Abstract
OBJECTIVES
The incidence of recurrent aortic arch obstruction after Norwood procedure and other types of aortic arch reconstruction in newborns remains high. Biological and synthetic materials are used to enlarge the aorta. We report our experience using autologous pericardium to reconstruct the aortic arch in patients with hypoplastic left heart syndrome, aortic arch interruption and hypoplastic aortic arch.
METHODS
A retrospective analysis of 39 consecutively operated patients evaluated after an initial Norwood and other types aortic arch repair was performed. The presence of recurrent arch obstruction (mean gradient ≥20 mmHg) and its management were noted. The mean weight of our patients was 3.2 ± 0.7 kg.
RESULTS
The mean age at primary surgical correction was 7.4 ± 6.8 (range 1–35 days). All patients were discharged without a significant residual gradient at the aortic arch except 4 who had a peak gradient of ≥30 mmHg. The overall incidence of recurrent arch obstruction was 28.2% (11 patients). Four (12.1%) patients had a distal obstruction, 1 (3%) had proximal obstruction and 1 had a mid-transverse arch obstruction. All patients underwent aortic arch reintervention consisting of balloon dilatation, and only after unsuccessful dilatation, 3 underwent surgical patch aortoplasties.
CONCLUSIONS
The use of autologous pericardium in aortic arch reconstruction procedure is effective and associated with an acceptable incidence of recurrent arch obstruction. Its availability and characteristics make it an attractive alternative to other materials.
Keywords: Congenital heart disease, Aortic arch plasty, Autologous pericardium, Hypoplastic left heart syndrome, Aortic interruption, Hypoplastic aortic arch
INTRODUCTION
The reconstruction of the aortic arch has been performed by several [1–3] groups of authors without the use of any prosthetic material. Despite the techniques of surgical reconstruction, the aortic arch underwent multiple modifications, and the incidence of reintervention remains the same [2–4]. The materials used to enlarge the aorta could range from animal-derived tissue [4] to extracellular matrix-based scaffolds, which are cellularized by autologous progenitors and eventually digested [5]. Although these solutions are fascinating and have the advantage of being ‘off-the-shelf’, there are many drawbacks, such as the limited application range and the high costs. An autologous pericardial patch is a ready-to-use and abundant resource directly available in the surgical field that can be tailored according to the pathology and surgeons’ choice. Notwithstanding, there is scarce literature on autologous patch aortoplasty and its use for reconstructive techniques. We report our experience on aortic arch reconstruction with the autologous pericardial patch.
MATERIALS AND METHODS
Patients' characteristics
The study was approved by the ethical committee of our institution. From January 2006 to January 2010, 86 patients underwent aortic arch reconstruction; of those, 39 had repair with the autologous pericardial patch. Patients' characteristics are outlined in Table 1. The mean age was 7.41 ± 6.82 days, with an average weight of 3.22 ± 0.7 kg at the time of intervention. Fourteen (35%) patients had prenatal diagnosis of congenital heart disease (CHD). Modality of birth was natural delivery in 30 (76.9%) patients and caesarean section in 9 (23.1%). Eighteen (46.1%) patients were diagnosed with hypoplastic left heart syndrome (HLHS), 17 (43.6%) had hypoplastic aortic arch (HAA) and 4 (10.2%) had aortic arch interruption (AAI).
Table 1:
Descriptive statistics by diagnostic groups
| N | AAI (N = 4) (a, b, c) | HLHS (N = 18) (a, b, c) | HAA (N = 17) (a, b, c) | Test statistic (P-value) | |
|---|---|---|---|---|---|
| Months to reintervention (months) | 11 | 2.9, 2.9, 2.9 | 2.8, 4.7, 5.4 | 4.8, 6.1, 8.1 | 0.237* |
| Aortic arch diameter (mm) | 22 | 6.0, 6.0, 6.0 | 2.3, 2.8, 3.8 | 3.0, 3.3, 4.2 | 0.121* |
| Freedom from reoperation (months) | 28 | 2.9, 2.9, 2.9 | 5.2, 7.7, 36.8 | 6.9, 10.7, 25.5 | 0.296* |
| Aortic isthmus diameter (mm) | 13 | 1.8, 1.8, 1.8 | 2.0, 2.5, 3.8 | 1.8, 2.5, 2.7 | 0.355* |
| Patient's age (days) | 39 | 9.5, 12.0, 13.0 | 4.2, 7.0, 8.8 | 4.0, 5.0, 7.0 | 0.116* |
| Patient's weight (kg) | 35 | 3.1, 3.3, 3.5 | 2.8, 2.9, 3.6 | 3.0, 3.2, 3.6 | 0.568* |
| Patient's BSA (mm2) | 24 | 0.2, 0.2, 0.2 | 0.2, 0.2, 0.2 | 0.2, 0.2, 0.2 | 0.668* |
| Prematurity: yes | 39 | 0% (0) | 33% (6) | 18% (3) | 0.28** |
| Prenatal diagnosis: yes | 39 | 25% (1) | 39% (7) | 35% (6) | 0.87** |
BSA: body surface area; AAI: aortic arch interruption; HLHS: hypoplastic left heart syndrome; HAA: hypoplastic aortic arch.
a, b, c represent the lower quartile a, the median b and the upper quartile c for continuous variables. N is the number of non-missing values. Numbers after percents are frequencies.
Tests used: *Kruskal–Wallis test.
**Pearson test.
Surgical technique
The surgical approach was through a median sternotomy in all patients. A large patch of antephrenic pericardium was excised and underwent fixation with glutaraldehyde (0.6% for 5 min). Cardiopulmonary bypass (CPB) was established interposing a 3–5-mm polytetrafluoroethylene (PTFE) conduit to the innominate artery as aortic cannulation site, and venous drainage was accomplished with either atrial or bicaval venous cannulation. After starting the CPB, the patients were cooled to reach moderate hypothermia (25°C) [6]; the operation was performed in circulatory arrest with selective antegrade cerebral perfusion for cerebral protection. After ductal isolation and ligation, all neck vessels were clamped. Patients with HLHS underwent either the ‘classic’ Norwood procedure (n = 7) or Norwood with Sano modification (n = 10) with arch reconstruction. In HAA patients, the aortic arch and the isthmus were opened longitudinally and reconstructed with autologous pericardium. In AAI patients, the proximal part of the arch and the distal portion of the interrupted arch were opened longitudinally; the ventral portion was then anastomosed using a running 7–0 polypropylene suture; finally, the dorsal portion of the arch was reconstructed with autologous pericardium. In the case of recurrent arch obstruction, balloon angioplasty was considered as a first option when the peak systolic gradient in the re-stenotic area was judged >20 mmHg in the catheter lab, associated with diastolic run-off on Doppler echocardiography. If the balloon angioplasty was ineffective in reducing the gradient, the patients were referred to redo surgical correction. The surgical approach in these patients was resternotomy and routine cardiopulmonary set-up with hypothermic circulatory arrest and antegrade cerebral perfusion. In the case of limited availability of autologous pericardium, a PTFE membrane was used as an enlargement patch.
Postoperative care
All patients were transferred to the paediatric intensive care unit (PICU) after the operation. The PICU discharge to the ward was accomplished after careful multidisciplinary judgment. They were discharged home from the hospital. Postoperative anticoagulant therapy was not used. All patients received 3–6 months of antiplatelet therapy according to Congenital Heart Disease/Paediatric Cardiology guidelines immediately after intervention [7].
Follow-up
Follow-up studies were performed routinely by the referral paediatric cardiologist by physical examination and echocardiographic assessment; magnetic resonance imaging and/or haemodynamic assessment were used to further investigate the clinical conditions when necessary.
Data analysis
Continuous data are reported with the mean and the standard deviation and 95% confidence interval (CI) where appropriate. For testing the difference between the continuous variables, we have used Student's t-test or Wilcoxon test when appropriate, for categorical data, Pearson's χ2 analysis or Fisher's exact test were done. The follow-up data for survival were estimated using the Kaplan–Meier method.
RESULTS
All patients had arch reconstruction with autologous pericardium during surgical correction of the CHD. The postoperative aortic arch gradient was 7.8 ± 9.75 mmHg. All patients were discharged without a significant residual gradient at the aortic arch except 4 who had peak gradient of ≥30 mmHg (median 33 mmHg, range 30–36 mmHg). The mean PICU stay was 11.8 ± 10.36. The mean hospital stay was 27.4 ± 20.97 days and was not significantly different between the diagnostic groups (pairwise Wilcoxon test HAA vs HLHS P <0.05; analysis of variance P = 0.066, Table 2). All patients were discharged without a significant residual gradient at the aortic arch except 4 who had a peak gradient on anastomosis of ≥30 mmHg (median 33 mmHg, range 30–36 mmHg). Eleven (28.2%) patients had recurrence of the aortic arch obstruction (5.3 ± 2.59 months; range 0.8–9.2) and underwent balloon angioplasty (3 HLHS—16.7%; 7 HAA—41.1% and 1 AAI—25%). In 3 cases (1 HLHS and 2 HHA), angioplasty was not effective and patients were referred to surgical reintervention. Preangioplastic evaluation of mean systolic gradient in the restenotic aortic site was 40.6 ± 17.26 mmHg. Percutaneous angioplasty was effective in 8 subjects, with a significant decrease in the systolic pressure to 16.2 ± 10.5 (mean gradient 40.6 ± 17.4 vs 16.2 ± 10.5 mmHg; P = 0.008). Intraoperatively, an overgrowth of neo-intima was found to be responsible for the progressive lumen narrowing. Overall in-hospital mortality was 17.9% (7 of 39 patients).
Table 2:
Hospital stay after operation
| N | Mean | Standard deviation | Lower 95% CI | Upper 95% CI | |
|---|---|---|---|---|---|
| Diagnosis at intervention | |||||
| AAI | 4 | 20.75 | 15.22 | 10.30 | 39.25 |
| HLHS | 18 | 35.78 | 26.49 | 3.70 | 78.70 |
| HAA | 17 | 20.12 | 10.44 | 12.60 | 34.20 |
| Overall | 39 | 27.41 | 20.97 | 5.80 | 63.10 |
AAI: aortic arch interruption; HLHS: hypoplastic left heart syndrome; HAA: hypoplastic aortic arch.
Follow-up data
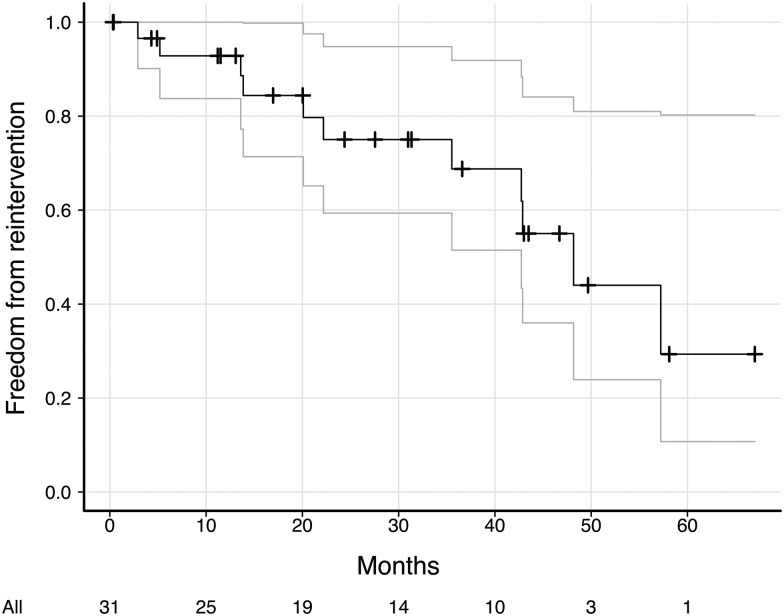
Follow-up was completed for 80% of patients with a median time of 24.4 (range 0.32–67 months). All patients were in Class II of New York Heart Association Functional Classification (NYHA) or less (23 of them had ≤ Class I NYHA). The actuarial freedom from reoperation was 92.8 and 68.8% at 12 and 36 months, respectively (Figs 1 and 2). Late mortality was 9.3% (3 of 32 patients).
Figure 1:
Actuarial freedom from a reintervention curve with 95% Cl.
Figure 2:
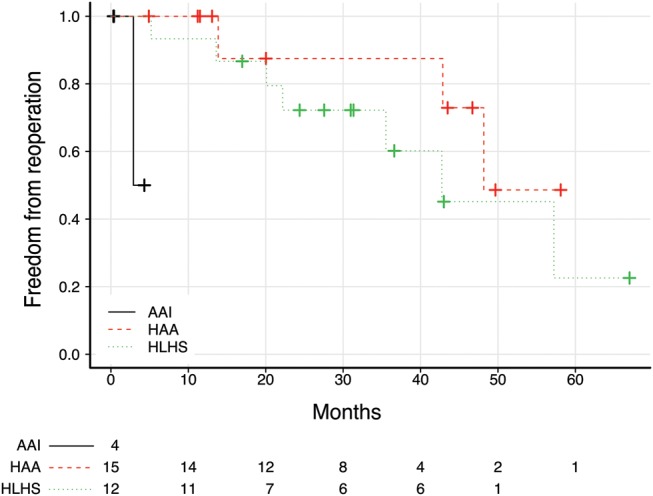
Kaplan–Meier survival curves for freedom from reintervention by the diagnostic group. There were significant differences between diagnostic groups in freedom from reintervention (log-rank test, P < 0.001).
DISCUSSION
In the original description of the Norwood procedure [1], the reconstruction of the aortic arch was performed without the use of any prosthetic material. Historically, prosthetic materials other than pericardium have been used to repair the ascending aorta and the arch. In the paper of Pigott et al. [8], an allograft patch is used for effective augmentation. A similar rate of recurrent arch obstruction in patients with or without extra material patch augmentation has also been found [9]. In cases of HAA or AAI, extended aortic arch anastomosis and end-to-side anastomosis are the standard surgical options for correction if anatomical conditions are favourable [10, 11]. Nevertheless, the use of the enlargement patch in complex coarctation has been described and is associated with a low incidence of stenosis recurrence [12, 13]. In our series, all patients (18 HLHS, 17 HAA and 4 AAI) had the aortic arch reconstructed with autologous pericardium. All patients recovered uneventfully from the operation and with low postoperative pressure gradient. The latter has been found to be predictive of recoarctation if higher than 13 mmHg. Our patients had a median/mean of 5/7.8 mmHg. We had a restenosis rate of 28%, being more frequent in HAA (41.1%), compared with AAI and HLHS. This is not surprising, since along with the type of surgical correction, HAA is a risk factor of recurrent aortic arch stenosis per se [14]. Eight patients underwent successful aortic balloon angioplasty, but 3 (7%) required surgical reintervention. The surgical procedure was effective in relieving stenosis as many authors have recognized and, in some cases, is mandatory when restenosis occurs in HAA [15, 16]. Even in the case of a redo operation, residual autologous pericardium has been used, due to its advantages over bioprosthetic material and allografts, i.e. availability, ease of tailoring and highly haemostatic nature; furthermore, it has a low tendency to calcification, due to low immunogenicity. In this study, we have observed only one proximal restenosis, probably due to the particular shape of the aortic arch ('Gothic’ arch). Furthermore, with the use of patch enlargement with autologous pericardium, we have never observed bronchi or trachea compression described as possible complication of end-to end anastomosis [17, 18].
An alternative patch material, such as CorMatrix submucous extracellular matrix (CorMatrix Cardiovascular, Atlanta, GA, USA), in aortic arch reconstruction has been reported by Quarti et al. [5] as a vascular patch in pulmonary artery reconstruction. This material has the advantage of being a scaffold for autologous cells that eventually will engraft and replace the patch, with the potential of growing with the patient. In conclusion, our experience outlines that autologous patch enlargement and reconstructive techniques are effective and suitable for a tailor-made approach in different pathologies that share the common feature of aortic arch abnormalities. Moreover, this approach will provide good freedom from stenosis recurrence and is also effective in complex redo cases.
ACKNOWLEDGEMENTS
We thank Susan E. Gwynne for undertaking the reviewing and syntax corrections of the manuscript.
REFERENCES
- 1.Norwood WI, Kirklin JK, Sanders SP. Hypoplastic left heart syndrome: experience with palliative surgery. Am J Cardiol. 1980;45:87–91. doi: 10.1016/0002-9149(80)90224-6. [DOI] [PubMed] [Google Scholar]
- 2.Tworetzky W, McElhinney DB, Burch GH, Teitel DF, Moore P. Balloon arterioplasty of recurrent coarctation after the modified Norwood procedure in infants. Catheter Cardiovasc Interv. 2000;50:54–8. doi: 10.1002/(sici)1522-726x(200005)50:1<54::aid-ccd11>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 3.Burkhart HM, Ashburn DA, Konstantinov IE, De Oliveira NC, De Oliviera NC, Benson L, et al. Interdigitating arch reconstruction eliminates recurrent coarctation after the Norwood procedure. J Thorac Cardiovasc Surg. 2005;130:61–5. doi: 10.1016/j.jtcvs.2005.02.060. [DOI] [PubMed] [Google Scholar]
- 4.Morell VO, Wearden PA. Experience with bovine pericardium for the reconstruction of the aortic arch in patients undergoing a Norwood procedure. Ann Thorac Surg. 2007;84:1312–5. doi: 10.1016/j.athoracsur.2007.05.036. [DOI] [PubMed] [Google Scholar]
- 5.Quarti A, Nardone S, Colaneri M, Santoro G, Pozzi M. Preliminary experience in the use of an extracellular matrix to repair congenital heart diseases. Interact CardioVasc Thorac Surg. 2011;13:569–72. doi: 10.1510/icvts.2011.280016. [DOI] [PubMed] [Google Scholar]
- 6.Imoto Y, Kado H, Shiokawa Y, Minami K, Yasui H. Experience with the Norwood procedure without circulatory arrest. J Thorac CardioVasc Surg. 2001;122:879–82. doi: 10.1067/mtc.2001.116948. [DOI] [PubMed] [Google Scholar]
- 7.Baumgartner H, Bonhoeffer P, De Groot NMS, de Haan F, Deanfield JE, Galie N, et al. ESC guidelines for the management of grown-up congenital heart disease (new version 2010) Eur Heart J. 2010;31:2915–57. doi: 10.1093/eurheartj/ehq249. [DOI] [PubMed] [Google Scholar]
- 8.Pigott JD, Murphy JD, Barber G, Norwood WI. Palliative reconstructive surgery for hypoplastic left heart syndrome. Ann Thorac Surg. 1988;45:122–8. doi: 10.1016/s0003-4975(10)62420-4. [DOI] [PubMed] [Google Scholar]
- 9.Griselli M, McGuirk SP, StÃijmper O, Clarke AJB, Miller P, Dhillon R, et al. Influence of surgical strategies on outcome after the Norwood procedure. J Thorac Cardiovasc Surg. 2006;131:418–26. doi: 10.1016/j.jtcvs.2005.08.066. [DOI] [PubMed] [Google Scholar]
- 10.Lansman S, Shapiro AJ, Schiller MS, Ritter S, Cooper R, Galla JD, et al. Extended aortic arch anastomosis for repair of coarctation in infancy. Circulation. 1986;74:I37–41. [PubMed] [Google Scholar]
- 11.Elliott MJ. Coarctation of the aorta with arch hypoplasia: improvements on a new technique. Ann Thorac Surg. 1987;44:321–3. doi: 10.1016/s0003-4975(10)62087-5. [DOI] [PubMed] [Google Scholar]
- 12.Ashcraft TM, Jones K, Border WL, Eghtesady P, Pearl JM, Khoury PR, et al. Factors affecting long-term risk of aortic arch recoarctation after the Norwood procedure. Ann Thorac Surg. 2008;85:1397–1401. doi: 10.1016/j.athoracsur.2007.11.054. discussion 1401–2. [DOI] [PubMed] [Google Scholar]
- 13.Vitullo DA, DeLeon SY, Graham LC, Eidem BW, Roughneen PT, Javorski JJ, et al. Extended end-to-end repair and enlargement of the entire arch in complex coarctation. Ann Thorac Surg. 1999;67:528–31. doi: 10.1016/s0003-4975(98)01254-5. [DOI] [PubMed] [Google Scholar]
- 14.Dodge-Khatami A, Backer CL, Mavroudis C. Risk factors for recoarctation and results of reoperation: a 40-year review. J Card Surg. 2000;15:369–77. doi: 10.1111/j.1540-8191.2000.tb01295.x. [DOI] [PubMed] [Google Scholar]
- 15.Zoghbi J, Serraf A, Mohammadi S, Belli E, Lacour Gayet F, Aupecle B, et al. Is surgical intervention still indicated in recurrent aortic arch obstruction? J Thorac Cardiovasc Surg. 2004;127:203–12. doi: 10.1016/s0022-5223(03)01290-x. [DOI] [PubMed] [Google Scholar]
- 16.Brown JW, Ruzmetov M, Hoyer MH, Rodefeld MD, Turrentine MW. Recurrent coarctation: is surgical repair of recurrent coarctation of the aorta safe and effective? Ann Thorac Surg. 2009;88:1923–30. doi: 10.1016/j.athoracsur.2009.07.024. discussion 1930-1. [DOI] [PubMed] [Google Scholar]
- 17.Jhang WK, Park J, Seo D, Goo HW, Gwak M. Perioperative evaluation of airways in patients with arch obstruction and intracardiac defects. Ann Thorac Surg. 2008;85:1753–8. doi: 10.1016/j.athoracsur.2008.01.059. [DOI] [PubMed] [Google Scholar]
- 18.Watanabe T, Hoshino S, Iwaya F, Igari T, Ono T, Takahashi K. A case of tracheo-bronchial stenosis after extended-to-end aortic arch anastomosis for interrupted aortic arch treated with suspension of the ascending artery and pulmonary artery. Jap J Thorac Surg. 2001;54:151–3. [PubMed] [Google Scholar]