Abstract
Whether ventilator-associated pneumonia is a manifestation of severity of illness or an independent cause of mortality in ventilator-dependent patients is not known. In this complex area, which cannot be readily subjected to randomized controlled trials, studies should focus on the underlying questions of relevance, how to improve care of ventilated patients.
In this issue of Critical Care, Forel and colleagues [1], in a substudy of the ACURASYS trial of neuromuscular blockade in patients with severe acute respiratory distress syndrome (ARDS) [2], explore the association between mortality and incident ventilator-associated pneumonia (VAP) in ARDS, a problem of causal inference that has remained unresolved despite multiple attempts to clarify the question [3]. Forel and colleagues' careful study, employing a rigorous definition of incident VAP [4] and multistate regression to attempt to control for the time-dependence of VAP, is probably the best observational study of its kind on the topic. According to their analysis, VAP was not independently associated with mortality after controlling for severity of illness in a multi-state model.
This study is of excellent quality on many fronts. Ascertainment of VAP was rigorous and prospective, outcomes were established prospectively. Unlike many prior studies pertaining to VAP in ARDS patients [5-7], Forel and colleagues employed lung-protective ventilation strategies [8], which improves the signal-to-noise ratio and the likelihood of ascertaining actual relationships.
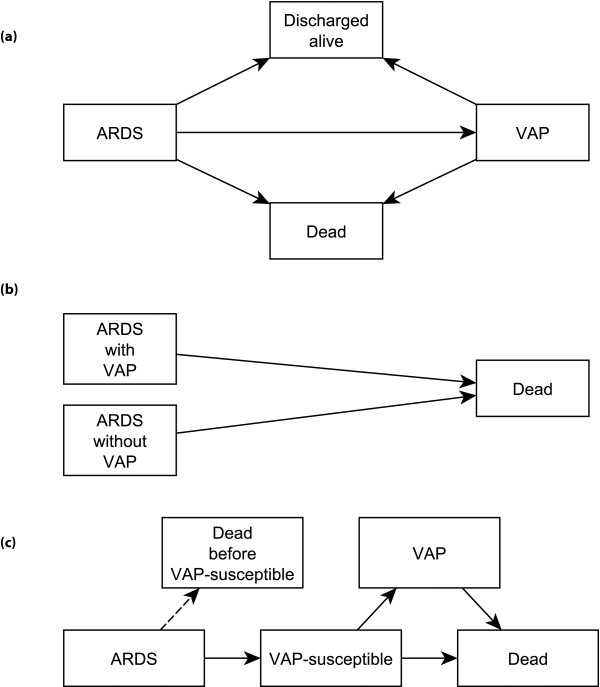
At the same time, most studies of the relationship between mortality and VAP [3,5-7], including the present one, suffer from some combination of length bias and time-dependent bias. In order to acquire VAP, a patient must survive on the ventilator for a period of time, probably somewhere between 4 and 12 days, and this time lag in susceptibility to VAP will vary from patient to patient [5,9]. The model Forel and colleagues employed does not fully overcome length- and time-dependent bias, as we depict in Figure 1a,b. Figure 1c depicts a more accurate multi-state model for this problem, but this non-biased schematic obfuscates the fact that 'VAP-susceptibility' is currently an essentially unmeasurable state. The fact that the authors' sensitivity analysis restricted to those patients surviving at least 9 days almost achieved statistical significance (P = 0.055), in the face of a small sample size, suggests the possibility that VAP is, in fact, independently associated with mortality. The fact that this extremely well designed study was unable to answer the question definitively suggests that this methodology has provided all that it can offer.
Figure 1.
Schematics for possible multi-state models to describe the possible association between ventilator-associated mortality (VAP) among patients who die. (a,b) The schematic of Forel and colleagues [1] (a), which we have simplified into more standard notation in (b). This schematic demonstrates the presence of length and time-dependent bias in the analysis. (c) Schematic that eliminates the length and time-dependence. By VAP-susceptible, we mean that a patient has been ventilated long enough to be susceptible to VAP. Our depiction is modeled on the descriptions in Wolkewitz and colleagues [10]. ARDS, acute respiratory distress syndrome; VAP, ventilator-associated pneumonia.
The limitations of observational methods raise the issue of which problems matter most about clinical out-comes in VAP. The purest methodology for determining whether VAP increases mortality in humans is unethical to the point of being criminal: instill bacteria directly into the trachea of a ventilated patient, as is standard in animal studies of pneumonia. But such an experiment is not only criminal, it is also scientifically pointless. Direct induction of pneumonia in ventilated patients is a bad idea, regardless of whether antibiotic therapy could avert a fatal outcome.
If the pure methodology for assessing the relationship between VAP and death is unethical and irrelevant, it is worth asking what questions matter most. With trends toward penalties for healthcare facilities based on VAP incidence, one important question is whether VAP rates are surrogates for quality of clinical care. Recognizing that facilities with high early mortality rates may have lower VAP rates - an effect that could be compounded by early transition to comfort care in high-risk patients - simple comparisons of VAP rates, even after standard severity-adjustment, may be highly misleading. A metric that incorporates process measures (for example, elevation of the head of the bed, oral care, possibly alimentary tract decontamination) and severity-adjusted mortality rates will almost certainly outperform even severity-adjusted VAP rates.
A more important question is whether interventions to improve care of patients with respiratory failure targeted at prevention or improved treatment of VAP will improve mortality. Such interventions will almost certainly need to be multi-factorial and multi-disciplinary. Studies of relevant interventions are urgently needed and should be supported by healthcare facilities and research-funding bodies. Equivocal findings from observational studies about associations between mortality and VAP should not diminish our enthusiasm for high-quality interventional trials aimed at improving outcomes for patients with ventilator-dependent respiratory failure and/or VAP.
Abbreviations
ARDS: acute respiratory distress syndrome; VAP: ventilator-associated pneumonia.
Competing interests
The authors declare that they have no competing interests.
See related research by Forel et al., http://ccforum.com/content/16/2/R65
Contributor Information
Michael J Lanspa, Email: michael.lanspa@imail.org.
Samuel M Brown, Email: samuel.brown@imail.org.
References
- Forel JM, Voillet F, Pulina D, Gaucouin A, Perrin G, Barrau K, Jaber S, Arnal JM, Fathallah M, Auquier P, Roch A, Azoulay E, Papazian L. Ventilator-associated pneumonia and ICU mortality in severe ARDS patients ventilated according to a lung-protective strategy. Crit Care. 2012;16:R65. doi: 10.1186/cc11312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papazian L, Forel JM, Gacouin A, Penot-Ragon C, Perrin G, Loundou A, Jaber S, Arnal JM, Perez D, Seghboyan JM, Constantin JM, Courant P, Lefrant JY, Guérin C, Prat G, Morange S, Roch A. ACURASYS Study Investigators. Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med. 2010;363:1107–1116. doi: 10.1056/NEJMoa1005372. [DOI] [PubMed] [Google Scholar]
- Melsen WG, Rovers MM, Bonten MJ. Ventilator-associated pneumonia and mortality: a systematic review of observational studies. Crit Care Med. 2009;37:2709–2718. doi: 10.1097/CCM.0b013e3181ab8655. [DOI] [PubMed] [Google Scholar]
- Craven DE, Hjalmarson KI. Ventilator-associated tracheobronchitis and pneumonia: thinking outside the box. Clin Infect Dis. 2010;51(Suppl 1):S59–66. doi: 10.1086/653051. [DOI] [PubMed] [Google Scholar]
- Markowicz P, Wolff M, Djedaïni K, Cohen Y, Chastre J, Delclaux C, Merrer J, Herman B, Veber B, Fontaine A, Dreyfuss D. Multicenter prospective study of ventilator-associated pneumonia during acute respiratory distress syndrome. Incidence, prognosis, and risk factors. ARDS Study Group. Am J Respir Crit Care Med. 2000;161:1942–1948. doi: 10.1164/ajrccm.161.6.9909122. [DOI] [PubMed] [Google Scholar]
- Sutherland KR, Steinberg KP, Maunder RJ, Milberg JA, Allen DL, Hudson LD. Pulmonary infection during the acute respiratory distress syndrome. Am J Respir Crit Care Med. 1995;152:550–556. doi: 10.1164/ajrccm.152.2.7633706. [DOI] [PubMed] [Google Scholar]
- Meduri GU, Reddy RC, Stanley T, El-Zeky F. Pneumonia in acute respiratory distress syndrome. A prospective evaluation of bilateral bronchoscopic sampling. Am J Respir Crit Care Med. 1998;158:870–875. doi: 10.1164/ajrccm.158.3.9706112. [DOI] [PubMed] [Google Scholar]
- Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- Cook DJ, Walter SD, Cook RJ, Griffith LE, Guyatt GH, Leasa D, Jaeschke RZ, Brun-Buisson C. Incidence of and risk factors for ventilator-associated pneumonia in critically ill patients. Ann Intern Med. 1998;129:433–440. doi: 10.7326/0003-4819-129-6-199809150-00002. [DOI] [PubMed] [Google Scholar]
- Wolkewitz M, Allignol A, Schumacher M, Beyersmann J. Two pitfalls in survival analyses of time-dependent exposure: a case study in a cohort of oscar nominees. Am Stat. 2010;64:205–211. doi: 10.1198/tast.2010.08259. [DOI] [Google Scholar]