Abstract
Cancer metastasis becomes an initial cause of cancer death in human population. In many cancers, it has been shown that the high levels of matrix metalloproteinase (MMP)-2 and/or MMP-9 are associated with the invasive phenotypes of cancer cells. In this study, we investigated the effects of cantharidin, a derivative of blister beetles which is one of the traditional Chinese medicines, on the adhesion, migration, and invasion of human bladder cancer TSGH-8301 cells. Cantharidin effectively suppressed TSGH-8301 cell adhesion, migration, and invasion in a concentration-dependent manner. Results from Western blotting, RT-PCR, and gelatin zymography assays indicated that cantharidin blocked the protein levels, gene expression (mRNA), and activities of MMP-2 and -9 in TSGH-8301 cells. Cantharidin also significantly suppressed the protein expressions of p-p38 and p-JNK1/2 in TSGH-8301 cells. Taken together, cantharidin was suggested to present antimetastatic potential via suppressing the levels of MMP-2 and MMP-9 expression that might be mediated by targeting the p38 and JNK1/2 MAPKs pathway in TSGH-8301 human bladder cancer cells.
1. Introduction
In genitourinary tumor, bladder cancer is a significant cause of morbidity and mortality [1]. In the United States, bladder cancer is the fourth most common malignancy, and new cases about 70,530 (52,760 men and 17,770 women) and deaths for the year 2010 were 14,680 (10,410 men and 4270 women) [2]. In Taiwan, about 2.3 individuals per 100,000 die annually from bladder cancer on the basis of the 2011 report from the Department of Health, Taiwan. In bladder cancer of patients, 75% present with superficial disease and 25% with invasive disease [3].
During the metastasis development, there are about 50% of patients with muscle invasive bladder cancer within 2 years of cystectomy [4, 5]. Muscle-invasive bladder cancer is an aggressive epithelial tumor; almost 50% of these patients develop metastases and ultimately succumb to their disease with poor long-term survival [6, 7]. Invasion and metastasis are predominant properties in cancer cells that led to hard-to-cure patients [8, 9]. It is well documented that the activities of matrix metalloproteinases (MMPs) play an important role in the cancer cell's metastasis process, including cell adhesion, migration, and invasion [10–12]. Therefore, blockage of the activities of MMPs may be a strategy to inhibit the cancer cell metastasis.
Cantharidin, a derivative of Blister Beetles, is protein phosphatase 1 (PP1) and protein phosphatase 2A (PP2A) inhibitors [13, 14] and has been used in traditional Chinese medicine [15]. Cantharidin induced cell cycle arrest [16, 17] and triggered apoptosis in various types of tumor cells, including hepatoma [18], myeloma [19], oral buccal carcinoma [20], leukemia cells [21, 22], and intestinal epithelial cells [23]. Recently, cantharidin was found in our laboratory to provoke apoptosis in human bladder carcinoma TSGH-8301 and colorectal cancer colo 205 cells [24, 25] but there is no report to show that cantharidin inhibited the migration and invasion of TSGH-8301 cells. Therefore, the current study investigated the effects of cantharidin on migration and invasion and explored its signaling molecules in in vitro study. Our results demonstrated that cantharidin potently inhibited the migration and invasion of TSGH-8301 human bladder carcinoma cells through inhibiting the p38 and JNK1/2-modulated MMP-2/-9 signaling in vitro.
2. Materials and Methods
2.1. Chemicals and Reagents
Cantharidin, dimethyl sulfoxide (DMSO), propidium iodide (PI) and anti-β-Actin were purchased from Sigma-Aldrich Corp. (St. Louis, MO, USA). Cantharidin was dissolved in DMSO at a stock concentration of 50 mM and followed to dilute in further experiments. RPMI-1640 medium, L-glutamine, fetal bovine serum (FBS), penicillin-streptomycin, and trypsin-EDTA were obtained from Gibco/Life Technologies (Grand Island, NY, USA). Anti-MMP-9 (Cat. AB19016) and Millicell Hanging Cell Culture Inserts (Cat. PIEP12R48) were brought from Merck Millipore Corp. (Billerica, MA, USA). The antibodies to p-p38, p-JNK1/2, p-ERK1/2, and MMP-2 and horseradish-peroxidase- (HRP-) conjugated secondary antibodies were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA).
2.2. Cell Culture
The human bladder carcinoma TSGH-8301 cell line was purchased from the Food Industry Research and Development Institute (Hsinchu, Taiwan). TSGH-8301 cells were maintained in RPMI-1640 medium supplemented with 10% FBS, 100 Units/mL penicillin, and 100 μg/mL streptomycin in 75 cm2 tissue culture flasks and grown at 37°C under a humidified atmosphere with 5% CO2 as previously described [24, 26].
2.3. Assessment for Cell Viability
TSGH-8301 cells were seeded at a density of 2 × 105 cells/well in 12-well plates and were incubated with 0, 0.25, 0.5, 1, 2, and 2.5 μM of cantharidin for 24 h. DMSO at the concentration of 0.5% served as a vehicle control. Cells were harvested and were stained with PI (5 μg/mL) and then were analyzed by flow cytometry (BD Biosciences, FACSCalibur, San Jose, CA, USA) for viability determinations as previously described [24, 27].
2.4. Adhesion Assay
TSGH-8301 cells at the density of 5 × 104 cells/well were preincubated with cantharidin (0, 1, and 2.5 μM) and 0.5% DMSO (vehicle control) for 24 or 48 h at 37°C in 96-well plates precoated with type I collagen (10 μg/mL) (EMD Millipore) for 60 min at 37°C. After a 3 h incubation, the unattached cells were removed, and attached cells were fixed in 1% glutaraldehyde in PBS for 20 min. Then cells were stained with 0.02% crystal violet solution for 5 min at room temperature. For quantification of the attached cells, 70% ethanol was used to dissolve the crystal violet, and O.D. was measured at 570 nm by using microplate reader and reference 405 nm. The percentage of adhesion was calculated based on the adhesion cells compared to control [28, 29].
2.5. Wound Healing Assay
TSGH-8301 cells at the density of 5 × 105 cells/well were maintained in 6-well plates and incubated at 37°C for 24 h. After cells were grown in confluent then cells were scratched with a 200-μL pipette tip, cells in the plate were washed with PBS, and then added the new complete medium then were treated with or without 1 and 2.5 μM of cantharidin for 24 h and 0.5% DMSO served as a vehicle control. At the end of incubation, the cells were examined and were photographed under a fluorescence microscope. The number of cells that migrated into the scratched area was calculated as described elsewhere [28, 30].
2.6. In Vitro Migration and Invasion Assays
TSGH-8301 cell migration or invasion was conducted using 24-well Transwell inserts (8 μm pore filters, Merck Millipore) individually coated with 30 μg type I collagen (Merck Millipore) (for migration) or Matrigel (BD Biosciences, Bedford, MA, USA) (for invasion) [28]. In brief, TSGH-8301 cells (2 × 104 cells/well) were cultured for 24 h in serum-free RPMI-1640 medium, and then cells were placed in the upper chamber of the Transwell insert and treated with 0.5% DMSO (as a control) or cantharidin (1 or 2.5 μM) for 24 h. In the lower chamber, the medium containing 10% FBS was placed. At the end of incubation, the nonmigrated cells were removed using a cotton swab; the invaded cells maintained in the upper chamber were fixed with 4% formaldehyde and stained with 2% crystal violet. In the lower surface of the filter, cells penetrated were counted and photographed under a phase-contract microscope at a 200x magnification. Three independent experiments were performed as described elsewhere [31, 32].
2.7. Western Blotting Analysis
For investigating the protein levels associated with migration and invasion, whether are affected or not by cantharidin, we determined related signaling molecules by Western blotting as described elsewhere [27, 33, 34]. Briefly, TSGH-8301 cells (1 × 106 cells/well) were placed in 6-well plates for 24 h and then were incubated with cantharidin (0, 1, or 2.5 μM) for 24 h. At the end of incubation, cells were harvested from each treatment and were individually lysed in lysis buffer (PRO-PREP protein extraction solution, iNtRON Biotechnology, Seongnam-si, Gyeonggi-do, Korea). The total protein amount was individually determined using a Bio-Rad protein assay kit (Bio-Rad Laboratories, Hercules, CA, USA). The protein abundance of p-p38, p-JNK1/2, p-ERK1/2, MMP-2, and MMP-9 was examined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting as previously described [33, 34]. The relative abundance of each band which represents associated protein expression was quantified using the NIH ImageJ [35].
2.8. Gelatin Zymography Assay
TSGH-8301 cells at the density of 1 × 106 cells/well were plated in 12-well plates and then were incubated in serum-free RPMI-1640 medium in the presence of 0, 1, or 2.5 μM of cantharidin for 24 and 48 h. In the end of incubation, the conditioned medium was harvested, placed on 10% SDS-PAGE containing 0.2% gelatin (Sigma-Aldrich Corp.), and then separated by electrophoresis. The gels were soaked in 2.5% Triton X-100 in dH2O twice for a total of 60 min at 25°C to remove SDS. Gels were incubated at 37°C with substrate buffer (50 mM Tris HCl, 5 mM CaCl2, 0.02% NaN3, and 1% triton X-100, pH 8.0) for 18 h. The gel was stained using 0.2% Coomassie blue for 1 h, was destained in water containing 10% acetic acid and 50% methanol, and bands corresponding to the activity of MMP-2 and -9 were quantified with the NIH ImageJ software as previously described [28, 36].
2.9. Real-Time PCR of MMP-2 and -9 mRNA Expressions
TSGH-8301 cells at the density 1 × 106 cells/well were placed in 6-well plates for 24 h and then were incubated with cantharidin (0, 1, or 2.5 μM) for 24 h. Cells from each treatment were harvested and total RNA was extracted as previously described [33]. RNA samples were reverse-transcribed at 42°C with High Capacity cDNA Reverse Transcription Kit for 30 min according to the protocol of the supplier (Applied Biosystems, Foster City, CA, USA). The primers were set as MMP-2F: CCCCAGACAGGTGATCTTGAC; MMP-2R: GCTTGCGAGGGAAGAAGTTG; MMP-7F: GGATGGTAGCAGTCTAGGGATTAACT; MMP-9F: CGCTGGGCTTAGATCATTCC; MMP-9R: AGGTTGGATACATCACTGCATTAGG; GAPDH-F: ACACCCACTCCTCCACCTTT; GAPDH-R: TAGCCAAATTCGTTGTCATACC. Each assay was performed in triplicate by using the Applied Biosystems 7300 Real-Time PCR system, and the expression fold changes were performed by using the comparative CT (threshold cycle) method [31, 34].
2.10. Statistical Analysis
Statistical differences were determined using one-way analysis of variance (ANOVA) followed by Dunnett's posttest and considered significant at the P < 0.05 between experimental and control samples. All data are presented as means ± standard deviation (SD) in triplicate of at least three independent experiments.
3. Results
3.1. Cantharidin Has No Effect on Percentage of Viable TSGH-8301 Cells
It is well documented that cantharidin decreased the percentage of viable cells in many types of human cancer cell lines [18–20, 22, 23]. TSGH-8301 cells were treated with various concentrations of cantharidin in serum-containing medium for 24 and 48 h, and cell viability was determined by flow cytometric assay. Results are shown in Figure 1 and indicated that cantharidin slightly decreased cell viability at the concentration of 1 μM but cantharidin at 2.5 μM decreased cell viability by approximately 17% and 30% at 24 and 48 h, respectively (Figure 1).
Figure 1.
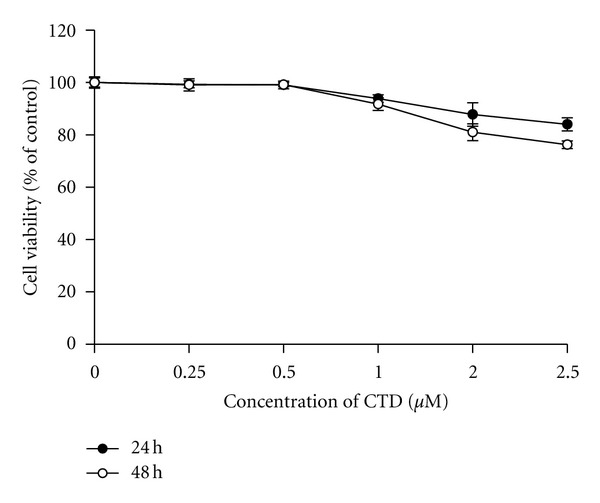
Cantharidin has no dramatic influence on the percentage of viable TSGH-8301 cells. Cells in 12-well plate were incubated with or without 0, 0.25, 0.5, 1, 2, and 2.5 μM of cantharidin for 24 h and 48 h and then harvested for determination of the percentage of viable cells by flow cytometry as described in Section 2. Data represents mean ± S.D. in triplicate.
3.2. Cantharidin Decreases Cell Adhesion of TSGH-8301 Cells
To investigate the effects of cantharidin on the adhesion of TSGH-8301 cells, adhesive cells were quantified, and results are demonstrated in Figure 2. TSGH-8301 cells after incubation with cantharidin at the final concentrations (0, 1, and 2.5 μM) for 24 and 48 h indicated that cantharidin significantly inhibited cell adhesion in a concentration- and time-dependent manner. Approximately 52% and 58% reduction were seen within 2.5 μM treatment for 24 and 48 h, respectively.
Figure 2.
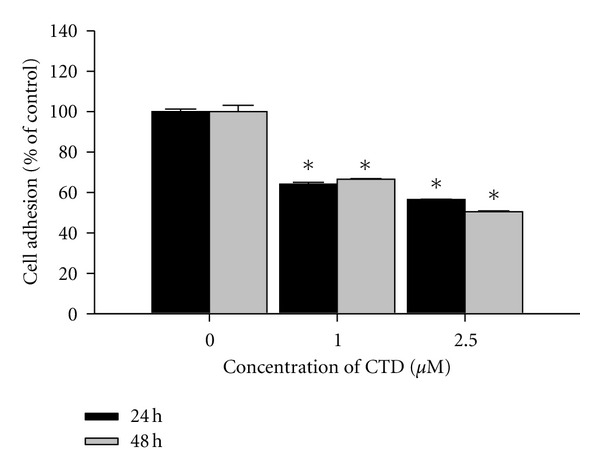
Cantharidin decreases cell adhesion of TSGH-8301 cells. Cells were incubated with or without 0, 1, and 2.5 μM of cantharidin for 24 h and 48 h; then cells were measured the percentage of cell adhesion as described in Section 2. Data represents mean ± SD in triplicate and *P < 0.05, significant difference between cantharidin-treated groups and the control as analyzed by Dunnett's posttest.
3.3. Cantharidin Blocks TSGH-8301 Cell Migration by Wound Healing Examination
Since data in Figure 2 indicated that cantharidin inhibited the adhesion of TSGH-8301 cells, we used wound-healing assay to examine the inhibition of cell migration of TSGH-8301 in vitro. Figure 3 displays that the migration distance between the leading edge and the wound line was compared between cantharidin-treated and untreated cells (Figure 3(a)). The results demonstrated that cantharidin suppressed the migration of TSGH-8301 cells in a concentration-dependent manner (Figure 3(b)).
Figure 3.
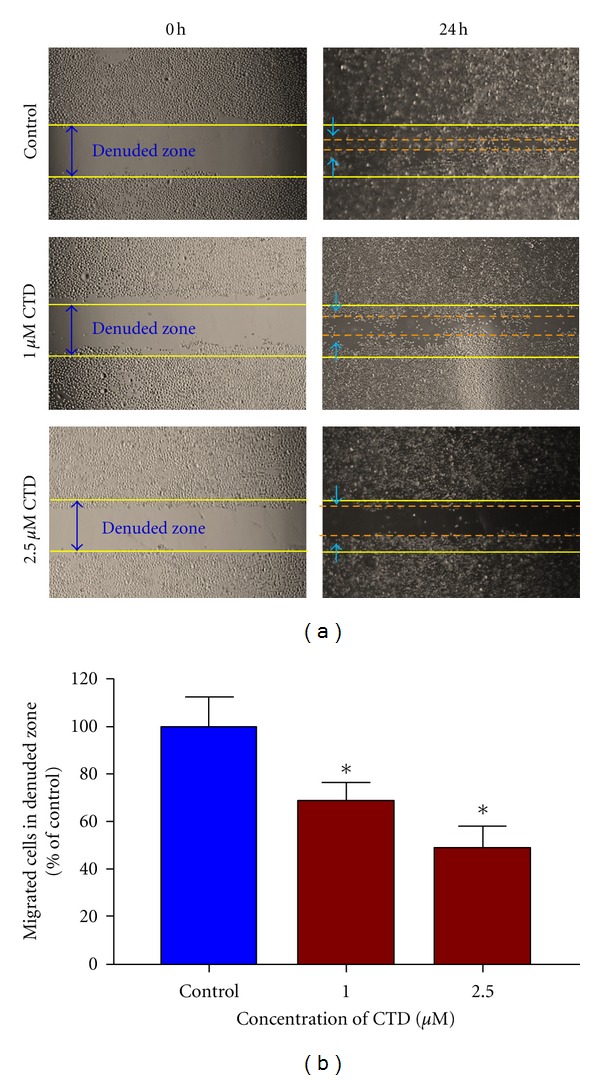
Wound healing assay for the effects of cantharidin on the migration of TSGH-8301 cells. Cells were placed for 24 h; then a wound was performed by scraping confluent cell layers with a pipette tip. Cantharidin was added to the cells at the final concentration were 0, 1 and 2.5 μM and then incubated for 24. (a) Some of the representative photographs of invading treated and untreated cells are presented. Three separate experiments with similar results were carried out. (b) The migrated cells in the five random fields after exposure for 24 h were counted to quantify, and data was expressed as mean ± S.D. on the basis of untreated cells (control) represented as 100%. *P < 0.05, significant difference between cantharidin-treated groups and the control as analyzed by Dunnett's posttest.
3.4. Cantharidin Inhibits the Migration and Invasion of TSGH-8301 Cells In Vitro
For further investigating if cantharidin inhibits the migration and invasion of TSGH-8301 cells, Boyden chamber assay was performed and results are shown in Figures 4(a), 4(b), 4(c), and 4(d). These results were obtained due to the effects of cantharidin on cell migration (Figures 4(a) and 4(b)) and invasion (Figures 4(c) and 4(d)) in TSGH-8301 cells that were treated with 0, 1, and 2.5 μM of cantharidin for 24 and 48 h (cell migration and invasion). Results indicated that cantharidin reduced the migration and invasion of TSGH-8301 cells substantially in a concentration-dependent manner.
Figure 4.
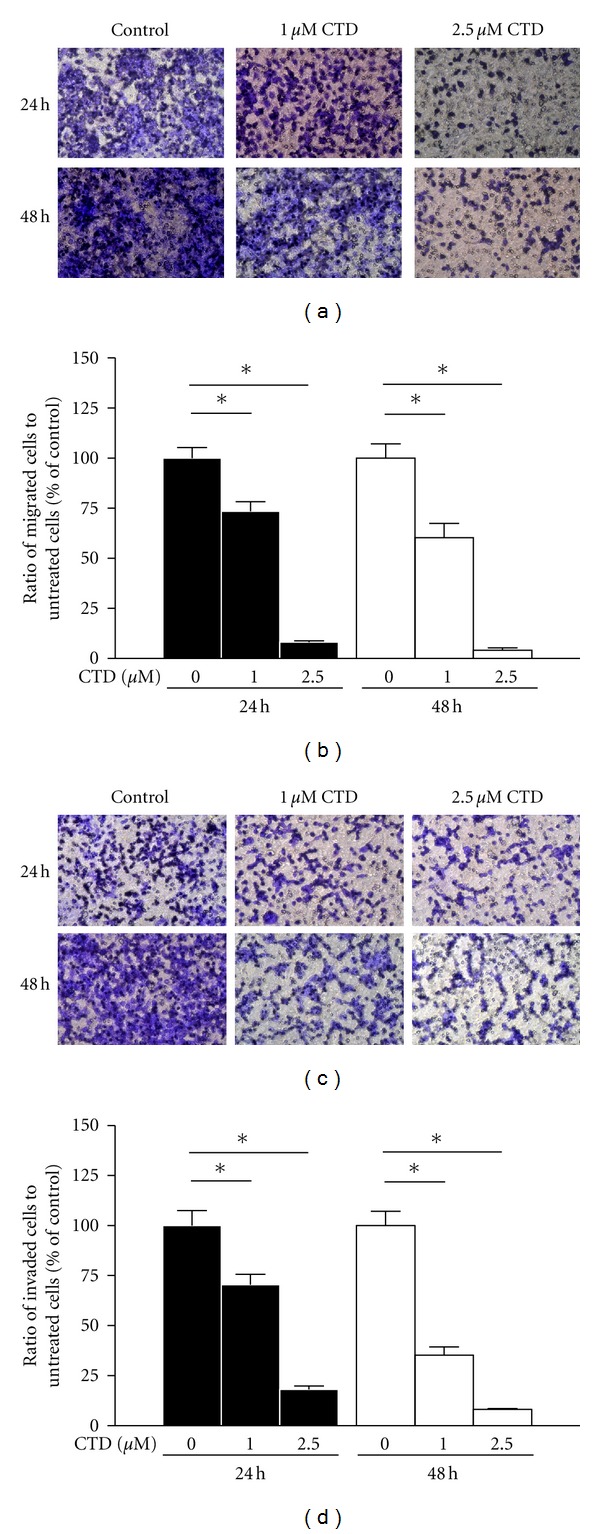
Cantharidin inhibits the migration and invasion of TSGH-8301 cells in vitro. Cells were placed in the well at the density of 5 × 104 cells/well and then were treated with 0, 1, and 2.5 μM cantharidin then that penetrated through with or without the Matrigel to the lower surface of the filter were examined. Cells were stained with crystal violet and then were examined and photographed under a light microscope at 200x (a and c). The quantification of cells from each treatment in the lower chambers was counted at 200x (b) and (d). Columns repeat the mean from three independent experiments. *P < 0.05, significant difference between cantharidin-treated groups and the control as analyzed by Dunnett's posttest.
3.5. Cantharidin Affects the Levels of Associated Protein and Gene Levels for Migration and Invasion of TSGH-8301 Cells
We further examined the effects of cantharidin on the inhibition of migration and invasion of TSGH-8301 cells, which are involved in the effects of associated protein levels of migration and invasion; those changes of associated protein were measured by SDS-PAGE and Western blotting. TSGH-8301 cells were treated with cantharidin (0, 1, and 2.5 μM) for 24 h and then subjected to Western blotting, and results are shown in Figure 5(a). Results from Western blotting showed that cantharidin could reduce the phosphorylation of p38 and JNK1/2 as well as MMP-2 and -9 in TSGH-8301 cells. However, the protein level of p-ERK1/2 was no significant alteration in comparison to untreated control. Figure 5(b) indicated that cantharidin suppressed the gene expression of MMP-2 and -9 in TSGH-8301 cells.
Figure 5.
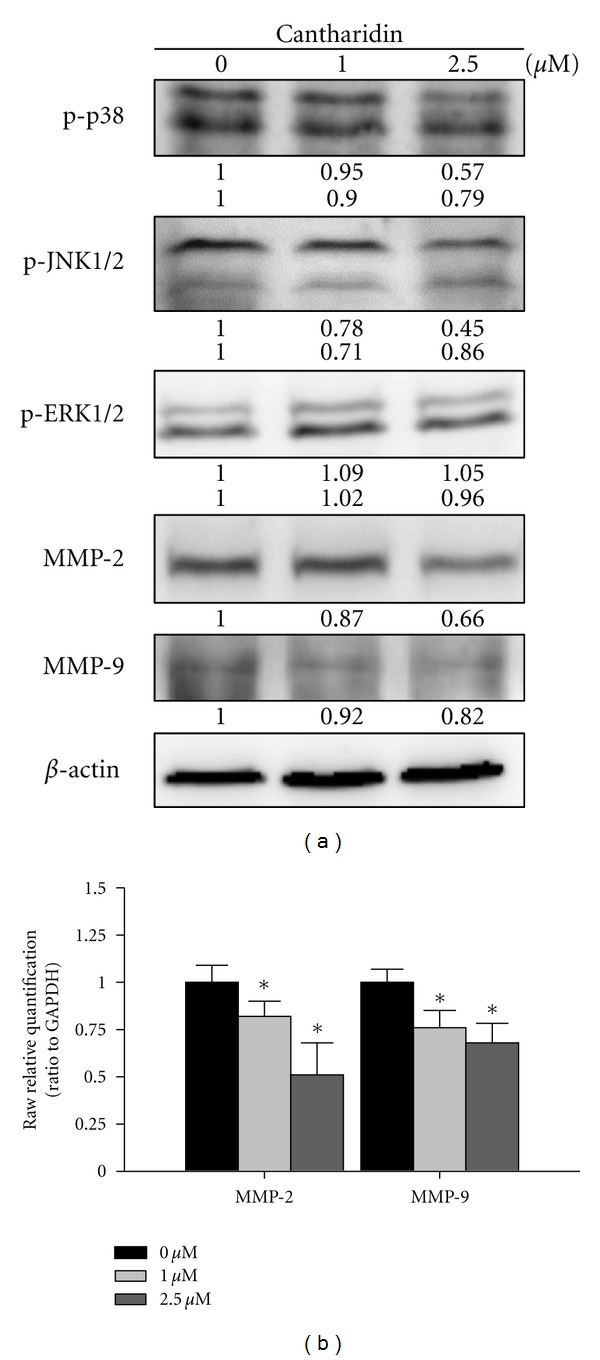
Cantharidin affects the levels of associated proteins and gene levels in migration and invasion of TSGH-8301 cells. Cells were treated with cantharidin (0, 1, and 2.5 μM) for 24 h, and then cells were collected. The total protein extract was quantified and determined as described in Section 2. The levels of p-p38, p-JNK1/2, p-ERK1/2, MMP-2, and MMP-9 protein expressions (a) were estimated by Western blotting as described in Section 2. (b) The total RNA was extracted from cantharidin-treated cells, and the RNA samples were reverse-transcribed to cDNA for real-time PCR as described in Section 2. The ratios between MMP-2, MMP-9, and GAPDH mRNA are used and data represents mean ± SD in duplicate of at least three independent experiments. *P < 0.05 was considered significantly as analyzed by Dunnett's posttest.
3.6. Cantharidin Suppresses the Activities of Matrix Metalloproteinases (MMPs) in TSGH-8301 Cells
Gelatin zymography was used for analysis of MMP-2 and -9 activities. As shown in Figure 6, cantharidin treatment may lead to reduced activity of MMP-2 and -9 in a dose-dependent manner. This also confirmed that cantharidin inhibited the gene expression (mRNA) of MMP-2 and -9 in TSGH-8301 cells (Figure 5(b)).
Figure 6.
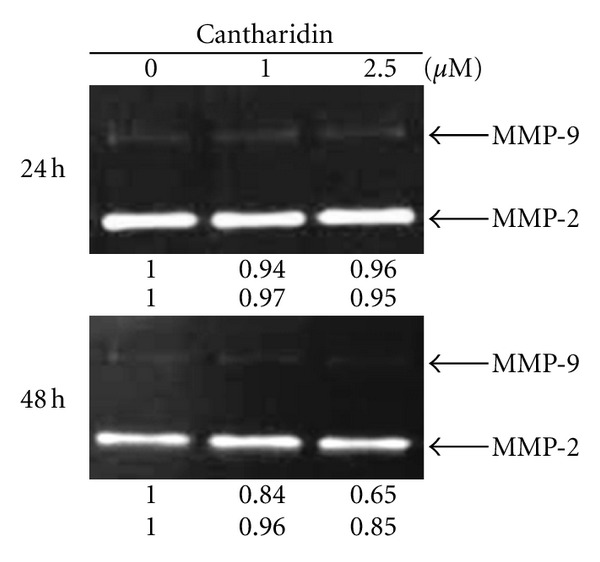
Cantharidin suppresses the activities of matrix metalloproteinases (MMPs) in TSGH-8301 cells. Gelatin zymography was used to evaluate the activities of MMP-2 and MMP-9 as described in Section 2. The different activity of MMP-2 and -9 was determined by densitometry analysis, and results are expressed as % of control. Similar results were obtained from three independent experiments.
4. Discussion
Numerous reports have demonstrated that cantharidin processes antitumor properties [16–23] but there is no report to show the inhibition of migration and invasion of human bladder cancer cells. In this study, we investigated the inhibitory effects of cantharidin on the adhesion, invasion, and migration of TSGH-8301 cells. Results indicated that cantharidin inhibited the cell adhesion, invasion and migration on TSGH-8301 cells (Figures 2 and 3). Cantharidin decreased the protein expressions, gene expression (mRNA) and activities of MMP-2 and -9 in TSGH-8301 (Figures 5(a) and 5(b) and 6). In general, metastasis of cancer cells involves multiple processes, proteins function, and various physiological changes. Furthermore, the degradation or breakdown of the ECM through protease is a critical step in tumor invasion or migration [29, 30]. The involved proteases in migration and invasion, in particular, MMP-2 and MMP-9 were reported to play an important role in cancer invasion and metastasis [37, 38].
Mitogen-activated protein kinases (MAPKs) include p38, c-Jun-N-terminal kinase (JNK), and extracellular signal-regulated kinase (ERK) [35], and MAPKs activation is followed by phosphorylation of a variety of cytosolic substrates associated with cell proliferation, cell differentiation, cell invasion, cell migration, and cell death [39, 40]. It was reported that MAPK pathways were involved in the regulation of MMPs and uPA expression in tumor-cell invasion [41, 42]. Herein, we verified that cantharidin has an inhibitory effect on migration and invasion through the suppression of MMP-2 and -9 in TSGH-8301 cells. We further found that cantharidin inhibited the p-JNK1/2 and p-p38. Thus, our results suggested that cantharidin downregulated MMP-2 and MMP-9 protein expression and suppressed metastatic effect through JNK1/2 and p38 MAPKs signals but not ERK1/2 molecule on TSGH-8301 cells.
Taken together, the present study showed novel findings addressing that cantharidin exerts an inhibitory effect on several essential steps of cancer cell metastasis, including cell adhesion, invasion, and migration via regulating the activities of metastasis-associated proteases such as MMP-2 and -9. Based on those observations, we suggest that cantharidin could be a powerful candidate for development of preventive agents against bladder cancer metastasis in the future. Overall, we showed that cantharidin effectively inhibits the expressions of p-p38 and p-JNK1/2, causing downregulation of MMP-2 and -9 in TSGH-8301 cells that can be seen in Figure 7. Thus, cantharidin could be tested further in vivo to justify its effectiveness in the prevention of bladder tumor cell invasion or migration during cancer treatment.
Figure 7.
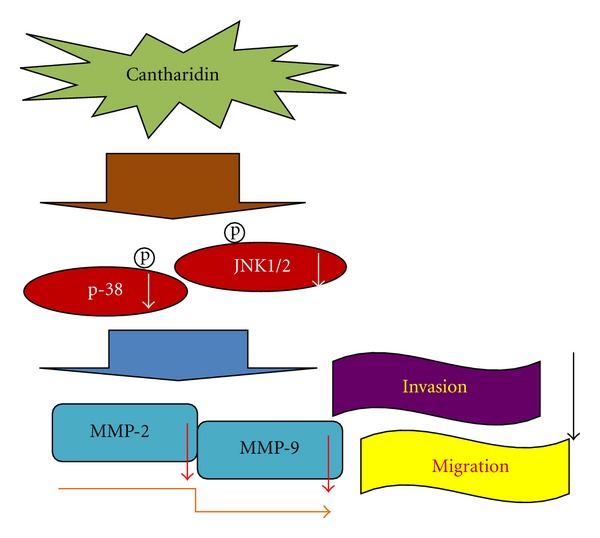
The possible working model and signaling transduction molecules for cantharidin-inhibited cell invasion and migration of TSGH-8301 human bladder cancer cells.
Authors' Contribution
S. J. Chang and J. G. Chung contributed equally to this work.
Acknowledgment
This work was supported by the research Grants CMU99-COL-40-1 and CMU99-COL-40-2 from China Medical University, Taichung, Taiwan.
References
- 1.Hussain SA, James ND. The systemic treatment of advanced and metastatic bladder cancer. Lancet Oncology. 2003;4(8):489–497. doi: 10.1016/s1470-2045(03)01168-9. [DOI] [PubMed] [Google Scholar]
- 2.Hernandez BY, Green MD, Cassel KD, Pobutsky AM, Vu V, Wilkens LR. Preview of Hawaii cancer facts and figures 2010. Hawaii Medical Journal. 2010;69(9):223–224. [PMC free article] [PubMed] [Google Scholar]
- 3.Messing EM, Young TB, Hunt VB, et al. Comparison of bladder cancer outcome in men undergoing hematuria home screening versus those with standard clinical presentations. Urology. 1995;45(3):387–397. doi: 10.1016/s0090-4295(99)80006-5. [DOI] [PubMed] [Google Scholar]
- 4.Whitmore WF., Jr. Management of invasive bladder neoplasms. Seminars in Urology. 1983;1(1):34–41. [PubMed] [Google Scholar]
- 5.Zhang X, Shi X, Li J, et al. A novel therapeutic vaccine of mouse GM-CSF surface modified MB49 cells against metastatic bladder cancer. Journal of Urology. 2012;187(3):1071–1079. doi: 10.1016/j.juro.2011.10.126. [DOI] [PubMed] [Google Scholar]
- 6.Raghavan D, Shipley WU, Garnick MB, Russell PJ, Richie JP. Biology and management of bladder cancer. New England Journal of Medicine. 1990;322(16):1129–1138. doi: 10.1056/NEJM199004193221607. [DOI] [PubMed] [Google Scholar]
- 7.Stein JP, Lieskovsky G, Cote R, et al. Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1,054 patients. Journal of Clinical Oncology. 2001;19(3):666–675. doi: 10.1200/JCO.2001.19.3.666. [DOI] [PubMed] [Google Scholar]
- 8.Liu PL, Tsai JR, Charles AL, et al. Resveratrol inhibits human lung adenocarcinoma cell metastasis by suppressing heme oxygenase 1-mediated nuclear factor-κB pathway and subsequently downregulating expression of matrix metalloproteinases. Molecular Nutrition and Food Research. 2010;54(supplement 2):S196–S204. doi: 10.1002/mnfr.200900550. [DOI] [PubMed] [Google Scholar]
- 9.Christofori G. New signals from the invasive front. Nature. 2006;441(7092):444–450. doi: 10.1038/nature04872. [DOI] [PubMed] [Google Scholar]
- 10.Folgueras AR, Pendas AM, Sanchez LM, Lopez-Otin C. Matrix metalloproteinases in cancer: from new functions to improved inhibition strategies. The International Journal of Developmental Biology. 2004;48(5-6):411–424. doi: 10.1387/ijdb.041811af. [DOI] [PubMed] [Google Scholar]
- 11.Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141(1):52–67. doi: 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coussens LM, Fingleton B, Matrisian LM. Matrix metalloproteinase inhibitors and cancer: trials and tribulations. Science. 2002;295(5564):2387–2392. doi: 10.1126/science.1067100. [DOI] [PubMed] [Google Scholar]
- 13.Li W, Xie L, Chen Z, et al. Cantharidin, a potent and selective PP2A inhibitor, induces an oxidative stress-independent growth inhibition of pancreatic cancer cells through G2/M cell-cycle arrest and apoptosis. Cancer Science. 2010;101(5):1226–1233. doi: 10.1111/j.1349-7006.2010.01523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCluskey A, Ackland SP, Bowyer MC, et al. Cantharidin analogues: synthesis and evaluation of growth inhibition in a panel of selected tumour cell lines. Bioorganic Chemistry. 2003;31(1):68–79. doi: 10.1016/s0045-2068(02)00524-2. [DOI] [PubMed] [Google Scholar]
- 15.Honkanen RE. Cantharidin, another natural toxin that inhibits the activity of serine/threonine protein phosphatases types 1 and 2A. FEBS Letters. 1993;330(3):283–286. doi: 10.1016/0014-5793(93)80889-3. [DOI] [PubMed] [Google Scholar]
- 16.Clarke PR, Hoffmann I, Draetta G, Karsenti E. Dephosphorylation of cdc25-C by a type-2A protein phosphatase: specific regulation during the cell cycle in Xenopus egg extracts. Molecular Biology of the Cell. 1993;4(4):397–411. doi: 10.1091/mbc.4.4.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ishida Y, Furukawa Y, Decaprio JA, Saito M, Griffin JD. Treatment of myeloid leukemia cells with the phosphatase inhibitor okadaic acid induces cell cycle arrest at either G1/S or G2/M depending on dose. Journal of Cellular Physiology. 1992;150(3):484–492. doi: 10.1002/jcp.1041500308. [DOI] [PubMed] [Google Scholar]
- 18.Chen YN, Chen JC, Yin SC, et al. Effector mechanisms of norcantharidin-induced mitotic arrest and apoptosis in human hepatoma cells. International Journal of Cancer. 2002;100(2):158–165. doi: 10.1002/ijc.10479. [DOI] [PubMed] [Google Scholar]
- 19.Kang HS, Lee BS, Yang Y, et al. Roles of protein phosphatase 1 and 2A in an IL-6-mediated autocrine growth loop of human myeloma cells. Cellular Immunology. 1996;168(2):174–183. doi: 10.1006/cimm.1996.0064. [DOI] [PubMed] [Google Scholar]
- 20.Kok SH, Cheng SJ, Hong CY, et al. Norcantharidin-induced apoptosis in oral cancer cells is associated with an increase of proapoptotic to antiapoptotic protein ratio. Cancer Letters. 2005;217(1):43–52. doi: 10.1016/j.canlet.2004.07.045. [DOI] [PubMed] [Google Scholar]
- 21.Dorn DC, Kou CA, Png KJ, Moore MAS. The effect of cantharidins on leukemic stem cells. International Journal of Cancer. 2009;124(9):2186–2199. doi: 10.1002/ijc.24157. [DOI] [PubMed] [Google Scholar]
- 22.Yi S, Wass J, Vincent P, Iland H. Inhibitory effect of norcantharidin on K562 human myeloid leukemia cells in vitro. Leukemia Research. 1991;15(10):883–886. doi: 10.1016/0145-2126(91)90163-n. [DOI] [PubMed] [Google Scholar]
- 23.Ray RM, Bhattacharya S, Johnson LR. Protein phosphatase 2A regulates apoptosis in intestinal epithelial cells. Journal of Biological Chemistry. 2005;280(35):31091–31100. doi: 10.1074/jbc.M503041200. [DOI] [PubMed] [Google Scholar]
- 24.Kuo JH, Chu YL, Yang JS, et al. Cantharidin induces apoptosis in human bladder cancer TSGH 8301 cells through mitochondria-dependent signal pathways. International Journal of Oncology. 2010;37(5):1243–1250. doi: 10.3892/ijo_00000775. [DOI] [PubMed] [Google Scholar]
- 25.Huang WW, Ko SW, Tsai HY, et al. Cantharidin induces G2/M phase arrest and apoptosis in human colorectal cancer colo 205 cells through inhibition of CDK1 activity and caspase-dependent signaling pathways. International Journal of Oncology. 2011;38(4):1067–1073. doi: 10.3892/ijo.2011.922. [DOI] [PubMed] [Google Scholar]
- 26.Chen NG, Lu CC, Lin YH, et al. Proteomic approaches to study epigallocatechin gallate-provoked apoptosis of TSGH-8301 human urinary bladder carcinoma cells: roles of AKT and heat shock protein 27-modulated intrinsic apoptotic pathways. Oncology Reports. 2011;26(4):939–947. doi: 10.3892/or.2011.1377. [DOI] [PubMed] [Google Scholar]
- 27.Lu CC, Yang JS, Chiang JH, et al. Novel quinazolinone MJ-29 triggers endoplasmic reticulum stress and intrinsic apoptosis in murine leukemia WEHI-3 cells and inhibits leukemic mice. PLoS ONE. 2012;7(5, article e36831) doi: 10.1371/journal.pone.0036831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu KC, Huang AC, Wu PP, et al. Gallic acid suppresses the migration and invasion of PC-3 human prostate cancer cells via inhibition of matrix metalloproteinase-2 and -9 signaling pathways. Oncology Reports. 2011;26(1):177–184. doi: 10.3892/or.2011.1264. [DOI] [PubMed] [Google Scholar]
- 29.Ma CY, Ji WT, Chueh FS, et al. Butein inhibits the migration and invasion of SK-HEP-1 human hepatocarcinoma cells through suppressing the ERK, JNK, p38, and uPA signaling multiple pathways. Journal of Agricultural and Food Chemistry. 2011;59(16):9032–9038. doi: 10.1021/jf202027n. [DOI] [PubMed] [Google Scholar]
- 30.Liao CL, Lai KC, Huang AC, et al. Gallic acid inhibits migration and invasion in human osteosarcoma U-2 OS cells through suppressing the matrix metalloproteinase-2/-9, protein kinase B (PKB) and PKC signaling pathways. Food and Chemical Toxicology. 2012;50(5):1734–1740. doi: 10.1016/j.fct.2012.02.033. [DOI] [PubMed] [Google Scholar]
- 31.Chen YY, Chiang SY, Lin JG, et al. Emodin, aloe-emodin and rhein inhibit migration and invasion in human tongue cancer SCC-4 cells through the inhibition of gene expression of matrix metalloproteinase-9. International Journal of Oncology. 2010;36(5):1113–1120. doi: 10.3892/ijo_00000593. [DOI] [PubMed] [Google Scholar]
- 32.Ho YT, Yang JS, Li TC, et al. Berberine suppresses in vitro migration and invasion of human SCC-4 tongue squamous cancer cells through the inhibitions of FAK, IKK, NF-κB, u-PA and MMP-2 and -9. Cancer Letters. 2009;279(2):155–162. doi: 10.1016/j.canlet.2009.01.033. [DOI] [PubMed] [Google Scholar]
- 33.Chiang JH, Yang JS, Ma CY, et al. Danthron, an anthraquinone derivative, induces DNA damage and caspase cascades-mediated apoptosis in SNU-1 human gastric cancer cells through mitochondrial permeability transition pores and Bax-triggered pathways. Chemical Research in Toxicology. 2011;24(1):20–29. doi: 10.1021/tx100248s. [DOI] [PubMed] [Google Scholar]
- 34.Lu CC, Yang JS, Huang AC, et al. Chrysophanol induces necrosis through the production of ROS and alteration of ATP levels in J5 human liver cancer cells. Molecular Nutrition and Food Research. 2010;54(7):967–976. doi: 10.1002/mnfr.200900265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lu CC, Yang JS, Chiang JH, et al. Inhibition of invasion and migration by newly synthesized quinazolinone MJ-29 in human oral cancer CAL 27 cells through suppression of MMP-2/9 expression and combined down-regulation of MAPK and AKT signaling. Anticancer Research. 2012;32(7):2895–2903. [PubMed] [Google Scholar]
- 36.Lai KC, Huang ANC, Hsu SC, et al. Benzyl Lsothiocyanate (BITC) inhibits migration and invasion of human colon cancer HT29 cells by inhibiting matrix metalloproteinase-2/-9 and urokinase plasminogen (uPA) through PKC and MAPK signaling pathway. Journal of Agricultural and Food Chemistry. 2010;58(5):2935–2942. doi: 10.1021/jf9036694. [DOI] [PubMed] [Google Scholar]
- 37.Yang SF, Chen MK, Hsieh YS, et al. Antimetastatic effects of Terminalia catappa L. on oral cancer via a down-regulation of metastasis-associated proteases. Food and Chemical Toxicology. 2010;48(4):1052–1058. doi: 10.1016/j.fct.2010.01.019. [DOI] [PubMed] [Google Scholar]
- 38.Gialeli C, Theocharis AD, Karamanos NK. Roles of matrix metalloproteinases in cancer progression and their pharmacological targeting. The FEBS Journal. 2011;278(1):16–27. doi: 10.1111/j.1742-4658.2010.07919.x. [DOI] [PubMed] [Google Scholar]
- 39.Chen PN, Hsieh YS, Chiou HL, Chu SC. Silibinin inhibits cell invasion through inactivation of both PI3K-Akt and MAPK signaling pathways. Chemico-Biological Interactions. 2005;156(2-3):141–150. doi: 10.1016/j.cbi.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 40.Hsiao YC, Kuo WH, Chen PN, et al. Flavanone and 2′-OH flavanone inhibit metastasis of lung cancer cells via down-regulation of proteinases activities and MAPK pathway. Chemico-Biological Interactions. 2007;167(3):193–206. doi: 10.1016/j.cbi.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 41.Rao JS. Molecular mechanisms of glioma invasiveness: the role of proteases. Nature Reviews Cancer. 2003;3(7):489–501. doi: 10.1038/nrc1121. [DOI] [PubMed] [Google Scholar]
- 42.Westermarck J, Kähäri VM. Regulation of matrix metalloproteinase expression in tumor invasion. The FASEB Journal. 1999;13(8):781–792. [PubMed] [Google Scholar]


