Abstract
An 80-year old patient underwent a transapical aortic valve implantation. On the 28th postoperative day, the patient developed acute mitral valve endocarditis. Initially, the patient was unsuccessfully treated conservatively. After 71 days, the patient was operated on with mitral valve replacement. In this report, we discuss the potentially growing problem of complications related to transcatheter valve implantation.
Keywords: (Iatrogenic) Mitral valve endocarditis, Transcatheter aortic valve implantation
CASE REPORT
An 80-year old woman with symptomatic severe aortic valve stenosis (effective orifice area of 0.6 cm2, peak gradient 83 mmHg, mean gradient 58 mmHg) was presented for surgical treatment. The patient's estimated operative risk was significantly elevated because of her advanced age and multiple comorbidities: insulin-dependent diabetes mellitus, arterial hypertension, pulmonary hypertension, mitral valve insufficiency (II°) and peripheral atherosclerosis (bilateral internal carotid artery stenosis), resulting in logistic EuroSCORE of 14.24%. Additionally, the patient suffered from severe depression, which could negatively influence postoperative recovery. Thus, in spite of relatively low-estimated operative risk, our institutional ‘Heart Team’ voted for a transcatheter aortic valve implantation (TAVI), and she underwent initially uneventful transapical implantation of a 23-mm Sapien XT® (Edwards Lifesciences, Irvine, CA, USA) aortic bioprothesis. Perioperatively, the patient received intravenous (i.v.) antibiotics as per our institutional standard protocol (including 2 g of i.v. cefazolin for 8 days).
In the postoperative course, the patient suffered from transitory, right haemiparesis and symptomatic transitory psychotic syndrome for 6 days, which was treated in an intermediate care unit. After the stabilization of the patient's clinical status, she was transferred to a ward.
Until 1 week after the surgery, no clinical signs of infective endocarditis were seen. The body temperature was normal and inflammatory markers were within normal limits: white blood cell count (WBC) 10.6/µl, C-reactive protein (C-RP) 3.7 mg/dl. A routine transthoracic echocardiography performed 1 week after the procedure revealed a proper function of the aortic valve prosthesis with a low transvalvular gradient (20.5/12.7 mmHg), minimal central aortic regurgitation jet and a trivial paravalvular leak.
Because of a severe depression and symptomatic transitory psychotic syndrome abiding for the next 5 days, the patient was transferred to an intermediate care unit. The patient refused to take drugs and was highly uncooperative. After the stabilization of mental and clinical status, the patient was transferred once again to a ward for further rehabilitation.
However, on the 28th postoperative day, the patient developed an episode of fever. Blood cultures were immediately taken and were positive for Enterococcus faecalis. The transoesophageal echocardiography (TEE) showed an accessory, floating structure (1.4 × 0.7 cm) on the atrial side of the posterior mitral leaflet, suggesting vegetation with moderate mitral valve regurgitation (Fig. 1). Treatment with i.v. antibiotics (14 days gentamycin 4 × 20 mg, 6 weeks ampicillin/sulbactam 4 × 3 g) was initiated. Two consecutive TEE showed a regression of the vegetation, and the patient was discharged 12 days later (on Day 60 after TAVI) with no signs of infective endocarditis confirmed by negative blood cultures. Medical treatment with mezlocillin 4 g/day was continued for another 2 weeks after hospital discharge.
Figure 1:
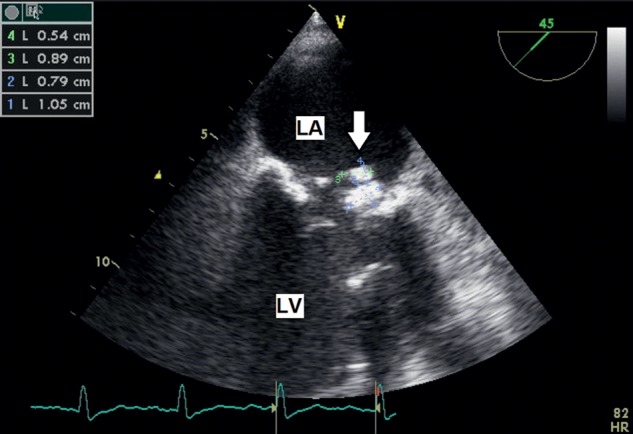
TEE, mid-oesophageal level. On the atrial surface of posterior mitral leaflet large vegetation (arrow). LA: left atrium; LV: left ventricle.
Two weeks after discharge, the patient was readmitted with symptoms of an acute congestive heart failure. The temperature was normal 36.8°C, WBC 6.7/µl, C-RP was slightly elevated at 2.4 mg/dl. TEE showed a recurrent large (18.9 mm) vegetation on the posterior leaflet of the mitral valve (Fig. 2).
Figure 2:
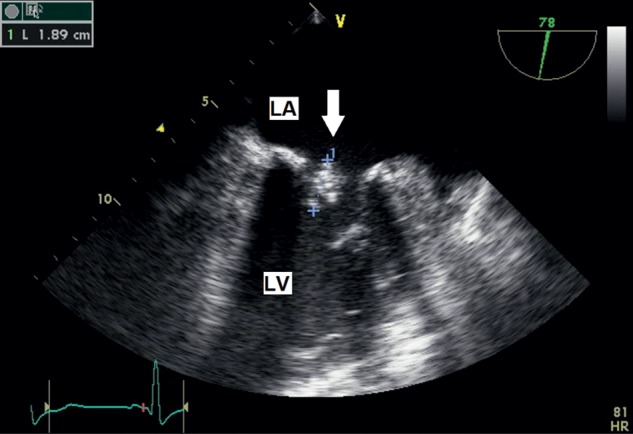
TEE, mid-oesophageal level. On the atrial surface of posterior mitral leaflet recurrent large vegetation (arrow). LA: left atrium; LV: left ventricle.
An emergency surgery was decided despite an excessively high risk (logistic EuroSCORE 47.88%). The patient reached two major and two minor Duke criteria for infective endocarditis. After a median sternotomy and the institution of a cardiopulmonary bypass, the heart was arrested with cold blood cardioplegia. The valve was exposed through a left atrial approach. The severely damaged mitral valve with large vegetations was excised and replaced with a pericardial tissue valve (25-mm SJM-Epic® prosthesis) with interrupted pledged sutures.
A postoperative antibiotic regimen included imipenem i.v. 4 × 500 mg for 1 month, vancomycin i.v. 4 × 500 mg for 2 weeks and gentamycin i.v. 4 × 20 mg for 2 weeks. In the postoperative course, the patient developed respiratory failure and required tracheotomy for prolonged ventilatory support.
The patient was transferred to a peripheral hospital on postoperative day 14 while still in respiratory weaning. At discharge, there were no signs of general infection, and three consecutive blood cultures had been negative. The postoperative TEE confirmed good function of the mitral valve prosthesis, the aortic valve prosthesis continued to show minimal central aortic regurgitation and small paravalvular leak, there were no signs of endocarditis.
DISCUSSION
The presented case depicts a good example of the decision-making process in which cardiac surgeons will be involved more often in the future: a patient who was initially evaluated as inoperable or at very high risk for open aortic valve replacement receiving a TAVI procedure suffering from a complication that ultimately requires open chest surgery. Possible examples include intraoperative problems such as valve dysfunction, high-grade paravalvular leak, annular rupture, pericardial tamponade with ventricle perforation, but also complications in the postoperative course such as prosthetic valve degeneration, or infective endocarditis of the prosthesis or other heart valves. In fact, patients chosen for TAVI are probably at a higher risk for any postoperative complication because of their comorbidities and advanced age [1]. Antibiotic prophylaxis is essential and strict sterile conditions during TAVI are crucial [2].
After conventional open aortic valve surgery, prosthetic valve infective endocarditis is known as a devastating complication occurring in 0.3–1% after the procedure [1] with in-hospital mortality reaching 30%. Mortality from native valve endocarditis approaches 30% at 1 year [3] and is even worse when caused by nosocomial infection reaching 50% in some series [4]. The most common micro-organisms responsible for infective endocarditis (IE) are coagulase-negative staphylococci (52%), Staphyloccocus aureus (10%), Enterococci (8%), Streptococcus viridans (5%), gram-negative organisms (6%), fungi (10%) for prosthetic valve endocarditis and S. viridans, S. aureus, Staphyloccocus epidermidis and Enteroccoci for native valve endocarditis [5].
The frequency of IE after TAVI is unknown, but can be expected to rise in the future because of the increasing number of patients treated.
CONCLUSION
In our case, IE occurred early after TAVI and was initially treated conservatively. Only after treatment failure, the operation was chosen as an ultima ratio solution. Until more reports and long-term studies are available, it will remain the joint responsibility of the Heart Team to individually tailor the treatment concept in these complex cases.
Conflict of interest: none declared.
REFERENCES
- 1.Wang A, Athan E, Pappas PA, Fowler VG, Jr, Olaison L, Paré C, et al. International Collaboration on Endocarditis-Prospective Cohort Study Investigators. Contemporary clinical profile and outcome of prosthetic valve endocarditis. J Am Med Assoc. 2007;297:1354–61. doi: 10.1001/jama.297.12.1354. [DOI] [PubMed] [Google Scholar]
- 2.Habib G, Hoen B, Tornos P, Thuny F, Prendergast B, Vilacosta I, et al. Guidelines on the prevention, diagnosis, and treatment of infective endocarditis (new version 2009): the task force on the prevention, diagnosis, and treatment of infective endocarditis of the European Society of Cardiology (ESC) Eur Heart J. 2009;30:2369–413. doi: 10.1093/eurheartj/ehp285. [DOI] [PubMed] [Google Scholar]
- 3.Prendergast BD. The changing face of infective endocarditis. Heart. 2006;92:879–85. doi: 10.1136/hrt.2005.067256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bouza E, Menasalvas A, Muñoz P, Vasallo FJ, del Mar Moreno M, García Fernández MA. Infective endocarditis–a prospective study at the end of the twentieth century: new predisposing conditions, new etiologic agents, and still a high mortality. Medicine (Baltimore) 2001;80:298–307. doi: 10.1097/00005792-200109000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Lytle BW. Prosthetic valve endocarditis. Thorac Cardiovasc Surg. 1995;7:1. [PubMed] [Google Scholar]


