Abstract
The diaphragm muscle (DIAm) is critically responsible for sustaining ventilation. Previously we showed in a commonly used model of spinal cord injury, unilateral spinal cord hemisection at C2 (SH), that there are minimal changes to muscle fiber cross-sectional area (CSA) and fiber type distribution following 14 days of SH-induced ipsilateral DIAm inactivity. In the present study, effects of long-term SH-induced inactivity on DIAm fiber size and force were examined. We hypothesized that prolonged inactivity would not result in substantial DIAm atrophy or force loss. Adult rats were randomized to control or SH groups (n = 34 total). Chronic bilateral DIAm electromyographic (EMG) activity was monitored during resting breathing. Minimal levels of spontaneous recovery of ipsilateral DIAm EMG activity were evident in 42% of SH rats (<25% of preinjury root mean square amplitude). Following 42 days of SH, DIAm specific force was reduced 39%. There was no difference in CSA for type I or IIa DIAm fibers in SH rats compared with age, weight-matched controls (classification based on myosin heavy chain isoform expression). Type IIx and/or IIb DIAm fibers displayed a modest 20% reduction in CSA (P < 0.05). Overall, there were no differences in the distribution of fiber types or the contribution of each fiber type to the total DIAm CSA. These data indicate that reduced specific force following prolonged inactivity of the DIAm is associated with modest, fiber type selective adaptations in muscle fiber size and fiber type distribution.
Keywords: fiber type, myosin heavy chain isoform, spinal cord injury
the diaphragm muscle (DIAm) is the major inspiratory pump muscle responsible for breathing in mammals (7, 42). The DIAm is continually active in sustaining ventilatory behaviors (i.e., normal resting breathing) and also participates in nonventilatory behaviors associated with clearance of the airway, such as coughing or sneezing (25, 28, 43). These high demands on the activation of the DIAm make it a likely candidate for inactivity-induced adaptations, especially in the long term. However, how the DIAm responds to chronic, long-term inactivity is not clear.
In a commonly used model of spinal cord injury, unilateral spinal cord hemisection at C2 (SH), there are minimal changes to the ipsilateral DIAm following 14 days of SH-induced inactivity (27, 30, 54). In this model, absence of DIAm activity ipsilateral to SH was verified at the time of surgery and at the terminal experiment using electromyographic (EMG) recordings. Importantly, there were limited changes in DIAm fiber cross-sectional area (CSA) compared with control (30), with fiber type classification based on the expression of specific myosin heavy chain (MyHC) isoforms (16, 38, 46). Specifically, there was slight (11%) hypertrophy of type I fibers and reduced proportion of type IIa fibers (17%). Following 14 days of SH, no change in DIAm force was evident (30). With SH the descending excitatory drive to phrenic motor neurons is interrupted, resulting in inactivation of DIAm motor units ipsilateral to SH. In several studies, SH-induced DIAm inactivity was shown to result in synaptic plasticity at DIAm neuromuscular junctions with increased presynaptic vesicle pool and terminal size, increased apposition of pre- and postsynaptic structures, and improved neuromuscular transmission (23, 27, 34). As such, it stands to reason that prolonged DIAm inactivity, when all components of the motor unit (i.e., the motoneuron, neuromuscular junction, and muscle fibers) are simultaneously inactive, may modestly impact DIAm fiber size and force.
In contrast, several other models of DIAm disuse, including unilateral denervation or tetrodotoxin conduction block of the phrenic nerve, and controlled mechanical ventilation, induce fiber type specific adaptations. For instance, following14 days of DIAm disuse imposed by either ipsilateral denervation or tetrodotoxin block, type I and IIa fibers displayed ∼70% hypertrophy, whereas the larger type IIx and/or IIb fibers displayed ∼50% atrophy (30). In contrast, after 18 h of mechanical ventilation there is substantial (15–57%) atrophy across all DIAm fiber types in both rats and humans (19, 29, 40). These models differ in the preservation of motoneuron-muscle fiber interactions (e.g., denervation vs. phrenic nerve conduction block), unloading as a result of changes in chest wall compliance and mechanics (e.g., mechanical ventilation) or other pathophysiological conditions (e.g., systemic inflammatory response). It is important to note that, in many studies examining adaptations associated with DIAm inactivity, the extent of inactivity was not measured directly. Thus the role of inactivity in DIAm adaptations remains unclear. Regardless, fiber type dependent adaptations to imposed disuse must be considered.
Following SH, there is gradual spontaneous recovery of rhythmic DIAm activity (8, 9, 14, 55) such that, by 6–8 wk post-SH, a substantial number of animals display ipsilateral DIAm activity. The extent of such activity seems minimal and its contribution to ventilatory behaviors is likely small (9, 51). Accordingly, the primary aim of the present study was to determine the effects of prolonged SH-induced inactivity of the ipsilateral DIAm by examining DIAm fiber size and force 42 days post-SH. Based on the matching inactivity of all motor unit components in the SH model, we hypothesized that there would be modest effects of long-term inactivity on the DIAm. Inactivity was determined using chronic DIAm EMG recordings, in accordance with a recent report (24).
METHODS
Animals.
Adult male Sprague-Dawley rats (n = 34) were purchased from Harlan (Indianapolis, IN). Upon arrival, rats were given 1 wk to acclimate to the facility and provided with food and water ad libitum. Rats were divided into body weight-matched groups of either control (n = 15) or spinal hemisection at C2 (SH; n = 19). A subset of SH rats (n = 12) underwent repeated bilateral DIAm EMG recordings under anesthesia (at 3 days and 42 days post-SH). Anesthesia for all surgical procedures and EMG recording sessions was achieved by intramuscular injection of ketamine (90 mg/kg) and xylazine (10 mg/kg). At the terminal experiment (42 days postintervention), anesthetized rats were euthanized by exsanguination. All protocols were approved by the Institutional Animal Care and Use Committee at the Mayo Clinic, in compliance with the American Physiological Society guidelines.
SH.
Surgical procedures for SH have been previously described and validated by our lab (22, 23, 30, 34). Briefly, 3 days following bilateral electrode placement in the DIAm rats underwent SH surgery. With sterile conditions and under ketamine and xylazine anesthesia, a dorsal laminectomy was performed. A cut was made with a surgical microknife at the right anterolateral C2 level of the spinal cord. The hemisection was completed anterior to the dorsal root entry zone fissure, preserving the dorsal funiculus. Rats were allowed to fully recover while body temperature was controlled. Rats were administered acetaminophen (100–300 mg/kg) orally for 3 days postsurgery and if necessary buprenorphine (0.1 mg/kg) via subcutaneous injection.
Implantation of DIAm electrodes and analysis of DIAm EMG activity were performed as previously reported (4, 5, 22, 24, 48). Briefly, while under ketamine and xylazine anesthesia, a pair of fine wire electrodes were implanted into the midcostal DIAm bilaterally to allow for chronic recordings of EMG activity. The electrodes (AS631; Cooner Wire, Chatsworth CA) had a 2-mm section stripe of insulation inserted in the DIAm. The remaining length of the electrode was tunneled subcutaneously to the dorsum of the rat and externalized for use in chronic EMG assessment.
Rhythmic DIAm EMG activity was assessed in anesthetized rats at the time of SH surgery and 3 days post-SH to confirm the absence of ipsilateral DIAm activity and thus the completeness of SH. Post-SH eupneic DIAm EMG activity was recorded in anesthetized rats to determine ipsilateral rhythmic recovery. EMG recordings were conducted using LabView data acquisition board and software (National Instruments, Austin, TX). The signal was amplified (2,000 ×), band-pass filtered (20–1,000 Hz), and analog-to-digital converted at 2 kHz (22, 28). Root mean square (RMS) was calculated to determine the EMG amplitude of DIAm activity using a sliding window period of 50 ms. In addition, the proportion of rats displaying rhythmic DIAm activity concurrent with contralateral ventilatory activity was determined. Recovery of DIAm EMG activity was classified as being present when the following criteria were met: 1) EMG signal was rhythmic, reflecting inspiratory activity in phase with contralateral DIAm activity, and was persistent across multiple inspiratory bursts; 2) EMG signal comprised more than one motor unit (multispike with different waveform profiles); and 3) EMG activity was at least 10% of peak RMS EMG amplitude prior to SH surgery (5). EMG measurements were analyzed for the duration of inspiration, respiratory rate, duty cycle (inspiratory time to total cycle time), and peak RMS EMG amplitude (25). Ventilatory patterns were determined from analyses of DIAm EMG recorded during normal, eupneic breathing in lightly anesthetized rats as in previous studies (24, 25).
DIAm in vitro contractility.
In a subset of control (n = 6) and SH rats (n = 7), DIAm isometric force was measured as previously described (1, 15, 21, 30, 49, 50). None of these rats underwent chronic EMG recordings to prevent any potential effects of EMG implantation. Briefly, 3-mm wide segments of the midcostal DIAm ipsilateral to SH (in experimental rats) were dissected with the rib and central tendon intact. Isolated muscle segments were mounted vertically in a glass chamber with oxygenated (95% O2-5% CO2) Rees-Simpson solution (pH 7.4) and maintained at 26°C. The maximum tetanic force of DIAm segments were determined using a force-frequency protocol of 1-s trains of stimuli at 5, 10, 20, 40, 50, 75, and 100 Hz. Force was normalized for DIAm physiological CSA [mass/(optimal length × muscle density)]. Fatigability of DIAm was determined with stimulation at 40 Hz in 330-ms trains each second over 2 min. Fatigue index was calculated as a ratio of the final force following 2 min of repetitive stimulation relative to the initial force.
DIAm histomorphological analysis.
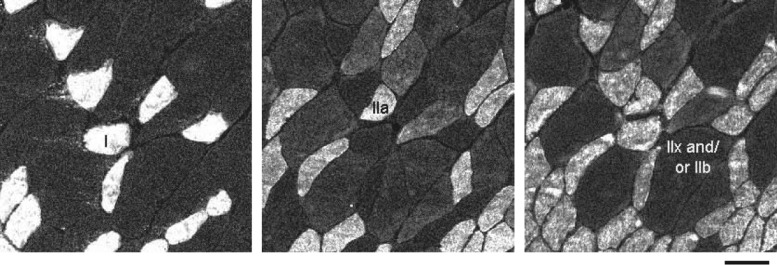
Samples of the DIAm were serially cut into 10 μm thick sections and fixed for further analysis. Serial DIAm sections were labeled using primary antibodies for MyHC isoforms: anti-MyHCSlow (NCL; Novocastra Antibodies, Leica Microsystems, Buffalo Grove, IL), anti-MyHC2A (A4–74), anti-MyHC2B (BF-F3), and an antibody reactive to all MyHC isoforms except MyHC2X (BF-35). Primary antibodies A4–74, BF-F3, and BF-35 were obtained from Developmental Studies Hybridoma Bank (Iowa City, IA). Sections were treated with appropriate fluorescently conjugated secondary antibodies. Muscle fibers were classified as type I, IIa, IIx and/or IIb based on expression of MyHCSlow, MyHC2A, MyHC2X and/or MyHC2B isoforms, respectively (Fig. 1). A single confocal image of each DIAm section was obtained using an Olympus Fluoview 300 laser-scanning confocal microscope (Olympus America, Melville, NY) mounted on an upright Olympus BX50WI microscope and equipped with Argon (488 nm) and HeNE (543 and 643 nm) lasers. As previously described (41), ∼250 type-identified fibers per DIAm sample were randomly sampled to determine fiber counts and measure fiber CSA using the morphometric tool in MetaMorph software (Universal Imaging, Downingtown, PA). All confocal images were saved using Fluoview software as 12-bit multi-TIFF files. All image stacks were separated in individual colors (channels) in MetaMorph software. Individual images were exported into Adobe Photoshop (Adobe Systems, San Jose, CA) as TIFF files, downconverted to 8-bit, and cropped to highlight representative areas for presentation only.
Fig. 1.
Representative serial cross-sections of type-identified rat diaphragm muscle (DIAm) fibers following 42 days of ipsilateral DIAm inactivity caused by unilateral spinal cord hemisection at C2 (SH). Fiber types were classified according to myosin heavy chain (MyHC) expression based on immunoreactivity to MyHC isoforms MyHCSlow, MyHC2A, and MyHC2B (not shown), and to all MyHC isoforms except MyHC2X. Notice expected differences in DIAm fiber cross-sectional area (CSA) across fiber types. Bar, 50 μm.
Statistical analysis.
All data were summarized and analyzed using JMP statistical software (JMP version 8.0; SAS Institute, Cary, NC). Repeated-measures ANOVA were used to determine group differences in DIAm EMG activity or ventilatory parameters over time. Differences in DIAm contractile properties and fiber type CSA and distribution between control and SH groups were compared using one-way ANOVA. As appropriate, post hoc analyses (Tukey-Kramer's honestly significant difference test) were conducted. When possible (i.e., ventilatory parameters and histomorphological analysis), additional analyses were conducted comparing SH animals that recovered any ipsilateral DIAm EMG activity vs. those that did not recover. Data are presented as means ± standard deviation (SD), unless otherwise specified.
RESULTS
Animals.
Following SH, animals recovered promptly and displayed only limited deficits in movement of their ipsilateral forelimb, as previously reported (30, 54). No animal required assisted ventilation and no deaths occurred in either experimental group. There was no difference in the body weight between SH and control rats following 42 days of intervention. By 42 days postintervention, rats weighed 383 ± 28 and 361 ± 35 g in the SH and control groups, respectively (P = 0.090).
Assessment of SH-induced DIAm inactivity.
In the SH group, DIAm EMG activity was lost immediately post-SH and was confirmed to be absent in all rats at day 3 post-SH, indicating the completeness of the hemisection. No technical failures in the SH procedure were evident in this study. In a few SH rats (5 of 12 rats where chronic EMG recordings were conducted), ipsilateral rhythmic DIAm activity became evident over time post-SH, reflecting recovery of DIAm activity post-SH. However, the extent of ipsilateral activity was greatly diminished compared with preinjury in all rats (Table 1). Accordingly, there was a significant difference in the RMS EMG amplitude between the contralateral (uninjured) and ipsilateral hemidiaphragms at 42 days post-SH. Of note, RMS EMG amplitude increased approximately twofold in the contralateral DIAm at day 42 post-SH, regardless of whether animals displayed recovery of ipsilateral DIAm activity. In contrast, there was an approximately eightfold reduction in RMS EMG amplitude ipsilaterally to SH overall, including both animals displaying recovery or those that did not, and, when examining only those rats that displayed recovery of ipsilateral DIAm activity, RMS EMG amplitude ipsilateral to SH was 26 ± 14% of the day 0 amplitude. In agreement with a lack of a substantial contribution of ipsilateral DIAm activity, there was no difference in contralateral DIAm EMG amplitude at day 42 across SH animals displaying recovery (n = 5) compared with those not displaying recovery (n = 7; P = 0.787).
Table 1.
Ventilatory parameters obtained in anesthetized rats during eupnea following 42 days of diaphragm muscle (DIAm) inactivity induced by unilateral spinal cord hemisection at C2 (SH)
| Contralateral (n = 12) | Ipsilateral (n = 12) | 2-Way Repeated Measures ANOVA Interaction P value | |
|---|---|---|---|
| DIAm EMG root mean square amplitude, mV | |||
| Day 0 | 135 ± 46 | 100 ± 33 | <0.001 |
| Day 42 | 234 ± 107* | 12 ± 26*† | |
| Burst duration, ms | |||
| Day 0 | 249 ± 32 | 249 ± 32 | <0.001 |
| Day 42 | 309 ± 66* | 78 ± 112*† | |
| Duty cycle, % | |||
| Day 0 | 29.7 ± 2.9 | 29.7 ± 2.9 | <0.001 |
| Day 42 | 39.1 ± 9.7* | 8.2 ± 11.6*† | |
Data were analyzed by 2-way repeated measures ANOVA with day as the repeated factor and are presented as means ± SD. Main effects: ipsilateral DIAm was different from contralateral DIAm for all reported data; day 0 and day 42 were different for burst duration and duty cycle.
Significantly different across time within same group;
Significantly different from contralateral at same day.
In lightly anesthetized rats, there was no evidence of tachypnea at 42 days post-SH (respiratory rate: 72 ± 7 min−1 in SH rats at day 0 vs. 75 ± 17 min−1 in SH rats at day 42; P = 0.537). Additional ventilatory parameters were significantly different between the contralateral and ipsilateral DIAm over the 42 days post-SH (Table 1). Inspiratory burst duration increased in the contralateral DIAm 42 days post-SH; however, burst duration decreased over time on the ipsilateral side. Accordingly, the duty cycle increased 32% in the contralateral DIAm post-SH and decreased 69% in the ipsilateral DIAm.
DIAm in vitro contractility.
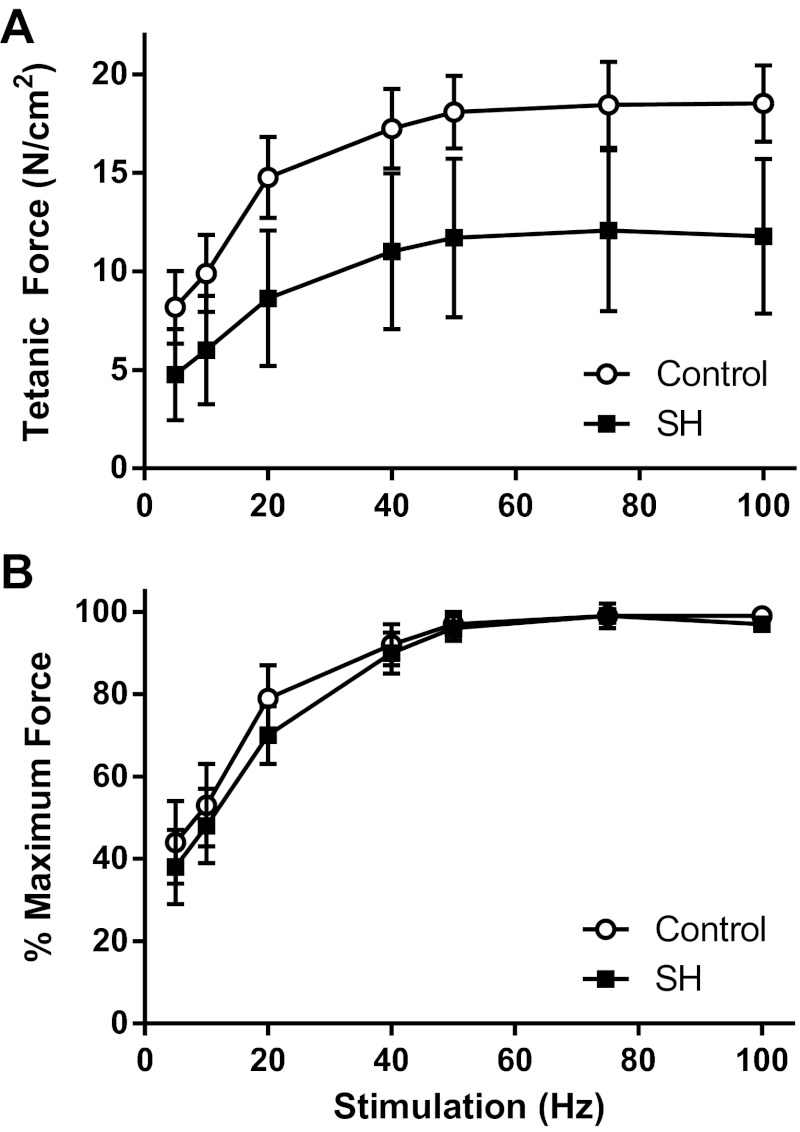
We previously found a modest decrease of 23% in specific force of the DIAm 14 days post-SH (30). In the current study, maximal isometric specific force in DIAm segments was ∼40% less following 42 days of SH-induced inactivity compared with control rats (P = 0.001; Table 2). The frequency at which maximal force is elicited was found to be 75 Hz, with the half-maximal force elicited at ∼10 Hz (Fig. 2). There was no difference in slope of the force-frequency relationship as a percentage of maximum force elicited between the SH and control groups (P = 0.367; Fig. 2). Consistent with measurements in DIAm segments, maximal isometric twitch force was ∼38% of tetanic force for both groups combined (P = 0.514). The DIAm fatigue index following 42 days of SH-induced inactivity was ∼50%, albeit with substantial variability, such that there was a trend increase in the DIAm fatigue index (P = 0.077; Table 2). The subset of rats that were analyzed for DIAm force did not undergo EMG measurements, and thus it was not possible to compare SH rats that had spontaneous recovery of ipsilateral EMG activity with those that did not recover for this measurement. Regardless, all of the SH rats displayed tetanic force levels (range: 6.7–16.3 N/cm2) that were below those of the control animals (range: 17.2–22.6 N/cm2).
Table 2.
Contractile properties in control DIAm and following 42 days of SH-induced DIAm inactivity
| n | Pt, N/cm2 | Po, N/cm2 | Fatigue Index, % | |
|---|---|---|---|---|
| Control | 6 | 7.7 ± 1.1 | 19.7 ± 1.9 | 28.5 ± 1.1 |
| SH | 7 | 4.5 ± 2.1* | 12.1 ± 4.0* | 47.6 ± 23.8 |
Data were analyzed by 1-way ANOVA and are presented as means ± SD. Pt, peak twitch force; Po, maximum tetanic force normalized for physiological cross-sectional area; Fatigue index is the ratio of force generated following repetitive stimulation (at 40 Hz in 330-ms trains each second) over 2 min to the initial force.
Significantly different from control (P ≤ 0.029).
Fig. 2.
Force-frequency relationship of DIAm in control rats and following 42 days of SH-induced DIAm inactivity. A: maximal isometric tetanic force generated by the DIAm at stimulation frequencies ≥20 Hz differed significantly between control (n = 6) and SH (n = 7) rats (one-way ANOVA with Bonferroni correction; P ≤ 0.006). B: there was no shift in the normalized force-frequency relationship (one-way ANOVA with Bonferroni correction; P ≥ 0.016). There was no difference in the slope of the force-frequency relationship between groups (one-way ANOVA; P = 0.367).
Histological characteristics of the DIAm.
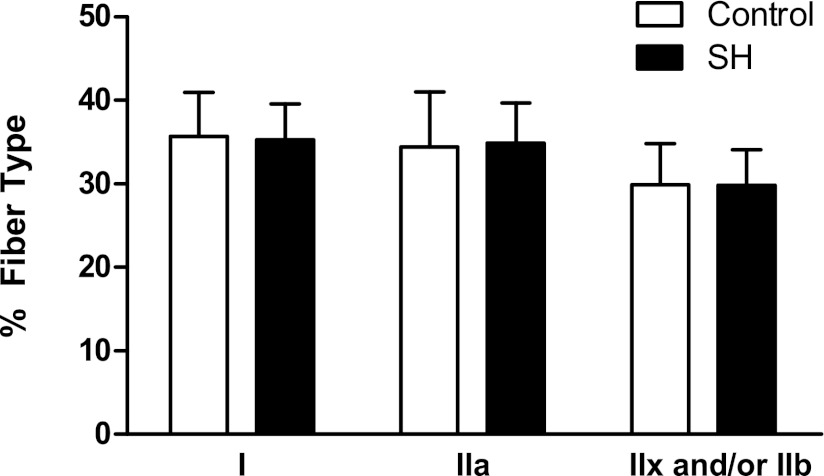
There was no difference in the distribution of DIAm fiber types between control and SH groups 42 days post-SH (P ≥ 0.841; Fig. 3). Overall, in control and SH DIAm, type I, type IIa, and type IIx and/or IIb fibers comprised 36%, 35%, and 30% of the total fibers, respectively (Fig. 3). Of note, there was no difference in the distribution of fiber types between SH rats that displayed spontaneous recovery of ipsilateral DIAm EMG activity and those that did not recover (P ≥ 0.333).
Fig. 3.
Distribution of fiber types in control DIAm and following 42 days of SH-induced DIAm inactivity. The proportion of type I, IIa, and IIx and/or IIb DIAm fibers was not different between the control (n = 9) and SH (n = 12) groups (one-way ANOVA; P ≥ 0.841 for all fiber type classifications).
There were modest, type-specific differences in fiber CSA following 42 days of SH-induced inactivity compared with controls (Fig. 4). As expected, there were fiber type differences in mean CSA, with type IIx and/or IIb fibers displaying larger CSA than type I or IIa fibers. The mean CSA of type I and IIa DIAm fibers was not different across groups (overall, type I = 956 ± 186 μm2 and type IIa = 1,011 ± 217 μm2; P ≥ 0.806). The CSA of type IIx and/or IIb DIAm fibers decreased 20% post-SH (2,821 ± 662 μm2 vs. 3,510 ± 580 μm2 in the control group; P = 0.023). Spontaneous recovery of ipsilateral activity in SH rats was not associated with differences in mean CSA for any fiber type compared with SH rats that did not recover ipsilateral activity (P ≥ 0.222). This lack of an effect of spontaneous recovery of ipsilateral DIAm activity on fiber CSA was evident even for type IIx and/or IIb DIAm fibers.
Fig. 4.
Fiber CSA of type-identified DIAm fibers in control DIAm and following 42 days of SH-induced DIAm inactivity. The CSA of type I and IIa DIAm fibers did not differ between control (n = 9) and SH (n = 12) groups (one-way ANOVA; P ≥ 0.806 for all fiber type classifications). However, there was a modest decrease in the CSA of type IIx and/or IIb DIAm fibers 42 days post-SH compared with control (one-way ANOVA; P = 0.023). Note differences in fiber CSA according to fiber type in both the control and SH groups. *Significant difference between control and SH group for same fiber type.
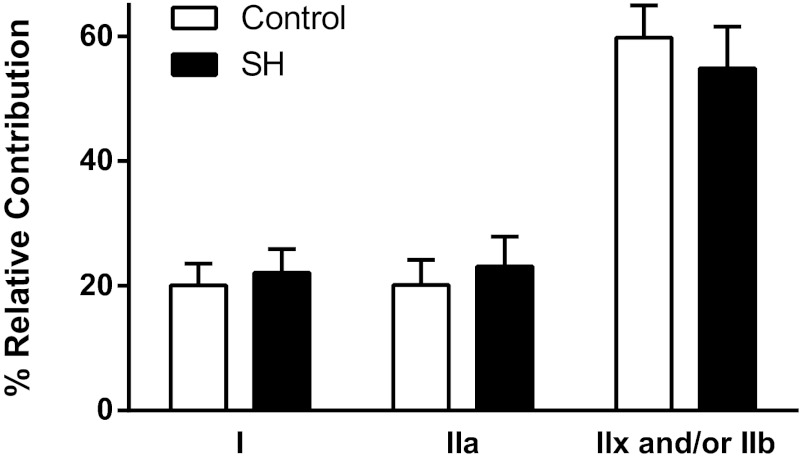
The contribution of each fiber type to total DIAm CSA was not affected by 42 days of SH-induced inactivity (P ≥ 0.080 for all fiber type classifications; Fig. 5). Both type I and IIa DIAm fibers account individually for ∼20% of the total DIAm CSA, whereas type IIx and/or IIb fibers account for ∼60% of the total CSA in the SH and control groups. Accordingly, there was no difference in the contribution of each fiber type to the total DIAm CSA when comparing SH rats that had spontaneous recovery and those that did not recover activity (P ≥ 0.494 for all fiber type classifications).
Fig. 5.
Contribution of each fiber type to the total DIAm CSA in control DIAm and following 42 days of SH-induced DIAm inactivity. There were no differences between control (n = 9) and SH (n = 12) groups (one-way ANOVA; P ≥ 0.080 for all fiber type classifications).
DISCUSSION
The results of this study provide novel information regarding the long-term effects of inactivity in the DIAm. Reduced specific force following ipsilateral SH was associated with modest, fiber type selective adaptations in DIAm fiber size, suggesting that changes in contractile protein content or force per cross-bridge may be present. Importantly, inactivity of the ipsilateral DIAm was verified by chronic EMG recordings, and although some animals displayed recovery of ipsilateral EMG activity, the amplitude of the DIAm EMG activity was substantially reduced compared with the initial (preinjury) activity or with the contralateral (uninjured) DIAm. Furthermore, there were no differences in any of the histomorphological measurements between SH animals displaying spontaneous recovery and those that did not recover ipsilateral DIAm activity. Overall, there were modest adaptations in DIAm fiber dimensions following prolonged inactivity, and thus the mechanisms underlying reduced force generation following inactivity do not reflect morphological changes in DIAm fibers.
Maximal force was reduced ∼40% with modest changes in DIAm fiber size following prolonged ipsilateral DIAm inactivity induced by SH. These results contrast previous reports using other models of DIAm disuse, such as controlled mechanical ventilation, which present both rapid and dramatic reductions in DIAm force and fiber dimensions (atrophy) (17, 19, 31–33, 36, 52). Several studies suggest that short periods of controlled mechanical ventilation result in significant reductions in both force and fiber dimensions across all fiber types. Following just 12 h of controlled mechanical ventilation, there is non-fiber type specific DIAm fiber atrophy, with reductions in DIAm fiber CSA of 25% in type IIx and/or IIb fibers and ∼15% in type I and IIa fibers with concomitant ∼20% decrease in DIAm force (17, 33, 52). Following 18 h of mechanical ventilation, DIAm fiber atrophy of up to 45% in type I fibers and 20–30% in type II fibers was present in rats, while DIAm force was reduced by ∼20% (29, 33, 40). Similarly, in brain dead patients that were mechanically ventilated between 18 and 69 h, there was DIAm fiber atrophy of 57% in type I fibers and 53% in type II fibers compared with patients exposed to 2–3 h of mechanical ventilation during lung surgery (19). However, DIAm force was not examined. Clearly, the relationship between DIAm force loss and fiber atrophy is not straightforward (44), especially since the loss in force appears to be similar between 12 and 18 h while a further loss in fiber size is occurring. Further reductions in DIAm force by 46% and 55% in rats, and 54% in rabbits were found following 24, 48, and 72 h of mechanical ventilation, respectively (18, 33, 36). Taken together, these findings suggest that the mechanisms accounting for force loss following controlled mechanical ventilation may differ from those associated with inactivity itself.
Other models of DIAm disuse, for example, unilateral phrenic nerve denervation (30) and chronic systemic corticosteroid administration (20), also cause selective atrophy of type II fibers, albeit to a much greater extent than that caused by SH-induced inactivity. For instance, type IIx and/or IIb fibers display substantial atrophy compared with controls [by 60% and 33% following 14 days of denervation (30) or corticosteroid administration (20), respectively]. However, these models have varying effects on DIAm force, with a denervation-induced force loss of 51% and no change in DIAm force following corticosteroid administration (20, 30). Coexpression of MyHC isoforms is found following denervation and hypothyroidism (10, 11, 27), and may contribute to reduced force. For instance, in the hypothyroidism model, the decrease in DIAm fiber force is greatest in type II fibers, consistent with the substantial MyHC isoform coexpression of MyHCSlow or MyHC2A found in type IIx and/or IIb fibers predominantly expressing MyHC2X and/or MyHC2B, respectively (10). MyHC coexpression was not examined directly in the current study. In studies using single, type-identified rat DIAm fibers, type I and IIa fibers exert ∼50% less specific force than type IIx and IIb fibers (12). It is possible to estimate the anticipated loss of total DIAm force based on the fiber type distributions, the CSA for each fiber type, and the specific force of type-identified single DIAm fibers (10–12). Using this estimate, the 20% decrease in CSA of type IIx and/or IIb fibers would result in ∼14% loss in total DIAm force with a minimal change in specific force (∼3%). Accordingly, the selective DIAm fiber atrophy does not account for the significant reduction in specific force following SH-induced DIAm inactivity (40% vs. uninjured controls).
In vitro measurements of fatigue resistance reflect the relative contribution of different fiber types to force generation in muscles of mixed fiber composition. As such, it is worth noting that the reduction of DIAm specific force following SH-induced inactivity was associated with a non-statistically significant increase in fatigue resistance (i.e., force following 2 min of 40-Hz repetitive stimulation). Clearly, in vitro fatigue measurements do not reflect in vivo fatigability of a given muscle (or muscle group).
In the present study, a subset of animals (5 of 12) displayed recovery of rhythmic, inspiratory activity in the DIAm ipsilateral to SH, as evidenced using chronic DIAm EMG recordings. These findings post-SH are in general agreement with previous studies that assessed ipsilateral DIAm (or phrenic nerve) activity at the terminal experiment only (8, 9, 13, 14, 55). Of note, the reliability of chronic DIAm EMG recordings was recently validated for longitudinal measurements of DIAm activity (24). The amplitude of DIAm EMG activity following SH remained consistently below preinjury (<25% of the initial RMS), even in those animals displaying recovery of ipsilateral activity. Importantly, there were no differences between animals displaying restored activity and those with no ipsilateral DIAm activity in any of the measurements obtained in this study, suggesting that the spontaneous recovery of DIAm activity following SH does not contribute to the differences in DIAm specific force or fiber size.
Changes in DIAm loading following SH could contribute to fiber atrophy and reduced force, as has been seen in limb muscles (47). However, there was no evidence of passive lengthening of the hemi-DIAm following ipsilateral denervation (53), indicating that paralysis resulted in minimal changes in load, despite contralateral activity. This is substantiated by a lack of DIAm fiber atrophy following exposure to microgravity compared with the atrophy of various limb muscles (10–35%) (39). In addition, SH, unilateral denervation and tetrodotoxin conduction block of the phrenic nerve all result in comparable ipsilateral DIAm inactivity and unloading (26, 27, 35), yet result in substantially different effects on muscle fibers (27, 30, 54).
It is evident that the effects of DIAm inactivity depend on the model examined, and the exact mechanism(s) of DIAm atrophy and force loss following inactivation are not well understood. For instance, increased reactive oxygen species and mitochondrial dysfunction (17, 52), local and systemic inflammation and cytokine production (37), and increased protein degradation and decreased protein synthesis (29) have been reported following controlled mechanical ventilation. In a rat model of cardiopulmonary bypass, a systemic inflammatory response contributes to reductions in DIAm force after only 1 h (6). Following phrenic nerve denervation, there are time-dependent changes in the net protein balance of the DIAm, reflecting a short-term increase in protein synthesis followed by a longer-term increase in degradation rates (2, 3). Certainly, reduced contractile protein expression in single fibers can contribute to reduced force, in agreement with findings in models of unilateral DIAm denervation (11, 45) or hypothyroidism (10). Alterations in force per cross-bridge and/or Ca2+ sensitivity can also contribute to reduced force, as found in a model of unilateral DIAm denervation (11).
Our results demonstrate a small but significant and selective decrease in the CSA of type IIx and/or IIb DIAm fibers following prolonged SH-induced inactivity, which would be consistent with a reduction in force without any change in specific force. However, it appears that the reduction in specific force exceeds that which can be attributed to the reduction in CSA alone. These data, as collected at the whole muscle level, are a necessary first step for the analysis of the impact of prolonged inactivity on DIAm force generation. Therefore, future studies should be designed to examine such changes at single type-identified DIAm fibers following prolonged inactivity. Specifically, the examination of force per cross-bridge and contractile protein content per half-sarcomere in single fiber studies will be useful future studies to pinpoint specific mechanisms in several models of inactivity.
GRANTS
This research was supported by National Institutes of Health Grants RO1-HL-096750 (GCS and CBM) and T32-HL-105355 (SMG), the Paralyzed Veterans of America Research Foundation (CBM and GCS), and the Mayo Clinic.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
C.B.M. and G.C.S. conception and design of research; C.B.M., S.M.G., W.Z.Z., and Y.B.S. analyzed data; C.B.M., S.M.G., W.Z.Z., Y.B.S., and G.C.S. interpreted results of experiments; C.B.M. and S.M.G. prepared figures; C.B.M. and S.M.G. drafted manuscript; C.B.M., S.M.G., W.Z.Z., Y.B.S., and G.C.S. edited and revised manuscript; C.B.M., S.M.G., W.Z.Z., Y.B.S., and G.C.S. approved final version of manuscript; W.Z.Z. and Y.B.S. performed experiments.
ACKNOWLEDGMENTS
We would like to acknowledge Jessica Stowe and Juan N. Hurtado-Palomino for technical assistance in the completion of this project.
REFERENCES
- 1. Ameredes BT, Zhan WZ, Prakash YS, Vandenboom R, Sieck GC. Power fatigue of the rat diaphragm muscle. J Appl Physiol 89: 2215–2219, 2000 [DOI] [PubMed] [Google Scholar]
- 2. Argadine HM, Hellyer NJ, Mantilla CB, Zhan WZ, Sieck GC. The effect of denervation on protein synthesis and degradation in adult rat diaphragm muscle. J Appl Physiol 107: 438–444, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Argadine HM, Mantilla CB, Zhan WZ, Sieck GC. Intracellular signaling pathways regulating net protein balance following diaphragm muscle denervation. Am J Physiol Cell Physiol 300: C318–C327, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dow DE, Mantilla CB, Zhan WZ, Sieck GC. EMG-based detection of inspiration in the rat diaphragm muscle. Conf Proc IEEE Eng Med Biol Soc 1: 1204–1207, 2006 [DOI] [PubMed] [Google Scholar]
- 5. Dow DE, Zhan WZ, Sieck GC, Mantilla CB. Correlation of respiratory activity of contralateral diaphragm muscles for evaluation of recovery following hemiparesis. Conf Proc IEEE Eng Med Biol Soc 2009: 404–407, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ermilov LG, Pulido JN, Atchison FW, Zhan WZ, Ereth MH, Sieck GC, Mantilla CB. Impairment of diaphragm muscle force and neuromuscular transmission after normothermic cardiopulmonary bypass: effect of low-dose inhaled CO. Am J Physiol Regul Integr Comp Physiol 298: R784–R789, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fournier M, Sieck GC. Mechanical properties of muscle units in the cat diaphragm. J Neurophysiol 59: 1055–1066, 1988 [DOI] [PubMed] [Google Scholar]
- 8. Fuller DD, Doperalski NJ, Dougherty BJ, Sandhu MS, Bolser DC, Reier PJ. Modest spontaneous recovery of ventilation following chronic high cervical hemisection in rats. Exp Neurol 211: 97–106, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fuller DD, Golder FJ, Olson EB, Jr, Mitchell GS. Recovery of phrenic activity and ventilation after cervical spinal hemisection in rats. J Appl Physiol 100: 800–806, 2006 [DOI] [PubMed] [Google Scholar]
- 10. Geiger PC, Cody MJ, Han YS, Hunter LW, Zhan WZ, Sieck GC. Effects of hypothyroidism on maximum specific force in rat diaphragm muscle fibers. J Appl Physiol 92: 1506–1514, 2002 [DOI] [PubMed] [Google Scholar]
- 11. Geiger PC, Cody MJ, Macken RL, Bayrd ME, Sieck GC. Effect of unilateral denervation on maximum specific force in rat diaphragm muscle fibers. J Appl Physiol 90: 1196–1204, 2001 [DOI] [PubMed] [Google Scholar]
- 12. Geiger PC, Cody MJ, Macken RL, Sieck GC. Maximum specific force depends on myosin heavy chain content in rat diaphragm muscle fibers. J Appl Physiol 89: 695–703, 2000 [DOI] [PubMed] [Google Scholar]
- 13. Golder FJ, Fuller DD, Davenport PW, Johnson RD, Reier PJ, Bolser DC. Respiratory motor recovery after unilateral spinal cord injury: eliminating crossed phrenic activity decreases tidal volume and increases contralateral respiratory motor output. J Neurosci 23: 2494–2501, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Goshgarian HG. The crossed phrenic phenomenon: a model for plasticity in the respiratory pathways following spinal cord injury. J Appl Physiol 94: 795–810, 2003 [DOI] [PubMed] [Google Scholar]
- 15. Gosselin LE, Zhan WZ, Sieck GC. Hypothyroid-mediated changes in adult rat diaphragm muscle contractile properties and MHC isoform expression. J Appl Physiol 80: 1934–1939, 1996 [DOI] [PubMed] [Google Scholar]
- 16. Greising SM, Gransee HM, Mantilla CB, Sieck GC. Systems biology of skeletal muscle: fiber type as an organizing principle. Wiley Interdiscip Rev Syst Biol Med 4: 457–473, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hudson MB, Smuder AJ, Nelson WB, Bruells CS, Levine S, Powers SK. Both high level pressure support ventilation and controlled mechanical ventilation induce diaphragm dysfunction and atrophy. Crit Care Med 40: 1254–1260, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Le Bourdelles G, Viires N, Boczkowski J, Seta N, Pavlovic D, Aubier M. Effects of mechanical ventilation on diaphragmatic contractile properties in rats. Am J Respir Crit Care Med 149: 1539–1544, 1994 [DOI] [PubMed] [Google Scholar]
- 19. Levine S, Nguyen T, Taylor N, Friscia ME, Budak MT, Rothenberg P, Zhu J, Sachdeva R, Sonnad S, Kaiser LR, Rubinstein NA, Powers SK, Shrager JB. Rapid disuse atrophy of diaphragm fibers in mechanically ventilated humans. N Engl J Med 358: 1327–1335, 2008 [DOI] [PubMed] [Google Scholar]
- 20. Lewis MI, Monn SA, Sieck GC. Effect of corticosteroids on diaphragm fatigue, SDH activity, and muscle fiber size. J Appl Physiol 72: 293–301, 1992 [DOI] [PubMed] [Google Scholar]
- 21. Lewis MI, Sieck GC, Fournier M, Belman MJ. Effect of nutritional deprivation on diaphragm contractility and muscle fiber size. J Appl Physiol 60: 596–603, 1986 [DOI] [PubMed] [Google Scholar]
- 22. Mantilla CB, Bailey JP, Zhan WZ, Sieck GC. Phrenic motoneuron expression of serotonergic and glutamatergic receptors following upper cervical spinal cord injury. Exp Neurol 234: 191–199, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mantilla CB, Rowley KL, Zhan WZ, Fahim MA, Sieck GC. Synaptic vesicle pools at diaphragm neuromuscular junctions vary with motoneuron soma, not axon terminal, inactivity. Neuroscience 146: 178–189, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Mantilla CB, Seven YB, Hurtado-Palomino JN, Zhan WZ, Sieck GC. Chronic assessment of diaphragm muscle EMG activity across motor behaviors. Respir Physiol Neurobiol 177: 176–182, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mantilla CB, Seven YB, Zhan WZ, Sieck GC. Diaphragm motor unit recruitment in rats. Respir Physiol Neurobiol 173: 101–106, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mantilla CB, Sieck GC. Invited review: mechanisms underlying motor unit plasticity in the respiratory system. J Appl Physiol 94: 1230–1241, 2003 [DOI] [PubMed] [Google Scholar]
- 27. Mantilla CB, Sieck GC. Neuromuscular adaptations to respiratory muscle inactivity. Respir Physiol Neurobiol 169: 133–140, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mantilla CB, Sieck GC. Phrenic motor unit recruitment during ventilatory and non-ventilatory behaviors. Respir Physiol Neurobiol 179: 57–63, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. McClung JM, Kavazis AN, Whidden MA, DeRuisseau KC, Falk DJ, Criswell DS, Powers SK. Antioxidant administration attenuates mechanical ventilation-induced rat diaphragm muscle atrophy independent of protein kinase B (PKB Akt) signalling. J Physiol 585: 203–215, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Miyata H, Zhan WZ, Prakash YS, Sieck GC. Myoneural interactions affect diaphragm muscle adaptations to inactivity. J Appl Physiol 79: 1640–1649, 1995 [DOI] [PubMed] [Google Scholar]
- 31. Mrozek S, Jung B, Petrof BJ, Pauly M, Roberge S, Lacampagne A, Cassan C, Thireau J, Molinari N, Futier E, Scheuermann V, Michel Constantin J, Matecki S, Jaber S. Rapid onset of specific diaphragm weakness in a healthy murine model of ventilator-induced diaphragmatic dysfunction. Anesthesiology 117: 560–567, 2012 [DOI] [PubMed] [Google Scholar]
- 32. Powers SK, Kavazis AN, Levine S. Prolonged mechanical ventilation alters diaphragmatic structure and function. Crit Care Med 37: S347–S353, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Powers SK, Shanely RA, Coombes JS, Koesterer TJ, McKenzie M, Van Gammeren D, Cicale M, Dodd SL. Mechanical ventilation results in progressive contractile dysfunction in the diaphragm. J Appl Physiol 92: 1851–1858, 2002 [DOI] [PubMed] [Google Scholar]
- 34. Prakash YS, Miyata H, Zhan WZ, Sieck GC. Inactivity-induced remodeling of neuromuscular junctions in rat diaphragmatic muscle. Muscle Nerve 22: 307–319, 1999 [DOI] [PubMed] [Google Scholar]
- 35. Rowley KL, Mantilla CB, Sieck GC. Respiratory muscle plasticity. Respir Physiol Neurobiol 147: 235–251, 2005 [DOI] [PubMed] [Google Scholar]
- 36. Sassoon CS, Caiozzo VJ, Manka A, Sieck GC. Altered diaphragm contractile properties with controlled mechanical ventilation. J Appl Physiol 92: 2585–2595, 2002 [DOI] [PubMed] [Google Scholar]
- 37. Schellekens WJ, van Hees HW, Vaneker M, Linkels M, Dekhuijzen PN, Scheffer GJ, van der Hoeven JG, Heunks LM. Toll-like receptor 4 signaling in ventilator-induced diaphragm atrophy. Anesthesiology 117: 329–338, 2012 [DOI] [PubMed] [Google Scholar]
- 38. Schiaffino S, Reggiani C. Fiber types in mammalian skeletal muscles. Physiol Rev 91: 1447–1531, 2011 [DOI] [PubMed] [Google Scholar]
- 39. Schuenke MD, Reed DW, Kraemer WJ, Staron RS, Volek JS, Hymer WC, Gordon S, Perry Koziris L. Effects of 14 days of microgravity on fast hindlimb and diaphragm muscles of the rat. Eur J Appl Physiol 106: 885–892, 2009 [DOI] [PubMed] [Google Scholar]
- 40. Shanely RA, Zergeroglu MA, Lennon SL, Sugiura T, Yimlamai T, Enns D, Belcastro A, Powers SK. Mechanical ventilation-induced diaphragmatic atrophy is associated with oxidative injury and increased proteolytic activity. Am J Respir Crit Care Med 166: 1369–1374, 2002 [DOI] [PubMed] [Google Scholar]
- 41. Sieck DC, Zhan WZ, Fang YH, Ermilov LG, Sieck GC, Mantilla CB. Structure-activity relationships in rodent diaphragm muscle fibers vs. neuromuscular junctions. Respir Physiol Neurobiol 180: 88–96, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sieck GC. Diaphragm muscle: structural and functional organization. Clin Chest Med 9: 195–210, 1988 [PubMed] [Google Scholar]
- 43. Sieck GC, Fournier M. Diaphragm motor unit recruitment during ventilatory and nonventilatory behaviors. J Appl Physiol 66: 2539–2545, 1989 [DOI] [PubMed] [Google Scholar]
- 44. Sieck GC, Mantilla CB. Effect of mechanical ventilation on the diaphragm. N Engl J Med 358: 1392–1394, 2008 [DOI] [PubMed] [Google Scholar]
- 45. Sieck GC, Zhan WZ, Han YS, Prakash YS. Effect of denervation on ATP consumption rate of diaphragm muscle fibers. J Appl Physiol 103: 858–866, 2007 [DOI] [PubMed] [Google Scholar]
- 46. Sieck GC, Zhan WZ, Prakash YS, Daood MJ, Watchko JF. SDH and actomyosin ATPase activities of different fiber types in rat diaphragm muscle. J Appl Physiol 79: 1629–1639, 1995 [DOI] [PubMed] [Google Scholar]
- 47. Talmadge RJ, Roy RR, Edgerton VR. Prominence of myosin heavy chain hybrid fibers in soleus muscle of spinal cord-transected rats. J Appl Physiol 78: 1256–1265, 1995 [DOI] [PubMed] [Google Scholar]
- 48. Trelease RB, Sieck GC, Harper RM. A new technique for acute and chronic recording of crural diaphragm EMG in cats. Electroencephalogr Clin Neurophysiol 53: 459–462, 1982 [DOI] [PubMed] [Google Scholar]
- 49. Van Balkom RH, Zhan WZ, Prakash YS, Dekhuijzen PN, Sieck GC. Corticosteroid effects on isotonic contractile properties of rat diaphragm muscle. J Appl Physiol 83: 1062–1067, 1997 [DOI] [PubMed] [Google Scholar]
- 50. Van Der Heijden HF, Zhan WZ, Prakash YS, Dekhuijzen PN, Sieck GC. Salbutamol enhances isotonic contractile properties of rat diaphragm muscle. J Appl Physiol 85: 525–529, 1998 [DOI] [PubMed] [Google Scholar]
- 51. Vinit S, Gauthier P, Stamegna JC, Kastner A. High cervical lateral spinal cord injury results in long-term ipsilateral hemidiaphragm paralysis. J Neurotrauma 23: 1137–1146, 2006 [DOI] [PubMed] [Google Scholar]
- 52. Whidden MA, Smuder AJ, Wu M, Hudson MB, Nelson WB, Powers SK. Oxidative stress is required for mechanical ventilation-induced protease activation in the diaphragm. J Appl Physiol 108: 1376–1382, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zhan WZ, Farkas GA, Schroeder MA, Gosselin LE, Sieck GC. Regional adaptations of rabbit diaphragm muscle fibers to unilateral denervation. J Appl Physiol 79: 941–950, 1995 [DOI] [PubMed] [Google Scholar]
- 54. Zhan WZ, Miyata H, Prakash YS, Sieck GC. Metabolic and phenotypic adaptations of diaphragm muscle fibers with inactivation. J Appl Physiol 82: 1145–1153, 1997 [DOI] [PubMed] [Google Scholar]
- 55. Zhou SY, Basura GJ, Goshgarian HG. Serotonin(2) receptors mediate respiratory recovery after cervical spinal cord hemisection in adult rats. J Appl Physiol 91: 2665–2673, 2001 [DOI] [PubMed] [Google Scholar]