive interventions before the full clinical picture has developed. We have regularly used CT, initially without contrast medium, directly followed by CT with ingestion of water-soluble contrast medium, at the time of clinical suspicion of anastomotic dehiscence. Even if the CT investigation is negative, a reasonable clinical suspicion of leakage has to be followed by immediate endoscopy [11]. If the endoscopic image is consistent with an anastomotic defect, we always insert a SEMS—at least on the oesophageal side. By such a proactive therapeutic intervention, the final outcome may ultimately be affected in a positive way. At the time of final diagnostic radiological and endoscopic investigation, all of our patients had produced bile-stained sputum. In order to obtain as much diagnostic information as possible, we recommend bronchoscopy which, in addition to the diagnostic information, also offers information of relevance to the design of the subsequent therapeutic strategy.
Whenever an anastomotic dehiscence engages the oesophageal replacement conduit, the question arises of how to most accurately bridge the defect from the alimentary side. Among the range of available SEMS, none has been designed for this particular situation. The minimal requirement is to deploy a stent with an external diameter of >30 mm at the ends. Most often, the clinician is facing a situation where the ideal stent should, for preference, have a shape with an even larger diameter on the gastric side [14]. Due to lack of optimum stent design, we often inserted a colonic stent. This strategy clearly requires a consistent approach, incorporating repetitive repositioning and replacement of the SEMS.
As a result of the incomplete sealing of the fistula that follows oesophageal stenting alone, we learned important lessons from the use of a variety of simultaneous airway stents [16, 17]. Tracheo-bronchoscopy at the outset will accurately delineate the extent of the damage to the respiratory tract. Of course there are technical challenges in adequately positioning a tracheal stent at the same time as performing, for instance, a tracheostomy. The expandable properties of the trachea, as well as the unilateral bronchial stents, often effectively blocked the fistula tract from the airway side. However, the most challenging situation, in our experience, is represented by an airway defect affecting the tracheal bifurcation, together with the proximal parts of the main bronchus. In such a situation a bifurcation stent has to be deployed. The caveat is, however, that the two bronchial limbs of such a stent are not expandable. In order to adapt to this situation, and accordingly to completely seal the fistula tract, separate expandable bronchial stents have to be inserted within each limb of the bifurcation stent.
Another pivotal aspect of the management of oesophageal-respiratory fistulas concerns the pathogenesis of the lesion, knowledge of which permits preventive measures to be taken. An absolute prerequisite is, of course, an anastomotic dehiscence but only a minority of those will progress to also contain a fistula to the respiratory tract. Obviously, concurrent damage to the latter is likely to be fundamental but we found it exceedingly difficult to identify such a common denominator. It can of course be alleged that surgical trauma must have been inflicted, but only half of the tumours were obviously bulky, necessitating a plane of dissection very close to the membranous part of the trachea and main bronchi. In no single case was direct damage recognized perioperatively, that required repair. Moreover, only one of our cases received preoperative neoadjuvant radiochemotherapy which, some have argued, predisposes the patient to such a complication [6]. Another issue is the value of elevating parts of the omentum to encircle the anastomoses, which would offer a biological membrane to balance the risk of penetrating injury to the airways.
The alternative therapy in oesophago-respiratory fistula has to be considered, based on considerations relevant to the level of emergency. For the acute situation, it can be argued that surgical diversion is the preferred approach [12]. On the other hand, in the majority of our patients, we could manage the situation by the stent-based strategy and thereby transfer the reconstruction to a single-stage, elective and controlled restorative procedure. In the long-term perspective, the message from this series is clear: these fistulas usually do not heal and require subsequent resection, the timing of which should be planned accordingly. Another important piece of clinical information is hidden in the fact that the airways are perfectly under control (by the prevailing airway stent) during the subsequent elective resection. Moreover, after removal of these SEMS we have never experienced any airway strictures during follow-up.
In conclusion, when an oesophago-respiratory fistula is diagnosed, even in a situation where no neoplastic tissue is prevailing, attempts should be made to close the fistula tract by SEMS from both directions, i.e. from the alimentary as well as the respiratory side. This strategy has to be proactive, including a commitment to repeatedly reposition and change stents and modify their sizes and designs. By doing this, the majority of patients with such a life-threatening complication can be managed conservatively in the acute phase. However, the long-term successful outcome is entirely dependent on the accurate timing and implementation of immediate damage control, as well as the subsequent reconstructive procedure.
Conflict of interest: none declared.
REFERENCES
- 1.Tomaselli F, Maier A, Sankin O, Woltsche M, Pinter H, Smolle-Juttner FM. Successful endoscopical sealing of malignant esophageotracheal fistulae by using a covered self-expandable stenting system. Eur J Cardiothorac Surg. 2001;20:734–38. doi: 10.1016/s1010-7940(01)00867-3. [DOI] [PubMed] [Google Scholar]
- 2.Martin R, Duvall R, Ellis S, Scoggins CR. The use of self-expanding silicone stents in esophageal cancer care: optimal pre-, peri-, and postoperative care. Surg Endosc. 2009;23:615–21. doi: 10.1007/s00464-008-9851-x. [DOI] [PubMed] [Google Scholar]
- 3.Shin JH, Kim JH, Song HY. Interventional management of esophagorespiratory fistula Korean. J Radiol. 2010;11:133–40. doi: 10.3348/kjr.2010.11.2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balasz A, Kupcsulik PK, Galambos Z. Esopghagorespiratory fistulas of tumorous origin. Nonoperative management of 264 cases in a 20-year period. Eur J Cardiothorac Surg. 2008;34:1103–07. doi: 10.1016/j.ejcts.2008.06.025. [DOI] [PubMed] [Google Scholar]
- 5.Lacey SJ, Michaletz PA, Tabibian N, Schwartz JT, Graham Dy. Improved palliation of a repiratory-esophageal fistula with a cuffed esophageal prosthesis. Am J Gastroenterol. 1987;82:1175–76. [PubMed] [Google Scholar]
- 6.Bona D, Sarli D, Saino G, Quarenghi M, Bonavina L. Successful conservative management of benign gastro-bronchial fistula after intrathoracic esophagogastrostomy. Ann Thorac Surg. 2007;84:1036–38. doi: 10.1016/j.athoracsur.2007.04.043. [DOI] [PubMed] [Google Scholar]
- 7.Zisis C, Guillin A, Heyries L, Lienne P, D'Journo XB, Doddoli C, et al. Stent placement in the management of oesophageal leaks. Eur J Cardiothorac Surg. 2008;33:451–56. doi: 10.1016/j.ejcts.2007.12.020. [DOI] [PubMed] [Google Scholar]
- 8.Agustsson T, Nilsson M, Henriksson G, Arnelo U, Juto JE, Lundell L. Treatment of postoperative esophagorespiratory fistulas with dual self-expanding metal stents. World J Surg. 2009;33:1224–28. doi: 10.1007/s00268-009-0030-6. [DOI] [PubMed] [Google Scholar]
- 9.Sharma A, Rehman MUR, Cowen ME. Management of a difficult malignant tracheoesophageal fistula. Interact CardioVasc Thorac Surg. 2003;2:665–67. doi: 10.1016/S1569-9293(03)00203-2. [DOI] [PubMed] [Google Scholar]
- 10.Urschel JD. Esophagogastrostomy anastomotic leaks complicating esophagectomy: a review. Am J Surg. 1995;169:634–40. doi: 10.1016/s0002-9610(99)80238-4. [DOI] [PubMed] [Google Scholar]
- 11.Page RD, Shackcloth MJ, Russell GN, Pennefather SH. Surgical treatment of anastomotic leaks after oesophagectomy. Eur J Cardiothorac Surg. 2005;27:337–43. doi: 10.1016/j.ejcts.2004.10.053. [DOI] [PubMed] [Google Scholar]
- 12.Messmann H, Schmidbaur W, Jäckle J, Fürst A, Iesalnieks I. Endoscopic and surgical management of leakage and mediastinitis after esophageal surgery. Best Pract Res Clin Gastroenterol. 2004;18:809–27. doi: 10.1016/j.bpg.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 13.Liedman BL, Bennegård K, Olbe LC, Lundell LR. Predictors of postoperative morbidity and mortality after surgery for gastro-oesophageal carcinomas. Eur J Surg. 1995;161:173–80. [PubMed] [Google Scholar]
- 14.Lindenmann J, Matzi V, Porubsky C, Anegg U, Sankin O, Gabor S, et al. Self-expandable covered metal tracheal type stent for sealing cervical anastomotic leak after esophagectomy and gastric pull-up: pitfalls and possibilities. Ann Thorac Surg. 2008;85:354–56. doi: 10.1016/j.athoracsur.2007.06.044. [DOI] [PubMed] [Google Scholar]
- 15.Atkins BZ, D'Amico TA. Respiratory complications after esophagectomy. Thorac Surg Clin. 2006;16:35–48. doi: 10.1016/j.thorsurg.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 16.Sihoe AD, Wan IY, Yim AP. Airway stenting for unresectable esophageal cancer. Surg Oncol. 2004;13:17–25. doi: 10.1016/j.suronc.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 17.Kim JH, Shin JH, Song HY, Choi CM, Shim TS. Esophagorespiratory fistula without stricture: palliative treatment with a barbed covered metallic stent in the central airway. J Vasc Interv Radiol. 2011;22:84–88. doi: 10.1016/j.jvir.2010.10.003. [DOI] [PubMed] [Google Scholar]