Abstract
OBJECTIVES
Cirrhosis represents a serious risk in patients undergoing cardiac surgery. Several preoperative factors identify cirrhotic patients as high risk for cardiac surgery; however, a patient's preoperative status may be modified by surgical intervention and, as yet, no independent postoperative mortality risk factors have been identified in this setting. The objective of this study was to identify preoperative and postoperative mortality risk factors and the scores that are the best predictors of short-term risk.
METHODS
Fifty-eight consecutive cirrhotic patients requiring cardiac surgery between January 2004 and January 2009 were prospectively studied at our institution. Forty-two (72%) patients were operated on for valve replacement, 9 (16%) for a CABG and 7 (12%) for both (CABG and valve replacement). Thirty-four (58%) patients were classified as Child-Turcotte-Pugh class A, 21 (36%) as class B and 3 (5%) as class C. We evaluated the variables that are usually measured on admission and during the first 24 h of the postoperative period together with potential operative predictors of outcome, such as cardiac surgery scores (Parsonnet, EuroSCORE), liver scores (Child-Turcotte-Pugh, model for end-stage liver disease, United Kingdom end-stage liver disease score) and ICU scores (acute physiology and chronic health evaluation II and III, simplified acute physiology score II and III, sequential organ failure assessment).
RESULTS
Seven patients (12%) died in-hospital, of whom 5 were Child-Turcotte-Pugh class B and 2 class C. Comparing survivors vs non-survivors, univariate analysis revealed that variables associated with short-term outcome were international normalized ratio (1.5 ± 0.24 vs 2.2 ± 0.11, P < 0.0001), presurgery platelet count (171 ± 87 vs 113 ± 52 l nl−1, P = 0.031), presurgery haemoglobin count (11.8 ± 1.8 vs 10.2 ± 1.4 g dl−1, P = 0.021), total need for erythrocyte concentrates (2 ± 3.4 vs 8.5 ± 8 units, P < 0.0001), PaO2/FiO2 at 12 h after ICU admission (327 ± 84 vs 257 ± 78, P = 0.04), initial central venous pressure (11 ± 3 vs 16 ± 4 mmHg, P = 0.02) and arterial blood lactate concentration 24 h after admission (1.8 ± 0.5 vs 2.5 ± 1.3 mmol l−1, P = 0.019). Multivariate analysis identified initial central venous pressure as the only independent factor associated with short-term outcome (P = 0.027). The receiver operating characteristic curve showed that the model for end-stage Liver disease score had a better predictive value for short-term outcome than other scores (AUC: 90.5 ± 4.4%; sensitivity: 85.7%; specificity: 83.7%), although simplified acute physiology score III was acceptable.
CONCLUSIONS
We conclude that central venous pressure could be a valuable predictor of short-term outcome in patients with cirrhosis undergoing cardiac surgery. The model for end-stage liver disease score is the best predictor of cirrhotic patients who are at high risk for cardiac surgery. Sequential organ failure assessment and simplified acute physiology score III are also valuable predictors.
Keywords: Liver cirrhosis, Cardiac surgery, Short-term outcome, Mortality scores
INTRODUCTION
Liver cirrhosis (LC) is a major preoperative risk factor in general surgery, especially in cardiac surgery, and the outcome is strongly related to the severity of liver disease in those patients [1]. While in patients without advanced cirrhosis, cardiac surgery can be done safely, the risk of mortality is higher in patients with Child-Turcotte-Pugh (CTP) class B and C or with a model for end-stage liver disease score (MELD) >13 [1, 2]. Preoperative total plasma bilirubin, cholinesterase concentrations, the European system for cardiac operative risk evaluation (EuroSCORE), and the cardiopulmonary bypass (CPB) time have all been identified as potential predictors of mortality after cardiac surgery in those patients [3]. However, evidence comes mainly from several small studies; due to the lack of evidence from larger pools of data, postoperative risk factors remain unidentified.
At the same time, the option of liver transplantation as a treatment for patients with LC has produced an increase in survival rate and the evaluation of concomitant cardiac diseases, which increase post-liver transplantation complications, is crucial for preoperative risk assessment [4]. Thus, cardiac surgery is increasing in those patients awaiting liver transplantation.
Consequently, identifying independent cardiac surgery postoperative risk factors for these patients is an area of interest if we want to optimize post-surgical management and improve outcome, especially post-surgical short-term outcome. In this study, we also wanted to evaluate different score systems to identify the best predictors of mortality.
MATERIALS AND METHODS
This study is a prospective single-centre observational study performed between January 2004 and January 2009. Data were included from 58 patients of 2825 (2.05%) consecutive patients with LC who underwent cardiac surgery in our hospital. The study was approved by the Institutional Ethics Committee. All of the patients had previously granted permission for their medical records to be used for research purposes.
LC was confirmed either by a liver biopsy or by clinical, laboratory and radiographical findings showing impaired hepatic function and portal hypertension. The CTP classification score was calculated for each patient (CTP A: 7 points; CTP B: 8–10 points; CTP C >11 points); 58.6% (n = 34) were classified as class A, 36.2% (n = 21) as class B and 5.2% (n = 3) as class C.
We evaluated demographical data and comorbidities, treatment before surgery, bedside variables currently measured during the first 24 h of postoperative clinical care and complications/mortality during their admission. We calculated different prognosis scores for each patient: cardiac surgery scores (Parsonnet and EuroSCORE), liver scores (CTP, MELD and United Kingdom end-stage liver disease (UKELD)), ICU scores (sequential organ failure assessment (SOFA), acute physiology and chronic health evaluation (APACHE II and III) and simplified acute physiology score (SAPS II and III). Finally, survival of the different CTP groups was shown to allow a comparison with previous studies.
Cardiac surgical procedures were performed in all patients using median sternotomy, standard cardiopulmonary bypass (CPB) with moderate hypothermia (34°C) and antegrade cardioplegia. A mean aortic pressure of >60 mmHg was maintained during surgery. For revascularization, we used the internal thoracic artery (or bilateral if possible) and saphenous vein grafts. Bypass graft flow was assessed for each graft by Doppler transit time flowmetry. Protamine was administered to reverse heparin according to standard practice. For CABG surgery, aspirin was routinely administered within the first 6 h after surgery following local protocol.
Statistical analysis
Statistical analysis was carried out using PASW statistics 13.0 (SPSS, Inc., Chicago, IL, USA). Data are expressed as mean ± standard deviation. We analyzed differences in data between survivors and non-survivors. For the comparisons between the two groups, the Mann–Whitney U-test was used or, when appropriate (after applying the one-sample Kolmogorov–Smirnov test), the two-sample t-test was used. The χ² test was used to evaluate categorical prognostic factors. A multivariate analysis was carried out using Cox regression model to show independent risk mortality factors for short-term outcome. Finally, the survival analysis of the CTP group was carried out with the Kaplan–Meier estimator for comparison with previous studies. Receiver operating characteristic (ROC) curve analyses were applied to determine optimal cut-off values of the different scores for short-term outcome and to further evaluate the predictive power between them, considering the differences of the areas under the empirical ROC curves. A P-value of 0.05 was considered statistically significant in all cases.
RESULTS
Forty-one patients (70.7%) were operated on for valve replacement, 10 (17.2%) for CABG, 6 of them off-pump, and 7 (12.1%) were both CABG and valve replacement. Only 3 patients underwent urgent surgery for CABG and there were no mortalities. All valve replacement operations were isolated: 34 (70.83%) were mitral valve and 14 (29.17%) aortic. None of the patients had previously undergone cardiac surgery.
Aetiologies for LC were predominantly infective hepatitis in 37.9% (hepatitis C, 31% (n = 18); hepatitis B, 6.9% (n = 4)), alcohol-induced in 34.48% (n = 20) and both hepatitis C and alcohol-induced in 13.8% (n = 8). The other were cryptogenic cirrhosis/others (13.8% (n = 8)) and in 10 patients, it was because of hepatocellular carcinoma.
The preoperative characteristics of the patients, including treatment before surgery, presented differences between groups in platelet and haemoglobin counts (see Table 1). Three patients were admitted previously at the cardiology department for acute myocardial infarction and underwent urgent cardiac surgery during the same admission. None of them died and their postoperative course did not differ from the other patients. Six patients (10.3%) were treated with aspirin before going into theatre. None of them died and there was no significant increase in terms of postoperative bleeding or the requirement for blood products. Despite there being a considerable prevalence of preoperative risk factors in these patients in terms of LC complications due to end-stage liver disease, there was no significant difference between survivors and non-survivors.
Table 1:
Demographics and baseline data
| All patients (n = 58) | Survivors (n = 51) | Non-survivors (n = 7) | P | |
|---|---|---|---|---|
| Sex (male) | 69% (40) | 70.6% (36) | 57.1% (4) | 0.66 |
| Age (years) | 64.9 ± 11.6 | 64.6 ± 9.6 | 66.9 ± 10.3 | 0.92 |
| Body mass index (kg m−2) | 27 ± 4.2 | 27.6 ± 4.6 | 26.6 ± 4.2 | 0.54 |
| Hypertension | 56.9% (33) | 54.9% (28) | 71.4% (5) | 0.68 |
| Diabetes mellitus | 32.8% (19) | 33.3% (17) | 28.6% (2) | 0.99 |
| Dyslipidaemia | 34.5% (20) | 33.3% (17) | 42.9% (3) | 0.68 |
| Chronic renal insufficiency | 8.6% (5) | 7.8% (4) | 14.3% (1) | 0.12 |
| Renal failure (on dialysis) | 19% (11) | 19.60% (10) | 14.3% (1) | 0.60 |
| Creatinine before surgery (mmol l−1) | 114.4 ± 100.8 | 106.4 ± 93.7 | 170.3 ± 136.3 | 0.15 |
| Previous stroke | 12.1% (7) | 12.1% (7) | 0% | 0.23 |
| Chronic obstructive pulmonary disease | 17.2% (10) | 17.6% (9) | 14.3% (1) | 0.85 |
| Active smokers | 19% (11) | 19.6% (10) | 14.3% (1) | 0.64 |
| Active alcohol consumption | 3.4% (2) | 3.9% (2) | 0% | 0.84 |
| Previous atrial fibrillation | 31% (18) | 33.3% (17) | 14.3% (1) | 0.78 |
| Previous myocardial infarction | 12.1% (7) | 11.8% (6) | 14.3% (1) | 0.53 |
| NYHA class III-IV | 34.5% (20) | 35.3% (18) | 28.6% (2) | 0.58 |
| On B-blockers | 39.7% (23) | 41.2% (21) | 28.6% (2) | 0.69 |
| On statins | 25.9% (15) | 25.50% (13) | 28.6% (2) | 0.92 |
| Ascites (moderate to severe) | 69% (40) | 70.6% (36) | 57.1% (4) | 0.45 |
| Oesophageal varices | 31% (18) | 25.5% (13) | 71.4% (5) | 0.26 |
| Variceal bleeding | 17.2% (10) | 17.6% (9) | 14.3% (1) | 0.14 |
| Encephalopathy | 34.5% (20) | 33.3%(17) | 42.9% (3) | 0.32 |
| Hypertrophic cardiomyopathy | 31% (18) | 31.4% (16) | 28.6% (2) | 0.68 |
| Dilated cardiomyopathy | 27.6% (16) | 27.5% (14) | 28.6% (2) | 0.91 |
| Left ventricular ejection fraction (%) | 60.3 ± 11.2 | 59.3 ± 11.7 | 62.6 ± 10.1 | 0.71 |
| Pulmonary arterial pressure (mmHg) | 48.7 ± 15.4 | 48.6 ± 15.6 | 49.4 ± 14.7 | 0.58 |
| Haemoglobin before surgery (g dl−1) | 11.67 ± 1.82 | 11.8 ± 1.8 | 10.2 ± 1.05 | 0.02 |
| Platelet count before surgery (1 nl−1) | 164 ± 85 | 171 ± 87 | 113 ± 52 | 0.03 |
| International normalized ratio before surgery | 1.5 ± 0.83 | 1.45 ± 0.15 | 1.85 ± 0.76 | 0.18 |
NYHA: New York Heart Association classification. Results are expressed as mean ± standard deviation or percentage.
There were no differences in intraoperative data, such as CPB time and aortic cross-clamping (ACC), between groups (see Table 2). Differences in postoperative data were observed for arterial oxygen pressure of O2 and the fraction of inspired oxygen ratio (PaO2/FiO2), which was higher in survivors, while central venous pressure (CVP) on admission and 24 h after admission and arterial lactate (AL) 24 h after admission were all lower in survivors. With regard to postoperative morbidities, patients who died required a large amount of erythrocyte concentrates during admission, but there were no differences in terms of post-surgical bleeding. They also required a longer period on mechanical ventilation, and had a greater need for renal replacement therapies (RRT) and an increased the need for vasopressors.
Table 2:
Intraoperative and postoperative data
| All patients (n = 58) | Survivors (n = 51) | Non-survivors (n = 7) | P | |
|---|---|---|---|---|
| Intraoperative data | ||||
| Isolated CABG | 15.5% (9) | 15.7% (8) | 14.3% (1) | 0.95 |
| Isolated valve surgery | 72.4% (42) | 72.50% (37) | 71.4% (5) | 0.97 |
| CABG + valve surgery | 12.1% (7) | 11.76% (6) | 14.3% (1) | 0.78 |
| Fluid balance during surgery (ml) | 1325 ± 850 | 1250 ± 980 | 1350 ± 785 | 0.58 |
| Aortic cross-clamping time (min) | 72 ± 44 | 74 ± 41 | 69 ± 50 | 0.85 |
| Cardiopulmonary bypass time (min) | 107 ± 37 | 106 ± 48 | 108 ± 53 | 0.35 |
| Postoperative data and major postoperative complications | ||||
| Ventilation time (days) | 5.3 ± 10.2 | 3.16 ± 7.7 | 21 ± 12 | 0.01 |
| PaO2/FiO2 on admission | 287 ± 95 | 293 ± 93 | 245 ± 110 | 0.28 |
| PaO2/FiO2 12 h after admission | 318 ± 86 | 327 ± 84 | 257 ± 78 | 0.04 |
| PaO2/FiO2 24 h after admission | 307 ± 75 | 315 ± 70 | 253 ± 96 | 0.23 |
| MAP on admission (mmHg) | 83 ± 15 | 85 ± 15 | 74 ± 18 | 0.72 |
| MAP 24 h after admission (mmHg) | 80 ± 10 | 80 ± 9 | 75 ± 11 | 0.51 |
| CVP on admission (mmHg) | 12 ± 3.6 | 11.4 ± 3 | 16.5 ± 4.4 | 0.02 |
| CVP 24 h after admission (mmHg) | 12.5 ± 3.6 | 12 ± 2.8 | 16.3 ± 6 | 0.002 |
| Need of vasoactive drugs (h) | 165 ± 197 | 112 ± 109 | 490 ± 304 | 0.016 |
| Low cardiac output syndrome | 31% (18) | 34% (17) | 14.3% (1) | 0.25 |
| Perioperative myocardial infarction | 7.1% (4) | 6.1% (3) | 14.3% (1) | 0.18 |
| Arterial lactate on admission (mmol l−1) | 2.6 ± 1.4 | 2.45 ± 1.3 | 3.6 ± 1.5 | 0.22 |
| Arterial lactate 24 h after admission (mmol l−1) | 1.9 ± 0.7 | 1.8 ± 0.5 | 2.5 ± 1.3 | 0.02 |
| Creatinine 24 h after surgery (mmol l−1) | 129 ± 108 | 118 ± 101 | 207 ± 138 | 0.15 |
| Urine output first 24 h (ml) | 1860 ± 650 | 1920 ± 570 | 1444 ± 1066 | 0.28 |
| Need for renal replacement therapy | 8.9% (5) | 2% (1) | 57.1% (4) | <0.0001 |
| Albumin (g l−1) | 27 ± 4 | 27.9 ± 4 | 27.8 ± 4.5 | 0.97 |
| International normalized ratio on admission | 1.8 ± 0.32 | 1.5 ± 0.24 | 2.2 ± 0.11 | <0.0001 |
| Drainage loss first 12 h (ml) | 464 ± 308 | 446 ± 299 | 595 ± 369 | 0.34 |
| Major bleeding | 1.7% (1) | 2% (1) | 0% | 0.85 |
| Re-exploration | 19% (11) | 21.6% (11) | 0% | 0.15 |
| Erythrocyte concentrates (units) | 3 ± 4.6 | 2 ± 30.4 | 8.5 ± 8 | <0.0001 |
CABG: coronary artery bypass graft; PaO2/FiO2: arterial partial pressure of O2 and fraction of inspired oxygen ratio; MAP: mean arterial pressure; CVP: central venous pressure. Results are expressed as mean ± standard deviation or percentage.
The median ICU stay was 9 ± 10 days, with a difference between groups (7.7 ± 1 in the survival group vs 13 ± 5 in the non-survival group, P = 0.002). However, the median hospital stay was 34 ± 20 days, and there were no differences between groups (21 ± 3 vs 14.8 ± 5.6 days).
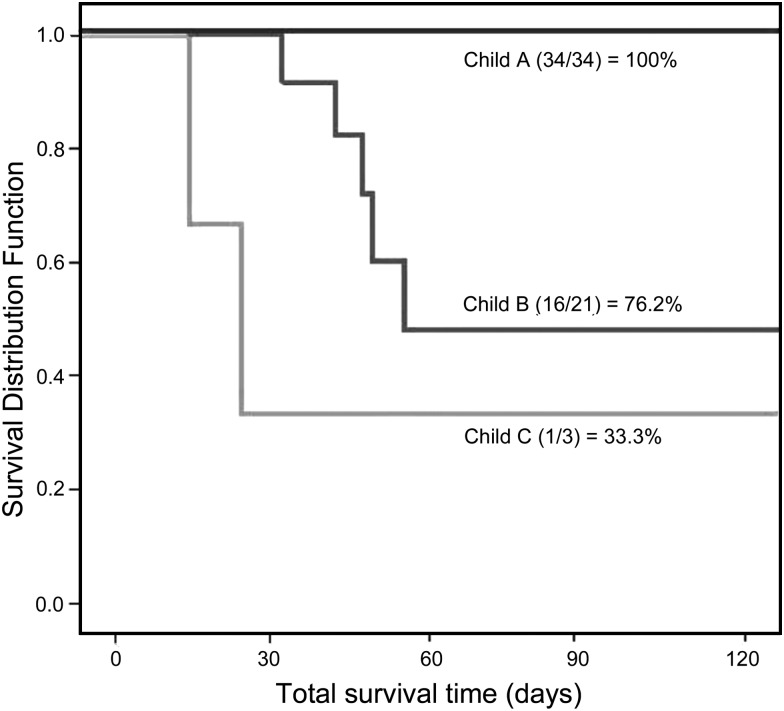
Mortality was 12.1% (n = 7); 5 patients were CTP class B and 2 class C. The class C died of multi-systemic organ failure (MSOF), and the class B MSOF (3 patients) and septic shock (2). Short-term survival evaluated by Kaplan–Meier in Fig. 1 showed differences between CTP class groups (log-rank test, P = 0.035). Some scores revealed significant differences between groups: only SAPS II and III and SOFA showed a significant predictive power similar to that of UKELD and CTP. However, the other ICU scores and cardiac surgery scores were not as useful (Table 3). In order to compare differences between potential preoperative (liver and cardiac surgery scores) and postoperative (ICU scores) predictions, predictors of outcome for short-term survival were analysed using the ROC curve. The MELD score was the most predictive for in-hospital mortality. The optimal cut-off level for the MELD score was 18.5, with a sensitivity of 85.7% and a specificity of 83.7% (Fig. 2).
Figure 1:
Short-term survival rate according to Child-Turcotte-Pugh score.
Table 3:
Evaluation scores for risk assessment
| All patients (n = 58) | Survivors (n = 51) | Non-survivors (n = 7) | P | |
|---|---|---|---|---|
| SAPS II | 25.2 ± 10.4 | 24 ± 9.4 | 33.7 ± 14 | 0.02 |
| SAPS III | 45.9 ± 10.8 | 44.7 ± 10.4 | 54.7 ± 10.4 | 0.045 |
| APACHE II | 13.9 ± 4.4 | 13.5 ± 4.1 | 16.8 ± 6 | 0.19 |
| APACHE III | 56.6 ± 18 | 55.2 ± 17.7 | 66.7 ± 19 | 0.17 |
| SOFA | 5.41 ± 2.72 | 6.6 ± 2.7 | 9.4 ± 1.8 | 0.005 |
| EuroSCORE | 6.48 ± 3 | 6.2 ± 2.9 | 8.8 ± 3.7 | 0.12 |
| Parsonnet score | 9.43 ± 6.42 | 9.2 ± 6.4 | 11.4 ± 6.8 | 0.43 |
| MELD | 16 ± 5.4 | 15 ± 4.57 | 23 ± 5.4 | 0.005 |
| UKELD | 49.8 ± 4 | 49.6 ± 4 | 52.6 ± 3.3 | 0.044 |
| CTP class A | 58.6% (n = 34) | 66.7% (n = 34) | 0% | <0.0001 |
| CTP class B | 36.2% (n = 21) | 31.4% (n = 16) | 71.4% (n = 5) | <0.0001 |
| CTP class C | 5.2% (n = 3) | 2% (n = 1) | 28.6% (n = 2) | 0.045 |
SAPS: simplified acute physiology score; APACHE: acute physiology and chronic health evaluation; SOFA: sequential organ failure assessment; EuroSCORE: European system for cardiac operative risk evaluation; MELD: model for end-stage liver disease score; UKELD: United Kingdom end-stage liver disease; CTP: Child-Turcotte-Pugh. Results are expressed as mean ± standard deviation or percentage.
Figure 2:
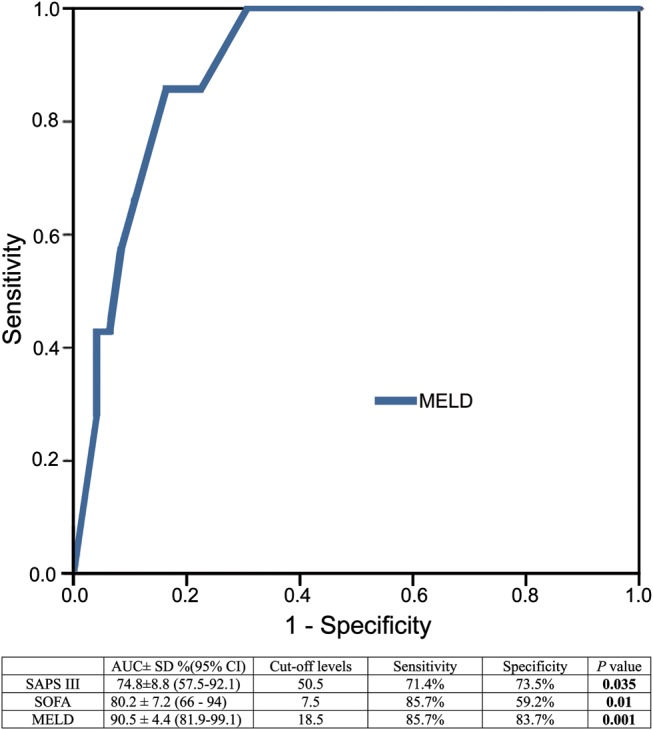
ROC curve for MELD. Comparison of AUC for MELD, SAPS III and SOFA scores. AUC: area under curve; ROC: receiver operating characteristic curve; SAPS: simplified acute physiology score; SOFA: sequential organ failure assessment; MELD: model for end-stage liver disease score; NS: non-statistically significant. Results are expressed as mean ± standard deviation or percentage.
To evaluate preoperative and postoperative predictors of death for all patients, a multivariate analysis was conducted (See Table 4). We included those univariate factors that showed significant differences between groups in a Cox regression model. After risk adjustment, the multivariate analysis revealed initial CVP as the only independent factor associated with short-term outcome.
Table 4:
Multivariate analysis-dependent variable deceased during admission
| Hazards ratio (95% CI) | P | |
|---|---|---|
| Age | 0.99 (0.94–1.036) | 0.69 |
| Platelets before surgery | 0.96 (0.79–1.164) | 0.68 |
| Haemoglobin before surgery | 1.13 (0.65–1.97) | 0.66 |
| INR after surgery | 0.65 (0.17–2.51) | 0.53 |
| CVP on admission | 0.88 (0.78–0.98) | 0.027 |
| SOFA score | 1.02 (0.86–1.195) | 0.82 |
| AL 24 h after admission | 0.81 (0.60–1.094) | 0.17 |
| PaO2/FiO2 12 h | 1.00 (0.99–1.004) | 0.91 |
| MELD score | 0.96 (0.87–1.068) | 0.48 |
PaO2/FiO2: arterial partial pressure of O2 and fraction of inspired oxygen ratio; AL: arterial lactate; INR: international normalized ratio; CVP: central venous pressure; SOFA: sequential organ failure assessment; MELD: model for end-stage liver disease score.
DISCUSSION
The most important finding of the current study was that in terms of predicting short-term mortality, both the CVP and the SAPS III and SOFA postoperative scores proved effective. We also confirm that the MELD score is the most effective predictor for the short-term outcome of these patients and that the CTP is a valuable score.
In view of the complexity of the procedure, the postoperative morbidity and mortality rates reported in the literature are considerably higher for cirrhotic patients undergoing cardiac surgery. [1]. The mortality risk in CTP class B patients is around 32.2% and increases to 66.6% in CTP class C patients [2]; even when there is a minimal degree of impaired liver function in combination with elective surgery, the incidence of complications significantly increases [5]. Careful patient selection is critical to improve surgical outcome in patients with cirrhosis [6]; however, there is a lack of factors that can be used to identify the mortality risk in those patients, especially after surgery. The lower incidence of comorbidities, the low number of urgent procedures and the low mortality rate found highlight the importance of our aim to select and prepare those patients for surgery carefully. Despite the differences in haemoglobin and platelets, the groups of survivors and non-survivors were comparable in almost all presurgery risk factors except the grade of liver disease. The major need for erythrocyte concentrates and RRT needs in non-survivors can be explained by initial presurgical lower haemoglobin, post-surgical INR differences and larger ICU admission and presence of MSOF as a cause of mortality, respectively. In any case, the risk of mortality increases with the deterioration of liver function [1–6].
In this scenario, INR progressively worsens during cirrhosis, also reflecting the current status of end-stage liver disease [7]. The replenishment of vitamin K-dependent factors beyond a normal INR has not proven its efficacy; however, individualized heparin and protamine dosing, antifibrinolytic drug administration, minimization of blood loss and dilution, and minimal CPB time could still potentially help achieve surgical homeostasis [8]. All these efforts are reflected in our results, in that drainage loss was similar between the groups despite postoperative INR differences.
Hyperlactataemia in the ICU, which is caused mainly by shock, is associated with increased mortality and is more frequent when respiratory and/or renal failures are/is present [9]. It predicts postoperative mortality after cardiac surgery with a maximum lactate threshold of ≥4.4 mmol l−1 in the first 10 h after operation [10]. Arterial lactate tends to be higher in non-survivors, though it could be a reflection of a presurgery poorer liver function or an exacerbation of liver dysfunction in the setting of CPB.
Arterial partial pressure of O2 and fraction of inspired oxygen ratio (PaO2/FiO2) is a new marker for outcome in some types of cardiac surgery [11]. Hypoxaemia depicted by low PaO2/FiO2 is common after CPB, and is associated with different variables, which are preoperative factors (age, obesity, chest X-ray with alveolar oedema 1 h after surgery, decreased baseline PaO2/FiO2, previous myocardial infarction), operative factors (emergency surgery, prolonged CPB) and postoperative factors (low cardiac output syndrome (LCOS), renal failure, persistent hypothermia 2–6 h after surgery, requirement for re-exploration). A lower PaO2/FiO2 ratio correlated significantly with the time required to carry out extubation and also to lung injury. However, in these patients, it had minimal effect on the postoperative clinical course [12]. Although PaO2/FiO2 12 h after admission was lower in non-survivors, it did not have an independent significant impact on the outcome of surgery.
Central venous pressure (CVP) is used almost universally to guide fluid therapy in hospitalized patients. Some authors argue that there is a very poor relationship between CVP and blood volume as well as the inability to predict the haemodynamic response to a fluid challenge, being a good indicator of blood volume only at the extreme values [13]. Nevertheless, the conditions that influence CVP are well known, and as such, CVP remains a useful tool for evaluating haemodynamic status if it is performed under controlled conditions. CVP has the great advantage of being able to be measured at the patient's bedside without the need of invasive methods [14]. Dynamic evaluation of CVP could be a reliable predictor of fluid responsiveness in patients under mechanical ventilation, similar to the variation of arterial pulse pressure after cardiac surgery [15]. The proper use of CVP requires a good understanding of the waveform because higher values and CVP tracing are concordant with rhythm disorders, tricuspid regurgitation, cardiac tamponade, cardiac restriction and decreased thoracic compliance [16]. Limitations of CVP as a surrogate variable of preload are caused by the influence of intrathoracic and intra-abdominal pressures. However, these limitations do not impair the importance of CVP as the downstream pressure of the systemic venous system [15, 16]. We found CVP on admission to be the only independent factor for short-term outcome in the multivariate analysis. We hypothesize that CVP could be a surrogate marker of underscored right ventricular failure, which can ultimately explain the higher mortality, but we cannot confirm our suspicions [17]. However, non-survivors did not receive larger amount of fluids in the operating theatre and did not have higher incidences of low cardiac output syndrome, which could have biased the CVP measurement.
Although EuroSCORE is widely accepted in Europe as a valuable score in cardiac surgery, in some populations, it does not have acceptable discriminatory ability. The development of local mortality risk scores corresponding to local epidemiological characteristics may improve the prediction of outcome [18]. In addition, it does not take into account surgical prognosis factors such as CPB time, and there is a lack of postoperative factors to determine short-term mortality [19]. Furthermore, the Parsonnet score does not consider specific liver variables. However, some authors suggest that it can be used to predict 3-month mortality, prolonged length of stay and specific postoperative complications such as renal failure, sepsis and respiratory failure in the whole context of cardiac surgery [20]. Because mortality in cirrhotic patients undergoing cardiac surgery is associated with liver function, liver scores such as the MELD or CTP score are associated with outcome [1–3]. Our results confirm that the MELD score most reliably identifies cirrhotic patients at high risk for cardiac surgery, with better results than in previous studies [1]. In our study, the MELD values are higher than in previous studies, which is likely due to the high number of patients awaiting liver transplantations. With regard to CTP class scores, mortality was higher in postoperative cardiac surgery in patients with a CTP score of class C [1–3, 6]. With a lack of a large data series in previous research or a significant number of CTP class C patients described in the literature, there is no basis for comparison. The UKELD score can be used as a local score for end-stage liver disease, but unlike the MELD, it has never been evaluated in cardiac surgery. It evaluates sodium as well as INR, creatinine and bilirubin, identifying cirrhotic patients with the poorest quality of life and the highest complication rates [21]. The results for UKELD were statistically significant in the univariate analysis, though the ROC analysis raised doubts about its clinical relevance. ICU scores such as SOFA have been previously evaluated in cardiac surgery for the same purpose [22]. We also evaluated other ICU scores such as SAPS and APACHE. SAPS scores provided an estimate of the risk of death without having to specify a primary diagnosis, including liver failure and cardiac insufficiency grade [23]. Furthermore, higher SAPS scores have been associated with a poor quality of life, with the worst outcome occurring both before and after general surgery [24]; additionally, a higher mortality rate was found in elderly patients (>70 years) who required dialysis after cardiac surgery [25]. In our series, SAPS III provided an acceptable level of sensitivity and specificity, comparable with MELD results of other series [1]. APACHE scores were not found to be valuable tools.
Our study presents certain limitations. The most important are that it was a single-centre observational study. Results should be viewed cautiously due to the low number of patients and events. However, we have shown a larger number of patients than any other study of this kind to date, and observed a low mortality rate despite the level of end-stage liver disease.
We conclude that cardiac surgery can be performed safely in CTP class A and in some class B patients. Regarding CTP class C patients, due to the higher mortality in these patients, we think that liver function should be optimized prior to cardiac surgery, perhaps even performing liver transplantation. Indeed, synchronous surgery has modestly improved survival in some patients with cirrhosis when cardiac surgery is needed [4]. We recommend proper preoperative selection of patients and apply careful operative and postoperative management, especially in terms of fluid balance, in order to increase the short-term survival rate. A higher CVP at ICU admission may make physicians aware of a patient's prognosis, but its efficacy as a valuable predictor of short-term outcome must be shown in future studies. MELD score and postoperative ICU scores such as SAPS III and SOFA can be used to predict short-term outcome in those patients. In our opinion, in the setting of end-stage liver disease and cardiac surgery, postoperative evaluation is as important as preoperative evaluation in terms of predicting short-term outcome.
Acknowledgements
We thank Toral and Ortiz of the Department of Cardiothoracic Surgery who both supported and corrected this manuscript, especially regarding surgical issues and also the ICU nurses who have contributed with their efforts in the care of the patients reported in this manuscript.
Conflict of interest: none declared.
REFERENCES
- 1.Thielmann M, Mechmet A, Neuhäuser M, Wendt D, Tossios P, Canbay A, et al. Risk prediction and outcomes in patients with liver cirrhosis undergoing open-heart surgery. Eur J Cardiothorac Surg. 2010;38:592–9. doi: 10.1016/j.ejcts.2010.02.042. [DOI] [PubMed] [Google Scholar]
- 2.Modi A, Vohra HA, Barlow CW. Do patients with liver cirrhosis undergoing cardiac surgery have acceptable outcomes? Interact CardioVasc Thorac Surg. 2010;11:630–4. doi: 10.1510/icvts.2010.241190. [DOI] [PubMed] [Google Scholar]
- 3.An Y, Xiao YB, Zhong QJ. Open-heart surgery in patients with liver cirrhosis: indications, risk factors, and clinical outcomes. Eur Surg Res. 2007;39:67–74. doi: 10.1159/000099145. [DOI] [PubMed] [Google Scholar]
- 4.Lima B, Nowicki ER, Miller CM, Hashimoto K, Smedira NG, Gonzalez-Stawinski GV. Outcomes of liver transplantation and elective cardiac surgical procedures. Ann Thorac Surg. 2011;92:1580–4. doi: 10.1016/j.athoracsur.2011.06.056. [DOI] [PubMed] [Google Scholar]
- 5.Bizouarn P, Ausseur A, Desseigne P, Le Teurnier Y, Nougarede B, Train M, et al. Early and late outcome after elective cardiac surgery in patients with cirrhosis. Ann Thorac Surg. 1999;67:1334–8. doi: 10.1016/s0003-4975(99)00226-x. [DOI] [PubMed] [Google Scholar]
- 6.Filsoufi F, Salzberg SP, Rahmanian PB, Schiano TD, Elsiesy H, Squire A, et al. Early and late outcome of cardiac surgery in patients with liver cirrhosis. Liver Transpl. 2007;13:990–5. doi: 10.1002/lt.21075. [DOI] [PubMed] [Google Scholar]
- 7.Hayashida N, Aoyagi S. Cardiac operations in cirrhotic patients. Ann Thorac Cardiovasc Surg. 2004;10:140–7. [PubMed] [Google Scholar]
- 8.Milas BL, Jobes DR, Gorman RC. Management of bleeding and coagulopathy after heart surgery. Semin Thorac Cardiovasc Surg. 2000;12:326–36. doi: 10.1053/stcs.2000.20511. [DOI] [PubMed] [Google Scholar]
- 9.Juneja D, Singh O, Dang R. Admission hyperlactatemia: causes, incidence, and impact on outcome of patients admitted in a general medical intensive care unit. J Crit Care. 2011;26:316–20. doi: 10.1016/j.jcrc.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 10.Kogan A, Preisman S, Bar A, Sternik L, Lavee J, Malachy A, et al. The impact of hyperlactatemia on postoperative outcome after adult cardiac surgery. J Anesth. 2012;26:174–8. doi: 10.1007/s00540-011-1287-0. [DOI] [PubMed] [Google Scholar]
- 11.Kurabayashi M, Okishige K, Azegami K, Ueshima D, Sugiyama K, Shimura T, et al. Reduction of the PaO2/FiO2 ratio in acute aortic dissection—relationship between the extent of dissection and inflammation. Circ J. 2010;74:2066–73. doi: 10.1253/circj.cj-10-0336. [DOI] [PubMed] [Google Scholar]
- 12.Weiss YG, Merin G, Koganov E, Ribo A, Oppenheim-Eden A, Medalion B, et al. Postcardiopulmonary bypass hypoxemia: a prospective study on incidence, risk factors, and clinical significance. J Cardiothorac Vasc Anesth. 2000;14:506–13. doi: 10.1053/jcan.2000.9488. [DOI] [PubMed] [Google Scholar]
- 13.Marik PE, Baram M, Vahid B. Does central venous pressure predict fluid responsiveness? A systematic review of the literature and the tale of seven mares. Chest. 2008;134:172–8. doi: 10.1378/chest.07-2331. [DOI] [PubMed] [Google Scholar]
- 14.Gelman S. Venous function and central venous pressure. Anesthesiology. 2008;108:735–48. doi: 10.1097/ALN.0b013e3181672607. [DOI] [PubMed] [Google Scholar]
- 15.Westphal GA, Silva E, Caldeira Filho M, Roman Gonçalves AR, Poli-de-Figueiredo LF. Variation in amplitude of central venous pressure curve induced by respiration is a useful tool to reveal fluid responsiveness in postcardiac surgery patients. Shock. 2006;26:140–5. doi: 10.1097/01.shk.0000227439.76418.7d. [DOI] [PubMed] [Google Scholar]
- 16.Magder S. Central venous pressure monitoring. Curr Opin Crit Care. 2006;12:219–27. doi: 10.1097/01.ccx.0000224866.01453.43. [DOI] [PubMed] [Google Scholar]
- 17.Richter HP, Petersen C, Goetz AE, Reuter DA, Kubitz JC. Detection of right ventricular insufficiency and guidance of volume therapy are facilitated by simultaneous monitoring of static and functional preload parameters. J Cardiothorac Vasc Anesth. 2011;25:1051–5. doi: 10.1053/j.jvca.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 18.Sadeghi MM, Arasteh M, Gharipour M, Nilfroush P, Shamsolketabi H, Etesampour A, et al. Evaluation of accuracy of Euroscore risk model in prediction of perioperative mortality after coronary bypass graft surgery in Isfahan. J Res Med Sci. 2011;16:787–92. [PMC free article] [PubMed] [Google Scholar]
- 19.Roques F, Nashef SA, Michel P, Gauducheau E, de Vincentiis C, Baudet E, et al. Risk factors and outcome in European cardiac surgery: analysis of the EuroSCORE multinational database of 19030 patients. Eur J Cardiothorac Surg. 1999;15:816–22. doi: 10.1016/s1010-7940(99)00106-2. [DOI] [PubMed] [Google Scholar]
- 20.Toumpoulis IK, Anagnostopoulos CE, Swistel DG, DeRose JJ., Jr Does EuroSCORE predict length of stay and specific postoperative complications after cardiac surgery? Eur J Cardiothorac Surg. 2005;27:128–33. doi: 10.1016/j.ejcts.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 21.Doerr F, Badreldin AM, Heldwein MB, Bossert T, Richter M, Lehmann T, et al. A comparative study of four intensive care outcome prediction models in cardiac surgery patients. J Cardiothorac Surg. 2011;6:21. doi: 10.1186/1749-8090-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Neuberger J, Gimson A, Davies M, Akyol M, O'Grady J, Burroughs A, et al. Selection of patients for liver transplantation and allocation of donated livers in the UK. Gut. 2008;57:252–7. doi: 10.1136/gut.2007.131730. [DOI] [PubMed] [Google Scholar]
- 23.Le Gall JR, Lemeshow S, Saulnier F. A new simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA. 1993;270:2957–63. doi: 10.1001/jama.270.24.2957. [DOI] [PubMed] [Google Scholar]
- 24.Abelha FJ, Santos CC, Barros H. Quality of life before surgical ICU admission. BMC Surg. 2007;7:23. doi: 10.1186/1471-2482-7-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van Den Noortgate N, Mouton V, Lamot C, Van Nooten G, Dhondt A, Vanholder R, et al. Outcome in a post-cardiac surgery population with acute renal failure requiring dialysis: does age make a difference? Nephrol Dial Transplant. 2003;18:732–6. doi: 10.1093/ndt/gfg043. [DOI] [PubMed] [Google Scholar]