Abstract
A considerable body of evidence links diminished brain-derived neurotrophic factor (BDNF) signaling to energy balance dysregulation and severe obesity in humans and rodents. Because BDNF exhibits broad neurotrophic properties, the underpinnings of these effects and its true role in the central regulation of food intake remain topics of debate in the field. Here, I discuss recent evidence supporting a critical role for this neurotrophin in physiological mechanisms regulating nutrient intake and body weight in the mature brain. They include reports of functional interactions of BDNF with central anorexigenic and orexigenic signaling pathways and evidence of recognized appetite hormones exerting neurotrophic effects similar to those of BDNF.
Keywords: BDNF, feeding, hypothalamus, anorexigenic, homeostatic, hedonic
Introduction
BDNF is a highly conserved member of the neurotrophin family of secreted proteins. It is synthesized at low levels in the central nervous system during development and at higher levels during the postnatal period [1, 2]. In the adult brain, BDNF is the most abundant and widely distributed neurotrophin [3]. It signals through the tropomyosin-related kinase B (TrkB) receptor and activates phospholipase C gamma (PLC-γ), mitogen-activated protein kinase (MAPK) and phosphatidylinositol-3 kinase (PI3-K) intracellular signaling cascades [4, 5]. BDNF is functionally versatile, facilitating neuronal survival, differentiation, and synaptic connectivity during development, as well as synaptic plasticity and efficacy in the mature brain. The serendipitous finding that intracerebroventricular (ICV) delivery of BDNF reduced food intake and body weight in rats [6, 7] provided the first indication that this neurotrophin might participate in the regulation of feeding.
In the last decade, ensuing investigations in humans and rodents have further reinforced a paramount role for BDNF and TrkB in the regulation of energy balance. In mice, global BDNF haploinsufficiency, brain-specific BDNF depletion, or TrkB hypomorphic expression, results in excessive feeding and body weight gain, accompanied by other features of the associated metabolic syndrome, including hyperleptinemia, hyperglycemia and hyperinsulinemia [8–11] (Table 1). Moreover, selective targeting of the truncated TrkB.T1 receptor isoform, which has been suggested to limit neuronal responsiveness to BDNF, attenuates obesity in BDNF haploinsufficient mice [12]. Human studies also underscore the importance of this neurotrophin pathway. A de novo missense mutation in the TrkB gene hindering MAPK activation was identified in an individual exhibiting hyperphagia and severe obesity [13]. Furthermore, 100% of individuals afflicted with Wilms’ tumor, aniridia, genitourinary anomalies, and mental retardation (WAGR) syndrome due to large truncations in chromosome 11 were obese by the time they reached 10 years of age when the deletion encompassed the Bdnf gene [14]. In contrast, only 20% of WAGR patients with intact Bdnf alleles exhibited obesity. Lastly, several studies have linked a common single nucleotide polymorphism in the human Bdnf gene (i.e. the Val66Met polymorphism), which impedes activity-dependent secretion and signaling of BDNF, to higher body mass index [15–19]. These investigations include an association study of nearly 250,000 individuals that identified Bdnf as a genetic locus linked to obesity susceptibility in humans [17].
Table 1.
Evidence from rodent studies in support of a role for BDNF and TrkB signaling on feeding behavior and body weight
| Manipulation | Description/Region | Functional effects | Refs |
|---|---|---|---|
| BDNF | |||
| Bdnf +/− | Global BDNF depletion (about 50% of normal levels) | ↑ Food intake ↑ Body weight Hyperleptinemia Hyperinsulinemia |
[8, 9] |
| Bdnf conditional KO | Selective depletion of BDNF throughout the brain (cerebellum exempted) during early postnatal period | ↑ Food intake ↑ Body weight Hyperleptinemia Hyperinsulinemia Hyperglycemia |
[10] |
| Knockdown of BDNF in VMN | Selective depletion of BDNF in the adult VMN via AAV-Cre recombinase delivery in floxed Bdnf mice | ↑ Food intake ↑ Body weight Hyperleptinemia Hyperinsulinemia Hyperglycemia |
[24] |
| Bdnf conditional KO | Selective depletion of BDNF in SF-1 neurons in the VMN | Normal food intake and body weight | [51] |
| Knockdown of BDNF in VTA | shRNA or cre-loxP-mediated knockdown of BDNF in VTA of adult rats or mice, respectively | ↑ Food intake ↑ Body weight |
[37, 52] |
| Mice harboring Bdnf alleles with truncated long 3′ UTR | Depletion of dendritically targeted BDNF mRNA | ↑ Food intake ↑ Body weight Hyperleptinemia Impaired glucose homeostasis Reduced anorexigenic response to leptin |
[47] |
| Exogenous BDNF delivery in wild type rodents | Selective delivery to the PVN, VMN or DVC | ↓ Food intake ↓ Body weight |
[20, 42, 43] |
| Exogenous BDNF delivery in Bdnf +/− mice | Chronic ICV delivery of BDNF | Reversal of hyperphagia and obesity | [8] |
| TrkB | |||
| TrkB hypomorphic expression | Global reduction in TrkB expression (by 75%) | ↑ Food intake ↑ Body weight |
[11] |
| TrkB.T1 knock out | Depletion of truncated TrkB.T 1 receptor isoform | Attenuates obesity when crossed with BDNF+/− mice | [12] |
| TrkBF616A knock in mice | Point mutation that blocks kinase activity of TrkB only in the presence of the pharmacological agent 1NaPP1 | Reduced anorexigenic effect of systemic delivery of CCK or MC4-R agonists when pre-treated with 1NaPP1 | [60] |
Despite this body of evidence, questions remain regarding the true contribution of BDNF to the regulation of energy homeostasis in the mature animal. Is the hyperphagia observed in several models of BDNF and TrkB deficiency the consequence of corrupted satiation signaling or the result of perturbing the broad neurotrophic effects of BDNF? Specifically, could aberrant development of the feeding neurocircuitry or alterations in basic components of neuronal function in BDNF mutants lead to obesity as a side effect? Here, I review evidence addressing these questions and supporting a critical role for this pleiotropic neurotrophin in physiological mechanisms controlling nutrient intake and body weight. They include reports of functional interactions of BDNF with anorexigenic and orexigenic signaling pathways in the brain and evidence of appetite hormones with well-established roles in the regulation of energy balance having neurotrophic effects similar to those of BDNF.
BDNF and TrkB expression and responses to energy status in feeding control centers of the brain
Food intake is a complex behavior governed by central homeostatic mechanisms balancing nutritional needs and caloric status. Consistent with a role in feeding regulation, BDNF and TrkB are expressed in several energy homeostasis centers within the hypothalamus and hindbrain of adult animals [11, 20–23]. These brain regions integrate acute satiety and hunger cues and long term adiposity signals from the periphery and, in response, regulate nutrient intake and energy utilization. Several interconnected hypothalamic nuclei influence feeding behavior including the arcuate nucleus (Arc), paraventricular nucleus (PVN), ventromedial nucleus (VMN), dorsomedial hypothalamus (DMH) and lateral hypothalamus (LH). BDNF and TrkB are expressed in the adult VMN, DMH, LH and PVN, with BDNF being most abundant in the VMN [11, 24, 25]. Additionally, TrkB is expressed in the Arc, which contains two functionally distinct populations of neurons that contain neuropeptide Y (NPY)+ or proopiomelanocortin (POMC)+ and promote and inhibit feeding, respectively [11, 26]. Interestingly, reduced expression of hypothalamic BDNF was reported in various obesity models, including leptin receptor-deficient db/db mice, the ty2576 Alzheimer’s disease mouse model, Rai1 and SF-1 deficient mice and Ay mutant mice with deficient melanocortin signaling [11, 23, 27–29], suggesting a functional role for BDNF in this region. Within the dorsal vagal complex (DVC) in the hindbrain, BDNF-containing cell bodies and fibers are present in the nucleus of tractus solitarious (NTS) and TrkB+ cells are located in the NTS and area postrema [21, 25].
In the absence of a homeostatic requirement, food intake can be driven by the highly rewarding qualities of palatable foods rich in sugar or fat. Brain systems involved in motivated and reward-seeking behaviors, including the mesolimbic dopamine pathway, are involved in this form of hedonic feeding [30, 31]. The mesolimbic system is composed of dopamine (DA) neurons in the ventral tegmental area (VTA) and their projections to the nucleus accumbens (NAc) and medial prefrontal cortex (mPFC). Consistent with a role in hedonic feeding, BDNF is expressed in DA neurons in the VTA and in the mPFC from which it is anterogradely transported to the NAc, a region with little or no BDNF expression [32–34]. TrkB is expressed in VTA DA neurons, PFC and GABAergic medium spiny-projection neurons in the NAc [25, 32, 33, 35].
Considering that BDNF is the most widely distributed neurotrophin in the brain, its expression in several appetite-regulating centers is not surprising. However, in agreement with a physiological role in food intake control, expression of BDNF and TrkB in some of these regions is exquisitely sensitive to energy status. Indeed, food deprivation results in a vast depletion of BDNF transcripts in the VMN [11, 24, 36]. Moreover, the caloric signal glucose acts centrally to induce rapid (within 30 minutes) and robust elevations in BDNF and TrkB mRNA content in this hypothalamic region [24]. Among 8 alternative promoters in the Bdnf gene, metabolic signals regulate BDNF mRNA expression in the VMN primarily through regulation of promoter 1 [24, 36]. BDNF content in the DVC is also influenced by energy signals [20]. Whereas extended fasting depleted BDNF protein in this region, 1 hour of re-feeding following a 15 hour fast resulted in elevated levels [20]. Furthermore, another study found that 30 minutes of palatable food consumption was sufficient to increase BDNF/TrkB signaling in the VTA of wild type mice [37].
It is important to note that BDNF is also expressed during developmental periods of neuronal differentiation and synaptic connectivity, raising the possibility that aberrant organization of the feeding neurocircuitry underlies the hyperphagia triggered by perturbed BDNF signaling. BDNF transcripts are detectable in the fetal rat VMN, starting at embryonic day 17 (E17) with expression peaking at postnatal day 4 [22, 23]. BDNF mRNA expression then gradually decreases during the first postnatal week until reaching adult expression levels [22]. The finding that BDNF expression in the VMN is induced by steroidogenic factor 1 (SF-1), a transcription factor required for normal differentiation of this region, suggests a developmental neurotrophic role [36]. BDNF is also found early in development in other appetite-regulating areas, including the PVN, where it is already detectable by E15 in the fetal mouse brain and in the midbrain [1, 38, 39].
Although the consequence of BDNF signaling during formation of feeding circuits remains to be fully elucidated, the data so far does not indicate a required developmental role for this neurotrophin. Anatomical examination of BDNF mutant mice showed normal cytoarchitecture of the VMN [40] and normal density of VTA DA neurons [41]. Moreover, a previous analysis of mice that are dramatically obese due to central BDNF depletion failed to uncover defects in the overall organization of the hypothalamus or in the hypothalamic pattern of distribution of NPY, melanin concentrating hormone (MCH), orexin, AGRP (agouti-related protein), α-MSH, and TRH (thyrotropin-releasing hormone)-containing cell bodies and fibers [10]. Importantly, ICV delivery of BDNF was found to reverse the obesity phenotype in adult BDNF+/− mice [8]. These results show that the hyperphagia triggered by perturbed BDNF signaling is not developmentally hardwired in central feeding circuits and support a physiological role for BDNF in appetite control in the mature brain.
Effects of loss or gain of BDNF function in the feeding circuitry
The functional significance of the spatiotemporal pattern of BDNF and TrkB expression described above is indicated by several studies assessing the effect of manipulating BDNF signaling in selective brain regions (Table 1). In addition to the effects in BDNF+/− mice described above, selective BDNF infusion into the VMN, PVN or DVC decreased food intake and cumulative body weight gain in adult rats [20, 42, 43]. In the VMN and PVN, local administration of BDNF also increased the resting basal metabolic rate [44, 45]. However, studies involving various lines of BDNF-deficient mice suggest that BDNF does not play an essential role in the regulation of energy expenditure [24, 46, 47]. They showed that pair feeding these BDNF mutants with food intake levels of wild types was sufficient to normalize their body weights, indicating that the obesity they exhibit arises primarily from uncontrolled feeding. Finally, BDNF administration was also efficacious in mitigating over eating, inducing weight loss and improving glucose homeostasis in leptin (ob/ob) and leptin receptor (db/db) deficient mutant mice, diet-induced obese rats and animals with diminished melanocortin signaling [11, 48–50].
Studies involving perturbations in BDNF signaling in the mature brain further inform the role of this neurotrophin (Table 1). Mice with intact levels of BDNF throughout development, but selective deletion of Bdnf in the adult VMN, exhibited increased food intake and body weight with concomitant metabolic dysregulation [24]. However, selective depletion of BDNF in SF-1-containing neurons in the VMN during early development did not elicit obesity in mice [51]. It is worth noting that SF-1 is expressed only in 60% of BDNF-containing cells in the VMN [36]. Therefore, these studies indicate that whereas BDNF expression in SF-1+ neurons is not required for normal energy homeostasis, its secretion by other cells within the adult VMN is essential. In the VTA, selective deletion of Bdnf in adult mice or knock down of BDNF via delivery of adeno-associated viral vectors containing short hairpin RNA (shRNA) against BDNF in rats resulted in increased food intake and excessive weight gain [37, 52]. The cumulative data indicate that, independently from effects that it might exert during development, BDNF acts as a required satiety factor in the adult brain and that the VMN and VTA are essential sources of this neurotrophin for food intake control.
Functional interactions of BDNF with anorexigenic and orexigenic signaling pathways in the brain
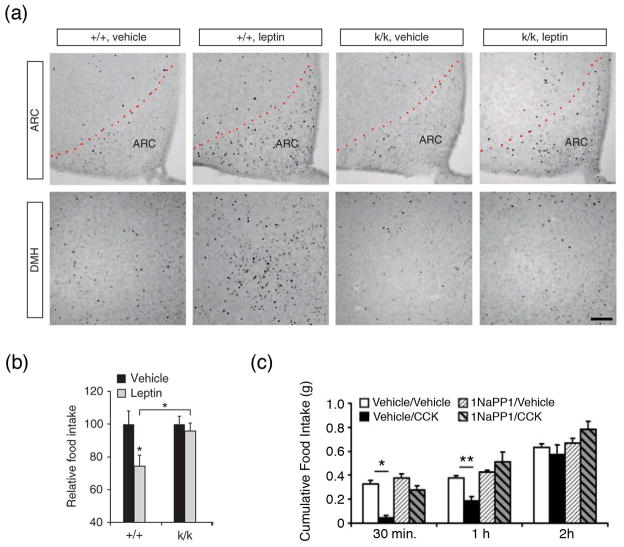
To further evaluate the putative role of BDNF in feeding, several studies have interrogated its involvement with signaling cascades that have ascribed roles in food intake regulation, including the leptin, melanocortin, corticotropin releasing hormone (CRH) and NPY pathways. A required facilitatory effect of BDNF on leptin-induced anorexia was reported [47, 53]. Leptin is a multifunctional adipostatic hormone released into the circulation in proportion to adipose tissue content. It increases the anorexigenic tone and energy expenditure primarily by activating the Janus kinase 2/STAT3 and PI3 kinase pathways in energy balance-regulating regions in the brain [54–56]. The first line of evidence suggesting a functional interaction of leptin and BDNF came from expression studies showing a positive effect of leptin administration on BDNF content in the VMN and DVC and reduced hypothalamic BDNF expression in db/db mice [20, 29, 57]. The effects of leptin on BDNF expression in the VMN appear to be mediated by hypothalamic network activity as leptin receptors and BDNF do not co-localize in this region [47]. A recent study provided mechanistic insight into BDNF-leptin interactions leading to appetite suppression by showing that leptin (and insulin) induces local translation of BDNF mRNA in dendrites of hypothalamic neurons [47]. Mice engineered to be depleted of dendritically-targeted BDNF mRNA develop hyperphagia and obesity and show blunted responses to leptin administration when treated at a young age before they become obese. Accordingly, these animals exhibit reduced neuronal activity, as marked by c-fos immunoreactivity, in the DMH, Arc and VMN and diminished appetite suppression compared to wild types following leptin treatment (Fig. 1a and b). Because leptin receptor and STAT3 activation was normal in the Arc, DMH and VMN of these mutants, BDNF appears to act downstream of this pathway [47]. Further evidence of BDNF involvement in leptin signaling comes from studies in the DVC. These investigations showed that TrkB receptor blockade abolishes the anorexigenic effects of leptin delivery to the NTS [53].
Figure 1. Interactions of BDNF and TrkB signaling with known anorexigenic pathways.
(a) Representative brain sections containing arcuate nucleus (ARC) or dorsomedial hypothalamus (DMH) from wild type (+/+) and knock-in mice (k/k) with depletion of dendritically-targeted BDNF mRNA. They show decreased neuronal activity as marked by c-fos immunoreactivity in k/k compared to WT mice following systemic vehicle and leptin treatment. Scale bar, 100 μM. (b) Food intake following systemic vehicle or leptin administration in wild type (+/+) and young non-obese knock-in mice (k/k) with depletion of dendritically-targeted BDNF transcripts. *, p < 0.05. (c) Cumulative food intake was measured in fasted TrkBF616A mice after various pharmacological treatments. These mice harbor a knock-in mutation in TrkB that results in the receptor being fully functional, but its kinase activity can be blocked by use of pharmacological agents such as the analog-sensitive kinase allele (ASKA) inhibitor 1NaPP1. The anorexigenic effects of CCK were reversed in the presence of 1NaPP1 in TrkBF616A mice. These findings demonstrate that CCK-induced suppression of feeding requires TrkB activation. *, p < 0.0001; **, p < 0.05. Adapted, with permission, from [47] (a and b) and [60] (c).
BDNF also acts as a downstream effector of anorexigenic melanocortin signaling in the VMN and DVC. The melanocortin receptors and their endogenous ligands have recognized roles in the homeostatic control of food intake and body weight [58]. In vitro, the selective melanocortin receptor 4 (MC4-R) agonist MK1 elicited BDNF secretion in isolated rat hypothalamus [59]. In vivo studies showed that ICV administration of another MC4-R agonist, MTII, partially reversed the reduction in BDNF mRNA content induced by food deprivation in the VMN [11]. Further, impaired melanocortin signaling in Ay mutant mice was associated with reduced expression of lacZ driven by endogenous Bdnf promoters in the VMN, particularly in the caudal aspect of this nucleus [11]. BDNF treatment abrogated the over-eating and excessive weight gain exhibited by Ay mutant mice that were administered a moderate fat diet. In rat DVC, acute delivery of MC4-R agonists and antagonists induced and inhibited expression of BDNF, respectively [60]. These studies also examined the effect of MC4-R stimulation in TrkBF616A knock-in mice expressing TrkB receptors with a point mutation that allows TrkB kinase activity blockade by the pharmacological agent 1NaPP1. They found that these mice exhibited blunted anorexigenic responses to MC4-R agonists only when acutely pre-treated with 1NaPP1, indicating a TrkB kinase activity requirement for the effects of melanocortins. Finally, these investigations demonstrated that BDNF infusion counteracted the hyperphagia induced by delivery of MC4-R antagonists to the fourth ventricle [60].
In the PVN, BDNF regulates CRH and urocortin signaling (Fig. 2). CRH is a stress response hormone that inhibits food intake and increases energy expenditure via augmentation of sympathetic nervous system activity [61]. Urocortin is a member of the CRH family of peptides and suppresses appetite with higher potency than CRH [62]. TrkB receptors co-localize with CRH in the PVN and chronic ICV infusion of BDNF results in dramatic increases in CRH and urocortin mRNA levels in this region [63]. Co-administration of the CRH receptor 1 and 2 antagonist, α-helical-CRH9–41, prevents the appetite suppression and weight loss induced by selective BDNF delivery to the PVN, indicating a functional interaction between these two pathways.
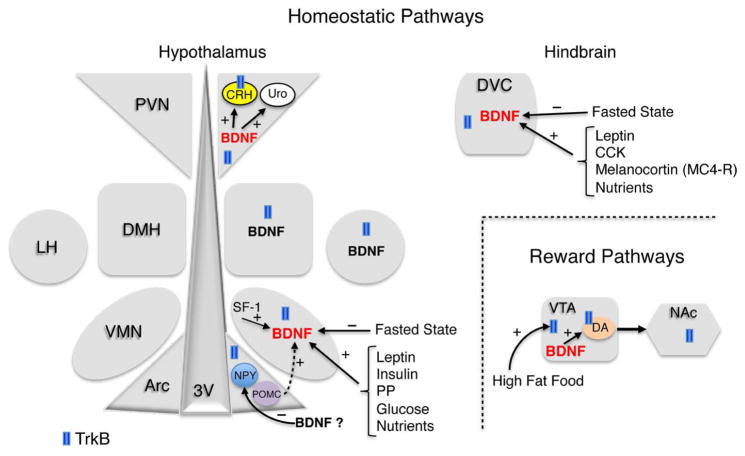
Figure 2. BDNF and TrkB expression and signaling in the feeding neurocircuitry.
Diagram depicts BDNF and TrkB expression in brain regions involved in the regulation of homeostatic and hedonic food intake. BDNF is shown in black font in regions where it is expressed and in red font in areas where it is synthesized and has been demonstrated to induce appetite suppression. Peripheral and central factors regulating expression of BDNF and TrkB in homeostatic and reward feeding pathways are also shown. Dashed arrow represents a previously proposed positive effect of POMC neurons in the Arc on BDNF expression in the VMN. Finally, putative mechanisms downstream of BDNF facilitating its satiety effects are shown in the VTA, PVN and the Arc, where the question mark reflects that the endogenous source of BDNF for this negative effect on NPY expression is unknown. Abbreviations: Arc, arcuate nucleus; CCK, cholecystokinin; CRH, corticotropin releasing hormone; DA, dopamine; DMH, dorsomedial hypothalamus; DVC, dorsal vagal complex; LH, lateral hypothalamus; MC4-R, melanocortin 4 receptor; NAc, nucleus accumbens; PP, pancreatic polypeptide; PVN, paraventricular nucleus; SF-1, steroidogenic factor 1; Uro, urocortin; VMN, ventromedial nucleus; VTA, ventral tegmental area; 3V, third ventricle.
Anorexigenic gut peptides released into the circulation during a state of positive energy balance have reported effects on central expression of BDNF. Whereas pancreatic polypeptide induces BDNF expression in the VMN [64], cholecystokinin (CCK) transiently elevates BDNF content both in the DVC and hypothalamus [20]. The effect of CCK on BDNF expression appears to have functional significance because TrkB kinase activity is required for the appetite-suppressing effects of peripheral CCK administration (Fig. 1c) [60]. Indeed, CCK treatment did not influence food intake of fasted TrkBF616A mice treated with 1NaPP1, in far contrast to the robust satiety effects observed in vehicle/CCK-treated controls. In addition to facilitating anorexigenic signaling, BDNF has been shown to impede orexigenic pathways. Specifically, BDNF delivery to the PVN prevented elevations in NPY expression in the Arc induced by fasting and reduced NPY-induced feeding [42]. Studies in rats indicate that NPY signaling reciprocates by decreasing hypothalamic expression of BDNF [65]. The collective results indicate that BDNF contributes prominently to the control of appetite in the mature animal, at least in part, through interactions with signaling pathways with recognized roles in energy balance regulation (Fig. 2).
Leptin and ghrelin exhibit broad neurotrophic effects similar to those of BDNF
In addition to its actions during development, BDNF regulates expression of neurotransmitter receptors and biosynthetic enzymes, regulates synaptic vesicle pools and participates in plasticity processes impacting synaptic strength in the mature brain [5]. In light of these effects, one view is that perturbations in basic components of neuronal function (rather than direct actions on anorexigenic or orexigenic pathways) underlie the obesity observed in animal models of BDNF or TrkB insufficiency. In that context, it is important to note that similar to BDNF, the appetite hormones leptin and ghrelin exert broad effects on neuronal development and neurotransmission. For instance, profound alterations in neural projections, originating in the Arc of ob/ob mice have been observed, indicating an essential role for leptin in targeting α-MSH and AGRP-containing fibers to the PVN [66]. Leptin also regulates the excitability of hypothalamic and VTA neurons via activation and trafficking of potassium channels [67, 68]. Reminiscent of the effects of selective deletion of Bdnf in the brain [37], mice depleted of leptin exhibit significant decreases in evoked release of DA from terminals in the NAc [69], indicating an important role in mesolimbic dopamine transmission. Finally, leptin was shown to promote proliferation of hippocampal neuronal progenitors and to exert neuroprotective effects in a mouse model of Alzheimer’s disease [70].
Ghrelin, a gastric peptide and the only identified orexigenic hormone in the circulation, acts in several brain nuclei and also parallels some of the neurotrophic effects of BDNF. It regulates membrane excitability, neurotransmitter release and neuronal survival and proliferation [71]. Ghrelin decreases and increases the firing rate of POMC+ and NPY+ neurons in the Arc, respectively [72, 73], and positively regulates the frequency of action potentials in VTA dopamine cells [74]. Similar to the neuroprotective actions of BDNF, it exhibits anti-apoptotic effects. Specifically, ghrelin protects hypothalamic neurons during oxygen-glucose deprivation by inhibiting production of reactive oxygen species, increasing the Bcl-2/Bax ratio and inhibiting caspase 3 activation [75].
Both leptin and ghrelin participate in synaptic remodeling processes proposed to contribute to the control of food intake [76, 77]. Indeed, ob/ob mutant mice exhibit aberrant numbers of excitatory and inhibitory synapses onto NPY+ and POMC+ cell bodies in the Arc in a pattern expected to promote over eating and reduce energy expenditure [76]. Remarkably, these alterations can be reversed within hours by exogenous leptin treatment. Ghrelin exerts effects opposite to those of leptin on the synaptic organization in the Arc and increases excitatory synaptic input onto dopamine neurons in the VTA [74, 76, 78].
Finally, it is worth noting that leptin and ghrelin do not exclusively regulate feeding behavior but also have antidepressant effects and influence learning and memory, reminiscent of the actions of BDNF [79–82]. The effects of leptin on cognition appear to be mediated by enhancement of hippocampal synaptic plasticity events, including facilitation of CA1 long term potentiation (LTP) and of Ca2+/calmodulin protein kinase II activity [80, 83]. Ghrelin, for its part, promotes dendritic spine synapse formation and LTP in the hippocampus [79]. This body of data suggests that the broad effects of BDNF on neurotransmission and synaptic efficacy do not preclude it from having an active and direct role in anorexigenic signaling.
Summary
In aggregate, the findings support an essential role for BDNF in energy balance regulation in the adult brain. Much remains to be unraveled regarding the cellular and molecular mechanisms underlying its effects. Because activation of Ras-MAPK pathways by neurotrophins regulates gene expression [84], one possibility is that it regulates transcriptional programs that enhance known and novel anorexigenic signaling pathways. BDNF is known to regulate local protein translation at the synapse [85], and thus, it could facilitate rapid post pandrial changes in the synaptic proteome of neurons involved in feeding regulation. Considering its well-established roles in structural plasticity in the hippocampus and cortex [86, 87], BDNF might also facilitate rewiring of feeding circuits in response to nutritional cues to increase the anorexigenic tone. Future investigations should include examination of these possibilities and defining the anatomical circuitry mediating the effects of BDNF. Of particular interest is identifying hypothalamic and hindbrain cell types expressing BDNF and TrkB, their targets and the precise role of BDNF in these cell populations. Considering the widespread expression of BDNF and TrkB and their multitude of actions in the brain, pharmacological approaches targeting this pathway might not represent an optimal intervention strategy to tackle the obesity problem. However, defining the mechanistic consequences of BDNF/TrkB signaling, including downstream target pathways, should prove an useful step toward developing novel treatment strategies for obesity and its many associated medical complications.
Acknowledgments
This work was supported by the National Institutes of Health (grant DK073311 to M.R.). The author thanks Jennifer Felsted, Jesus Mena and Leila Bradley for critical reading of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Maisonpierre PC, et al. NT-3, BDNF, and NGF in the developing rat nervous system: parallel as well as reciprocal patterns of expression. Neuron. 1990;5:501–509. doi: 10.1016/0896-6273(90)90089-x. [DOI] [PubMed] [Google Scholar]
- 2.Leibrock J, et al. Molecular cloning and expression of brain-derived neurotrophic factor. Nature. 1989;341:149–152. doi: 10.1038/341149a0. [DOI] [PubMed] [Google Scholar]
- 3.Kolbeck R, et al. Brain-derived neurotrophic factor levels in the nervous system of wild-type and neurotrophin gene mutant mice. Journal of neurochemistry. 1999;72:1930–1938. doi: 10.1046/j.1471-4159.1999.0721930.x. [DOI] [PubMed] [Google Scholar]
- 4.Patapoutian A, Reichardt LF. Trk receptors: mediators of neurotrophin action. Curr Opin Neurobiol. 2001;11:272–280. doi: 10.1016/s0959-4388(00)00208-7. [DOI] [PubMed] [Google Scholar]
- 5.Reichardt LF. Neurotrophin-regulated signalling pathways. Philos Trans R Soc Lond B Biol Sci. 2006;361:1545–1564. doi: 10.1098/rstb.2006.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pelleymounter MA, et al. Characteristics of BDNF-induced weight loss. Exp Neurol. 1995;131:229–238. doi: 10.1016/0014-4886(95)90045-4. [DOI] [PubMed] [Google Scholar]
- 7.Lapchak PA, Hefti F. BDNF and NGF treatment in lesioned rats: effects on cholinergic function and weight gain. Neuroreport. 1992;3:405–408. doi: 10.1097/00001756-199205000-00007. [DOI] [PubMed] [Google Scholar]
- 8.Kernie SG, et al. BDNF regulates eating behavior and locomotor activity in mice. Embo J. 2000;19:1290–1300. doi: 10.1093/emboj/19.6.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lyons WE, et al. Brain-derived neurotrophic factor-deficient mice develop aggressiveness and hyperphagia in conjunction with brain serotonergic abnormalities. Proc Natl Acad Sci U S A. 1999;96:15239–15244. doi: 10.1073/pnas.96.26.15239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rios M, et al. Conditional deletion of brain-derived neurotrophic factor in the postnatal brain leads to obesity and hyperactivity. Mol Endocrinol. 2001;15:1748–1757. doi: 10.1210/mend.15.10.0706. [DOI] [PubMed] [Google Scholar]
- 11.Xu B, et al. Brain-derived neurotrophic factor regulates energy balance downstream of melanocortin-4 receptor. Nat Neurosci. 2003;6:736–742. doi: 10.1038/nn1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carim-Todd L, et al. Endogenous truncated TrkB.T1 receptor regulates neuronal complexity and TrkB kinase receptor function in vivo. J Neurosci. 2009;29:678–685. doi: 10.1523/JNEUROSCI.5060-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yeo GS, et al. A de novo mutation affecting human TrkB associated with severe obesity and developmental delay. Nat Neurosci. 2004;7:1187–1189. doi: 10.1038/nn1336. [DOI] [PubMed] [Google Scholar]
- 14.Han JC, et al. Brain-derived neurotrophic factor and obesity in the WAGR syndrome. N Engl J Med. 2008;359:918–927. doi: 10.1056/NEJMoa0801119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beckers S, et al. Association of the BDNF Val66Met variation with obesity in women. Mol Genet Metab. 2008;95:110–112. doi: 10.1016/j.ymgme.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 16.Skledar M, et al. Association between brain-derived neurotrophic factor Val66Met and obesity in children and adolescents. Progress in neuro-psychopharmacology & biological psychiatry. 2011;36:136–140. doi: 10.1016/j.pnpbp.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 17.Speliotes EK, et al. Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nat Genet. 2010;42:937–948. doi: 10.1038/ng.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thorleifsson G, et al. Genome-wide association yields new sequence variants at seven loci that associate with measures of obesity. Nature genetics. 2009;41:18–24. doi: 10.1038/ng.274. [DOI] [PubMed] [Google Scholar]
- 19.Chen ZY, et al. Genetic variant BDNF (Val66Met) polymorphism alters anxiety-related behavior. Science. 2006;314:140–143. doi: 10.1126/science.1129663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bariohay B, et al. Brain-derived neurotrophic factor plays a role as an anorexigenic factor in the dorsal vagal complex. Endocrinology. 2005;146:5612–5620. doi: 10.1210/en.2005-0419. [DOI] [PubMed] [Google Scholar]
- 21.Conner JM, et al. Distribution of brain-derived neurotrophic factor (BDNF) protein and mRNA in the normal adult rat CNS: evidence for anterograde axonal transport. J Neurosci. 1997;17:2295–2313. doi: 10.1523/JNEUROSCI.17-07-02295.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sugiyama N, et al. Temporal changes in the expression of brain-derived neurotrophic factor mRNA in the ventromedial nucleus of the hypothalamus of the developing rat brain. Brain Res Mol Brain Res. 2003;115:69–77. doi: 10.1016/s0169-328x(03)00184-0. [DOI] [PubMed] [Google Scholar]
- 23.Tran PV, et al. Requirement of the orphan nuclear receptor SF-1 in terminal differentiation of ventromedial hypothalamic neurons. Mol Cell Neurosci. 2003;22:441–453. doi: 10.1016/S1044-7431(03)00027-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Unger TJ, et al. Selective deletion of Bdnf in the ventromedial and dorsomedial hypothalamus of adult mice results in hyperphagic behavior and obesity. J Neurosci. 2007;27:14265–14274. doi: 10.1523/JNEUROSCI.3308-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yan Q, et al. Immunocytochemical localization of TrkB in the central nervous system of the adult rat. J Comp Neurol. 1997;378:135–157. [PubMed] [Google Scholar]
- 26.Cone RD, et al. The arcuate nucleus as a conduit for diverse signals relevant to energy homeostasis. International journal of obesity and related metabolic disorders. 2001;25(Suppl 5):S63–67. doi: 10.1038/sj.ijo.0801913. [DOI] [PubMed] [Google Scholar]
- 27.Burns B, et al. Rai1 haploinsufficiency causes reduced Bdnf expression resulting in hyperphagia, obesity and altered fat distribution in mice and humans with no evidence of metabolic syndrome. Human molecular genetics. 2010;19:4026–4042. doi: 10.1093/hmg/ddq317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kohjima M, et al. Increased food intake leads to obesity and insulin resistance in the tg2576 Alzheimer’s disease mouse model. Endocrinology. 2010;151:1532–1540. doi: 10.1210/en.2009-1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stranahan AM, et al. Lowering corticosterone levels reinstates hippocampal brain-derived neurotropic factor and Trkb expression without influencing deficits in hypothalamic brain-derived neurotropic factor expression in leptin receptor-deficient mice. Neuroendocrinology. 2011;93:58–64. doi: 10.1159/000322808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bassareo V, Di Chiara G. Modulation of feeding-induced activation of mesolimbic dopamine transmission by appetitive stimuli and its relation to motivational state. Eur J Neurosci. 1999;11:4389–4397. doi: 10.1046/j.1460-9568.1999.00843.x. [DOI] [PubMed] [Google Scholar]
- 31.Rada P, et al. Daily bingeing on sugar repeatedly releases dopamine in the accumbens shell. Neuroscience. 2005;134:737–744. doi: 10.1016/j.neuroscience.2005.04.043. [DOI] [PubMed] [Google Scholar]
- 32.Numan S, et al. Differential regulation of neurotrophin and trk receptor mRNAs in catecholaminergic nuclei during chronic opiate treatment and withdrawal. J Neurosci. 1998;18:10700–10708. doi: 10.1523/JNEUROSCI.18-24-10700.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Numan S, Seroogy KB. Expression of trkB and trkC mRNAs by adult midbrain dopamine neurons: a double-label in situ hybridization study. J Comp Neurol. 1999;403:295–308. doi: 10.1002/(sici)1096-9861(19990118)403:3<295::aid-cne2>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 34.Okazawa H, et al. Dopaminergic stimulation up-regulates the in vivo expression of brain-derived neurotrophic factor (BDNF) in the striatum. FEBS Lett. 1992;313:138–142. doi: 10.1016/0014-5793(92)81430-t. [DOI] [PubMed] [Google Scholar]
- 35.Freeman AY, et al. Tyrosine kinase B and C receptors in the neostriatum and nucleus accumbens are co-localized in enkephalin-positive and enkephalin-negative neuronal profiles and their expression is influenced by cocaine. Neuroscience. 2003;117:147–156. doi: 10.1016/s0306-4522(02)00802-3. [DOI] [PubMed] [Google Scholar]
- 36.Tran PV, et al. Diminished hypothalamic bdnf expression and impaired VMH function are associated with reduced SF-1 gene dosage. J Comp Neurol. 2006;498:637–648. doi: 10.1002/cne.21070. [DOI] [PubMed] [Google Scholar]
- 37.Cordeira JW, et al. Brain-derived neurotrophic factor regulates hedonic feeding by acting on the mesolimbic dopamine system. J Neurosci. 2010;30:2533–2541. doi: 10.1523/JNEUROSCI.5768-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Friedman WJ, et al. Cells that Express Brain-Derived Neurotrophic Factor mRNA in the Developing Postnatal Rat Brain. The European journal of neuroscience. 1991;3:688–697. doi: 10.1111/j.1460-9568.1991.tb00854.x. [DOI] [PubMed] [Google Scholar]
- 39.McClellan KM, et al. Roles for gamma-aminobutyric acid in the development of the paraventricular nucleus of the hypothalamus. The Journal of comparative neurology. 2010;518:2710–2728. doi: 10.1002/cne.22360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McClellan KM, et al. Development of the ventromedial nucleus of the hypothalamus. Front Neuroendocrinol. 2006;27:193–209. doi: 10.1016/j.yfrne.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 41.Baquet ZC, et al. Brain-derived neurotrophic factor is required for the establishment of the proper number of dopaminergic neurons in the substantia nigra pars compacta. J Neurosci. 2005;25:6251–6259. doi: 10.1523/JNEUROSCI.4601-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang C, et al. Brain-derived neurotrophic factor in the hypothalamic paraventricular nucleus reduces energy intake. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1003–1012. doi: 10.1152/ajpregu.00011.2007. [DOI] [PubMed] [Google Scholar]
- 43.Wang C, et al. Brain-derived neurotrophic factor in the ventromedial nucleus of the hypothalamus reduces energy intake. Am J Physiol Regul Integr Comp Physiol. 2007:R1037–45. doi: 10.1152/ajpregu.00125.2007. [DOI] [PubMed] [Google Scholar]
- 44.Wang C, et al. Brain-derived neurotrophic factor in the hypothalamic paraventricular nucleus increases energy expenditure by elevating metabolic rate. Am J Physiol Regul Integr Comp Physiol. 2007:R992–1002. doi: 10.1152/ajpregu.00516.2006. [DOI] [PubMed] [Google Scholar]
- 45.Wang C, et al. Brain-derived neurotrophic factor (BDNF) in the hypothalamic ventromedial nucleus increases energy expenditure. Brain research. 2010;1336:66–77. doi: 10.1016/j.brainres.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Coppola V, Tessarollo L. Control of hyperphagia prevents obesity in BDNF heterozygous mice. Neuroreport. 2004;15:2665–2668. doi: 10.1097/00001756-200412030-00022. [DOI] [PubMed] [Google Scholar]
- 47.Liao GY, et al. Dendritically targeted Bdnf mRNA is essential for energy balance and response to leptin. Nature medicine. 2012;18:564–571. doi: 10.1038/nm.2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nakagawa T, et al. Brain-derived neurotrophic factor regulates glucose metabolism by modulating energy balance in diabetic mice. Diabetes. 2000;49:436–444. doi: 10.2337/diabetes.49.3.436. [DOI] [PubMed] [Google Scholar]
- 49.Tonra JR, et al. Brain-derived neurotrophic factor improves blood glucose control and alleviates fasting hyperglycemia in C57BLKS-Lepr(db)/lepr(db) mice. Diabetes. 1999;48:588–594. doi: 10.2337/diabetes.48.3.588. [DOI] [PubMed] [Google Scholar]
- 50.Wang C, et al. Chronic administration of brain-derived neurotrophic factor in the hypothalamic paraventricular nucleus reverses obesity induced by high-fat diet. American journal of physiology Regulatory, integrative and comparative physiology. 2010;298:R1320–1332. doi: 10.1152/ajpregu.00844.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tong Q, et al. Synaptic glutamate release by ventromedial hypothalamic neurons is part of the neurocircuitry that prevents hypoglycemia. Cell metabolism. 2007;5:383–393. doi: 10.1016/j.cmet.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fanous S, et al. Viral depletion of VTA BDNF in rats modulates social behavior, consequences of intermittent social defeat stress, and long-term weight regulation. Neuroscience letters. 2011;502:192–196. doi: 10.1016/j.neulet.2011.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Spaeth AM, et al. TrkB receptor signaling in the nucleus tractus solitarius mediates the food intake-suppressive effects of hindbrain BDNF and leptin. Am J Physiol Endocrinol Metab. 2012;302:E1252–1260. doi: 10.1152/ajpendo.00025.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bahrenberg G, et al. Identification of the critical sequence elements in the cytoplasmic domain of leptin receptor isoforms required for Janus kinase/signal transducer and activator of transcription activation by receptor heterodimers. Molecular endocrinology. 2002;16:859–872. doi: 10.1210/mend.16.4.0800. [DOI] [PubMed] [Google Scholar]
- 55.Kloek C, et al. Regulation of Jak kinases by intracellular leptin receptor sequences. The Journal of biological chemistry. 2002;277:41547–41555. doi: 10.1074/jbc.M205148200. [DOI] [PubMed] [Google Scholar]
- 56.Hill JW, et al. Acute effects of leptin require PI3K signaling in hypothalamic proopiomelanocortin neurons in mice. The Journal of clinical investigation. 2008;118:1796–1805. doi: 10.1172/JCI32964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Komori T, et al. Induction of brain-derived neurotrophic factor by leptin in the ventromedial hypothalamus. Neuroscience. 2006;139:1107–1115. doi: 10.1016/j.neuroscience.2005.12.066. [DOI] [PubMed] [Google Scholar]
- 58.Cone RD. Anatomy and regulation of the central melanocortin system. Nature neuroscience. 2005;8:571–578. doi: 10.1038/nn1455. [DOI] [PubMed] [Google Scholar]
- 59.Nicholson JR, et al. Melanocortin-4 receptor activation stimulates hypothalamic brain-derived neurotrophic factor release to regulate food intake, body temperature and cardiovascular function. J Neuroendocrinol. 2007;19:974–982. doi: 10.1111/j.1365-2826.2007.01610.x. [DOI] [PubMed] [Google Scholar]
- 60.Bariohay B, et al. Brain-derived neurotrophic factor/tropomyosin-related kinase receptor type B signaling is a downstream effector of the brainstem melanocortin system in food intake control. Endocrinology. 2009;150:2646–2653. doi: 10.1210/en.2008-1184. [DOI] [PubMed] [Google Scholar]
- 61.Richard D. Involvement of corticotropin-releasing factor in the control of food intake and energy expenditure. Annals of the New York Academy of Sciences. 1993;697:155–172. doi: 10.1111/j.1749-6632.1993.tb49930.x. [DOI] [PubMed] [Google Scholar]
- 62.Spina M, et al. Appetite-suppressing effects of urocortin, a CRF-related neuropeptide. Science. 1996;273:1561–1564. doi: 10.1126/science.273.5281.1561. [DOI] [PubMed] [Google Scholar]
- 63.Toriya M, et al. Long-term infusion of brain-derived neurotrophic factor reduces food intake and body weight via a corticotrophin-releasing hormone pathway in the paraventricular nucleus of the hypothalamus. Journal of neuroendocrinology. 2010;22:987–995. doi: 10.1111/j.1365-2826.2010.02039.x. [DOI] [PubMed] [Google Scholar]
- 64.Sainsbury A, et al. Y4 receptors and pancreatic polypeptide regulate food intake via hypothalamic orexin and brain-derived neurotropic factor dependent pathways. Neuropeptides. 2010;44:261–268. doi: 10.1016/j.npep.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 65.Gelfo F, et al. Intraperitoneal injection of neuropeptide Y (NPY) alters neurotrophin rat hypothalamic levels: Implications for NPY potential role in stress-related disorders. Peptides. 2011;32:1320–1323. doi: 10.1016/j.peptides.2011.03.023. [DOI] [PubMed] [Google Scholar]
- 66.Bouret SG, et al. Trophic action of leptin on hypothalamic neurons that regulate feeding. Science. 2004;304:108–110. doi: 10.1126/science.1095004. [DOI] [PubMed] [Google Scholar]
- 67.Hommel JD, et al. Leptin receptor signaling in midbrain dopamine neurons regulates feeding. Neuron. 2006;51:801–810. doi: 10.1016/j.neuron.2006.08.023. [DOI] [PubMed] [Google Scholar]
- 68.Spanswick D, et al. Leptin inhibits hypothalamic neurons by activation of ATP-sensitive potassium channels. Nature. 1997;390:521–525. doi: 10.1038/37379. [DOI] [PubMed] [Google Scholar]
- 69.Fulton S, et al. Leptin regulation of the mesoaccumbens dopamine pathway. Neuron. 2006;51:811–822. doi: 10.1016/j.neuron.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 70.Perez-Gonzalez R, et al. Leptin induces proliferation of neuronal progenitors and neuroprotection in a mouse model of Alzheimer’s disease. J Alzheimers Dis. 2011;24(Suppl 2):17–25. doi: 10.3233/JAD-2011-102070. [DOI] [PubMed] [Google Scholar]
- 71.Ferrini F, et al. Ghrelin in central neurons. Curr Neuropharmacol. 2009;7:37–49. doi: 10.2174/157015909787602779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.van den Top M, et al. Orexigen-sensitive NPY/AgRP pacemaker neurons in the hypothalamic arcuate nucleus. Nature neuroscience. 2004;7:493–494. doi: 10.1038/nn1226. [DOI] [PubMed] [Google Scholar]
- 73.Cowley MA, et al. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron. 2003;37:649–661. doi: 10.1016/s0896-6273(03)00063-1. [DOI] [PubMed] [Google Scholar]
- 74.Abizaid A, et al. Ghrelin modulates the activity and synaptic input organization of midbrain dopamine neurons while promoting appetite. J Clin Invest. 2006;116:3229–3239. doi: 10.1172/JCI29867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chung H, et al. Ghrelin inhibits apoptosis in hypothalamic neuronal cells during oxygen-glucose deprivation. Endocrinology. 2007;148:148–159. doi: 10.1210/en.2006-0991. [DOI] [PubMed] [Google Scholar]
- 76.Pinto S, et al. Rapid rewiring of arcuate nucleus feeding circuits by leptin. Science. 2004;304:110–115. doi: 10.1126/science.1089459. [DOI] [PubMed] [Google Scholar]
- 77.Sternson SM, et al. Topographic mapping of VMH --> arcuate nucleus microcircuits and their reorganization by fasting. Nat Neurosci. 2005;8:1356–1363. doi: 10.1038/nn1550. [DOI] [PubMed] [Google Scholar]
- 78.Andrews ZB, et al. UCP2 mediates ghrelin’s action on NPY/AgRP neurons by lowering free radicals. Nature. 2008;454:846–851. doi: 10.1038/nature07181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Diano S, et al. Ghrelin controls hippocampal spine synapse density and memory performance. Nature neuroscience. 2006;9:381–388. doi: 10.1038/nn1656. [DOI] [PubMed] [Google Scholar]
- 80.Oomura Y, et al. Leptin facilitates learning and memory performance and enhances hippocampal CA1 long-term potentiation and CaMK II phosphorylation in rats. Peptides. 2006;27:2738–2749. doi: 10.1016/j.peptides.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 81.Lutter M, et al. The orexigenic hormone ghrelin defends against depressive symptoms of chronic stress. Nature neuroscience. 2008;11:752–753. doi: 10.1038/nn.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lu XY, et al. Leptin: a potential novel antidepressant. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:1593–1598. doi: 10.1073/pnas.0508901103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shanley LJ, et al. Leptin enhances NMDA receptor function and modulates hippocampal synaptic plasticity. Journal of Neurosci. 2001;21:RC186. doi: 10.1523/JNEUROSCI.21-24-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Segal RA, Greenberg ME. Intracellular signaling pathways activated by neurotrophic factors. Annu Rev Neurosci. 1996;19:463–489. doi: 10.1146/annurev.ne.19.030196.002335. [DOI] [PubMed] [Google Scholar]
- 85.Liao L, et al. BDNF induces widespread changes in synaptic protein content and up-regulates components of the translation machinery: an analysis using high-throughput proteomics. J Proteome Res. 2007;6:1059–1071. doi: 10.1021/pr060358f. [DOI] [PubMed] [Google Scholar]
- 86.McAllister AK, et al. Opposing roles for endogenous BDNF and NT-3 in regulating cortical dendritic growth. Neuron. 1997;18:767–778. doi: 10.1016/s0896-6273(00)80316-5. [DOI] [PubMed] [Google Scholar]
- 87.Korte M, et al. Hippocampal long-term potentiation is impaired in mice lacking brain-derived neurotrophic factor. Proc Natl Acad Sci U S A. 1995;92:8856–8860. doi: 10.1073/pnas.92.19.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]