Abstract
Background. The emergence of a commercially prepared citrate solution has revolutionized the use of RCA in the intensive care unit (ICU). The aim of this study was to evaluate the safety profile of a commercially prepared citrate solution. Method. Predilution continuous venovenous hemofiltration (CVVH) was performed using Prismocitrate 10/2 at 2500 mL/h and a blood flow rate of 150 mL/min. Calcium chloride solution was infused to maintain ionized calcium within 1.0–1.2 mmol/L. An 8.4% sodium bicarbonate solution was infused separately. Treatment was stopped when the predefined clinical target was reached or the filter clotted. Result. 58 sessions of citrate RCA were analyzed. The median circuit lifetime was 26.0 h (interquartile range IQR 21.2–44.3). The percentage of circuits lasting more than 12 h, 24 h, and 48 h was 94.6%, 58.9%, and 16.1%, respectively. There was no incidence of hypernatremia and median pH was <7.5. Hypomagnesemia and hypophosphatemia were detected in 41.6% and 17.6% of blood samples taken, respectively. Although 16 episodes had a total calcium/ionized calcium (total Ca/iCa) >2.5, only four patients had evidence of citrate accumulation. Conclusion. The commercially prepared citrate solution could be used safely in critically ill patients who required CVVH with no major adverse events.
1. Introduction
Regional citrate anticoagulant (RCA) has been widely used in continuous renal replacement therapy (CRRT) [1–16]. Its use has been greatly simplified by the development of a commercially prepared citrate solution [11–16]. Shum et al. [14] reported a simple citrate regime in 10 critically ill patients using the commercial citrate solution. We would like to expand the use of this regime to a larger group of patients and in an intensive care unit (ICU) with limited prior experience in the use of RCA.
We chose this regime because it is simple to set up. First of all, the blood flow and the substitution fluid rate were fixed to give a constant blood citrate concentration and to eliminate regular measurement of prefilter ionized calcium (iCa). The procedure involved only one replacement solution, Prismocitrate 10/2 (Gambro-Hospal, Stockholm, Sweden). As the amount of base produced was only 30 mmol/L bicarbonate, an external pump was used to infuse the sodium bicarbonate via the heparin port of the circuit to supplement the base required (Figure 1).
Figure 1.
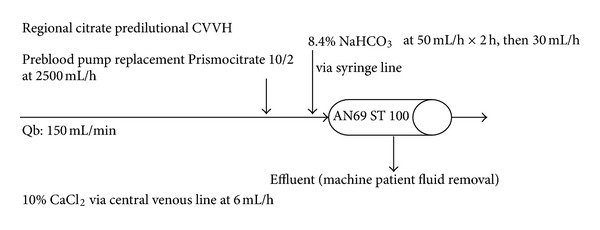
Diagram of predilutional continuous venovenous hemofiltration using Prismocitrate 10/2 solution and Prismaflex machine.
Following 3 months of staff training, citrate CRRT was first implemented in our unit on July 1, 2010. The initial response to this regime was suboptimal. This was related to the fact that there was only one dialysis machine that matched this commercialized citrate solution and the unfamiliar use of this new machine by the nursing staff. We subsequently applied this regime to all existing dialysis machines in the unit and the utilization rate rapidly improved.
2. Materials and Method
After obtaining approval from our regional Ethics Committee, we conducted a retrospective analysis of ICU patients who underwent citrate CRRT during the period from July to December 2010. Any ICU patients older than 18 years of age who required citrate CRRT for more than 4 hours were included. Indications for starting CRRT included fluid overload unresponsive to diuretic treatment, hyperkalemia with K > 6.5 mmol/L or rapidly rising K, severe acidemia with pH < 7.1, oliguria (urine output <200 mL/12 h), or anuria (urine output <50 mL/12 h). Those with a contraindication for RCA were excluded, including patients with liver disease and bilirubin ≥80 μmol/L and patients requiring massive blood transfusion or high volume hemofiltration ≥3 L/h. All dialyses were performed through a double lumen 12-F hemodialysis catheter (ARROW, Arrow International Inc., USA) inserted into either the femoral or internal jugular vein. Prismocitrate 10/2 (citrate of 10 mmol/L, citric acid 2 mmol/L, sodium 136 mmol/L, and chloride 106 mmol/L) was used as a predilutional replacement solution at a rate of 2500 mL/h. Blood flow was 150 mL/min. A solution of 8.4% sodium bicarbonate was infused at a rate of 50 mL/h for 2 hours, then at 30 mL/h till the end of the treatment. A 10% calcium chloride solution was infused through the central line at a rate of 6 mL/h to replace the loss of the citrate-calcium complex through the circuit. A titration table was used to adjust the rate of CaCl2 infusion (5–7 mL/h) to target an ionized Ca of 1.0–1.2 mmol/L. Any negative fluid balance was titrated according to the clinical status of the patient. With the Prismaflex machine, we used the Prismaflex control unit with an AN69 ST 100 hemofilter (Gambro Industries, France). With the MultiFiltrate machine (Fresenius Medical Care, Bad Homburg, Germany), we used the MultiFiltrate HV-CVVHD 600 kit with the Ultraflux AV 600 S hemofilter (Fresenius Medical Care, 1.4 m2 surface area, polysulfone membrane).
We monitored electrolytes, arterial blood gas, and iCa every 4 hours during treatment. Total calcium, phosphate, and magnesium levels were measured at least daily. As the median pre- and postfilter iCa in Shum et al.'s work [14] was consistently within the range of effective anticoagulation (0.24 and 0.25 mmol/L), we did not perform the pre-filter iCa check in this study. If the patient had worsening metabolic acidosis that exceeded the previous value by 3–5 mmol/L, the total Ca and anion gap were measured to detect any citrate accumulation.
Demographic data including age, gender, ideal body weight, APACHE II and IV scores, comorbidity, and reason for RRT support were collected. Circuit effectiveness was measured in terms of circuit lifetime, reasons for stopping CVVH and types of dialysis machine used. Complications related to the use of RCA were defined as metabolic alkalosis with pH > 7.5, hypernatremia with Na ≥ 150 mmol/L, hypokalemia with K ≤ 3.0 mmol/L, hypophosphatemia with PO4 ≤ 0.8 mmol/L, hypomagnesemia with Mg ≤ 0.66 mmol/L, low ionized Ca with iCa ≤ 0.85, and total-to-ionized calcium ratio >2.5. Lastly, daily bilirubin level, ICU, and hospital mortality were also recorded.
2.1. Statistical Analysis
All continuous variables were compared using Student's t-test and the analysis was performed using the Statistical Package for Social Science for Windows, version 16.0 (SPSS, Chicago, IL, USA). The results were displayed as the median with interquartile range (IQR) included. The trend in pH, electrolytes, and base excess was displayed using a standard box plot. This trial was also registered with the Australian and New Zealand Clinical Trials Registry, number ACTRN12611000360910.
3. Results
A total of 44 patients received 58 sessions of citrate CVVH. Two sessions were not analyzed as the duration of CRRT was less than 4 hours. The median age was 64 (IQR 57.5–74.5) and the median ideal body weight was 55.5 kg (IQR 51.6–60.0). The median APACHE II score was 29 (IQR 24.8–33) and the APACHE IV was 101.5 (79.3–125.3). Thirty-six patients had preexisting comorbidity and 11 had end-stage renal failure. The three most common reasons for starting RRT were due to sepsis, fluid overload, and after major surgery (Table 1).
Table 1.
Patients' characteristics.
| IRQ (interquartile range) | ||
|---|---|---|
| No. of patients included | 44 | |
| No. of treatment episodes | 56 | |
| Age (yr) (median) | 64.0 | 57.5–74.5 |
| Gender | ||
| Female | 14 | |
| Male | 30 | |
| Body weight (Kg) (median) | 55.5 | 51.6–60 |
| APACHE II score (median) | 29.0 | 24.8–33.0 |
| APACHE IV score (median) | 101.5 | 79.3–125.3 |
| No. of patients with comorbidities | 36 | |
| DM | 25 | |
| HT | 30 | |
| IHD | 6 | |
| ESRF | 11 | |
| Reasons for starting dialysis | ||
| Sepsis | 34 | |
| Fluid overload | 10 | |
| Major surgery | 6 | |
| Prerenal | 3 | |
| Others | 3 | |
| Baseline blood parameters | ||
| pH (median) | 7.35 | 7.25–7.42 |
| Base excess (median) | −4.5 | −9.0 to −2.0 |
| Sodium level (mmol/L, median) | 137.5 | 136–141.8 |
| Potassium level (mmol/L, median) | 4.2 | 3.6–5.1 |
| Phosphate level (mmol/L, median) | 1.68 | 1.30–2.42 |
| Magnesium level (mmol/L, median) | 0.87 | 0.71–0.95 |
| iCa (mmol/L, median) | 1.08 | 0.96–1.14 |
| Total Ca (mmol/L, median) | 1.99 | 1.87–2.10 |
| Urea (umol/L, median) | 22.9 | 17.3–32.2 |
| Creatinine (mmol/L, median) | 398.5 | 284.0–616.8 |
The median circuit lifetime was 26 h (IQR 21.1–44.3). The maximum duration was up to 62 h. Circuits lasted more than 12 h, 24 h, and 48 h in 94.6%, 58.9%, and 16.1% of cases respectively. Twenty-seven sessions (46.6%) were stopped as the predefined clinical target was reached. Nine were stopped due to circuit failure, three due to cannula problems and four due to citrate accumulation (Figure 2). For circuit with the clotted filter, the median circuit lifetime was 28.0 h (IQR 22.3–44.6 h). Thirty sessions of citrate CVVH were conducted using a Prismaflex machine and 26 sessions with a MultiFiltrate. There was no difference in terms of circuit patency or metabolic control between the two machines.
Figure 2.
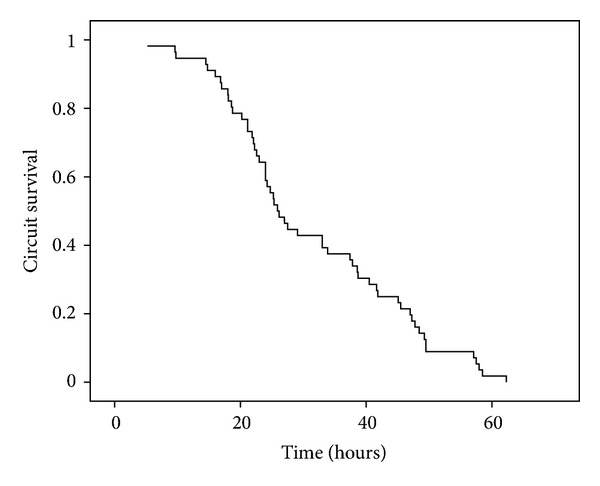
Circuit duration over time.
No patient developed hypernatremia. The median pH was less than 7.5, although 13.3% of the blood samples had a pH > 7.5. The most common electrolyte disturbance was hypomagnesemia (41.6% of blood samples), followed by hypophosphatemia (17.6%). The time taken to correct the metabolic acidosis as defined by zero-base excess was around 20 to 24 hours (Figures 3, 4, 5, 6, 7, and 8).
Figure 3.
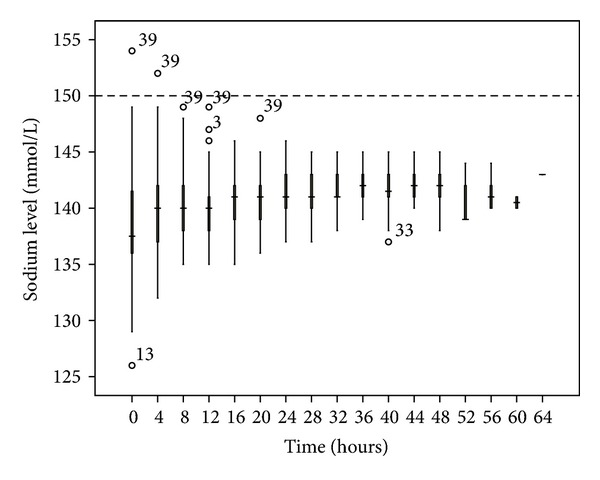
Sodium changes over time during citrate CRRT. Standard box plot in which the horizontal line represents the median, the thick line represents the interquartile range, and the thin line represents the maximum and minimum values. The circular dots represent the outliers.
Figure 4.
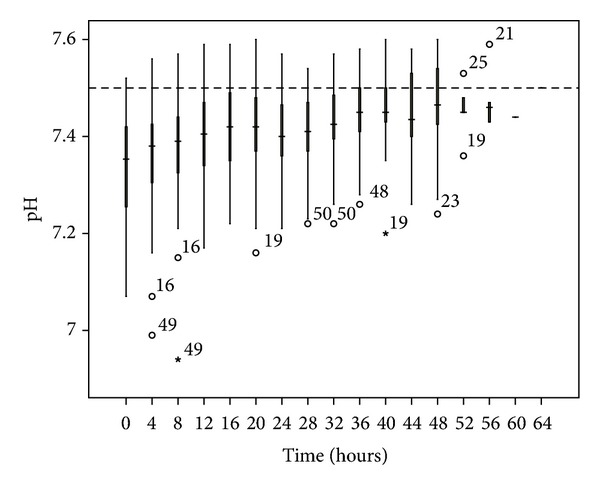
pH changes over time during citrate CRRT. Standard box plot in which the horizontal line represents the median, the thick line represents the interquartile range, and the thin line represents the maximum and minimum values. The circular dots represent the outliers.
Figure 5.
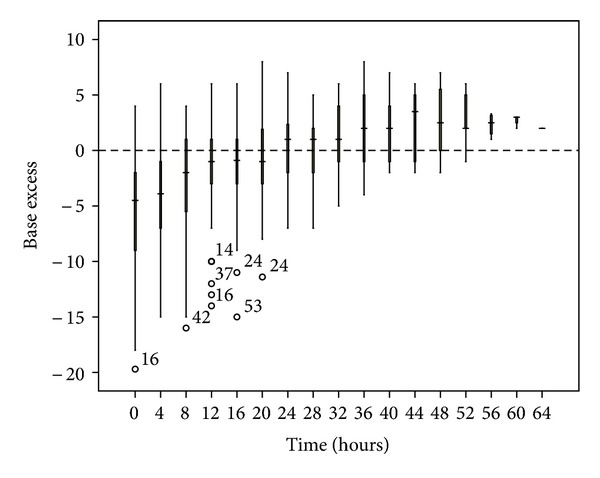
Base excess changes over time during citrate CRRT. Standard box plot in which the horizontal line represents the median, the thick line represents the interquartile range, and the thin line represents the maximum and minimum values. The circular dots represent the outliers.
Figure 6.
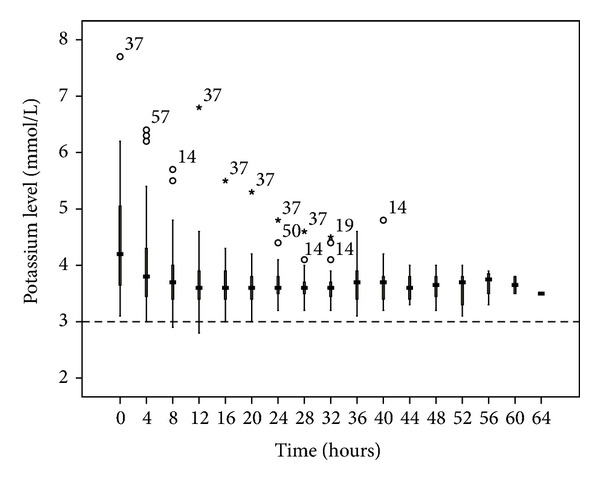
Potassium changes over time during citrate CRRT. Standard box plot in which the horizontal line represents the median, the thick line represents the interquartile range, and the thin line represents the maximum and minimum values. The circular dots represent the outliers.
Figure 7.
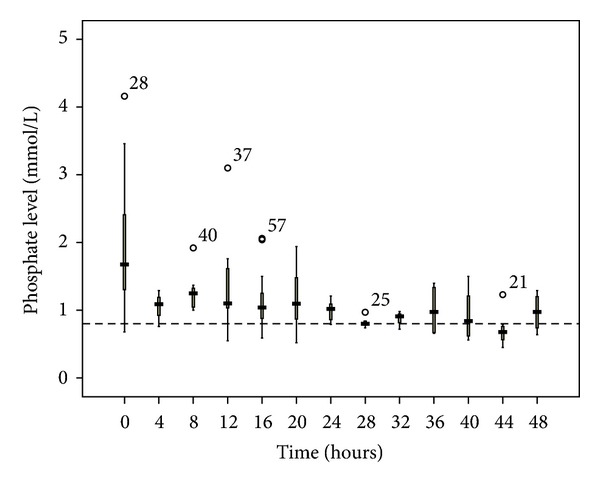
Phosphate changes over time during citrate CRRT. Standard box plot in which the horizontal line represents the median, the thick line represents the interquartile range, and the thin line represents the maximum and minimum values. The circular dots represent the outliers.
Figure 8.
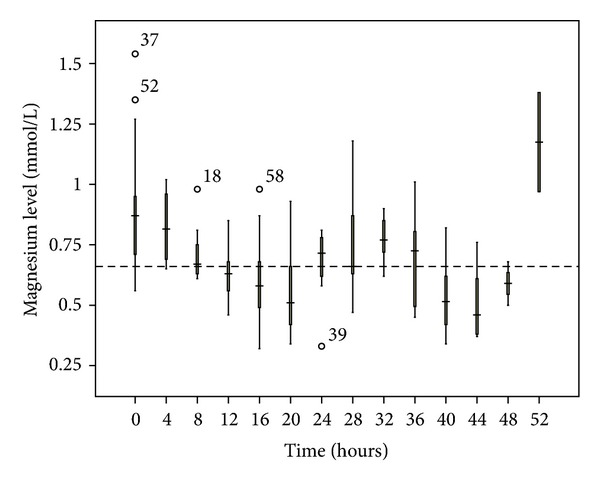
Magnesium changes over time during citrate CRRT. Standard box plot in which the horizontal line represents the median, the thick line represents the interquartile range, and the thin line represents the maximum and minimum values. The circular dots represent the outliers.
The median iCa was above 0.85, and only 4.1% of blood samples had values <0.85 (Figure 9). There were 16 episodes of total Ca/iCa > 2.5; but only four patients had CVVH terminated due to citrate accumulation. Three patients had either slow correction or worsening of metabolic control; all had elevated Total Ca/iCa > 2.5 and an increase in anion gap metabolic acidosis. The onset time was 10 to 25 hours after commencement of therapy (Table 2). There were no untoward side effects among these four patients and the metabolic acidosis resolved spontaneously after stopping the citrate CVVH. The ICU mortality rate of this cohort was 23% and the hospital mortality rate was 54.5%.
Figure 9.
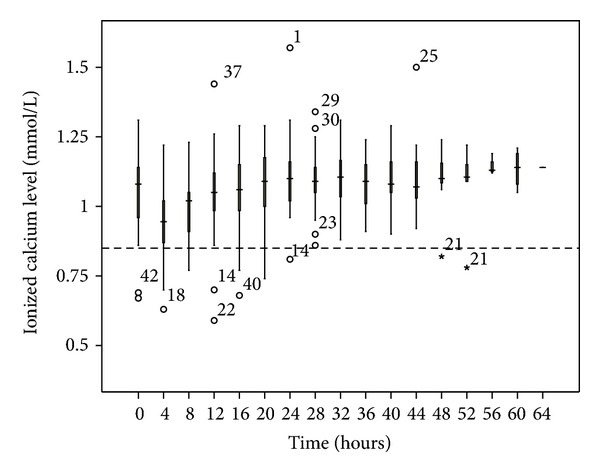
Ionized calcium changes over time during citrate CRRT. Standard box plot in which the horizontal line represents the median, the thick line represents the interquartile range, and the thin line represents the maximum and minimum values. The circular dots represent the outliers.
Table 2.
Acid-base profile of the four patients with citrate accumulation during citrate CRRT.
| Circuit time (hr) | Base excess changes over time |
Anion gap |
Total Ca/iCa |
Bilirubin (umol/L) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline BE |
4 hrs | 8 hrs | 12 hrs | 16 hrs | 20 hrs | 24 hrs | |||||
| Patient 1 | 9.6 | −12 | −6 | −8 | −10 | 29 | 4.1 | 27 | |||
| Patient 2 | 24 | −3 | −5 | −3 | −3 | −5 | −4 | −1.2 | 27 | 2.9 | 61 |
| Patient 3 | 9.8 | −17 | −15 | −16 | 32 | 2.4 | 54 | ||||
| Patient 4 | 16 | −14 | −11 | −11 | −13 | 36 | 2.5 | 5 | |||
4. Discussion
Various citrate CVVH regimes have been reported, using either trisodium citrate (TSC) [1–7] or Anticoagulant citrate dextrose solution (ACDA) [8–10]. These preparations are usually tailored made, limiting the widespread use of citrate CVVH. Since 2005, commercially prepared citrate solutions have been available on the market and various studies have reported the use of these products [11–16].
Similar to Bihorac and Ross [5] and Schmitz et al.'s studies, [12], Shum et al. [14] used a fixed blood flow to give a constant blood citrate concentration. It has been shown that a blood citrate level of 3–6 mmol/L is required to achieve a systemic ionized calcium of <0.35 [17]. The good anticoagulation efficacy of this regime was demonstrated by the pre- and postfilter iCa of 0.24 and 0.25 mmol/L, respectively [14]. As the citrate is mixed with the replacement solution, we needed to perform predilutional CVVH in our study. As the median body weight in this study was 55.5 Kg, the dose of CVVH was 45.0 mL/Kg/h. Despite the loss of efficiency with predilutional CVVH, it still exceeds the recommended dose of 25–35 mL/Kg/h [18–20].
4.1. Citrate Concentration and Circuit Efficacy
The blood citrate concentration of this study was 3.3 mmol/L, which was within the range of 3–6 mmol/L required to achieve effective anticoagulation [17]. The circuit lifetime reported by Shum and another study using a similar preparation in the form of CVVHDF [15] was 50.4 and 58.8 h, respectively. These results compared favorably to the reported circuit lifetime of 48.6–61.5 h [5, 8, 12, 13]. Straaten et al. [21] used a 3-mmol/L blood citrate level and achieved a circuit lifetime of 27 h. Similarly, Brain et al. [16] used a blood citrate level of 2.4 mmol/L and achieved a filter lifetime of 23.6 h. We reported a median circuit lifetime of 26.0 h, which was comparable with the results of these latter two studies. One reason for the shorter circuit lifetime in our study was that we stopped CRRT once the predefined clinical target was reached (e.g., correction of acid-base disturbance or achieving the target negative fluid balance); in contrast, most of the reported studies would run the circuit for 72 hours or until the filter clotted. The circuit failure rate in this study was 16%, which was less than the reported range of 23–49% [1, 5, 8, 12, 21]. Even with a clotted filter, the circuit lasted for a median of 28.0 h (IQR 22.3–444.6).
4.2. Complications Related to the Use of RCA
In terms of metabolic control, we encountered no hypernatremia and only occasional episodes of metabolic alkalosis. This might be related to the use of the exogenous infusion of the sodium bicarbonate. Both hypophosphatemia and hypomagnesemia occurred more frequently in our cohort, highlighting the importance of electrolyte monitoring during the extended period of dialysis after the use of citrate. As the mean treatment dose in this study was higher than the recommended dose (45.0 mL/Kg/h versus 25–30 mL/Kg/h), this might lead to increased loss of the electrolytes through the circuit. Hence, this observation might be a dose-related rather than a citrate-related complication. The addition of 0.75 mmol/L Mg into the substitution solution may help alleviate this problem [13].
In citrate CVVH, a total Ca/iCa of 2.5 has been used as a surrogate of citrate toxicity [22, 23]. The total Ca/iCa was 2.33 in Shum's study [14] and Tolwani et al. [7] reported a maximum total Ca/iCa of 2.8 in a 0.67% TSC group and 2.7 in a 0.5% TSC group. We reported 16 episodes (12.7% blood samples) of total Ca/iCa of >2.5 with four CVVH sessions terminated due to citrate accumulation. Similarly, Morgera et al. [13] reported seven such episodes, four being transient and three persistent. The latter three patients died of hepatic or multiple-organ failure. One interesting finding in our cohort was that the four transiently affected patients had no preexisting liver disease. The onset of citrate accumulation occurs from 10 to 24 hours the after commencement of citrate CVVH. This might be related to the relative hypoperfusion of the liver in critically ill patients with decreased metabolism of citrate and hence a relative accumulation of citrate in the body. All four patients had an increased anion gap and three had either slow correction or worsening of the preexisting metabolic acidosis. None of these four patients had any untoward side effects and the metabolic acidosis resolved spontaneously after stopping the citrate.
A drawback of this study was that it was a retrospective analysis and might, thus, have incurred bias during data correction. In addition, fixing the blood flow and substitution solution rate did not cater for different body weights. It also limited the ability to fine tune the control of metabolic disturbances. Furthermore, an external syringe pump was used to infuse sodium bicarbonate via the heparin port of the circuit to compensate for the inadequate bicarbonate in this regime. As this regime involved the use of the Prismocitrate solution 10/0 only, we preferred to reserve the postfilter pump for another infusion if necessary. The newer models of dialysis machine incorporate software that couples citrate dose and calcium infusion with different blood flows. This advancement will further enhance the control of the dosing of CRRT treatment according to the different needs of patients. Lastly, we had no citrate measurements from patients suspected of citrate accumulation and, thus, were unable to confirm accumulation unequivocally.
5. Conclusion
The modified use of this commercially prepared citrate solution in critically ill patients carried a low risk of hypernatremia and metabolic alkalosis. The relatively high incidence of hypophosphatemia and hypomagnesemia might be related to the higher than recommended dosing of RRT given in this study. Lastly, in circumstances in which there is slow correction or worsening of control of metabolic acidosis, the combined use of total Ca/iCa > 2.5 and increased anion gap can help to detect patients with citrate accumulation that may warrant an early termination of RCA.
Conflict of Interests
The authors declare that they have no conflict of interests.
Acknowledgment
This trial is registered, with the Australian and New Zealand Clinical Trials Registry, no. ACTRN12611000360910.
References
- 1.Mehta RL, McDonald BR, Aguilar MM, Ward DM. Regional citrate anticoagulation for continuous arteriovenous hemodialysis in critically ill patients. Kidney International. 1990;38(5):976–981. doi: 10.1038/ki.1990.300. [DOI] [PubMed] [Google Scholar]
- 2.Palsson R, Niles JL. Regional citrate anticoagulation in continuous venovenous hemofiltration in critically ill patients with a high risk of bleeding. Kidney International. 1999;55(5):1991–1997. doi: 10.1046/j.1523-1755.1999.00444.x. [DOI] [PubMed] [Google Scholar]
- 3.Kutsogiannis DJ, Mayers I, Chin WDN, Gibney RTN. Regional citrate anticoagulation in continuous venovenous hemodiafiltration. American Journal of Kidney Diseases. 2000;35(5):802–811. doi: 10.1016/s0272-6386(00)70248-4. [DOI] [PubMed] [Google Scholar]
- 4.Tolwani AJ, Campbell RC, Schenk MB, Allon M, Warnock DG. Simplified citrate anticoagulation for continuous renal replacement therapy. Kidney International. 2001;60(1):370–374. doi: 10.1046/j.1523-1755.2001.00809.x. [DOI] [PubMed] [Google Scholar]
- 5.Bihorac A, Ross EA. Continuous venovenous hemofiltration with citrate-based replacement fluid: efficacy, safety, and impact on nutrition. American Journal of Kidney Diseases. 2005;46(5):908–918. doi: 10.1053/j.ajkd.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 6.Kutsogiannis DJ, Gibney RTN, Stollery D, Gao J. Regional citrate versus systemic heparin anticoagulation for continuous renal replacement in critically ill patients. Kidney International. 2005;67(6):2361–2367. doi: 10.1111/j.1523-1755.2005.00342.x. [DOI] [PubMed] [Google Scholar]
- 7.Tolwani AJ, Prendergast MB, Speer RR, Stofan BS, Wille KM. A practical citrate anticoagulation continuous venovenous hemodiafiltration protocol for metabolic control and high solute clearance. Clinical Journal of the American Society of Nephrology. 2006;1(1):79–87. doi: 10.2215/CJN.00040505. [DOI] [PubMed] [Google Scholar]
- 8.Tobe SW, Aujla P, Walele AA, et al. A novel regional citrate anticoagulation protocol for CRRT using only commercially available solutions. Journal of Critical Care. 2003;18(2):121–129. doi: 10.1053/jcrc.2003.50006. [DOI] [PubMed] [Google Scholar]
- 9.Cointault O, Kamar N, Bories P, et al. Regional citrate anticoagulation in continuous venovenous haemodiafiltration using commercial solutions. Nephrology Dialysis Transplantation. 2004;19(1):171–178. doi: 10.1093/ndt/gfg488. [DOI] [PubMed] [Google Scholar]
- 10.Leung AKH, Yan WW. Renal replacement therapy in critically ill patients. Hong Kong Medical Journal. 2009;15(2):122–129. [PubMed] [Google Scholar]
- 11.Egi M, Naka T, Bellomo R, et al. A comparison of two citrate anticoagulation regimens for continuous veno-venous hemofiltration. International Journal of Artificial Organs. 2005;28(12):1211–1218. doi: 10.1177/039139880502801203. [DOI] [PubMed] [Google Scholar]
- 12.Schmitz M, Taskaya G, Plum J, et al. Continuous venovenous haemofiltration using a citrate buffered substitution fluid. Anaesthesia and Intensive Care. 2007;35(4):529–535. doi: 10.1177/0310057X0703500411. [DOI] [PubMed] [Google Scholar]
- 13.Morgera S, Schneider M, Slowinski T, et al. A safe citrate anticoagulation protocol with variable treatment efficacy and excellent control of the acid-base status. Critical Care Medicine. 2009;37(6):2018–2024. doi: 10.1097/CCM.0b013e3181a00a92. [DOI] [PubMed] [Google Scholar]
- 14.Shum HP, Chan KC, Yan WW. Regional citrate anti-coagulation in predilution continuous venovenous hemofiltration using Prismocitrate 10/2 solution. Therapeutic Apheresis and Dialysis. 2012;16(1):81–86. doi: 10.1111/j.1744-9987.2011.01001.x. [DOI] [PubMed] [Google Scholar]
- 15.Ooi E, Lim T, Lim N. Comparison of the efficacy and safety of two regional citrate anticoagulation protocols using acid citrate dextrose A or Prismocitrate 10/2, in patients with acute renal failure undergoing continuous venovenous hemodiafiltration. Critical Care. 2010;14(Supplement 11):p. S515. [Google Scholar]
- 16.Brain M, Parkes S, Fowler P, Robertson I, Brown A. Calcium flux in continuous venovenous haemodiafiltration with heparin and citrate anticoagulation. Critical Care and Resuscitation. 2011;13(2):72–81. [PubMed] [Google Scholar]
- 17.Flanigan MJ, Pillsbury L, Sadewasser G, Lim VS. Regional hemodialysis anticoagulation: hypertonic tri-sodium citrate or anticoagulant citrate dextrose-A. American Journal of Kidney Diseases. 1996;27(4):519–524. doi: 10.1016/s0272-6386(96)90162-6. [DOI] [PubMed] [Google Scholar]
- 18.Ronco C, Bellomo R, Homel P, et al. Effects of different doses in continuous veno-venous haemofiltration on outcomes of acute renal failure: a prospective randomised trial. The Lancet. 2000;356(9223):26–30. doi: 10.1016/S0140-6736(00)02430-2. [DOI] [PubMed] [Google Scholar]
- 19.Palevsky PM, Zhang JH, O'Connor TZ, et al. Intensity of renal support in critically ill patients with acute kidney injury. The New England Journal of Medicine. 2008;359(1):7–20. doi: 10.1056/NEJMoa0802639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bellomo R, Cass A, Cole L, et al. Intensity of continuous renal-replacement therapy in critically ill patients. The New England Journal of Medicine. 2009;361(17):1627–1638. doi: 10.1056/NEJMoa0902413. [DOI] [PubMed] [Google Scholar]
- 21.Straaten HMO, Bosman RJ, Koopmans M, et al. Citrate anticoagulation for continuous venovenous hemofiltration. Critical Care Medicine. 2009;37(2):545–552. doi: 10.1097/CCM.0b013e3181953c5e. [DOI] [PubMed] [Google Scholar]
- 22.Meier-Kriesche HU, Gitomer J, Finkel K, DuBose T. Increased total to ionized calcium ratio during continuous venovenous hemodialysis with regional citrate anticoagulation. Critical Care Medicine. 2001;29(4):748–752. doi: 10.1097/00003246-200104000-00010. [DOI] [PubMed] [Google Scholar]
- 23.Schultheiss C, Saugel B, Phillip V. Continuous venovenous hemodialysis with regional citrate anticoagulation in patients with liver failure: a prospective observational study. Critical Care. 2012;16(4, article R162) doi: 10.1186/cc11485. [DOI] [PMC free article] [PubMed] [Google Scholar]


