Abstract
Mammalian cells respond to amino acid deprivation through multiple signaling pathways referred to as the amino acid response (AAR). Transcription factors mediate the AAR after their activation by several mechanisms; examples include translational control (activating transcription factor 4, ATF4), phosphorylation (p-cJUN), and transcriptional control (ATF3). ATF4 induces ATF3 transcription through a promoter-localized C/EBP-ATF response element (CARE). The present report characterizes an ATF/CRE site upstream of the CARE that also contributes to AAR-induced ATF3 transcription. ATF4 binds to the ATF/CRE and CARE sequences and both are required for a maximal response to ATF4 induction. ATF3, which antagonizes ATF4 and represses its own gene, also exhibited binding activity to the ATF/CRE and CARE sequences. The AAR resulted in elevated total cJUN and p-cJUN protein levels and both forms exhibited binding activity to the ATF/CRE and CARE ATF3 sequences. Knockdown of AAR-enhanced cJUN expression blocked induction of the ATF3 gene and mutation of either the ATF/CRE or the CARE site prevented the cJUN-dependent increase in ATF3-driven luciferase activity. The results indicate that both increased cJUN and the cis-acting ATF/CRE sequence within the ATF3 promoter contribute to the transcriptional activation of the gene during the AAR.
Keywords: ATF4, nutrition, amino acid limitation, gene expression, liver
dynamic regulation of gene expression in mammalian cells is an important aspect of the cellular response to environmental nutrient changes. Amino acid limitation of cells or tissue can be caused by dietary protein insufficiency or by a protein diet that contains an amino acid imbalance. Amino acid deficiency triggers a series of signal transduction pathways collectively referred to as the amino acid response (AAR). A number of genes critical to the AAR have been identified (6, 21), but the mechanistic details of their transcriptional control are not fully understood. The general control nonderepressible 2 (GCN2) kinase acts as an sensor for amino acid levels by binding uncharged transfer tRNA, which leads to phosphorylation of the alpha subunit of the translation initiation factor eIF2 and increased translation of the activating transcription factor 4 (ATF4). Accumulation of ATF4 protein activates a broad spectrum of downstream target genes (17), including ATF3 (22, 28). In addition to the GCN2/eIF2/ATF4 pathway, mitogen-activated protein kinase (MAPK) pathways are activated by the AAR, which leads to phosphorylation/activation of critical transcription factors such as ATF2 (7) and cJUN (10).
The ATF3 gene is amino acid responsive and exhibits increased transcription in response to ATF4 (28), but the mechanisms are not fully resolved. It is known that the histone acetyltransferase (HAT) ATF2 (7) and ATF4-C/EBPβ (CCAAT-enhancer binding protein) binding to a proximal promoter enhancer site are required (28). The ATF3 enhancer sequence that is ATF4 responsive is composed of a half-site for the C/EBP family and a half-site for the ATF family of transcription factors and was first identified by Wolfgang et al. (39) as a sequence necessary for ATF3 autorepression. Identification of many C/EBP-ATF composite sequences has led to the consensus sequence of 5′-TGATGXAAX-3′ and the demonstration that these sequences mediate gene activation in response to a wide spectrum of stimuli that result in increased ATF4 synthesis (18, 38). To retain the original nomenclature of “C/EBP-ATF,” but also to indicate the broad array of genes that contain a variant of this sequence, we will refer to these sequences collectively as C/EBP-ATF response elements (CARE). The same CARE site within a particular gene can mediate ATF4-dependent transcriptional activation in response to many different stimuli. For example, the ATF3 CARE sequence has been implicated in activation of the gene by endoplasmic reticulum (ER) stress (19), amino acid deprivation (28), arsenite exposure(9), and treatment with an anti-inflammatory drug (24). ATF4 appears to bind to the ATF3 CARE site as a heterodimer with members of the C/EBP family, and subsequently, the resulting elevated ATF3 protein itself serves as a feedback suppressor of ATF4 action (28).
ATF3 is a member of the ATF subfamily of the basic region-leucine zipper (bZIP) superfamily of transcription factors. Other subfamilies include the JUN/FOS, C/EBP, and MAF proteins (16). Although many nonconsensus sequences have been identified for the bZIP members, the bZIP proteins generally bind as either homo- or heterodimers to sequences encompassed by the ATF/CRE (TGACGTCA), AP-1 (TGAC/GTCA), and C/EBP-ATF response element (CARE, TGATGXAAX) genomic elements. The “AP-1” family of transcriptional regulators includes complexes composed of homo- or heterodimers of cJUN, JUN-B, JUN-D, cFOS, FOS-B, Fra1, and Fra2 (31). Some of the JUN/FOS proteins are induced during the AAR (30), and the increase in cJUN expression occurs by a process that is independent of ATF4 signaling (10). ATF/CRE elements have been identified within the ATF3 gene (16, 37), and in particular, an ATF/CRE site (nt −93/−85) exists upstream of the ATF3 CARE site (nt −23/−15). One member of the JUN family, JunB, has been reported to act via this site to regulate ATF3 expression (13). Pan et al. (28) reported that while AAR activation of ATF3 transcription still occurs without this ATF/CRE sequence, the degree of ATF4-driven induction was greater if both the ATF/CRE and CARE sites were present.
In the present study, we report that the ATF3 ATF/CRE site at nt −93/−85 is necessary to achieve full transcriptional induction during the AAR and that ATF4-mediated regulation of the ATF3 gene occurs through both the CARE and ATF/CRE sites. Furthermore, cJUN is a component of the complexes that interact with the ATF/CRE and CARE sites within the ATF3 proximal promoter.
EXPERIMENTAL PROCEDURES
Cell culture.
HepG2 human hepatoma cells were cultured in Dulbecco's modified Eagle's medium (DMEM, pH 7.4) (Mediatech, Manassas, VA) at 37°C in a humidified atmosphere of 5% CO2 and 95% air. The medium was supplemented with 1× nonessential amino acids, 2 mM glutamine, 100 mg/ml streptomycin sulfate, 100 units/ml penicillin G, 0.25 mg/ml amphotericin B, and 10% fetal bovine serum. The cultures were replenished with fresh medium 12 h prior to initiating all treatments to ensure that the cells were in the basal state. The AAR was activated by incubation in culture medium containing 2 mM histidinol (HisOH), which blocks charging of histidine onto the corresponding tRNA and thus activates the AAR pathways by mimicking histidine deprivation without depleting the cellular histidine level (35).
Reagents.
Antibodies against the following molecules were obtained from Santa Cruz Biotechnology (Santa Cruz, CA): cJUN (sc-1694), Ser-63 p-cJUN (sc-822), ATF2 (sc-187), Thr-71 p-ATF2 (sc-8398), ATF3 (sc-188), C/EBPβ (sc-7962 and sc-150). The asparagine synthetase (ASNS) and ATF4 antibodies as well as the ATF4 wild-type and dominant negative (DN)-ATF4 expression plasmids were described previously (33). The cJUN and DN-cJUN expression plasmids were kindly provided by Dr. Harry Nick, University of Florida. The pTRIPZ-shRNA plasmid constructs against cJUN (RHS4696–996383) or ATF4 (RHS4696–99703331) were purchased from Open Biosystems (Huntsville, AL). The viral packaging vectors, pMD2g and psPAX2, were generously provided by Dr. Jianrong Lu, University of Florida.
RNA isolation and quantitative RT-PCR.
Total RNA was isolated with TRIzol Reagent (Invitrogen) following the manufacturer's protocol. Within an experiment, RNA samples were prepared from triplicate cultures for each condition to measure variation, and multiple experiments were performed to assess reproducibility. To measure steady-state mRNA for a specific gene, real-time quantitative reverse transcriptase PCR (qPCR) analysis was performed with SYBR Green detection using a DNA Engine Opticon 2 system (Bio-Rad). To monitor transcription activity from the ATF3 gene, we measured the short-lived heterogeneous nuclear RNA (hnRNA) by qPCR with primers that cross an exon-intron boundary. The validity of using hnRNA as a measure of transcription activity was originally documented by Lipson and Baserga (26). To ensure that DNA contamination was not responsible for detected products, each sample was tested in the absence of reverse transcriptase. The PCR primers used for ATF3 were as follows: for steady-state mRNA, forward 5′-GAAGAGCTGAGGTTTGCCATCCA-3′, reverse 5′-TGATTCCAGCGCAGAGGACATC-3′; for transcription activity, forward 5′-CCCTCGGGGTGTCCATCACAAA-3′, reverse 5′-TGGCTGCGAGCGAAACATG-3′. The primers for steady-state cJUN and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA were described previously (10).
Protein isolation and immunoblotting.
To obtain protein extracts, cells were washed with ice-cold PBS and then lysed with RIPA buffer (50 mM Tris, 150 mM NaCl, 0.1% SDS, 0.5% sodium deoxycholate, and 1% Triton X-100) supplemented with a protease and phosphatase inhibitor mix (Roche Applied Science). The whole cell lysate was sonicated, and protein was quantified before separation on a 10.5–14% Tris·HCl polyacrylamide gel and electro-transfer to a Trans-Blot PVDF membrane (Bio-Rad). The membrane was blocked in TBST (30 mM Tris, pH 7.5, 200 mM NaCl, and 0.1% Tween 20) containing 5 or 10% Carnation nonfat dry milk for 1 h at room temperature. Each primary antibody was diluted in blocking solution and used to incubate the blots for 1–2 h at room temperature or overnight at 4°C. The blots were washed and then incubated with the appropriate peroxidase-conjugated secondary antibody (Bio-Rad Laboratories, Hercules, CA) in blocking buffer for 1 h at room temperature. Bound secondary antibody was detected with an enhanced chemiluminescence kit (Pierce) after washing with TBST solution.
Coimmunoprecipitation assay.
HepG2 cells were treated with 2 mM HisOH for 3 or 12 h, washed with ice-cold PBS, and then incubated for 15 min on ice in cell lysis buffer (10 mM HEPES, 10 mM KCl, and 0.1 mM EDTA) containing a protease/phosphatase inhibitor mixture. After adding NP-40 to a final concentration of 0.3%, we centrifuged the samples at 2,700 g for 5 min at 4°C. The pellets were washed with cell lysis buffer three times and then incubated in nuclear extraction buffer (20 mM HEPES, 400 mM NaCl, and 1.0 mM EDTA) with protease/phosphatase inhibitors on ice for 30 min or −80°C overnight. The extracts were centrifuged at 21,000 g for 15 min, and the supernatant was collected as a nuclear protein extract. A 500 μg aliquot of the extract was precleared with 50 μl of a 50% slurry of recombinant protein G-Sepharose beads 4B (GE Healthcare) for 60 min at 4°C. After removing the beads by centrifugation, we incubated the supernatant with 4 μg of primary antibody or nonspecific IgG (negative control) overnight at 4°C. The protein-antibody complex was collected with recombinant protein G-Sepharose 4B beads, washed with buffer (50 mM Tris·HCl, 150 mM NaCl, 1% NP-40, pH 8.0) five times at 4°C, eluted by boiling in Laemmli buffer (Bio-Rad, #1610737), and then processed for immunoblot analysis. For the coimmunoprecipitation (co-IP) immunoblot analysis, protein detection involved horseradish peroxidase (HRP)-conjugated protein A (Upstate/Millipore, Billerica, MA) or HRP-conjugated anti-rabbit IgG light chain specific antibody (Jackson ImmunoResearch Laboratories, West Grove, PA).
DNA affinity precipitation assay.
The DNA affinity precipitation assay (DAPA) protocol was described previously (10). The sense and anti-sense 5′ biotin-labeled oligonucleotides corresponding to the wild-type CARE-binding site (5′-ATAAAAGGGGTGATGCAACGCTCTCCAAG-3′), a CARE binding site mutant (5′-GGGTTACTCGCCGCT-3′), the wild-type ATF/CRE-binding site (5′-ATTAATAGCATTACGTCAGCCTGGGACTG-3′), and a ATF/CRE-binding site mutant (5′-ATTAATAGCACCAAGACCGCCTGGGACTG-3′) within the ATF3 promoter were purchased from Sigma-Genosys (Woodlands, TX). A longer probe (nt −104/+27), containing both the ATF3 CARE and ATF/CRE sites, was synthesized by PCR with biotin-labeled primers (forward 5′-TATTAATAGCATTACGTCAGCC-3′ and reverse 5′-GCAGTGCGCGCCTGGCTGCGTGCGACTGTGGCTT-3′). The sense and anti-sense oligonucleotides were annealed by combining, heating to 95°C for 5 min, and then cooling by −1°C per min to 25°C. A 100 μg aliquot of nuclear protein extract was mixed with 1 μg of biotin-labeled DNA probe in 400 μl of buffer D (20 mM HEPES, pH 7.9, 10% glycerol, 50 mM KCl, 0.2 mM EDTA, 1.5 mM MgCl2, 1 mM dithiothreitol, and 0.25% Triton X-100) and incubated at 4°C for 1 h with rotation. A 50 μl aliquot of streptavidin-agarose beads (Sigma) was added to the samples for 1 h at 4°C with rotation. For a DNA competition assay, 100 μg of nuclear protein extract was preincubated with 10 μg of nonbiotin-labeled DNA competitor sequence for 30 min at 4°C, and then the biotin-labeled DNA probe was added to the sample for the binding reaction. The agarose bead-protein complexes were collected by brief centrifugation and washed five times with buffer D. Proteins were eluted from the DNA probes with 40–50 μl of Laemmli buffer and heated at 96°C for 5–10 min. The resulting supernatants were analyzed by immunoblot analysis as described above.
Chromatin immunoprecipitation analysis.
HepG2 cells were seeded at 1.5 ×107 per 150-mm dish with complete DMEM, cultured for 24 h, and then given fresh DMEM for 12 h before transfer to either complete DMEM or DMEM containing 2 mM HisOH for 1, 3, 6, or 12 h. Chromatin immunoprecipitation (ChIP) analysis was performed as described previously (10). Purified, immunoprecipitated DNA was analyzed by qPCR with a DNA Engine Opticon 3 system using the primers covering the ATF3 promoter region (forward 5′-CTGGCTTGGGCACCATTGGT-3′, reverse 5′-CCCGCCCTCTCTCTCCATATCA-3′) and downstream of the coding region (nt +6469, within exon 2) of the gene (forward 5′-CCCTCGGGGTGTCCATCACAAA-3′, reverse 5′-TGGCTGCGAGCGAAACATG-3′). The PCR product was detected with SYBR Green I (Applied Biosystems, Carlsbad, CA), and the ChIP results are expressed as the ratio to input DNA. Samples were prepared in triplicate for each condition and each experiment was performed at least twice.
Transient transfection and firefly luciferase assay.
For transient protein expression studies, HepG2 cells (0.4 × 106 cells per well in six-well plates or 0.8 × 106 cells per 60-mm dish) were seeded 18–24 h before transfection with expression constructs in pcDNA3.1 using GenJet Reagent II (SignaGen Laboratories, Gaithersburg, MD), following the manufacturer's protocol. At 8 h posttransfection, we enriched the cells by selecting with 2 μg/ml puromycin for 40 h. Following selection, the cells were transferred to fresh control DMEM for 12 h and then transferred to control DMEM or DMEM containing 2 mM HisOH for 4–16 h prior to collecting RNA and protein for analysis. For Firefly luciferase reporter assays, HepG2 cells (0.05–0.1 × 106 cells per well) were seeded on 24-well plates 18–24 h before transfection with Superfect reagent (Qiagen, Valencia, CA) at a ratio of 6 μl per μg plasmid DNA. For each transfection, 0.5 μg of plasmid containing the Firefly luciferase reporter gene (pGL3), driven by amino acid-responsive genomic fragments from the ATF3 gene, was used along with the indicated amount of transcription factor expression plasmids (28). The background values for empty pGL3 plasmid without or with each of the overexpressed transcription factors were determined to be small (an example for cJUN and DN-cJUN is given in Fig. 2C). The pGL3 constructs with the ATF3 promoter region (nt −107/+35) containing wild-type CARE or ATF/CRE sites as well as constructs with mutations obtained using the QuikChange II XL Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA). Final constructs were verified by DNA sequencing. The total amount of transfected DNA was kept constant at 600 ng/well among experimental groups by the addition of empty pcDNA3.1 plasmid. At 24–36 h following transfection, cells were incubated in control DMEM or DMEM containing 2 mM HisOH for the time indicated. We prepared cell extracts for analysis of luciferase activity by washing the cells with PBS and incubating them in 200 μl of lysis buffer (Promega, Madison, WI). The lysates were collected and stored at −80°C until luciferase assays were performed. The Firefly luciferase assays were performed in triplicate to establish the variation within an experiment, and at least two independent experiments were done to establish reproducibility between different batches of cells. The results were normalized to protein content for each sample rather than an internal plasmid control. This was done for two reasons. First, nonspecific effects of overexpressed transcription factor driving the control reporter gene, through the plasmid backbone or the constitutive promoter, will compromise the normalization. Second, with 6–12 individual assays for each condition, collected during several independent experiments, any unusually high variation due to transfection efficiency for a given sample would be obvious from the statistical analysis.
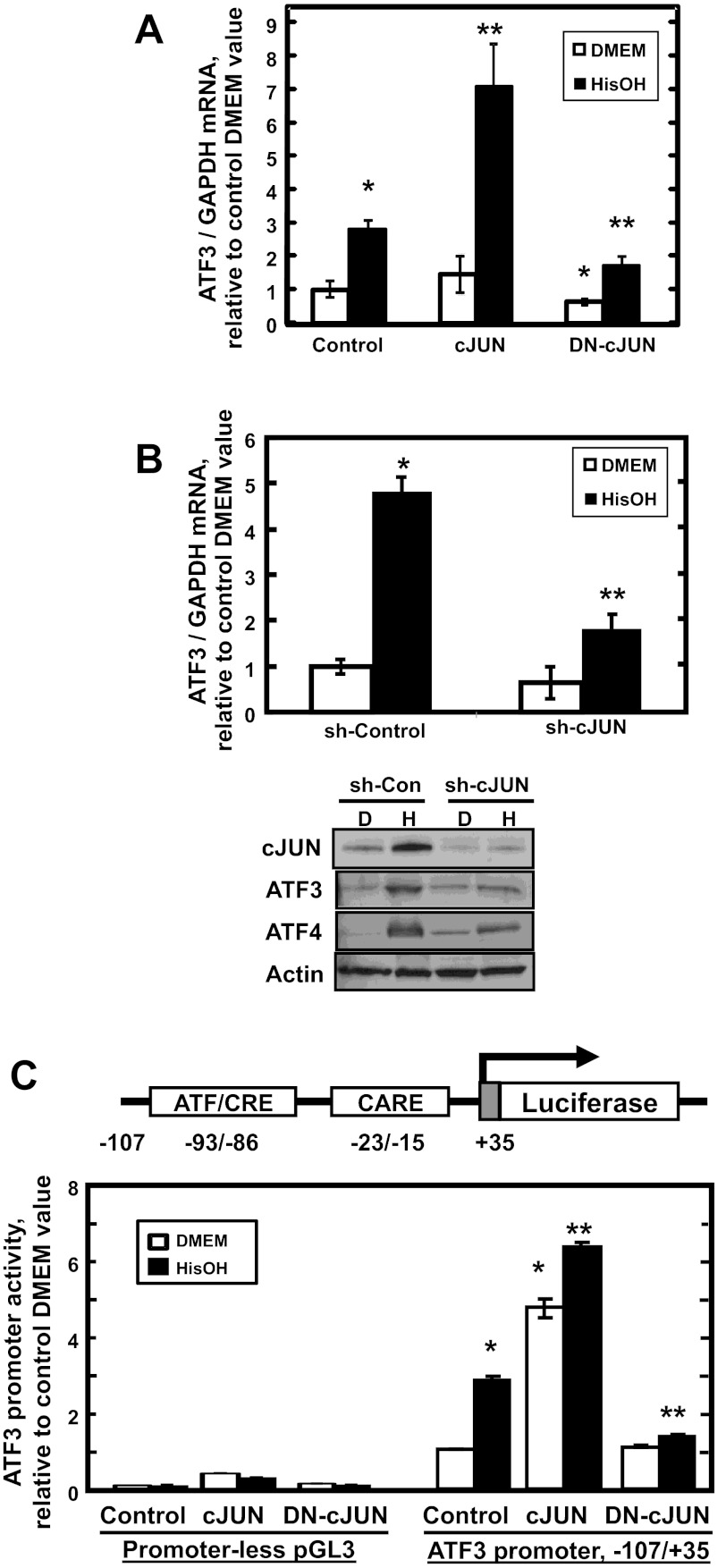
Fig. 2.
cJUN transcriptionally regulates ATF3 expression during the AAR. A: HepG2 cells were transiently transfected with plasmids containing green fluorescent protein (Control), wild-type cJUN (cJUN), or a dominant negative form of cJUN (DN-cJUN). B: in a 2nd series of studies, the cells were transfected with nontarget control shRNA (sh-Control) or cJUN shRNA (sh-cJUN). For A and B, after culture for 48 h, the endogenous ATF3 mRNA content was analyzed by qPCR after the transfected cells were treated with DMEM ± 2 mM HisOH for 6 h. Parallel incubations in DMEM (D) or DMEM + HisOH (H) were used to prepare samples for protein immunoblotting using the primary antibodies indicated. C: HepG2 cells were cotransfected with the pGL3 Firefly luciferase reporter plasmid with no promoter or with the human ATF3 promoter (nt −107/+35) and expression plasmids encoding wild-type (WT) cJUN or a DN-cJUN form. After 48 h, transfected cells were incubated in DMEM ± HisOH for 15 h, and then cell extracts were assayed for luciferase activity and normalized to cell protein content. The data are presented as the averages ± SD for a single experiment containing 3–4 replicates per condition and each experiment was repeated with 2–3 independent batches of cells. CARE, C/EBP-ATF response element. For A–C, *P value of ≤ 0.05 relative to the Control DMEM value, which was set to 1.0, and **P value of ≤ 0.05 relative to the Control HisOH-treated value.
Inducible ATF4 or cJUN shRNA expression.
The ATF4 or cJUN shRNA constructs were packaged in 293FT cells with Lenti virus packaging vectors (pMD2.g and psPAX.2) following the protocol of Barde et al. (3). HepG2 cells were incubated at 37°C for 6 h with 8 μg/ml polybrene and lentiviral particles containing the sh-ATF4 or sh-cJUN construct. The infected cells were cultured with fresh culture medium for 48 h before puromycin selection (2 μg/ml) for at least 14 days. After puromycin selection, infected HepG2 cells were incubated with 1 μg/ml doxycycline (DOX) for 48 h to induce ATF4 or cJUN shRNA expression, and the DOX was also present during the 2 mM HisOH treatment for the time indicated in each experiment.
Statistical analysis.
The results obtained were analyzed by Student's t-test. The data are expressed as the averages ± standard deviations within an individual experiment containing three or four replicates and the results with P ≤ 0.05 were considered statistically significant. Each experiment was performed with two or three independent batches of cells to establish reproducibility.
RESULTS
Transcriptional activation of the ATF3 gene by the AAR.
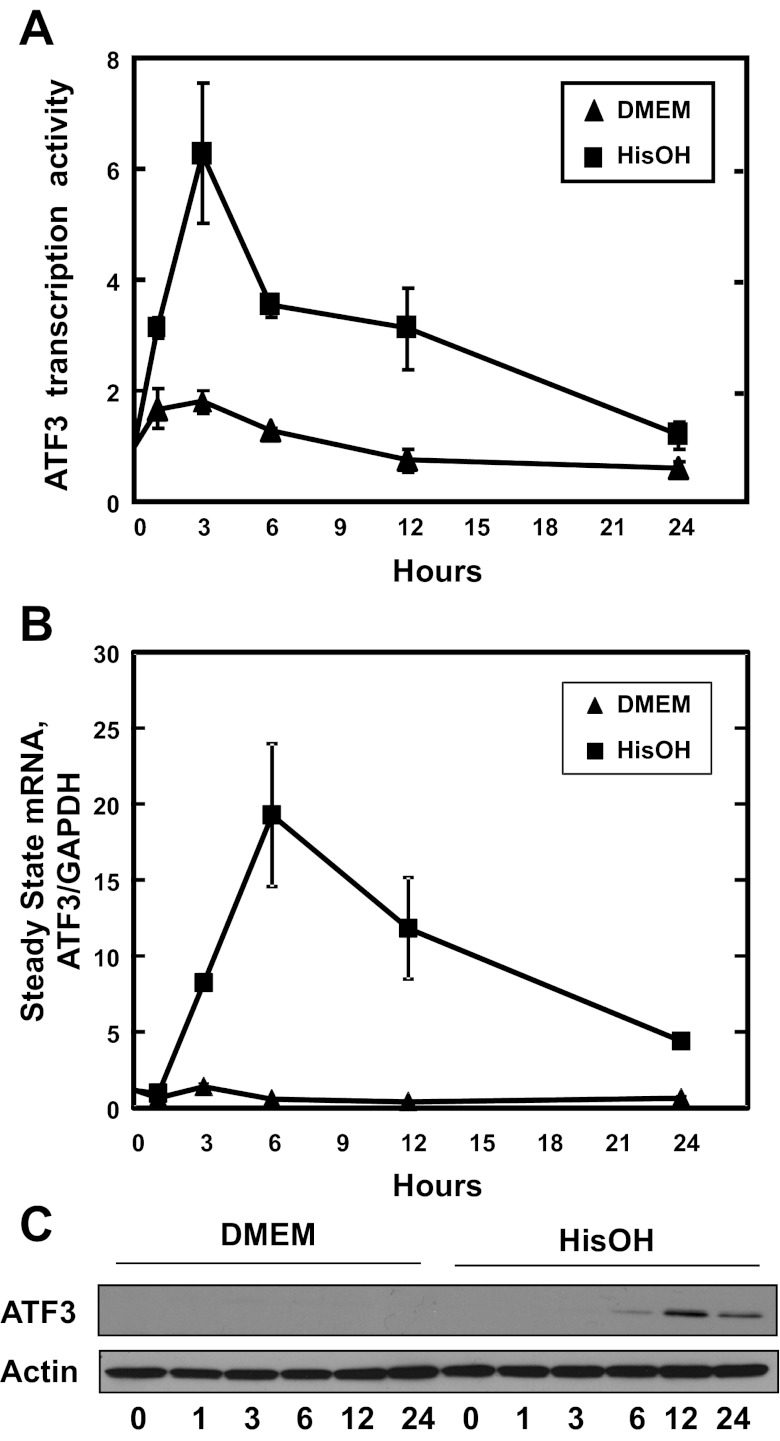
To analyze the time course of ATF3 expression, the AAR was activated by HisOH treatment of HepG2 human hepatoma cells for 0–24 h. The ATF3 transcription activity, monitored by measuring ATF3 hnRNA, was induced within 1 h after the addition of HisOH, peaked at the 3 h time point, and then declined slowly toward the basal level by 24 h (Fig. 1A). Although delayed by a few hours, the increase in steady-state mRNA was detected by 6 h, peaked at 12 h, and remained elevated at 24 h (Fig. 1B). Increased ATF3 protein content was detectable at 3 and 6 h, but peaked at the 12 h time point (Fig. 1C).
Fig. 1.
Expression of activating transcription factor (ATF)3 in HepG2 hepatocellular carcinoma cells after activation of the amino acid response (AAR). A: the hnRNA content of ATF3 was analyzed by qPCR to estimate the transcription activity in HepG2 human hepatocellular cells incubated in control medium (DMEM) or medium containing 2 mM HisOH for 0–24 h. GAPDH mRNA, which is unchanged by the AAR, was used as the internal control. The results are the averages ± SD of 3 or more assays and where not shown, the SD bars are contained within the symbol. B: the steady-state mRNA content of ATF3 was analyzed by qPCR. C: after the cells were treated with HisOH as described above, a 40 μg aliquot of whole cell extract was subjected to gel electrophoresis, transferred to nitrocellulose paper, and then immunoblotted with primary antibody against ATF3 or actin.
cJUN contributes to AAR activation of the ATF3 gene.
Activation of the AAR triggers MAPK activity that results in increased phosphorylation of existing cJUN protein, autoactivation of the cJUN gene, and subsequent cJUN-dependent stabilization of ATF4 protein by an unknown posttranscriptional mechanism (10). Whether or not cJUN contributes to the regulation of AAR target genes other than itself through more direct transcriptional mechanisms is unknown. To determine if cJUN contributes to the regulation of the ATF3 gene, HepG2 cells were transiently transfected with wild-type cJUN or a dominant negative cJUN form (DN-cJUN), subsequently treated with HisOH to activate the AAR, and then analyzed for endogenous ATF3 mRNA expression (Fig. 2A). Expression of exogenous cJUN did not significantly affect the amount of ATF3 mRNA in cells maintained in the basal state (DMEM alone), suggesting that the abundance of cJUN in the absence of other AAR signals has no effect on the ATF3 gene or that the relatively small transfection efficiency of HepG2 cells does not allow for detection of a cJUN-induced change in ATF3 mRNA. However, the transient expression of cJUN further enhanced the ATF3 induction by HisOH by more than twofold (Fig. 2A). Consistent with the positive effect of cJUN overexpression, transient expression of a DN form of cJUN partially blocked the induction by HisOH treatment (Fig. 2A). As an independent confirmation of the latter observation, inhibition of the AAR induction of ATF3 was also observed if the cells were transfected with an shRNA specific for cJUN (sh-cJUN) (Fig. 2B).
To test the effect of cJUN on ATF3 promoter activity, HepG2 cells were cotransfected with wild-type cJUN or DN-cJUN expression vectors and a Firefly luciferase reporter driven by the −107/+35 promoter fragment of the ATF3 gene, which contains both the ATF/CRE and CARE sequences. Exogenous cJUN expression resulted in a four- to fivefold increase in basal (DMEM medium alone) ATF3-driven transcription (Fig. 2C). While exogenous cJUN had a nonspecific effect on the plasmid backbone (Fig. 2C “Promoter-less pGL3”), the activity was negligible compared to that with the ATF3 promoter present. These results show that elevated cJUN has the ability to increase ATF3 transcription without additional signals from the AAR, including the AAR-induced MAPK-mediated phosphorylation cascade, which suggests that increased phosphorylation of cJUN may not be necessary. Furthermore, combining exogenous cJUN and HisOH treatment enhanced transcription beyond that observed for HisOH alone (Fig. 2C). Consistent with the hypothesis that cJUN can enhance ATF3 expression, transfection of the cells with DN-cJUN caused a complete inhibition of the HisOH-induced ATF3-driven transcription (Fig. 2C).
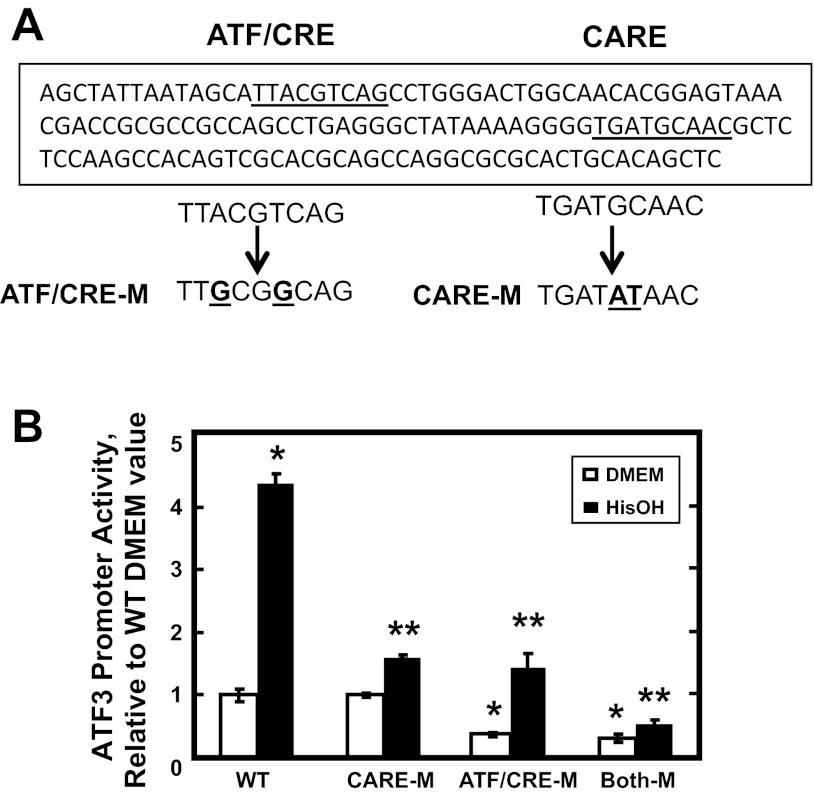
An ATF/CRE site within the ATF3 promoter is necessary for complete activation by the AAR.
An amino acid-responsive CARE sequence (nt −23/−16) has been identified within the ATF3 proximal promoter, but some evidence also suggests that an ATF/CRE site just upstream (nt −93/−86) may contribute to ATF4-dependent induction of the gene (28). To test whether or not the ATF/CRE site is required for the response to amino acid limitation, cells were transiently transfected with a Firefly luciferase reporter driven by the ATF3 promoter (nt −107/+35) containing the ATF/CRE and CARE sites as either wild-type or mutated sequences (Fig. 3A). After the AAR was activated with HisOH, the analysis of luciferase activity showed that mutation of either the CARE or ATF/CRE site individually resulted in a partial, but significant, reduction in AAR-induced transcription (Fig. 3B). Mutation of both sites simultaneously caused a complete inhibition of the activated transcription. Interestingly, mutation of the ATF/CRE site, either alone or in combination with the CARE site, resulted in a reduction in the basal activity as well. Collectively, these data show that the ATF/CRE site contributes to the maintenance of basal transcription from the ATF3 promoter and is also necessary for AAR induction of the gene to the fullest extent.
Fig. 3.
Both the CARE and ATF/CRE sites contribute to ATF3 promoter activation during the AAR. A: the proximal promoter sequence of the ATF3 gene containing the ATF/CRE and CARE sites (underlined) is shown. The sequences for mutations of the ATF/CRE (CRE-M) or the CARE (CARE-M) sites are shown below the WT promoter sequence. B: HepG2 cells were transiently transfected with a Firefly luciferase reporter gene driven by a human WT ATF3 promoter fragment (nt −107/+35) or the same fragment containing the ATF/CRE-M and/or CARE-M mutations described in A. To activate the AAR, at 36 h posttransfection, the cells were incubated in DMEM ± HisOH for 15 h, and then luciferase activity was analyzed and normalized to protein content. The data are presented as the averages ± SD for a single experiment containing 3–4 replicates per condition, and each experiment was repeated with 2–3 independent batches of cells. *P value of ≤ 0.05 relative to the WT DMEM value, which was set to 1.0, and **P value of ≤ 0.05 relative to the WT HisOH-treated value.
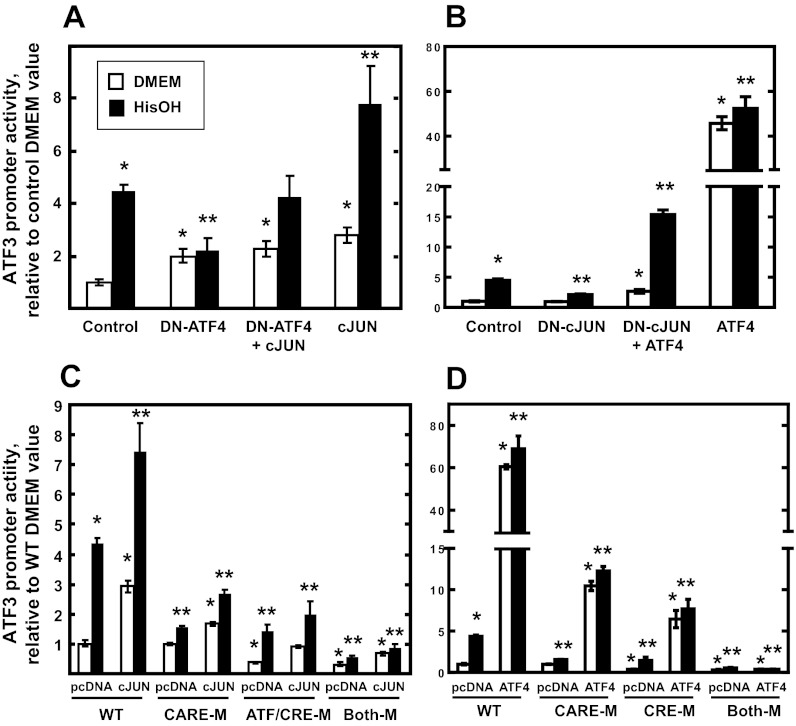
Activation of ATF3 transcription during the AAR is dependent on the ATF/CRE.
To further explore the contribution of cJUN and ATF4 to ATF3 regulation, cells were cotransfected with the −107/+35 ATF3-luciferase reporter and either wild-type or DN forms of ATF4 or cJUN (Fig. 4). As expected, exogenous expression of DN-ATF4 caused a strong inhibition of HisOH-induced transcription, but cotransfection with cJUN reversed that inhibition (Fig. 4A). Consistent with the data presented in Fig. 2, expression of cJUN alone caused an increase in basal and HisOH-induced transcription activity. Conversely, transfection of cells with DN-cJUN resulted in inhibition of AAR-enhanced transcription, whereas coexpression with ATF4 overrode the negative DN-cJUN effect (Fig. 4B). Thus, the data demonstrate that both cJUN and ATF4 contribute to the AAR enhancement of ATF3 transcription. To determine the role of the CARE and ATF/CRE sites, HepG2 cells were cotransfected with cJUN and ATF4 expression vectors and ATF3 promoter-luciferase reporter constructs with the two enhancer sites either as wild-type or as mutated sequences. The increase of both basal and HisOH-induced ATF3 transcription by cJUN was partially, but significantly, suppressed by mutation of either the ATF/CRE or the CARE site, whereas mutation of both completely blocked the positive cJUN effect (Fig. 4C). Note that the cJUN induction of basal activity (DMEM only) was suppressed by mutation of either site. These results suggest that both the ATF/CRE and CARE enhancer sites are necessary for cJUN action on both basal and AAR-induced transcription from the ATF3 gene. Surprisingly, parallel studies expressing exogenous ATF4 illustrated that not only is the CARE site necessary for AAR activation, as previously reported (28), but the ATF/CRE site is also required to obtain the level of induction observed for the wild-type ATF3 promoter (Fig. 4D).
Fig. 4.
Both the ATF/CRE site and elevated cJUN protein content contribute to ATF3 promoter activation during the AAR. HepG2 cells were transiently transfected with a Firefly luciferase reporter gene driven by an ATF3 promoter fragment (nt −107/+35). The cells were cotransfected with expression plasmids encoding WT cJUN and dominant negative ATF4 (DN-ATF4) (A) or WT ATF4 and dominant negative cJUN (DN-cJUN) (B). For C and D, HepG2 cells were transiently transfected with a Firefly luciferase reporter gene driven by the ATF3 promoter fragment (nt −107/+35) containing the WT sequence, ATF/CRE site mutated (ATF/CRE-M), CARE site mutated (CARE-M), or both sites mutated (Both-M). The cells were cotransfected with an expression plasmid encoding either cJUN (C) or ATF4 (D). For A–D, at 36 h posttransfection, the cells were incubated in DMEM ± HisOH for 15 h to activate the AAR, and then luciferase activity was analyzed and normalized to protein content. The data are presented as the averages ± SD for a single experiment containing 3–4 replicates per condition, and each experiment was repeated with 2–3 independent batches of cells. *P value of ≤ 0.05 relative to the control (A and B) or WT (C and D) DMEM value, and **P value of ≤ 0.05 relative to the control (A and B) or WT (C and D) HisOH-treated value.
Transcription factor binding activity within the ATF3 promoter.
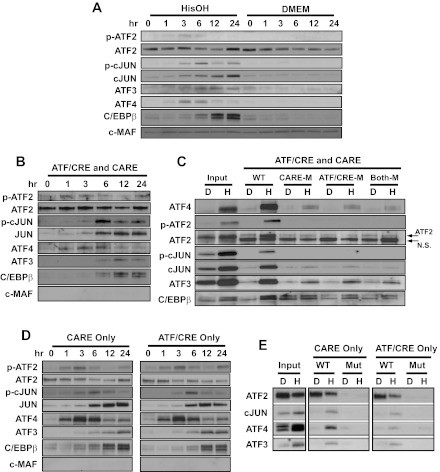
The AAR involves activation and/or increased synthesis of a network of transcription factors that belong to the bZIP superfamily, as reviewed previously (21). Immunoblotting of nuclear extracts established the time course of factor synthesis and phosphorylation after activating the AAR (Fig. 5A). The results revealed that an increase in ATF4 and p-ATF2 is detectable 1 h after HisOH addition, and they both peak at ∼3–6 h before declining to lower levels by the 12 h time point tested. The abundance of ATF3, total cJUN, and p-cJUN began to increase at the 3 h time point. Although the p-cJUN was transient and declined after 6 h, the total cJUN, ATF3, and C/EBPβ protein content remained elevated through the last time point tested at 24 h (Fig. 5A).
Fig. 5.
ATF4 and cJUN bind to both the CARE and ATF/CRE sites in the ATF3 promoter during the AAR. A: the cellular protein content of the indicated bZIP transcription factors was analyzed by immunoblot in HepG2 human hepatoma cells incubated for 0–24 h in control medium (DMEM) or DMEM containing 2 mM HisOH. The cMAF protein is not regulated by the AAR and was used a negative/loading control. B: DNA affinity precipitation assay (DAPA) analysis for protein binding to sequences within the human ATF3 promoter was measured using a oligonucleotide containing the WT ATF3 promoter sequence covering both the CARE and ATF/CRE regions (nt −104/+27). Nuclear protein extracts from HepG2 cells maintained for 0–24 h in DMEM + 2 mM HisOH were incubated with the ATF3 promoter DNA sequence bound to agarose beads. After protein elution from the agarose-bound DNA, the proteins were analyzed by immunoblot for the basic region leucine zipper (bZIP) proteins indicated. Equal loading of the samples was established by Fast Green staining of the blot (not shown). C: DAPA analysis for protein binding was measured using the following oligonucleotides: WT nt −104/+27 (WT), CARE site mutated (CARE-M), ATF/CRE site mutated (ATF/CRE-M), or both sites mutated (Both-M). The nuclear extracts were from cells that had been incubated in DMEM (D) or DMEM + 2 mM HisOH (H) for 6 h. After protein elution, bound proteins were analyzed by immunoblotting along with an aliquot of the starting nuclear extract (Input). D: DAPA analysis for protein binding was measured using an oligonucleotide covering the region of the human ATF3 promoter containing either the CARE sequence only (nt −33/−5) or the ATF/CRE sequence only (nt −103/−75). Nuclear protein extracts from HepG2 cells maintained for 0–24 h in DMEM + 2 mM HisOH were incubated with the indicated ATF3 promoter DNA sequence. After protein elution, bound proteins were analyzed by immunoblotting. E: DAPA analysis for transcription factor binding was performed using the oligonucleotides described for D, except that a 2nd oligonucleotide for each contained mutations in the CARE or ATF/CRE sequence. The nuclear protein extracts were prepared from HepG2 cells maintained for 6 h in DMEM (D) or DMEM + 2 mM HisOH (H). After the binding incubation, the eluted proteins were subjected to immunoblotting along with an aliquot of the starting nuclear extract (Input). Fast Green staining of the blots established equal loading of the lanes (not shown). Each panel shows a representative blot from 2–3 independent experiments.
To investigate the in vitro binding activity of the known AAR-associated transcription factors, we performed DAPA by incubating nuclear extracts with agarose-bound oligonucleotides corresponding to segments of the ATF3 proximal promoter. Previously, we have used the CARE sequence from the ASNS gene in DAPA assays to analyze the CARE binding activity of ATF4 and specific isoforms of C/EBPβ during activation of the AAR (35). To determine if the abundance of these factors correlated with their binding activity, DAPA assays were performed with an oligonucleotide (nt −104/+27) containing both the ATF/CRE and the CARE sites, and oligonucleotides containing the ATF/CRE site only (nt −102/−76) or the CARE site only (nt −32/−6). When we used the −104/+27 oligonucleotide, in general, the time course of binding mirrored the absolute abundance of the protein (Fig. 5B). These results provide evidence that factor association with the ATF3 promoter can be divided into three phases, 1–3 h (ATF4 and p-ATF2), 3–6 h (cJUN, p-cJUN), and 6–24 h (cJUN, ATF3, and C/EBPβ). Total ATF2 binding activity remained relatively constant during the entire time course, with the exception of a small decline at 12 h. Three variants of the −104/+27 oligonucleotide for DAPA analysis were generated that contained mutation of each enhancer site individually or together (Fig. 5C). The data illustrate that the binding of ATF4, ATF2/p-ATF2, and cJUN/p-cJUN was largely blocked by mutation of either element, a result consistent with the luciferase reporter data (Fig. 4). The data for ATF3 and C/EBPβ were less clear in that mutation of one or both sites caused a detectable reduction in binding, but some association of each factor with the DNA probe still remained even when both sites were mutated (Fig. 5C). The residual binding may reflect weak but specific binding to the mutated sequences or to cryptic sites within the flanking sequence on each end of the element. The data reveal that total cJUN and p-cJUN binding requires both ATF/CRE sequence as well as the CARE sequence (Fig. 5C). A necessary role for the ATF3 CARE site in cJUN binding is in contrast to published electrophoresis mobility shift analysis results for the ASNS CARE to which cJUN does not bind (12, 32). This result may suggest that the flanking sequences around each CARE site may be important as well.
The studies with the ATF3-derived −104/+27 oligonucleotide do not address whether there is a single complex that requires both sites to assemble or there are two separate complexes that are redundant in their composition for these particular factors. To investigate this issue, DAPA experiments were performed with shorter, site-specific oligonucleotides covering the ATF/CRE site only (nt −102/−76) or the CARE site only (nt −32/−6). The time course of binding activity and protein complex composition was similar for either the ATF/CRE or the CARE sequences when assayed individually (Fig. 5D) and largely reflected the results obtained for the longer oligonucleotide that contained both sites. To further confirm that transcription factor assembly represented specific binding, site-specific oligonucleotides were generated that represented the wild-type sequence or that contained mutations within the ATF/CRE and CARE sequences (Fig. 5E). Nuclear extracts from HepG2 cells incubated with HisOH for 6 h were used because this time point allows detection of at least some binding for all of the factors being investigated. The data revealed that mutation of either the ATF/CRE or the CARE sequence blocked transcription factor binding. Collectively, the time course data are consistent with the current working model for an AAR target gene such as ATF3, which proposes that within the first hour after amino acid deprivation, ATF4 binding is likely the initial triggering event and is associated with rapid histone modifications, including acetylation by p-ATF2 (5, 7, 8, 28). However, amino acid deprivation also triggers a self-limiting transcriptional program because ATF4 causes the induction of ATF3 and C/EBPβ, which subsequently antagonize ATF4 action (8, 27, 28). The present DAPA data are the first to demonstrate that cJUN is a component of the AAR-induced protein complexes that assemble on the ATF3 gene.
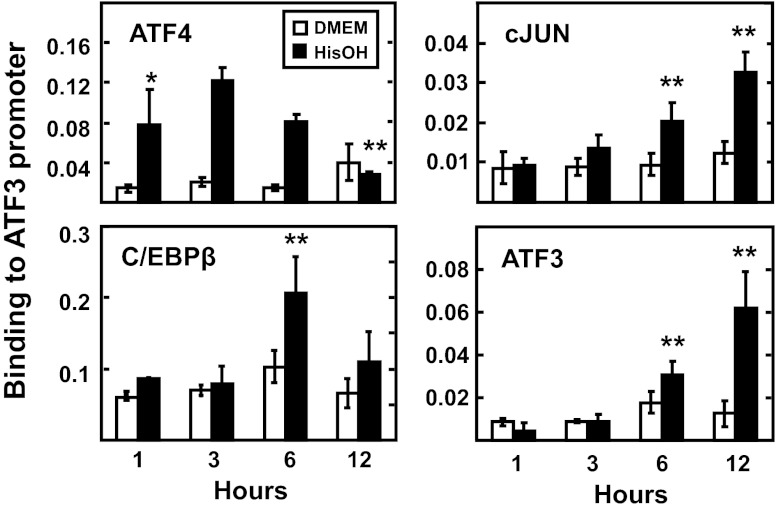
To investigate transcription factor binding in intact cells, ChIP was performed for the ATF3 promoter (Fig. 6). Negative controls, using primers specific for a region outside of the promoter, exon 2, after immunoprecipitation with each antibody or using promoter-specific primers after immunoprecipitating with a nonspecific IgG, revealed little or no binding (data not shown). The ChIP results showed a time-dependent recruitment of ATF4, cJUN, ATF3, and C/EBP to the ATF3 promoter, whereas little or no binding occurred within the body of the gene (data not shown). ATF4 recruitment was rapid and the amount associated with the ATF3 promoter was increased by several-fold after only 1 h of HisOH treatment (Fig. 6). Consistent with previous reports, the ATF4 binding returned to the basal level by 12 h (8). In contrast, cJUN and ATF3 rose steadily, and the greatest association was at the 12 h time point. C/EBPβ binding was also increased after 6 h of HisOH treatment, but the level at 12 h was not statistically different from the control value. Several experiments revealed that detection of p-ATF2 and p-cJUN binding was weak, and despite trends, it was difficult to establish a statistically significant increase, presumably because of relatively poor precipitation by the respective antibodies (data not shown). Collectively, by ChIP analysis of intact cells or by DAPA in vitro, the binding assays indicate that in addition to the bZIP factors previously implicated in the AAR control of the ATF3 gene, cJUN is also associated with the protein complexes that are recruited in response to amino acid deprivation.
Fig. 6.
Chromatin immunoprecipitation (ChIP) analysis reveals temporal binding of regulatory factors at the ATF3 promoter during the AAR. HepG2 cells were maintained in DMEM ± 2 mM HisOH for 1, 3, 6, or 12 h, and then ATF4, cJUN, C/EBPβ, and ATF3 binding to the ATF3 proximal promoter was measured by ChIP analysis. The data are presented as the averages ± SD for a single experiment containing 3–4 replicates per condition, and each experiment was repeated with 2–3 independent batches of cells. *P value of ≤ 0.05 relative to the 1 h DMEM value, and **P value of ≤ 0.05 relative to the 1 h HisOH-treated value. Nonspecific IgG and primers specific for exon 2 of the ATF3 gene were used as negative controls and showed little or no binding (data not shown).
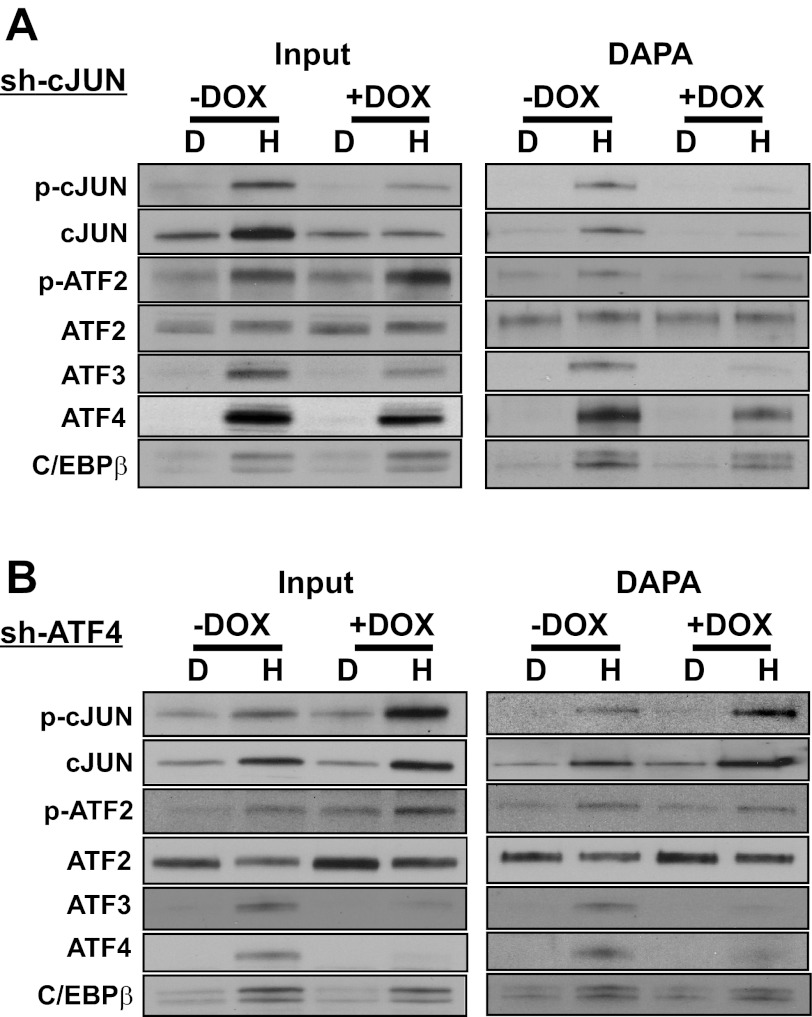
Binding of cJUN to the ATF3 promoter occurs independently of ATF4.
To determine the possible interaction of ATF4 and cJUN with regard to binding at the ATF3 promoter elements, DAPA was performed using the −104/+27 ATF3 promoter fragment and nuclear extracts from HepG2 cells that stably express DOX-inducible shRNA against either cJUN (Fig. 7A) or ATF4 (Fig. 7B). Immunoblotting (“Input”) established the abundance of the factors within the nuclear extract. The data reveal that following cJUN knockdown, there was little or no change in abundance or binding activity for total ATF2 or the HisOH-induced amount of p-ATF2, whereas the AAR-dependent increase in abundance and binding of ATF3 was significantly reduced in the absence of cJUN (Fig. 7A). Knockdown of cJUN produced a partial reduction of ATF4 protein abundance and binding activity, consistent with our previously published data indicating that ATF4 protein is stabilized by AAR-induced cJUN (10). Collectively, these results suggest that cJUN has a significant role in supporting the abundance, and therefore function, of ATF4 and ATF3 during the AAR. Parallel studies were performed in HepG2 cells after inducible knockdown of ATF4 (Fig. 7B). Reduced ATF4 expression caused little or no change in the amount of or binding activity of total ATF2 or the HisOH-induced p-ATF2. The reduction in ATF4 caused a strong decline in ATF3 abundance and binding activity, whereas C/EBPβ was largely unchanged. Interestingly, immunoblotting suggested that the amount of total cJUN and p-cJUN protein content after HisOH treatment was increased in the ATF4-deficient cells (Fig. 7B), but the increase in the amount of p-cJUN following HisOH treatment was much stronger. The increases in p-cJUN content were reflected as increases in ATF3 promoter binding activity as well (Fig. 7B). The data indicate that the binding of cJUN to the ATF3 promoter is not dependent on ATF4 and that the AAR-induced MAPK phosphorylation cascade that results in p-cJUN activation is actually increased in the absence of ATF4.
Fig. 7.
AAR-induced cJUN binding to the ATF3 promoter is independent of ATF4. HepG2 cells stably expressing a doxycycline (DOX)-inducible shRNA against either cJUN (A) or ATF4 (B) were treated with DMEM or DMEM containing 2 mM HisOH for 6 h after the shRNA expression was induced by DOX for 48 h. Nuclear protein extracts were assayed for transcription factor binding by DAPA with a DNA oligonucleotide (nt −104/+27) containing both the ATF/CRE and CARE sites of ATF3 promoter region. An aliquot of the nuclear extract was subjected to immunoblotting with the indicated primary antibody (“Input”). Equal loading of the samples was established by Fast Green staining of the blot (not shown). The blots shown are representative of multiple experiments.
bZIP factor composition of protein complexes following AAR activation.
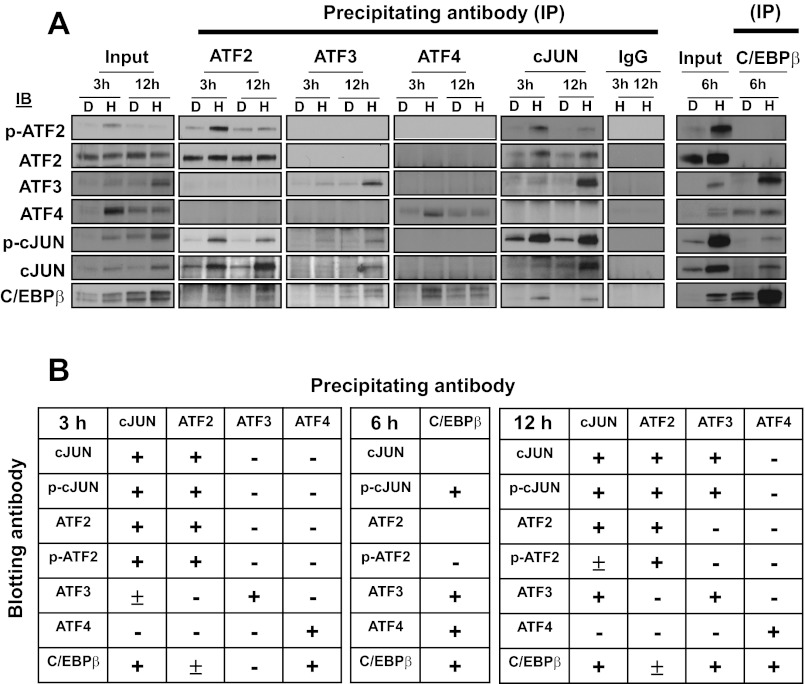
To determine the possible association between the bZIP transcription factors involved in mediating the AAR, co-IPs were performed using cell extracts from HepG2 cells incubated in HisOH for 3, 6, or 12 h. The data from a representative experiment are shown in Fig. 8A, and the results from multiple experiments are summarized in Fig. 8B. The phospho-specific antibodies for both p-ATF2 and p-cJUN were not strong enough to give reliable results when used as the precipitating antibody, so the summary data for precipitation are for total cJUN and ATF2 antibodies, whereas the immunoblotting results include the phosphorylated forms as well. The study indicates that of the proteins tested ATF4 is associated with C/EBPβ only and that ATF2 associates weakly with C/EBPβ, but more strongly with cJUN. ATF3 is bound to complexes containing C/EBPβ and cJUN, and, as expected, this interaction is greater at 12 h when C/EBPβ and ATF3 levels are high. Collectively, the results show that cJUN can be detected in complexes containing each of the other factors tested except for ATF4, with which cJUN has repeatedly shown no association in the present experiments and our earlier studies (10). C/EBPβ associates with all of the other bZIP proteins tested, but the interaction with complexes containing cJUN and ATF4 appears to be the strongest. Note that these two particular C/EBPβ-containing complexes appear to be different populations given that no association of cJUN and ATF4 is detected. If ATF2 functions as a HAT and is not part of the enhancer complex per se, perhaps it is understandable that it does not associate with the ATF4-containing complexes. Consistent with this proposal, ATF2 does associate with cJUN, which also does not interact with the ATF4-containing complexes, and may function as a cofactor that does not bind DNA directly (11).
Fig. 8.
Selective heterodimerization of bZIP transcription factors after induction by the AAR. A: HepG2 cells were incubated in DMEM (D) or DMEM + 2 mM HisOH (H) for 3, 6 or 12 h and then analyzed for protein-protein interactions by immunoprecipitation (IP) and immunoblotting (IB) with the indicated antibodies against specific members of the ATF, cJUN, or C/EBP families. The blots shown are representative of multiple experiments. B: the coimmunoprecipitation data from multiple experiments are summarized as follows: +, strong association; ±, weak but detectable association, −, little or no interaction. Note that for any pair of antibodies the result may vary depending on the strength of the antibody when it is used for the IP or IB portion of the study.
DISCUSSION
The present report characterizes the cis-genomic elements required for activation of the ATF3 gene in response to amino acid deprivation. The following novel observations have been made. 1) In addition to the known amino acid-responsive CARE site within the ATF3 promoter, an ATF/CRE site upstream of the CARE site also contributes to activation of the gene by the AAR. 2) Induction of transcription by ATF4 involves binding of this factor not only to the CARE site, but also to the ATF/CRE site. 3) Several bZIP transcription factors associate with the ATF/CRE sequence in the same time-dependent sequence, ATF4 (1–6 h) and C/EBPβ/ATF3 (6–24 h), that has been described previously for the CARE site (8). 4) The binding of cJUN to the ATF3 CARE site is in contrast to published evidence that the AAR does not lead to cJUN association with the ASNS CARE site (12, 32). 5) The time course of p-cJUN vs. total cJUN protein association was distinct, with p-cJUN peaking at about 6 h and then declining, whereas total cJUN protein binding continued to increase throughout the 24 h period studied.
ATF3 expression is highly regulated and transcription from the ATF3 gene is induced in response to a wide variety of stress signals (14, 16, 36). Among these are ER stress, which triggers the signal transduction pathway called the unfolded protein response, and the AAR, both of which activate ATF3 transcription through increased expression of ATF4 and binding to a C/EBP-ATF composite site (CARE) within the ATF3 promoter (19, 28). However, ATF4 can also bind to ATF/CRE sequences (TGACGTCA) (15, 23) and, as shown by the DAPA data, binding of ATF4 to the −93/−85 ATF/CRE site within the ATF3 promoter is enhanced in response to the AAR. Mutation of the ATF/CRE site, even in the presence of a functional CARE site, blunts the ATF4-dependent induction of ATF3-driven transcription activity by >80%. Unfortunately, ChIP analysis cannot confirm this observation in intact cells because of the close proximity of the ATF/CRE and CARE sites. Both the CARE and ATF/CRE elements are required for a maximal response to either AAR activation by HisOH or to exogenously supplied ATF4. These results are consistent with data by Wolfgang et al. (39), from the Hai laboratory, who showed that both the ATF/CRE and the CARE sites were required for ATF3 auto-repression of its own gene. With regard to the −93/−85 ATF/CRE site within the ATF3 gene, Liang et al. (25) were the first to show that exogenous co-expression of ATF2 and cJUN strongly activated transcription from an ATF3 promoter fragment. More recently, Tamura et al. (34) used ChIP and EMSA to document that serum-induction of the ATF3 gene required the ATF/CRE site and involved ATF2/cJUN binding. In that study, an ATF3 promoter fragment lacking the ATF/CRE sequence, but retaining the CARE site, was unresponsive to serum stimulation. Collectively, the present data illustrate that activation of the gene by the AAR involves combinatorial interactions of multiple bZIP transcription factors and at least two cis-acting promoter elements.
The DAPA, co-IP, and ChIP analysis revealed a temporal relationship for factor recruitment and binding to the ATF3 promoter and, for the factors tested, the initial events involve binding of p-ATF2 and ATF4 within the first hour after AAR activation. Consistent with that interpretation, Bruhat et al. (5) of the Fafournoux laboratory showed that p-ATF2, a known HAT (20), mediates an AAR-induced histone acetylation at the ATF3 promoter. However, data from the same laboratory indicate that p-ATF2 cannot activate transcription in the absence of ATF4 (5, 7). Conversely, in mouse embryonic fibroblasts (MEF), the lack of Atf4 blocks AAR-induced Atf3 transcription but does not prevent the increase in p-Atf2 content, p-Atf2 recruitment to the Atf3 gene, and histone acetylation near the Atf3 promoter. Thus, AAR-driven p-Atf2 association and its corresponding histone modification are not sufficient for transcriptional activation. The present results for ATF4, illustrating rapid association with the ATF3 gene, are consistent with our previously published ChIP analysis (28). Recently, we have discovered that while transcription from the ATF3 gene is severely reduced in Atf4-deficient MEF, the elevated transcription activity can be rescued by chemical inhibition of deacetylation to increase histone H4 acetylation at the ATF3 gene (29). Those observations, consistent with the present data, suggest that a primary function of ATF4 action on the ATF3 gene is to enhance acetylation by enhancing a HAT or inhibiting a histone deacetylase.
During the 3–6 h time period of the AAR, p-ATF2 and ATF4 levels and binding activity declined, whereas p-cJUN was near its maximum. The peak of p-cJUN expression occurred during a period when the transcription activity from the ATF3 gene had peaked. Strictly based on this temporal correlation one might argue that p-cJUN may contribute to the suppression phase of the transient ATF3 induction. However, exogenous expression of cJUN caused an increase in both basal and AAR-induced endogenous ATF3 mRNA and ATF3-driven luciferase activity, suggesting that cJUN serves as an activator of the process. The possible role of nonphosphorylated cJUN must also be considered. Whereas the increase in p-cJUN was transient, total cJUN abundance and binding activity continued to increase beyond 6 h. This discontinuity between p-cJUN and total cJUN may have significance as it has been reported that the nonphosphorylated form of cJUN has specific functions. cJun-deficient mice die as the result of liver failure, but they can be rescued by exogenous expression of a Ser63/73Ala form of cJUN that cannot be phosphorylated (4). In fact, nonphosphorylated cJUN can activate transcription in the absence of a naturally occurring unidentified repressor of p-cJUN function, and HepG2 cells appear to lack this repressor activity (1, 2). Conversely, cJUN can serve as a coactivator rather than as a DNA binding enhancer-protein by associating with C/EBPβ-PU.1 complexes via protein-protein interactions (11). This coactivator action of cJUN is also independent of Ser63/73 phosphorylation.
The DNA binding analysis indicates that, in vitro, the enhancer-protein complexes associated with regulation of the ATF3 gene can assemble on oligonucleotides containing either the CARE or ATF/CRE sequence alone, but this assembly appeared to be considerably stronger when a longer oligonucleotide containing both sequences was used. These results are consistent with the proposal that the two elements work in combination to control ATF3 transcription. This hypothesis is underscored by the observation that when the longer oligonucleotide was used mutation of either element nearly completely eliminated complex assembly. The interdependence of the two elements is not only true for formation of what is likely to be the “activating” ATF4-containing complex, but also the “repressing” ATF3-containing complex. Wolfgang et al. (39) have shown that the ATF/CRE and the CARE sites are both required for the ATF3 autorepression. ChIP analysis indicates that ATF2, cJUN, and C/EBPβ maintain a basal level of binding to the ATF3 promoter prior to and independently of activation by the AAR. This observation is consistent with the knowledge that their trans-activating capability is linked, at least in part, to phosphorylation of existing protein. The ATF4 binding activity within the initial hour or so after AAR induction, and that of ATF3, at 12 h or later, is more closely aligned with absolute protein abundance. Deciphering the exact function of these proteins within the CARE and ATF/CRE complexes within the ATF3 promoter must await further analysis. It is also clear that identification of the AAR-associated coactivators and corepressors will be necessary to provide additional understanding into transcriptional control by amino acid availability. An additional area for future investigation is the comparison of these pathways and control mechanisms in hepatoma cells such as HepG2 and normal human hepatocytes. The activation of the ATF4-dependent AAR pathway is known to exist in primary human hepatocytes (29), but that for cJUN is suppressed relative to transformed cells (10).
GRANTS
This research was supported by Institute of Diabetes, Digestive and Kidney Diseases Grants (to M. S. Kilberg) DK-92062, DK-94729.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: L.F. and M.S.K. conception and design of research; L.F. performed experiments; L.F. and M.S.K. analyzed data; L.F. and M.S.K. interpreted results of experiments; L.F. and M.S.K. prepared figures; L.F. and M.S.K. drafted manuscript; L.F. and M.S.K. edited and revised manuscript; L.F. and M.S.K. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Elizabeth Butterworth for editorial suggestions and other members of the laboratory for technical advice, reagents, and helpful discussion
REFERENCES
- 1. Baichwal VR, Park A, Tjian R. The cell-type-specific activator region of c-Jun juxtaposes constitutive and negatively regulated domains. Genes Dev 6: 1493–1502, 1992 [DOI] [PubMed] [Google Scholar]
- 2. Baichwal VR, Tjian R. Control of c-Jun activity by interaction of a cell-specific inhibitor with regulatory domain delta: differences between v- and c-Jun. Cell 63: 815–825, 1990 [DOI] [PubMed] [Google Scholar]
- 3. Barde I, Salmon P, Trono D. Production and titration of lentiviral vectors. Curr Protoc Neurosci 4: 4.21.21–24.21.23, 2010 [DOI] [PubMed] [Google Scholar]
- 4. Behrens A, Sibilia M, Wagner EF. Amino-terminal phosphorylation of c-Jun regulates stress-induced apoptosis and cellular proliferation. Nat Genet 21: 326–329, 1999 [DOI] [PubMed] [Google Scholar]
- 5. Bruhat A, Cherasse Y, Maurin AC, Breitwieser W, Parry L, Deval C, Jones N, Jousse C, Fafournoux P. ATF2 is required for amino acid-regulated transcription by orchestrating specific histone acetylation. Nucl Acids Res 35: 1312–1321, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Carraro V, Maurin AC, Lambert-Langlais S, Averous J, Chaveroux C, Parry L, Jousse C, Ord D, Ord T, Fafournoux P, Bruhat A. Amino acid availability controls TRB3 transcription in liver through the GCN2/eIF2alpha/ATF4 pathway. PloS One 5: e15716, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chaveroux C, Jousse C, Cherasse Y, Maurin AC, Parry L, Carraro V, Derijard B, Bruhat A, Fafournoux P. Identification of a novel amino acid response pathway triggering ATF2 phosphorylation in mammals. Mol Cell Biol 29: 6515–6526, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen H, Pan YX, Dudenhausen EE, Kilberg MS. Amino acid deprivation induces the transcription rate of the human asparagine synthetase gene through a timed program of expression and promoter binding of nutrient-responsive bZIP transcription factors as well as localized histone acetylation. J Biol Chem 279: 50829–50839, 2004 [DOI] [PubMed] [Google Scholar]
- 9. Fawcett TW, Martindale JL, Guyton KZ, Hai T, Holbrook NJ. Complexes containing activating transcription factor (ATF)/cAMP-responsive-element-binding protein (CREB) interact with the CCAAT enhancer-binding protein (C/EBP)-ATF composite site to regulate Gadd153 expression during the stress response. Biochem J 339: 135–141, 1999 [PMC free article] [PubMed] [Google Scholar]
- 10. Fu L, Balasubramanian M, Shan J, Dudenhausen EE, Kilberg MS. Auto-activation of c-JUN gene by amino acid deprivation of hepatocellular carcinoma cells reveals a novel c-JUN-mediated signaling pathway. J Biol Chem 286: 36724–36738, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Grondin B, Lefrancois M, Tremblay M, Saint-Denis M, Haman A, Waga K, Bedard A, Tenen DG, Hoang T. c-Jun homodimers can function as a context-specific coactivator. Mol Cell Biol 27: 2919–2933, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Guerrini L, Gong SS, Mangasarian K, Basilico C. Cis- and trans-acting elements involved in amino acid regulation of asparagine synthetase gene expression. Mol Cell Biol 13: 3202–3212, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gurzov EN, Barthson J, Marhfour I, Ortis F, Naamane N, Igoillo-Esteve M, Gysemans C, Mathieu C, Kitajima S, Marchetti P, Orntoft TF, Bakiri L, Wagner EF, Eizirik DL. Pancreatic beta-cells activate a JunB/ATF3-dependent survival pathway during inflammation. Oncogene 31: 1723–1732, 2012 [DOI] [PubMed] [Google Scholar]
- 14. Hai T. The ATF transcription factors in cellular adaptive responses. In: Gene Expression and Regulation. Beijing, China and New York: Higher Education Press and Springer, 2006, p. 322–333 [Google Scholar]
- 15. Hai T, Curran T. Cross-family dimerization of transcription factors Fos/Jun and ATF/CREB alters DNA binding specificity. Proc Natl Acad Sci USA 88: 3720–3724, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hai T, Wolford CC, Chang YS. ATF3, a hub of the cellular adaptive-response network, in the pathogenesis of diseases: is modulation of inflammation a unifying component? Gene Expr 15: 1–11, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Harding HP, Zhang Y, Bertolotti A, Zeng H, Ron D. Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol Cell 5: 897–904, 2000 [DOI] [PubMed] [Google Scholar]
- 18. Harding HP, Zhang Y, Zeng H, Novoa I, Lu PD, Calfon M, Sadri N, Yun C, Popko B, Paules R, Stojdl DF, Bell JC, Hettmann T, Leiden JM, Ron D. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell 11: 619–633, 2003 [DOI] [PubMed] [Google Scholar]
- 19. Jiang HY, Wek SA, McGrath BC, Lu D, Hai TW, Harding HP, Wang XZ, Ron D, Cavener DR, Wek RC. Activating transcription factor 3 is integral to the eukaryotic initiation factor 2 kinase stress response. Mol Cell Biol 24: 1365–1377, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kawasaki H, Schiltz L, Chiu R, Itakura K, Taira K, Nakatani Y, Yokoyama KK. ATF-2 has intrinsic histone acetyltransferase activity which is modulated by phosphorylation. Nature 405: 195–200, 2000 [DOI] [PubMed] [Google Scholar]
- 21. Kilberg MS, Balasubramanian M, Fu L, Shan J. The transcription factor network associated with the amino Acid response in Mammalian cells. Adv Nutr 3: 295–306, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kilberg MS, Shan J, Su N. ATF4-dependent transcription mediates signaling of amino acid limitation. Trends Endocrinol Metab 20: 436–443, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Koyanagi S, Hamdan AM, Horiguchi M, Kusunose N, Okamoto A, Matsunaga N, Ohdo S. cAMP-response element (CRE)-mediated transcription by activating transcription factor-4 (ATF4) is essential for circadian expression of the Period2 gene. J Biol Chem 286: 32416–32423, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lee SH, Bahn JH, Whitlock NC, Baek SJ. Activating transcription factor 2 (ATF2) controls tolfenamic acid-induced ATF3 expression via MAP kinase pathways. Oncogene 29: 5182–5192, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liang G, Wolfgang CD, Chen BP, Chen TH, Hai T. ATF3 gene. Genomic organization, promoter, regulation. J Biol Chem 271: 1695–1701, 1996 [DOI] [PubMed] [Google Scholar]
- 26. Lipson KE, Baserga R. Transcriptional activity of the human thymidine kinase gene determined by a method using the polymerase chain reaction and an intron-specific probe. Proc Natl Acad Sci USA 86: 9774–9777, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lopez AB, Wang C, Huang CC, Yaman I, Li Y, Chakravarty K, Johnson PF, Chiang CM, Snider MD, Wek RC, Hatzoglou M. A feedback transcriptional mechanism controls the level of the arginine/lysine transporter cat-1 during amino acid starvation. Biochem J 402: 163–173, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pan YX, Chen H, Thiaville MM, Kilberg MS. Activation of the ATF3 gene through a co-ordinated amino acid-sensing response programme that controls transcriptional regulation of responsive genes following amino acid limitation. Biochem J 401: 299–307, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shan J, Fu L, Balasubramanian MN, Anthony T, Kilberg MS. ATF4-dependent regulation of the JMJD3 gene during amino acid deprivation can be rescued in Atf4-deficient cells by inhibition of deacetylation. J Biol Chem 287: 36393–36403, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shan J, Lopez MC, Baker HV, Kilberg MS. Expression profiling after activation of the amino acid deprivation response in HepG2 human hepatoma cells. Physiol Genomics 41: 315–327, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shaulian E. AP-1–the Jun proteins: oncogenes or tumor suppressors in disguise? Cell Signal 22: 894–899, 2010 [DOI] [PubMed] [Google Scholar]
- 32. Siu FY, Chen C, Zhong C, Kilberg MS. CCAAT/enhancer-binding protein beta (C/EBPb) is a mediator of the nutrient sensing response pathway that activates the human asparagine synthetase gene. J Biol Chem 276: 48100–48107, 2001 [DOI] [PubMed] [Google Scholar]
- 33. Su N, Kilberg MS. C/EBP homology protein (CHOP) interacts with activating transcription factor 4 (ATF4) and negatively regulates the stress-dependent induction of the asparagine synthetase gene. J Biol Chem 283: 35106–35117, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tamura K, Hua B, Adachi S, Guney I, Kawauchi J, Morioka M, Tamamori-Adachi M, Tanaka Y, Nakabeppu Y, Sunamori M, Sedivy JM, Kitajima S. Stress response gene ATF3 is a target of c-myc in serum-induced cell proliferation. EMBO J 24: 2590–2601, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Thiaville MM, Dudenhausen EE, Zhong C, Pan YX, Kilberg MS. Deprivation of protein or amino acid induces C/EBP beta synthesis and binding to amino acid response elements, but its action is not an absolute requirement for enhanced transcription. Biochem J 410: 473–484, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Thompson MR, Xu D, Williams BR. ATF3 transcription factor and its emerging roles in immunity and cancer. J Mol Med 87: 1053–1060, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Weidenfeld-Baranboim K, Hasin T, Darlyuk I, Heinrich R, Elhanani O, Pan J, Yokoyama KK, Aronheim A. The ubiquitously expressed bZIP inhibitor, JDP2, suppresses the transcription of its homologue immediate early gene counterpart, ATF3. Nucl Acids Res 37: 2194–2203, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wek RC, Jiang HY, Anthony TG. Coping with stress: eIF2 kinases and translational control. Biochem Soc Trans 34: 7–11, 2006 [DOI] [PubMed] [Google Scholar]
- 39. Wolfgang CD, Liang G, Okamoto Y, Allen AE, Hai T. Transcriptional autorepression of the stress-inducible gene ATF3. J Biol Chem 275: 16865–16870, 2000 [DOI] [PubMed] [Google Scholar]