Abstract
We report the case of a 65-year-old woman, diagnosed with a breast cancer human epidermal growth factor receptor (HER2) previously negative, who developed leptomeningeal carcinomatosis and was treated with intrathecal (IT) trastuzumab (TST). After five doses of IT trastuzumab, at escalading doses, once weekly, the patient's neurological status stabilised, and that result was maintained for two months. There is evidence in the literature that breast cancer receptor status may change over time, and when it occurs, it may modify the therapeutical approach. We reviewed the pertinent literature and concluded that IT trastuzumab might be a promising treatment for patients with HER2-positive breast cancer leptomeningeal carcinomatosis.
1. Case Report
A 65-year-old woman was diagnosed in 1996 to have an invasive ductal breast carcinoma with positive local lymph nodes. Tumor HER2 receptors were negative; estrogen receptor (ER) and progesterone receptor (PgR) were positive. She was treated by a combination of doxorubicin and docetaxel followed by radiotherapy and five years of hormonotherapy with tamoxifen.
From 2004 until August 2011 multiple bone, lymph node, liver, and parenchyma brain metastases were treated with various chemotherapeutic regimens and focal radiation therapy. Brain metastases appeared in August 2008 and were treated by gamma-knife followed by whole brain radiation therapy (WBRT) one month later with partial response. Twenty-eight months later a second course of WBRT was applied for parenchymal progression.
In August 2011 a biopsy of liver metastases revealed the presence of HER2 receptor, contrary to the first diagnostic of breast biopsy which did not showed the presence of the HER2 receptor [1, 2]. On this liver biopsy the status of ER and PgR was not determined.
The patient was treated by trastuzumab (4 mg/kg at loading dose, then 2 mg/kg weekly) and vinorelbine. After one cycle, the patient developed ataxia, vertigo, low back pain, headache, and mild cognitive changes. On brain MRI, parenchymal metastatic lesions were stable with no signs of leptomeningeal disease. However spinal MRI showed multiple leptomeningeal lesions (see Figure 1).
Figure 1.
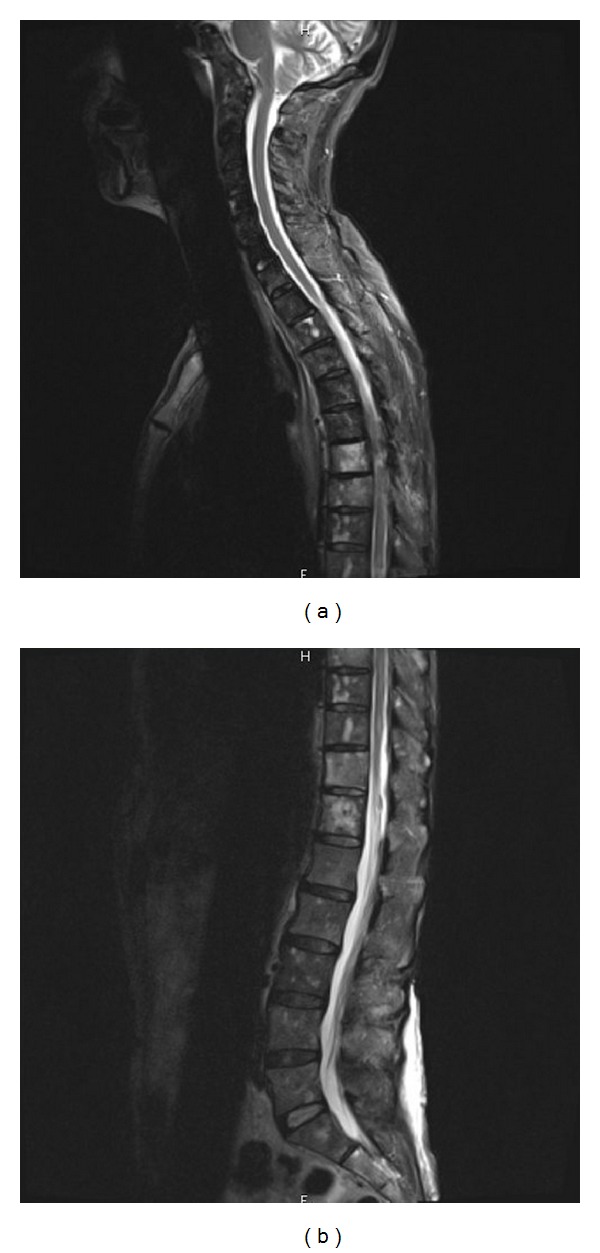
MRI evidence of leptomeningeal carcinomatosis before treatment with IT trastuzumab.
A lumbar punction was performed, and the CSF examination showed increased protein level (660 mg/dL), normal glycorrhachia, and the presence of malignant cells.
Intrathecal trastuzumab was given weekly through an Ommaya reservoir, at doses of 20 mg, 40 mg, and then three injections of 100 mg weekly. This treatment was associated with systemic trastuzumab 6 mg/Kg every 3 weeks. Lapatinib treatment was also started but rapidly discontinued because of digestive toxicity.
After the first dose of IT treatment no malignant cells were found in the CSF analysis. The protein level decreased until normal values (0.15–0.45 g/L) after the fourth dose (see Figures 2 and 3).
Figure 2.

Decreased values until normal ranges of the CSF protein level with IT trastuzumab. First value corresponds to the lumbar punction. The next values corresponds to the Ommaya reservoir functions.
Figure 3.
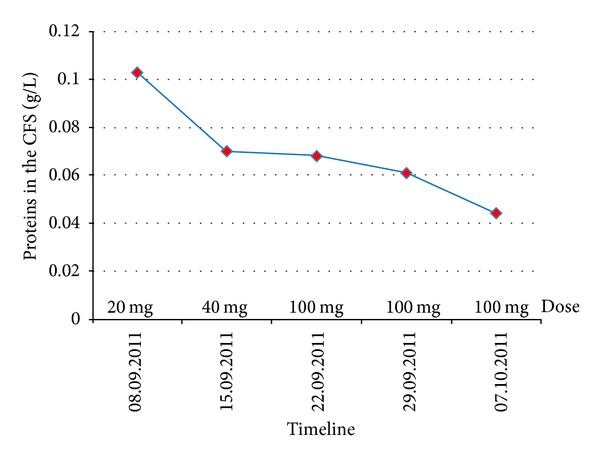
Decreased values of the proteins in the CSF by Ommaya punction, during the treatment with IT trastuzumab.
After the first two doses of intrathecal trastuzumab, the neurological status of the patient stabilised and did not deteriorate further. The MRI showed a reduction of the brain metastatic lesions and a stabilisation of the leptomeningeal infiltration.
The patient died from hepatic failure due to liver metastases eight weeks after the onset of the leptomeningeal disease symptoms.
2. Discussion
The first cause of leptomeningeal carcinomatosis is breast cancer (43%), followed by the lung cancer (31%) and melanoma (6%) [9, 10]. Only 5% of meningeal metastasis are diagnosed during the cancer evolution; meanwhile 20% are discovered at the autopsy [11].
Trastuzumab is a murine antibody that recognizes the extracellular domain of the HER2/Neu receptor and has been used successfully for the treatment of breast cancer. But this molecule is unable to penetrate through the blood-brain barrier (BBB) due to its 148000 kiloDa (kD) molecular weight. As usually reported, molecules must not exceed 200 Da to cross the BBB.
In 2000 Pestalozzi and Brignoli [12] measured the concentration of trastuzumab in the CSF in a 62-year-old woman treated with weekly intravenous injections. He demonstrated that a very small amount of the drug penetrates through the BBB: 210 ng/dL in the CSF versus 61392 ng/dL in the serum, 210 minutes after the administration of 120 mg of trastuzumab. In 2007 Stemmler et al. [13] showed that values of CSF trastuzumab were two times higher after radiotherapy and three times higher in presence of leptomeningeal carcinomatosis and radiotherapy [14].
The first treatment using trastuzumab as IT chemotherapy in HER2-positive breast cancer leptomeningeal carcinomatosis was reported by Laufman and Forsthoefel in 2001 [15] in a 48-year-old woman, with no immediate positive therapeutic effect neither any neurological nor site toxicity.
Following this, in 2005 Stemmler et al. [3] highlighted the effectiveness of the IT trastuzumab after using this treatment for a 39-year-old woman with HER2-positive breast cancer who developed headache and dizziness. Five increasing doses, by steps of 5 mg, of IT trastuzumab were administered six months after WBRT. The patient condition improved, and no significant toxicity was observed.
In 2006 Platini et al. [4] published the encouraging results of a 17-month treatment with intrathecal trastuzumab without any clinical adverse events.
A paper published by Colozza et al. [5] described the case of a 38-year-old woman with HER2-positive breast cancer treated with 12.5 mg of IT trastuzumab every 3 weeks. After nineteen months the patient showed no toxicity but had an improved neurological status. A second paper published by Ferrario et al. [6] showed that combined treatment of IT trastuzumab and thiotepa is a promising and safe approach; this conclusion was based on the observation of 31-year-old woman with HER2-positive breast cancer treated with increased doses of IT trastuzumab and then IT trastuzumab with thiotepa (see Table 1).
Table 1.
Intrathecal treatments using trastuzumab, alone or in association with other agents.
| Reference | Symptoms | WBRT | CSF before ITT | MRI | Intrathecal therapy | CSF after ITT | MRI | Clinical response | Toxicity | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| before ITT | Proteins | Malignant C | before ITT | Proteins | Malignant C | after ITT | |||||
| Stemmler et al., 2006[3] |
♀ 39 Y Headache, dizziness |
+ | Increased | + | No data | MTX 6 × 15 mg + Ommaya TST 5 mg, 10 mg, 15 mg, 20 mg, 20 mg |
Low | − | No data | Improved | None |
|
| |||||||||||
| Platini et al., 2006 [4] |
♀ 36 Y Vertigo, amnesia, mental confusion |
− | Increased | + | No data | MTX Peridural PAC 46 × 25 mg TST (17 mo) THI |
Normal | − | No data | Stable | None |
|
| |||||||||||
|
Colozzaet al., 2009 [5] |
♀ 38 Y Headache, dizziness, gait disorder |
+ | No lumbar punction | LMC+ | Ommaya 23 × 12.5 mg/3 weeks TST |
No data | − | Stable | Improved | None | |
|
| |||||||||||
| Ferrario et al., 2009 [6] |
♀ 31 Y Visual impairment, right ptosis, paralysis of the right facial nerve, and left foot drop |
− | Normal | + | No data | Ommaya TST 2 × 20 mg, 2 × 25 mg, 3 × 30 mg, TST 40 mg q3w × 8 mo TST 40 mg q3w + thiotepa 10 mg × 6 mo TST 50 mg q3W + 12 mg THI × 13 mo |
No data | − | Improvement | Complete recovery | None |
|
| |||||||||||
|
Mego et al., 2011 [7] |
♀ 43 Y Dizziness, cranial nerves palsy. |
+ | Increased | − | LMC+ | MTX 15 mg + CYT 24 mg + HDC 24 mg + 3 × 20 mg TST, 3 × 40 mg TST | Increased | − | No data | Improved | None |
| ♀ 39 Y Headache, dizziness, cranial nerve palsies, vision disorders |
+ | Increased | + | LMC+ | MTX 15 mg + CYT 24 mg + HDC 24 mg + TST 1 × 20 mg, 1 × 40 mg, 1 × 80 mg, 3 × 100 mg | No data | − | Improved | None | ||
|
| |||||||||||
| Oliveira et al., 2011 [8] |
♀ 40 Y Headache, vomiting, gait disturbance |
+ | No lumbar punction | LMC+ (CT scan exam) |
TST 25 mg + PRD 25 mg × 25 doses (2 years) | No data | Partial response | Complete recovery | None | ||
TST: trastuzumab, MTX: methotrexate, THI: thiotepa, HDC: hydrocortisone, PRD: prednisolone, CYT: cytarabine, MRI: magnetic resonance imaging, CSF: cerebrospinal fluid, mo: months, CT: computed tomography, WBRT: whole brain radiotherapy, ITT: intrathecal treatment, “+”: presence, “−”: absence, malignant C: malignant cells.
The hypothesis of using a combined IT treatment was successfully applied by Mego et al. [7] in the case of two HER2-positive breast cancer patients; the treatment consisted in the association of trastuzumab with hydrocortisone and cytarabine. The neurological status improved in both patients, and no malignant cells were further detected in the CSF.
In a paper published by Lombardi et al. [9] in 2011, it is clearly indicated that IT trastuzumab therapy lacks od significant toxicity and can result in significant efficacy compared to other intrathecal drugs (topotecan, etoposide, gemcitabine, Α interferon, and alpha interleukin).
Finally in a paper published by Oliveira et al. [8] in 2001, sixty-seven administrations of 25 mg of IT trastuzumab with prednisolone resulted in complete recovery of neurological symptoms in a 40-year-old woman.
Since then trastuzumab had been iteratively used as intrathecal treatment for the HER2-positive breast cancer brain and leptomeningeal metastasis, with no toxicity, increased overall survival, and no chemical interactions [16–19].
There is evidence in the literature [20–24] that tumor receptor status may change over time, and when it occurs, it may modify the therapeutical approach.
In a recent paper published in 2011, Curigliano et al. [25] evaluated the discordance rate of ER, PgR, and HER2 status between primary tumor and liver metastases, with a potential impact on treatment choice. The study was conducted on 255 patients over a ten-year period. Changes in the HER2 status were observed in 172 patients as follows: 17 of 54 patients (31.5%) changed their status from positive to negative and 7 of 118 patients (5.9%) changed their HER2 status from negative to positive. The study also revealed that HER2 status changing from negative to positive was associated with a certain decrease in ER and PgR expression between primary tumor and liver biopsy. Regitnig et al. [22] explained this by a possible crosstalk between the pathways driven by HER2 and the hormone receptors.
Discordance in the receptor status between primary tumor and metastatic site could also be related to different factors: transcriptional and posttranscriptional modification in the gene expression level, that can occur spontaneously or as a consequence of clonal selection in response to chemotherapy, immunotherapy, and hormonal therapy; limited accuracy and reproducibility of receptor assay; errors due to biopsy procedure; real switch in the biology of the disease [25].
Our case report and the review of the literature lead us to conclude that IT administration of trastuzumab might improve or stabilise the consequences of leptomeningeal involvement by HER2-positive breast cancer with no toxicity.
Intrathecal trastuzumab might thus be a promising treatment for leptomeningeal involvement in HER2-positive breast cancer patients, and further perspective studies have to be done to better determine the efficacy, safety, and tolerability of this approach.
Conflict of Interests
The authors have no conflict of interests to declare.
Acknowledgments
The authors thank Professors Jerzy Hildebrand and Jean Klastersky for their contribution and review of the paper.
References
- 1.Wolff AC, Hammond ME, Schwartz JN, et al. ASCO/CAP guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. Archives of Pathology & Laboratory Medicine. 2007;131 doi: 10.5858/2007-131-18-ASOCCO. [DOI] [PubMed] [Google Scholar]
- 2.Colpaert C, Salgado R. Belgian guidelines for Her2/neu testing in breast cancer. Belgian Journal of Medical Oncology. 2007;1:22–29. [Google Scholar]
- 3.Stemmler HJ, Schmitt M, Harbeck N, et al. Application of intrathecal trastuzumab (Herceptin) for treatment of meningeal carcinomatosis in HER2-overexpressing metastatic breast cancer. Oncology Reports. 2006;15(5):1373–1377. doi: 10.3892/or.15.5.1373. [DOI] [PubMed] [Google Scholar]
- 4.Platini C, Long J, Walter S. Meningeal carcinomatosis from breast cancer treated with intrathecal trastuzumab. Lancet Oncology. 2006;7(9):778–780. doi: 10.1016/S1470-2045(06)70864-6. [DOI] [PubMed] [Google Scholar]
- 5.Colozza M, Minenza E, Gori S, et al. Extended survival of a HER-2-positive metastatic breast cancer patient with brain metastases also treated with intrathecal trastuzumab. Cancer Chemotherapy and Pharmacology. 2009;63(6):1157–1159. doi: 10.1007/s00280-008-0859-7. [DOI] [PubMed] [Google Scholar]
- 6.Ferrario C, Davidson A, Bouganim N, Aloyz R, Panasci LC. Intrathecal trastuzumab and thiotepa for leptomeningeal spread of breast cancer. Annals of Oncology. 2009;20(4):792–795. doi: 10.1093/annonc/mdp019. [DOI] [PubMed] [Google Scholar]
- 7.Mego M, Sycova-Mila Z, Obertova J, et al. Intrathecal administration of trastuzumab with cyrarabine and methotrexate in breast cancer patients with leptomeningeal carcinomatosis. The Breast. 2011;20(5):478–480. doi: 10.1016/j.breast.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 8.Oliveira M, Braga S, Passos-Coelho JL, Fonseca R, Oliveira J. Complete response in HER2+ leptomeningeal carcinomatosis from breast cancer with intrathecal trastuzumab. Breast Cancer Research and Treatment. 2011;127(3):841–844. doi: 10.1007/s10549-011-1417-2. [DOI] [PubMed] [Google Scholar]
- 9.Lombardi G, Zustovich F, Farina P, et al. Neoplastic meningitis from solid tumors: new diagnostic and therapeutic approaches. The Oncologist. 2011;16:1175–1188. doi: 10.1634/theoncologist.2011-0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Le Rhun E, Tailibert S, Zairi F, et al. Clinicopathological features of breast cancers predict the development of leptomeningeal metastases: a case-control study. Journal of Neuro-Oncology. 2011;105(2):309–315. doi: 10.1007/s11060-011-0592-7. [DOI] [PubMed] [Google Scholar]
- 11.Platini C. Trastuzumab and blood-brain barrier. Bulletin du Cancer. 2007;94(10):857–859. [PubMed] [Google Scholar]
- 12.Pestalozzi BC, Brignoli S. Trastuzumab in CSF. Journal of Clinical Oncology. 2000;18(11):2349–2351. doi: 10.1200/JCO.2000.18.11.2349. [DOI] [PubMed] [Google Scholar]
- 13.Stemmler HJ, Schmitt M, Willems A, Bernhard H, Harbeck N, Heinemann V. Ratio of trastuzumab levels in serum and cerebrospinal fluid is altered in HER2-positive breast cancer patients with brain metastases and impairment of blood-brain barrier. Anti-Cancer Drugs. 2007;18(1):23–28. doi: 10.1097/01.cad.0000236313.50833.ee. [DOI] [PubMed] [Google Scholar]
- 14.Gutierrez M, Lyazidi S, Brasseur L, Cvitkovic F, Le Scodan R. Méningites carcinomateuses des cancers du sein surexprimant HER2: pour un traitement spécifique? Bulletin du Cancer. 2011;98(4):417–424. doi: 10.1684/bdc.2011.1341. [DOI] [PubMed] [Google Scholar]
- 15.Laufman LR, Forsthoefel KF. Use of intrathecal trastuzumab in a patient with carcinomatous meningitis. Clinical Breast Cancer. 2001;2(3):p. 235. doi: 10.1016/S1526-8209(11)70419-0. [DOI] [PubMed] [Google Scholar]
- 16.Boogerd W, van den Bent MJ, Koehler PJ, et al. The relevance of intraventricular chemotherapy for leptomeningeal metastasis in breast cancer: a randomised study. European Journal of Cancer. 2004;40(18):2726–2733. doi: 10.1016/j.ejca.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 17.Gauthier H, Guilhaume MN, Bidard FC, et al. Survival of breast cancer patients with meningeal carcinomatosis. Annals of Oncology. 2010;21(11):2183–2187. doi: 10.1093/annonc/mdq232. [DOI] [PubMed] [Google Scholar]
- 18.Rudnicka H, Niwińska A, Murawska M. Breast cancer leptomeningeal metastasis—the role of multimodality treatment. Journal of Neuro-Oncology. 2007;84(1):57–62. doi: 10.1007/s11060-007-9340-4. [DOI] [PubMed] [Google Scholar]
- 19.Baculi RH, Suki S, Nisbett J, Leeds N, Groves M. Meningeal carcinomatosis from breast carcinoma responsive to trastuzumab. Journal of Clinical Oncology. 2001;19(13):3297–3298. doi: 10.1200/JCO.2001.19.13.3297. [DOI] [PubMed] [Google Scholar]
- 20.Simmons C, Miller N, Geddie W, et al. Does confirmatory tumor biopsy alter the management of breast cancer patients with distant metastases? Annals of Oncology. 2009;20(9):1499–1504. doi: 10.1093/annonc/mdp028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aitken SJ, Thomas JS, Langdon SP, Harrison DJ, Faratian D. Quantitative analysis of changes in ER, PR and HER2 expression in primary breast cancer and paired nodal metastases. Annals of Oncology. 2010;21(6):1254–1261. doi: 10.1093/annonc/mdp427. [DOI] [PubMed] [Google Scholar]
- 22.Regitnig P, Schippinger W, Lindbauer M, Samonigg H, Lax SF. Change of HER-2/neu status in a subset of distant metastases from breast carcinomas. Journal of Pathology. 2004;203(4):918–926. doi: 10.1002/path.1592. [DOI] [PubMed] [Google Scholar]
- 23.Lipton A, Leitzel K, Ali SM, et al. Serum HER-2/neu conversion to positive at the time of disease progression in patients with breast carcinoma on hormone therapy. Cancer. 2005;104(2):257–263. doi: 10.1002/cncr.21202. [DOI] [PubMed] [Google Scholar]
- 24.Amir E, Clemons M, Freedman OC, et al. Tissue confirmation of disease recurrence in patients with breast cancer: pooled analysis of two large prospective studies. Journal of Clinical Oncology. 2010;28:p. 15s. abstract no. 1007. [Google Scholar]
- 25.Curigliano G, Bagnardi V, Viale G, et al. Should liver metastases of breast cancer be biopsied to improve treatment choice? Annals of Oncology. 2011;22(10):2227–2233. doi: 10.1093/annonc/mdq751. [DOI] [PubMed] [Google Scholar]


