Abstract
We investigated whether combinatorial post-injury treatment with progesterone (P4) and vitamin D hormone (VDH) would reduce ischemic injury more effectively than P4 alone in an oxygen glucose deprivation (OGD) model in primary cortical neurons and in a transient middle cerebral artery occlusion (tMCAO) model in rats. In the OGD model, P4 and VDH each showed neuroprotection individually, but combination of the “best” doses did not show substantial efficacy; instead, the lower dose of VDH in combination with P4 was the most effective. In the tMCAO model, P4 and VDH were given alone or in combination at different times post-occlusion for 7 days. In vivo data confirmed the in vitro findings and showed better infarct reduction at day 7 and functional outcomes (at 3, 5 and 7 days post-occlusion) after combinatorial treatment than when either agent was given alone. VDH, but not P4, upregulated heme oxygenase-1, suggesting a pathway for the neuroprotective effects of VDH differing from that of P4. The combination of P4 and VDH activated brain-derived neurotrophic factor and its specific receptor, tyrosine kinase receptor-B. Under specific conditions VDH potentiates P4’s neuroprotective efficacy and should be considered as a potential partner of P4 in a low-cost, safe and effective combinatorial treatment for stroke.
1. Introduction
Despite extensive effort and costs, the results of well over one hundred industry-sponsored clinical trials for the treatment of stroke by medication have been disappointing, with genetically engineered tissue plasminogen activators (tPAs) still the only agents approved by the FDA. Unfortunately, tPA has a high risk-to-benefit ratio (causing intracranial hemorrhage in some patients) and is used in fewer than 5% of stroke victims. Several years ago a NIH-sponsored consensus meeting on neuroprotective treatments for brain injury specifically recommended directing therapeutic strategies toward combinations of neuroprotective agents acting on different pathways (Margulies and Hicks, 2009). The consensus report singled out the neurosteroid hormone progesterone (P4) as one of the pleiotropic agents particularly well-suited for studies of combination therapies for brain injury.
P4 treatment for traumatic brain injury (TBI), and more recently for stroke (Sayeed and Stein, 2009), is under investigation. In addition to leading to improved functional/behavioral outcomes in several injury models, P4 treatment reduces inflammatory cytokines, brain tissue necrosis, apoptosis, and cerebral edema (Stein, 2008). P4 is in national and international Phase III clinical trials for moderate to severe TBI (Stein, 2011). We and others have recently demonstrated substantial neuroprotection by P4 in several different stroke models (Liu et al., 2012; Dang et al., 2011; Gibson et al., 2011; Gibson et al., 2005; Gibson and Murphy, 2004; Ishrat et al., 2009; Sayeed and Stein, 2009; Sayeed et al., 2007; Sayeed et al., 2006).
Accumulating pre-clinical evidence of the complex systemic pathophysiology of stroke suggests that it is unrealistic to confine research to neuroprotective agents targeted primarily to a single, or a small number of, injury mechanisms. Drugs like P4 with pleiotropic consequences are more likely to provide effective neuroprotection. There are at least two good reasons to consider 1, 25-dihydroxyvitamin D3 hormone (VDH) as a potential combinatorial treatment with P4. First, it is well known that VDH insufficiency is common in acute stroke patients, and low levels of VDH are independently predictive for fatal strokes (Pilz et al., 2008). Second, both P4 and VDH are natural hormones known to have neuroprotective properties (Cekic et al., 2009). While both P4 and VDH can have neuroprotective effects, the latter exerts some of its actions on different signaling pathways from those of P4 (Cekic et al., 2009). Therapies for stroke that combine P4 with other agents that, like VDH, act on both similar and different injury pathways, may be able to improve stroke outcomes when given in the acute stage of injury. There is evidence to support this notion. We know that combinatorial treatment with P4 and VDH enhances the neuroprotective efficacy of P4 against excitotoxic cell death in vitro (Atif et al., 2009) and improves functional outcomes when given after TBI (Cekic et al., 2011).
P4 and VDH have high safety profiles, act on different injury and pathological mechanisms, and are clinically relevant, easy to administer and inexpensive, making them good choices for a new form of stroke therapy. Here, we investigated whether combinatorial treatment with P4 and VDH would produce better outcomes than P4 alone in reducing ischemic neuronal death in vitro and in reducing cerebral ischemia-induced brain infarction and restoring functional outcomes in vivo. We also explored some of their mechanisms of action by examining the effects of P4 and VDH on (1) growth factor signaling; (2) inflammatory markers (interleukin-6 (IL-6) and nuclear factor kappa B (NFκB); (3) apoptosis markers (cleaved caspase-3 and BCl-2); and (4) an oxidative injury marker (heme oxygenase-1 (HO-1)). For growth factor signaling, we examined the expression of brain-derived neurotrophic factor (BDNF) and its specific receptor tropomysin-related kinase B (TrkB), along with their downstream signaling mediated by extracellular, signal-regulated kinase1/2 (Erk1/2) and phosphoinositide 3-kinase/Protein kinase B (PI3K/Akt).
2. Materials and methods
2.1. Neuronal culture
NeuroPure™ E18 primary rat cortical cells were commercially procured (N200200, Genlantis, San Diego, CA) as micro-surgically dissected regions from day 18 embryonic Sprague-Dawley rat brains. Enzymatic pre-treatment of the cells was followed by mechanical dissociation by incubation in sterile NeuroPapain™ enzyme solution at 30° C for 30 min. The cells were then centrifuged and transferred to fresh plating medium and dissociated into isolated neurons using a P-1000 pipettor with a sterile 1-ml plastic tip (0.8-1.0-mm diameter opening). The cells were again centrifuged and seeded in multi-well plates pre-coated with poly-D-lysine (0.15 ml/cm2, 50 μg/ml) and maintained at 37° C in a humidified 5% CO 2 atmosphere. All experiments were performed on the neuronal cells after 9-10 days in culture.
2.2. Oxygen glucose deprivation (OGD) and drug treatment
OGD was carried out in 8-day in vitro (DIV) cultures, as required for the primary neurons to express all their cell surface receptors. The medium was replaced with pre-warmed Dulbecco’s Modified Eagle’s Medium (DMEM) without glucose. Cell cultures were then transferred into an anaerobic chamber equilibrated with 95% N2 and 5% CO2. The chamber was kept in a 37° C incubator. Sham OGD cultures were maintained in a normal oxygenated DMEM containing 25 mM glucose. After 2 h, cultures were placed back into the normoxic incubator with normal culture medium. For concurrent treatment, P4 (0.1, 1, 5, 10, 20, 40, 80 μM) and VDH (1, 20, 50, 75, 100, 500 nM and 1, 5 μM) were presented either individually or in different combinations (PROG: 10, 20 μM + VDH: 1, 20, 50 and 100 nM) in the culture medium during OGD and reoxygenation. For the controls, only vehicle was added to the culture medium.
2.3. MTT reduction assay
Neuronal death was assessed by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. The reaction is based on the cleavage of the tetrazolium ring of the pale yellow MTT into dark blue formazan crystals by mitochondrial dehydrogenase enzyme in viable cells. These blue formazan crystals accumulate within the cells due to their impermeability to the cell membrane, and are then solubilized by adding dimethyl sufoxide (DMSO;50 μl). The intensity of blue colored formazan solution is directly proportional to the number of surviving cells. Concentrations were determined by photometric analysis. Ten μl of MTT was added per well and incubated at 37° C for 4 h until purple precipitate was visible. DMSO was added to solubilize the crystals and the absorbance was read at 570 nm.
2.4. Propidium iodide (PI) staining
PI-staining of neuronal cell cultures was performed as described previously (Endres et al., 2004). Cortical neurons were incubated for 1 min with 0.02 mg/mL PI (stock solution 1 mg/mL, 1:50) in medium with gentle shaking and rinsed once with phosphate-buffered saline. Conditioned medium was reapplied and phase contrast and fluorescent pictures were taken immediately with an inverse fluorescence microscope attached to a digital camera.
2.5. Animals and treatment regimen
Thirty adult male Sprague-Dawley rats (300 to 325 gm; Charles River Laboratories, Wilmington, MA) were used according to procedures approved by the Institutional Animal Care and Use Committee, Emory University, Atlanta, GA, USA (protocol 151-2005). The rats were quarantined for 7 days before the experiment and housed in individual cages in a room maintained at 21-25° C, 45-50% humidity, a 12-h light/dark cycle and free access to pellet chow and water. Rats were randomized to the treatment conditions and the identity of the groups was coded to avoid experimenter bias while testing. There were 5 groups (n = 6/group). One group served as sham-operated, vehicle-treated controls (SHAM). Animals from the other groups were given middle cerebral artery occlusions (MCAO) followed by treatment with either vehicle (VEH), P4 (8 mg/kg), VDH (1 μg/kg body weight/day), or a combination of P4 (8 mg/kg) + VDH (1 μg/kg body weight/day) for 6 days. P4 (P-0130; Sigma-Aldrich Co., St. Louis, MO) was dissolved in 22.5% 2-hydroxypropyl-cyclodextrin (HBC). VDH stock was prepared in absolute ethanol and further diluted in 22.5% HBC. P4 was given intraperitoneally (i.p.) 5 min prior to reperfusion followed by subcutaneous (s.c.) injections at 6 h post-occlusion and then on days 1, 2, 3, 4, 5 and 6 post-occlusion. The i.p. injection of P4 provides rapid absorption, peak blood P4 levels, and quick access to the brain. Since P4 has a very short half-life, and washes out in a short period, remaining injections were s.c. for slow absorption/release to maintain high blood P4 levels for a longer period. The P4 8-mg/kg dose and routes of administration were determined from previous studies showing that this amount provided the maximal protective effects for stroke (Sayeed et al., 2007; Sayeed et al., 2006). One i.p. injection of VDH was given 5 min prior to reperfusion followed by daily s.c. injections for 6 days either alone or in combination with P4. On day 7, animals were killed and their brains removed after transcardial perfusion.
All efforts were made to minimize animal suffering and we conducted several experiments in vitro to reduce the number of animals. For each outcome measure, we calculated the starting sample sizes and power needed to reject the null hypothesis with a p-value of 0.05. The number of rats per group at these criteria was determined to be 6 to reject the null hypothesis (H0) at the 0.05 level at a power of 0.8.
2.6. Transient middle cerebral artery occlusion (tMCAO)
Focal cerebral ischemia was induced by the occlusion of the right middle cerebral artery as previously described (Longa et al., 1989). A midline incision was made on the ventral surface of the neck, and the right common carotid arteries were isolated and ligated with 6.0 silk suture. The internal carotid artery and the pterygopalatine artery were temporarily occluded using a microvascular clip. A 4-0 Doccol™ filament (Doccol Corporation, Redlands, CA) was introduced into the internal carotid artery through the incision in the external carotid artery. The filament was advanced approximately 20 mm distal to the carotid bifurcation. Relative cerebral blood flow was monitored by laser Doppler (LD) for the entire 90 min of occlusion. Drug treatment was randomly assigned 5 min before onset of reperfusion. After 90 min of MCAO, the occluding filament was withdrawn back into the common carotid artery to allow reperfusion. Relative cerebral blood flow was monitored for 5 min before the wound was sutured and rats permitted to recover from anesthesia. Rats subjected to MCAO with less than 40% of baseline LD flowmetry were randomly assigned to receive drug treatments.
2.7. Analysis of infarct volume
On day 7, animals were deeply anesthetized using isoflurane. After transcardial perfusion with 10% buffered formalin, brains were extracted and fixed in gradient sucrose solution and cut into 20-m sections for histological analysis. Brain sections were stained in 0.1% cresyl violet solution for 10 min at 45° C, and then rinsed in distilled water. Stained sections were fixed by serial dehydration in alcohol and xylene and mounted with xylene-based cytoseal. Fixed sections were coded for hiding group identity and then scanned. Infarct volume was calculated using NIH Image-J software.
2.8. Behavioral testing
2.8.1. Motor Coordination--Accelerating Rotarod
Motor impairment was assessed with the accelerating rotarod (Ishrat et al., 2009). Rats were given 3 training sessions 5 min apart before surgery. The animals were habituated to the stationary rod, and then exposed to the rotating rod. The rod was started at 2 rpm and then accelerated linearly to 20 rpm within 300 sec. Latency to fall off the rotarod was determined before ischemia (pre-surgery) and post-surgery.
2.8.2. Grip strength
Forelimb grip strength was measured pre-surgery and again at 3, 5 and 7 days post-surgery with a grip-strength meter (Columbus Instruments, Columbus, OH). A digital reading (in Newtons) of three successive trials was obtained for each rat, and then averaged for analysis.
2.8.3. Sticky-tape removal test
To assess somatosensory dysfunction after tMCAO, we used a modified adhesive removal (sticky-tape) test. A removable sticky label was placed under the animal’s paw contralateral to the stroke and the time taken for the animal to remove the label was recorded during a 60-sec observation period. Three trials per animal were averaged for analysis.
2.8.4. Spontaneous locomotor activity
Digiscan™ activity-monitoring boxes were used to assay spontaneous motor activity pre-surgery and then at 3, 5, and 7 days post-surgery. Each session lasted 5 min and was conducted under red-light conditions.
2.9. Blood collection and serum VDH assay
For serum isolation, blood (0.5–1.0ml) was collected from the right ventricle of the heart under deep isoflurane anesthesia and coagulated for 30 min at room temperature followed by centrifugation for 5 min at 1000×g. Serum VDH levels were measured by RIA assay with a kit produced by DiaSorin™ (65100E; Stillwater, MN). Assays were performed independently from anyone in the laboratory by the Biomarkers Core Laboratory at the Yerkes National Primate Research Center at Emory University.
2.10. Western blot analyses
Protein was determined in cell lysates by bicinchoninic acid protein assay (23225; Pierce, Rockford, IL). Forty-g protein samples were separated under reducing and denaturing conditions by 4-20% acrylamide Criterion™ gel (Bio-Rad, Hercules, CA) at 200V for 1 h and transferred to a polyvinylidene difluoride membrane at 100 V for 30 min. Nonspecific binding sites of the membrane were blocked with 5% nonfat dry milk in PBS-T (PBS containing 0.05% Tween-20). Membranes were probed with primary antibodies for Erk1/2 (sc-154, Santa Cruz Biotechnology, Santa Cruz, CA), pErk1/2 (sc-101761, Santa Cruz Biotechnology), BCl-2 (sc-7382, Santa Cruz Biotechnology), MMP-2 (sc-10736, Santa Cruz Biotechnology), Heme Oxygenase 1 (sc-136960, Santa Cruz Biotechnology), pro- and mature BDNF (sc-546, Santa Cruz Biotechnology), TrkB (sc-80401, Santa Cruz Biotechnology), cleaved Caspase-3 (Asp175) (9665S, Cell Signaling, Danvers, MA), Akt (9272, Cell Signaling), pAkt (Ser473) (9271, Cell Signaling), NFkB p65 (3034, Cell Signaling), pNFkB p65 (Ser276) (3037S, Cell Signaling), IL-6, and β-actin (Sigma) overnight at 4° C. Membranes were then incubated in horseradish peroxidase-conjugated secondary antibodies. β-Actin was probed as a loading control. Blots were developed using a chemiluminescent substrate (Pierce) for 5 min. Chemiluminescent bands were detected on Kodak autoradiography film in a dark room, and their densities were measured using Bio-Rad Gel-Doc software (Quantity-One 4.6.1).
2.11. Statistical analysis of data
All data were expressed as mean ± standard error of the mean (SEM). Statistical significance was set at P<0.05. All in vitro, brain infarction and Western blot data were analyzed using one-way analysis of variance (ANOVA) with the LSD and Tukey-HSD test. The behavioral data were analyzed using repeated measures ANOVA with a Greenhouse-Geisser correction for sphericity. Post-hoc tests, LSD and Tukey-HSD were employed using the Bonferroni correction for multiple comparisons. Analyses were calculated using SPSS™ 17.0 statistical analysis software (IBM, Armonk, NY). None of the animals that survived the stroke surgeries were excluded from the experiments.
3. Results
3.1. P4 and VDH attenuate in vitro ischemic cell death individually and VDH enhances the efficacy of P4 in combination
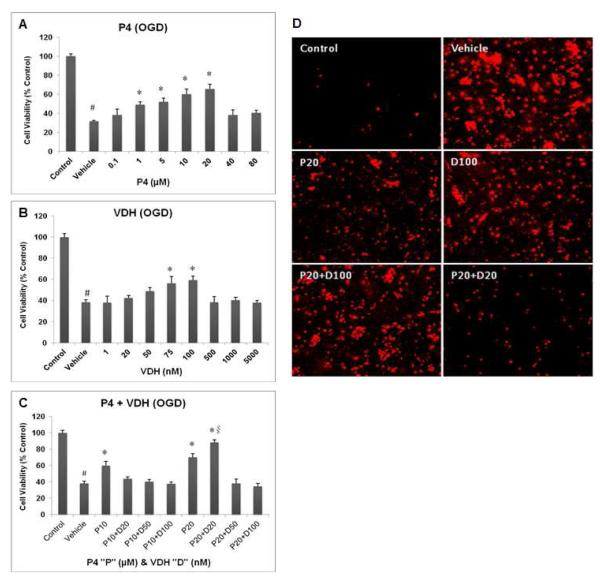
P4 (Fig. 1A) and VDH (Fig. 1B) significantly (P<0.05) reduced neuronal loss when tested independently. P4 at 20 M and VDH at 100 nM concentrations were the most neuroprotective. When the drugs were combined, the “best” dose of VDH as tested individually did not show substantial efficacy; rather, 20 nM was most effective (P<0.05) in combination with P4 (Fig. 1C). Figure 1D shows PI-stained cells following OGD and the extent of cell death in the different groups. The vehicle group had more PI-positive, dead/dying cells compared to controls (no OGD). The combination of P4 (20 μM) + VDH (20 nM) produced the minimum number of PI-positive cells compared to vehicle. Thus, in our hands, the best doses of two drugs given individually were not beneficial or optimally beneficial when given in combination.
Fig. 1.
Concurrent treatments of P4 (A) and VDH (B) and their combinations (C) on the viability of primary cortical neurons 24 h post-OGD. Panel (D) shows representative photomicrographs of cell death by PI staining 24 h post-OGD. Values are expressed as means±SEM of three experiments. Significant difference #P<0.05 compared to sham; *P<0.05 compared to vehicle; and §P<0.05 compared to P4.
3.2. In vivo physiological variables
Using a SurgiVet™ pulse oximeter, we monitored heartbeat and blood oxygen saturation levels (SpO2) and maintained them at levels greater than or equal to 90%. Body temperature was monitored throughout surgery (by rectal probe) and maintained at 36.5-37.5° C using an automated heat lamp (Harva rd Apparatus, South Natick, MA). No significant differences were observed in physiological parameters among the sham-operated and MCAO groups (data not shown).
3.3. Cerebral blood flow measurements
In all rats subjected to MCAO, cortical cerebral blood flow was reduced by at least 70% of pre-ischemic values. There were no significant differences in cortical cerebral blood flow after occlusion among rats given vehicle, P4, VDH or combination treatment. There were no significant differences in the increase in relative cerebral blood flow in drug-treated compared to vehicle-treated rats after reperfusion.
3.4. Combined treatment with P4 and VDH shows better functional outcomes than P4 alone
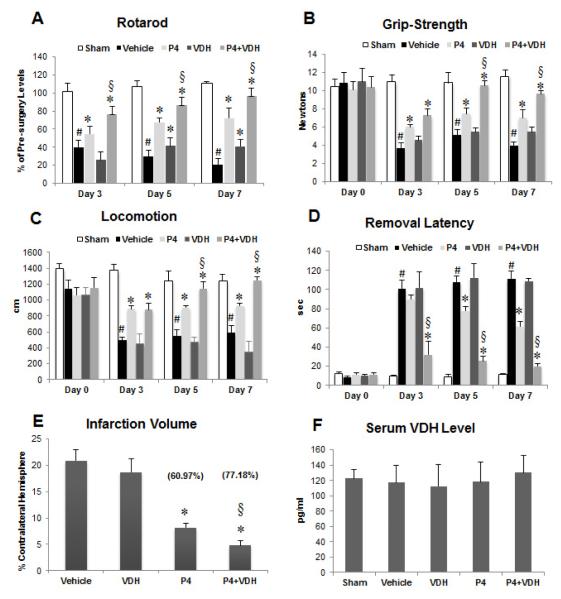
Rotarod
With repeated measures ANOVA and Greenhouse-Geisser correction, the mean scores for rotarod activity were significantly different (F(1.875, 48.762) = 13.971, P<0.0005) between time points and there were significant group effects (F(4, 26)=263.58, P<0.0005). Post-hoc tests using the Bonferroni correction revealed that the rats’ ability to remain on a moving rotarod was significantly impaired (P<0.001) in the vehicle-treated group compared to shams at days 3, 5 and 7 post-stroke (Fig. 2A). Treatment with P4 alone significantly (P<0.001) reduced motor deficits at days 3, 5 and 7 post-stroke compared to vehicle. VDH alone also showed a significant (P<0.01; P<0.001) improvement at 5 and 7 days post-stroke. Animals given P4+VDH performed significantly better (P<0.001) on the rotarod than the P4-alone group at days 3, 5 and 7. Combination treatment with VDH enhanced the beneficial effects of P4 by 34.1% at day 7 post-stroke.
Fig. 2.
Functional outcomes on days 3, 5 and 7 post-injury using rotarod (A), grip strength (B), locomotor activity (C), and sticky tape removal (D) tests; and effect of P4 and VDH on infarct volume (E) and serum VDH level (F) following tMCAO. Values are expressed as means ± SEM. Significant difference #P<0.05 compared to sham; *P<0.05 compared to vehicle; and §P<0.05 compared to P4. Vaues in parentheses represent percent reduction in infarct volume.
Grip Strength
A repeated measures ANOVA with a Greenhouse-Geisser correction revealed that mean grip strength differed significantly between time points (F(2.15, 56.06) = 25.337, P<0.0005) and there was a significant group effect (F(4, 26)=21.992, P<0.0005). Post-hoc analyses showed a significant (P<0.001) reduction in muscle strength in the vehicle-treated animals with stroke at all time points compared to shams (Fig. 2B). Compared to the vehicle group, P4 treatment significantly (P<0.05) improved grip strength at 3, 5 and 7 days post-stroke. No significant change in grip strength was observed in animals supplemented with VDH alone at any time point. Animals which received P4+VDH treatment showed better (P<0.003; P<0.005) recovery in grip strength than the P4-alone group at days 5 and 7 (36.6% better) post-stroke.
Locomotor Activity
A repeated measures ANOVA with Greenhouse-Geisser correction showed that mean locomotion differed significantly between time points (F(2.25, 56.25) = 22.800, P<0.0005) with a significant group effect (F(4, 25)=25.999, P<0.0005). Post-hoc analyses showed a significant (P<0.001) decrease in distance travelled in the vehicle-treated stroke group compared to shams at all time points (Fig. 2C). P4-alone treatment produced significant (P<0.001, P<0.006, P<0.01) recovery in locomotor activity at days 3, 5 and 7 respectively compared to the vehicle group, and this recovery was even better at days 5 (P<0.04) and 7 (P<0.01; 34.34% improvement) when P4 was combined with VDH. VDH alone did not show any positive effect.
Sticky-tape removal
A repeated measures ANOVA with a Greenhouse-Geisser correction determined that mean locomotion differed significantly between time points (F(1.81, 45.48) = 98.882, P<0.0005) with a significant group effect (F(4, 25)=61.375, P<0.0005). Post-hoc analyses showed a significant (P<0.001) increase in latency to remove the sticker from the contralateral forepaw in vehicle-treated stroke rats compared to shams at days 3, 5 and 7 (Fig. 2D). Treatment with VDH alone did not show improvement in removal latency compared to vehicle controls. P4 alone resulted in a significant (P<0.01, P<0.001) decrease in latency at 5 and 7 days following stroke. P4+VDH showed a significant (P<0.001) decrease in latency compared to P4 alone at days 3, 5, and 7 (67% improvement) post-injury.
3.5. Infarct reduction by P4 and VDH following cerebral ischemia
We examined the efficacy of a combinatorial treatment of P4+VDH against tMCAO-induced brain infarction 7 days post-injury in rats (Fig. 2E). One-way ANOVA showed a significant group effect (F(3, 20)=68.477, P<0.0005). P4 alone showed significant (P<0.001) reduction (60.97%) in infarct volume. VDH alone was not significantly different (10.59%). Rats given combination treatment had significantly better reduction (77.18%) in infarct volume than the P4-alone group. When given with P4, VDH significantly (P<0.001) enhanced P4’s efficacy in reducing infarct volume by 16.21%.
3.6. Serum VDH levels
Serum VDH assay data revealed no significant difference in VDH levels among the groups (Fig. 2F).
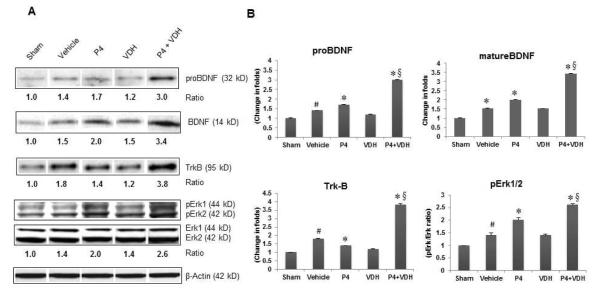
3.7. P4 and VDH trigger BDNF/TrkB/Erk1/2 signaling
MCAO caused a significant (P<0.05) increase in the expression of both pro- and mature BDNF and its specific receptor, TrkB, in the vehicle group compared to shams (Fig. 3). P4 alone showed a significant (P<0.05) increase in both pro- and mature BDNF and TrkB expression. After combination treatment there was a significant (P<0.05) increase in the expression of both pro- and mature BDNF (BDNF) and TrkB compared to vehicle, and there was a still greater increase with combination therapy (P<0.05) than in the P4-alone group. We observed a significant (P<0.05) increase in p-Erk1/2 level in the vehicle group compared to shams (Fig. 3). P4 and VDH independently showed a significant increase in p-Erk1/2 level compared to vehicle. When the hormones were combined, there was a significantly larger increase in p-Erk1/2 level than seen with P4 alone.
Fig. 3.
P4 and VDH effects on expression of proBDNF and mature BDNF (BDNF), its receptor TrkB, and downstream Erk1/2 phosphorylation in rat brain 24 h post-injury. (A) Representative protein bands of Western data. (B) Densitometry data. β-actin was used as the loading control. Values are expressed as means ± SEM. Significant difference #P<0.05 compared to sham; *P<0.05 compared to vehicle; and §P<0.05 compared to P4. Combination treatment with P4 and VDH triggered BDNF/TrkB/Erk1/2 signaling.
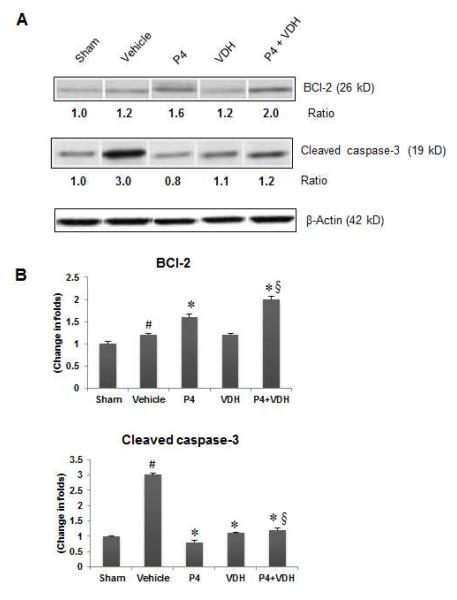
3.8. P4 and VDH inhibit apoptosis by modulating BCl-2 and cleaved caspase-3
We observed a significant (P<0.05) increase in BCl-2 expression in the vehicle group compared to shams (Fig. 4). P4 alone and P4+VDH, but not VDH alone, showed a significant (P<0.05) increase in BCl-2 expression compared to vehicle. We also observed a marked increase (P<0.05) in the expression of cleaved caspase-3 in the vehicle group compared to shams (Figure 4). P4 and VDH individually showed a significant (P<0.05) decrease in cleaved caspase-3 expression. In combination with VDH, P4 had significantly better (P<0.05) reduction in the level of cleaved caspase-3 than P4 alone.
Fig. 4.
B>. P4 and VDH effects on expression of anti-apoptotic protein BCl-2 and cleaved caspase-3 24 h post-injury. (A) Representative protein bands of Western data. (B) Densitometry data. β-actin was used as the loading control. Values are expressed as means ± SEM. Significant difference #P < 0.05 compared to sham; *P < 0.05 compared to vehicle; §P < 0.05 compared to P4. The P4 and VDH combination showed a synergistic effect on BCl-2 expression.
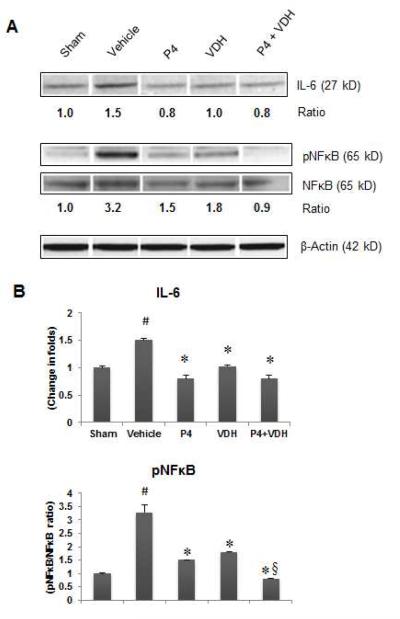
3.9. P4 and VDH suppress inflammation
We examined IL-6 expression and NFκB phosphorylation (pNFκB) as markers of neuronal inflammation (Fig. 5). In the vehicle group, a significant (P<0.05) increase in IL-6 and pNFκB expression indicated an inflammatory response. Both P4 and VDH alone significantly (P<0.05) reduced the levels of IL-6 and pNFκB compared to vehicle. P4 alone reduced IL-6 to sham levels. When P4 was combined with VDH, no additional reduction in IL-6 was observed. Combination of P4 and VDH produced significantly (P<0.05) better reduction in pNFκB expression compared to P4 alone.
Fig. 5.
P4 and VDH effects on expression of inflammatory markers IL-6 and NFκB 24 h post-injury. (A) Representative protein bands of Western data. (B) Densitometry data. β-actin was used as the loading control. Values are expressed as means ± SEM. Significant difference #P<0.05 compared to sham; *P<0.05 compared to vehicle; §P<0.05 compared to P4. VDH enhanced the anti-inflammatory efficacy of P4 in combination.
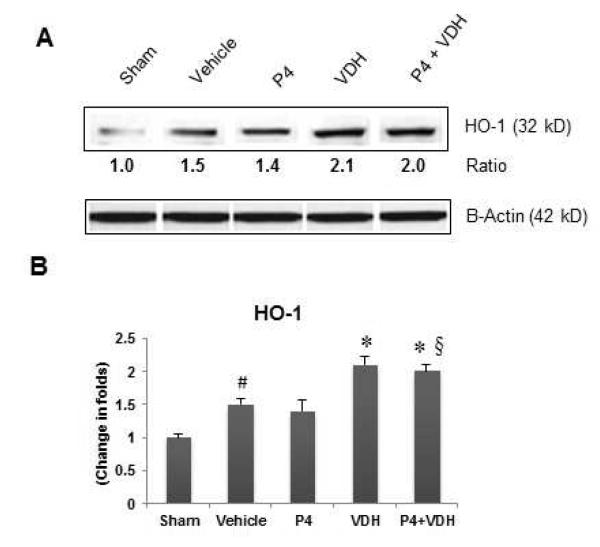
3.10. VDH but not P4 activates HO-1
MCAO led to a significant (P<0.05) increase in HO-1 expression in the vehicle group compared to shams, suggesting oxidative load following injury (Fig. 6). P4 alone did not reveal any increase in HO-1 expression, whereas VDH alone significantly (P<0.05) increased HO-1 levels compared to vehicle. Combination therapy provided no additional increase in HO-1 expression.
Fig. 6.
P4 and VDH effects on the expression of HO-1, a marker of neuronal oxidative damage, 24 h post-injury. (A) Representative protein bands of Western data. (B) Densitometry data. β-actin was used as the loading control. Values are expressed as means ± SEM. Significant difference #P<0.05 compared to sham; *P<0.05 compared to vehicle; §P<0.05 compared to P4. Data indicate that VDH, but not P4, increased the expression of HO-1, suggesting a neuroprotective response against oxidative damage.
4. Discussion
We evaluated the neuroprotective effects of combining P4 and VDH treatment in primary cortical neurons exposed to OGD, and following tMCAO in male rats. P4 treatment was beneficial in reducing ischemic cell death in vitro, and in reducing infarct volume and restoring behavioral function following tMCAO in vivo. This beneficial effect was stronger both in vitro and in vivo when P4 was combined with VDH.
Our in vitro data show that both P4 and VDH independently decrease OGD-induced neuronal death. P4 and VDH were most neuroprotective at 20 μM and 100 nM respectively. To explore a wider range of potential drug interactions, we combined 10 M and 20 M P4 with different doses of VDH. Interestingly, the combination of the most effective and higher) concentrations of P4 (20 M) and VDH (100 nM) appeared to reduce the individual protective effects of P4, but a combination with a lower concentration of VDH (20 nM) showed better neuroprotection than either hormone alone. In contrast, when P4 at 10 μM was combined with VDH (20, 50, 100 nM), P4 neuroprotection was inhibited. Our results are in agreement with another recent paper showing the neuroprotective effects of the hormones either alone or in combination against glutamate-induced excitotoxic cell death in primary cortical neurons (Atif et al., 2009). These findings indicate that there is perhaps a rather narrow range of optimal doses for combinations of these two drugs. The combination of VDH with P4 merits investigation as a therapy for stroke, but our findings also demonstrate the variability in individual and combinatorial effects of neuroprotective agents and the importance of carefully testing combination dose–response efficacy rather than selecting the best effective doses for single drugs.
Our in vitro and previous in vivo studies encouraged us to test a combination of P4 (8 mg/kg) and a lower dose of VDH (1 μg/kg/day) in a tMCAO model. P4 treatment before reperfusion reduced cortical infarct volume and attenuated functional deficits on various behavioral tests at different times over the 7 days of post-injury treatment. The data are consistent with our previous reports (Ishrat et al., 2009; Sayeed et al., 2007; Sayeed et al., 2006) and those of others (Gibson et al., 2005; Gibson and Murphy, 2004). Recently, Gibson et al. (2011) demonstrated that P4 treatment was beneficial following tMCAO in aged and ovariectomized female mice as assessed by infarct volume and motor functions. In our study, treatment with VDH alone did not affect brain infarct volume. Our findings agree with previous reports (Losem-Heinrichs et al., 2005; Oermann et al., 2004) showing VDH treatment alone (4 μg/kg and 1 μg/kg respectively) did not affect infarct size in a photothrombosis model of cortical ischemia. Here we show that P4 produced more reduction in infarct volume combined with VDH than when given alone.
VDH by itself did not produce beneficial effects in restoring functional outcomes except for performance on the rotarod, where there was improvement on days 5 and 7 post-surgery compared to vehicle. However, VDH combined with P4 led to better functional outcomes compared to P4 alone on rotarod, grip strength, locomotion and sensory neglect tests. On post-surgery day 7, VDH in combination enhanced the neuroprotective effects of P4 by 34% on the rotarod, 36% in grip strength, 34% in locomotion and 67% in sticky tape removal. To the best of our knowledge, we are the first to report the beneficial effects of VDH in combination with P4 on behavioral dysfunctions following ischemic stroke in rats.
Both P4 and VDH are pleiotropic agents affecting a number of mechanisms involved in CNS repair (Cekic et al., 2009). To explore some of these mechanisms, we investigated the individual and combined effects of P4 and VDH on the expression of BDNF and its specific receptor TrkB, and on activation of downstream Erk1/2 signaling 24 h post-occlusion. There is growing evidence that neurotrophic factors are involved in cellular and behavioral recovery following brain injury (Sofroniew et al., 2001). BDNF also plays a role in synaptic modification, the establishment of long-term memory through long-term potentiation (Greenberg et al., 2009; Lu, 2003), and in stabilizing cell signaling and synaptic plasticity in aged animals (Zeng et al., 2011). Through its specific receptor TrkB, BDNF promotes neuronal survival signaling by interacting with its low-affinity receptor p75NTR.
BDNF is known to protect against neuronal loss caused by cerebral ischemia (Mattson et al., 2004) and reduces infarct volume in different stroke models (Shi et al., 2009; Mizuno et al., 2000). P4 increases BDNF expression in various brain injury models (Coughlan et al., 2009; Jodha et al., 2009). We observed an increase in pro-BDNF and mature BDNF expression in the vehicle group. This may be a protective response following ischemic injury. The expression of several neurotrophic factors, including BDNF, is affected by ischemia (Abe and Hayashi, 1997). In our study P4 and P4+VDH, but not VDH alone, increased pro- and mature BDNF expression compared to vehicle. The effect was more pronounced in the P4+VDH group. There was also a marked increase in TrkB receptor in the P4+VDH group that was not as evident in the individual treatment groups.
We examined the activation of downstream Erk1/2 signaling, which transduces growth factor signals into the nuclei for regulation of gene expression. Erk1/2 phosphorylation plays a critical role in a number of intracellular activities like metabolism, mitosis, differentiation, inflammation, cell death, and survival (Roux and Blenis, 2004). We observed an increase in pErk1/2 levels induced by P4 that was more pronounced in the P4+VDH group. These in vivo results confirm our previous in vitro findings (Atif et al., 2009) of enhanced neuroprotection and Erk1/2 phosphorylation by the combination of P4+VDH compared to either drug alone. Activation of Erk1/2 induces expression of anti-apoptotic genes like BCl-2, which protects cells from toxic injury (Nilsen and Brinton, 2003). Increased BCl-2 protein expression in the vehicle group may be linked to increased expression of BDNF/TrkB/Erk1/2, a potential protective response following brain damage. P4 treatment enhanced BCl-2 expression, suggesting that this is one of its neuroprotective mechanisms of action. P4 is known to enhance BCl-2 expression in different injury models and our findings are in agreement with these reports (Liu et al., 2010; Yao et al., 2005; Nilsen and Brinton, 2002). VDH alone did not show any effect, but VDH+P4 enhanced BCl-2 expression better than P4 alone.
MCAO resulted in apoptosis in the vehicle group as measured by the expression of cleaved caspase-3. Individual treatments with P4 and VDH led to a marked decrease in cleaved caspase-3, but no additive or synergistic effect was observed for this biomarker in P4 combined with VDH. The combination of P4 and VDH may activate BDNF/TrkB/Erk1/2 signaling, which then leads to neuroprotection by enhancing anti-apoptotic protein BCl-2 and suppressing cleaved caspase-3.
Inflammation plays a pivotal role in the pathophysiology of brain injury. P4 has been reported to exert anti-inflammatory effects in a variety of brain injury models (Cekic et al., 2011; Hua et al., 2011; Wang et al., 2011; Gibson et al., 2005). VDH also plays an important role in modulating inflammation under both normal and pathologic conditions (Balden et al., 2012; Kojima et al., 2012; Makariou et al., 2012; Sun et al., 2012). In fact, Makariou and colleagues (2012) noted that very low levels of VDH are common in stroke patients and are associated with cardiovascular morbidity that can make stroke outcomes even worse. Along with others cited above, Makariou et al. (2012) propose that VDH supplementation could even help to prevent and treat cerebrovascular disease. Taking treatment protocol to the next level, in our hands, for the most part, combining VDH with P4 led to more effective amelioration of the injury cascade than either agent given alone. In the present study however, there were a few limitations to the extent of neuroprotection we were able to observe: both P4 and VDH individually decreased the levels of IL-6 but no additional effect was observed when the two hormones were combined, presumably because P4 had already reduced the levels of IL-6 to normal values. We noted a marked increase in pNFκB levels in the vehicle group, suggesting an inflammatory response following ischemic injury. Both hormones reduced pNFκB levels individually and had a synergistic effect in combination, suggesting that VDH can enhance P4 efficacy.
HO-1, a marker of oxidative injury, is one of the rapidly induced heat shock proteins (Hsp32) which catabolizes free heme to biliverdin, CO and Fe2+ (Maines, 1997). The products of HO-1 activity such as biliverdin and bilirubin are antioxidants with a high cytoprotective potential (Dore et al., 1999). Ischemic injury increased the expression of HO-1, and VDH increased this expression further. P4 did not affect HO-1 expression either alone or in combination with VDH. VDH is known to increase HO-1 expression in glial cells following photothrombotic lesions of the cerebral cortex (Oermann et al., 2004). These findings suggest a missing mechanism for P4 and indicate that VDH reduces oxidative brain injury by enhancing HO-1 expression in post-ischemic neurons, thus providing enhanced neuroprotection in combination with P4.
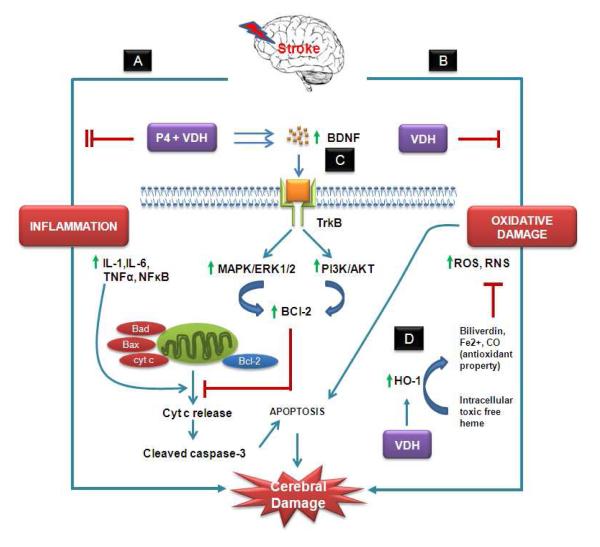
In conclusion, ischemic stroke induces multiple injury pathways including neuroinflammatory and oxidative stress reactions, which then trigger the secondary brain damage cascade leading to neuronal death. The combination of P4 and VDH protects the brain from ischemic damage better than P4 alone. This combination works by modulating neuroinflammation, oxidative damage and growth factors, especially by triggering BDNF/TrkB/Erk1/2 signaling, eventually leading to smaller infarct volume and better functional recovery (Fig. 7). Based on our results we suggest that stroke victims, especially the elderly and very young, be evaluated for VDH sufficiency and possibly supplementation in evaluating treatment options.
Fig. 7.
Hypothetical presentation of some possible mechanisms of action of P4 and VDH against ischemic stroke. We target two important detrimental pathways of ischemic injury: (A) inflammation, and (B) oxidative damage, and their modulation by P4 and VDH alone and in combination. P4 and VDH inhibited inflammatory response synergistically by suppressing IL-6 and NFκB. Both inflammation and oxidative damage cause mitochondrial damage. As a result, damaged mitochondria release cytochrome c (cyt c) into the cytosol, which further activates caspases and associated apoptosis. (C) Growth factor signaling (BDNF/TrkB/Erk1/2) is one of the neuroprotective mechanisms of P4 and VDH. BDNF promotes cell survival through activation of TrkB, a high-affinity receptor for BDNF. Upon activation, TrkB receptor signaling activates several signaling proteins and pathways regulated by MAP kinase (MAPK/Erk1/2) and PI3 kinase (PI3K/Akt). These pathways further activate the anti-apoptotic protein BCl-2, which prevents mitochondrial damage and cyt-c release-mediated caspase activation and apoptosis, thus promoting neuronal survival. Both P4 and VDH synergistically activate BDNF, TrkB, Erk1/2 signaling, and BCl-2, and suppress cleaved caspase-3 activation. VDH alone showed modulatory effects on oxidative damage as evidenced by the expression of HO-1. HO-1 is a rapidly induced heat shock protein which catabolizes free heme to biliverdin, CO, and Fe2+. These byproducts of HO-1 activity have potential antioxidant activity and provide cytoprotection (D).
Highlights.
Low-dose vitamin D enhanced progesterone (P4) neuroprotection after in vitro stroke.
P4+VDH reduced infarct in rats after transient stroke more than P4 alone.
P4+VDH showed better functional recovery than P4 alone at post-stroke day 7.
P4+VDH modulates neuroinflammation, oxidative damage, and growth factor signaling.
Acknowledgements
This research was supported by NIH grant RO1 HD061971. The authors would like to thank Leslie McCann for invaluable editorial assistance.
Footnotes
Disclosures DG Stein is entitled to royalties from products of BHR Pharmaceuticals Ltd related to the use of P4 in TBI and stroke, and may receive research funding from BHR Pharmaceuticals, which is developing products related to this research. In addition, he serves as a consultant to BHR Pharmaceuticals and receives compensation for these services. The terms of this arrangement have been reviewed and approved by Emory University, which receives the largest share of fees in accordance with its conflict of interest policies.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abe K, Hayashi T. Expression of the glial cell line-derived neurotrophic factor gene in rat brain after transient MCA occlusion. Brain Res. 1997;776:230–234. doi: 10.1016/s0006-8993(97)01041-x. [DOI] [PubMed] [Google Scholar]
- Atif F, Sayeed I, Ishrat T, Stein DG. Progesterone with vitamin D affords better neuroprotection against excitotoxicity in cultured cortical neurons than progesterone alone. Mol. Med. 2009;15:328–336. doi: 10.2119/molmed.2009.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balden R, Selvamani A, Sohrabji F. Vitamin D deficiency exacerbates experimental stroke injury and dysregulates ischemia-induced inflammation in adult rats. Endocrinol. 2012;153:2420–2435. doi: 10.1210/en.2011-1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cekic M, Cutler SM, VanLandingham JW, Stein DG. Vitamin D deficiency reduces the benefits of progesterone treatment after brain injury in aged rats. Neurobiol. Aging. 2011;32:864–874. doi: 10.1016/j.neurobiolaging.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cekic M, Sayeed I, Stein DG. Combination treatment with progesterone and vitamin D hormone may be more effective than monotherapy for nervous system injury and disease. Front. Neuroendocrinol. 2009;30:158–172. doi: 10.1016/j.yfrne.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coughlan T, Gibson C, Murphy S. Progesterone, BDNF and neuroprotection in the injured CNS. Int. J. Neurosci. 2009;119:1718–1740. doi: 10.1080/00207450903116430. [DOI] [PubMed] [Google Scholar]
- Dang J, Mitkari B, Kipp M, Beye r C. Gonadal steroids prevent cell damage and stimulate behavioral recovery after transient middle cerebral artery occlusion in male and female rats. Brain Behav. Immun. 2011;25:715–726. doi: 10.1016/j.bbi.2011.01.013. [DOI] [PubMed] [Google Scholar]
- Dore S, Sampei K, Goto S, Alkayed NJ, Guastella D, Blackshaw S, Gallagher M, Traystman RJ, Hurn PD, Koehler RC, Snyder SH. Heme oxygenase-2 is neuroprotective in cerebral ischemia. Mol. Med. 1999;5:656–663. [PMC free article] [PubMed] [Google Scholar]
- Endres M, Biniszkiewicz D, Sobol RW, Harms C, Ahmadi M, Lipski A, Katchanov J, Mergenthaler P, Dirnagl U, Wilson SH, Meisel A, Jaenisch R. Increased postischemic brain injury in mice deficient in uracil-DNA glycosylase. J. Clin. Invest. 2004;113:1711–1721. doi: 10.1172/JCI20926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson CL, Constantin D, Prior MJ, Bath PM, Murphy SP. Progesterone suppresses the inflammatory response and nitric oxide synthase-2 expression following cerebral ischemia. Exp. Neurol. 2005;193:522–530. doi: 10.1016/j.expneurol.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Gibson CL, Coomber B, Murphy SP. Progesterone is neuroprotective following cerebral ischaemia in reproductively ageing female mice. Brain. 2011;134:2125–2133. doi: 10.1093/brain/awr132. [DOI] [PubMed] [Google Scholar]
- Gibson CL, Murphy SP. Progesterone enhances functional recovery after middle cerebral artery occlusion in male mice. J. Cereb. Blood Flow Metab. 2004;24:805–813. doi: 10.1097/01.WCB.0000125365.83980.00. [DOI] [PubMed] [Google Scholar]
- Greenberg ME, Xu B, Lu B, Hempstead BL. New insights in the biology of BDNF synthesis and release: implications in CNS function. J. Neurosci. 2009;29:12764–12767. doi: 10.1523/JNEUROSCI.3566-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua F, Wang J, Ishrat T, Wei W, Atif F, Sayeed I, Stein DG. Genomic profile of Toll-like receptor pathways in traumatically brain-injured mice: effect of exogenous progesterone. J. Neuroinflammation. 2011;8:42. doi: 10.1186/1742-2094-8-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF. Trk receptors: roles in neuronal signal transduction. Annu. Rev. Biochem. 2003;72:609–642. doi: 10.1146/annurev.biochem.72.121801.161629. [DOI] [PubMed] [Google Scholar]
- Ishrat T, Sayeed I, Atif F, Hua F, Stein DG. Progesterone and allopregnanolone attenuate blood-brain barrier dysfunction following permanent focal ischemia by regulating the expression of matrix metalloproteinases. Exp Neurol. 2010;226:183–190. doi: 10.1016/j.expneurol.2010.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishrat T, Sayeed I, Atif F, Stein DG. Effects of progesterone administration on infarct volume and functional deficits following permanent focal cerebral ischemia in rats. Brain Res. 2009;1257:94–101. doi: 10.1016/j.brainres.2008.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jodha PK, Kaur P, Underwood W, Lydon JP, Singh M. The differences in neuroprotective efficacy of progesterone and medroxyprogesterone acetate correlate with their effects on brain-derived neurotrophic factor expression. Endocrinology. 2009;150:3162–3168. doi: 10.1210/en.2008-1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima G, Bell C, Abbott RD, Launer L, Chen R, Motonaga H, Ross GW, Curb JD, Masaki K. Low dietary vitamin d predicts 34-year incident stroke: the honolulu heart program. Stroke. 2012;43:2163–2167. doi: 10.1161/STROKEAHA.112.651752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu A, Margaill I, Zhang S, Labombarda F, Coqueran B, Delespierre B, Liere P, Marchand-Leroux C, O’Malley BW, Lydon JP, De Nicola AF, Sitruk-Ware R, Mattern C, Plotkine M, Schumacher M, Guennoun R. Progesterone receptors: a key for neuroprotection in experimental stroke. Endocrinol. 2012;153:3747–3757. doi: 10.1210/en.2012-1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Zhao L, She H, Chen S, Wang JM, Wong C, McClure K, Sitruk-Ware R, Brinton RD. Clinically relevant progestins regulate neurogenic and neuroprotective responses in vitro and in vivo. Endocrinology. 2010;151:5782–5794. doi: 10.1210/en.2010-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- Losem-Heinrichs E, Gorg B, Redecker C, Schleicher A, Witte OW, Zilles K, Bidmon HJ. 1alpha,25-dihydroxy-vitamin D3 in combination with 17beta-estradiol lowers the cortical expression of heat shock protein-27 following experimentally induced focal cortical ischemia in rats. Arch. Biochem. Biophys. 2005;439:70–79. doi: 10.1016/j.abb.2005.04.021. [DOI] [PubMed] [Google Scholar]
- Lu B. BDNF and activity-dependent synaptic modulation. Learn. Mem. 2003;10:86–98. doi: 10.1101/lm.54603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maines MD. The heme oxygenase system: a regulator of second messenger gases. Annu. Rev. Pharmacol. Toxicol. 1997;37:517–554. doi: 10.1146/annurev.pharmtox.37.1.517. [DOI] [PubMed] [Google Scholar]
- Makariou SE, Michel P, Tzoufi MS, Challa A, Milionis HJ. Vitamin D and Stroke: Promise for Prevention and Better Outcome. Curr. Vasc. Pharmacol. 2012 Jun 22; doi: 10.2174/15701611113119990119. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Margulies S, Hicks R. Combination therapies for traumatic brain injury: prospective considerations. J. Neurotrauma. 2009;26:925–939. doi: 10.1089/neu.2008.0794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Maudsley S, Martin B. BDNF and 5-HT: a dynamic duo in age-related neuronal plasticity and neurodegenerative disorders. Trends Neurosci. 2004;27:589–594. doi: 10.1016/j.tins.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Mizuno M, Yamada K, Olariu A, Nawa H, Nabeshima T. Involvement of brain-derived neurotrophic factor in spatial memory formation and maintenance in a radial arm maze test in rats. J. Neurosci. 2000;20:7116–7121. doi: 10.1523/JNEUROSCI.20-18-07116.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsen J, Brinton RD. Impact of progestins on estrogen-induced neuroprotection: synergy by progesterone and 19-norprogesterone and antagonism by medroxyprogesterone acetate. Endocrinology. 2002;143:205–212. doi: 10.1210/endo.143.1.8582. [DOI] [PubMed] [Google Scholar]
- Nilsen J, Brinton RD. Divergent impact of progesterone and medroxyprogesterone acetate (Provera) on nuclear mitogen-activated protein kinase signaling. Proc. Natl. Acad. Sci. U S A. 2003;100:10506–10511. doi: 10.1073/pnas.1334098100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oermann E, Bidmon HJ, Witte OW, Zilles K. Effects of 1alpha,25 dihydroxyvitamin D3 on the expression of HO-1 and GFAP in glial cells of the photothrombotically lesioned cerebral cortex. J. Chem. Neuroanat. 2004;28:225–238. doi: 10.1016/j.jchemneu.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Pilz S, Marz W, Wellnitz B, Seelhorst U, Fahrleitner-Pammer A, Dimai HP, Boehm BO, Dobnig H. Association of vitamin D deficiency with heart failure and sudden cardiac death in a large cross-sectional study of patients referred for coronary angiography. J. Clin. Endocrinol. Metab. 2008;93:3927–3935. doi: 10.1210/jc.2008-0784. [DOI] [PubMed] [Google Scholar]
- Roux PP, Blenis J. ERK and p38 MAPK-activated protein kinases: a family of protein kinases with diverse biological functions. Microbiol. Mol. Biol. Rev. 2004;68:320–344. doi: 10.1128/MMBR.68.2.320-344.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayeed I, Guo Q, Hoffman SW, Stein DG. Allopregnanolone, a progesterone metabolite, is more effective than progesterone in reducing cortical infarct volume after transient middle cerebral artery occlusion. Ann. Emerg. Med. 2006;47:381–389. doi: 10.1016/j.annemergmed.2005.12.011. [DOI] [PubMed] [Google Scholar]
- Sayeed I, Stein DG. Progesterone as a neuroprotective factor in traumatic and ischemic brain injury. Prog. Brain. Res. 2009;175:219–237. doi: 10.1016/S0079-6123(09)17515-5. [DOI] [PubMed] [Google Scholar]
- Sayeed I, Wali B, Stein DG. Progesterone inhibits ischemic brain injury in a rat model of permanent middle cerebral artery occlusion. Restor. Neurol. Neurosci. 2007;25:151–159. [PubMed] [Google Scholar]
- Shi Q, Zhang P, Zhang J, Chen X, Lu H, Tian Y, Parker TL, Liu Y. Adenovirus-mediated brain-derived neurotrophic factor expression regulated by hypoxia response element protects brain from injury of transient middle cerebral artery occlusion in mice. Neurosci. Lett. 2009;465:220–225. doi: 10.1016/j.neulet.2009.08.049. [DOI] [PubMed] [Google Scholar]
- Sofroniew MV, Howe CL, Mobley WC. Nerve growth factor signaling, neuroprotection, and neural repair. Annu. Rev. Neurosci. 2001;24:1217–1281. doi: 10.1146/annurev.neuro.24.1.1217. [DOI] [PubMed] [Google Scholar]
- Stein DG. Progesterone exerts neuroprotective effects after brain injury. Brain Res. Rev. 2008;57:386–397. doi: 10.1016/j.brainresrev.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein DG. Is progesterone a worthy candidate as a novel therapy for traumatic brain injury? Dialogues Clin. Neurosci. 2011;13:352–359. doi: 10.31887/DCNS.2011.13.2/dstein. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q, Pan A, Hu FB, Manson JE, Rexrode KM. 25-Hydroxyvitamin D levels and the risk of stroke: a prospective study and meta-analysis. Stroke. 2012;43:1470–1477. doi: 10.1161/STROKEAHA.111.636910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Zhao Y, Liu C, Jiang C, Zhao C, Zhu Z. Progesterone inhibits inflammatory response pathways after permanent middle cerebral artery occlusion in rats. Mol. Med. Report. 2011;4:319–324. doi: 10.3892/mmr.2011.418. [DOI] [PubMed] [Google Scholar]
- Yao XL, Liu J, Lee E, Ling GS, McCabe JT. Progesterone differentially regulates pro- and anti-apoptotic gene expression in cerebral cortex following traumatic brain injury in rats. J. Neurotrauma. 2005;22:656–668. doi: 10.1089/neu.2005.22.656. [DOI] [PubMed] [Google Scholar]
- Zeng Y, Tan M, Kohyama J, Sneddon M, Watson JB, Sun YE, Xie CW. Epigenetic enhancement of BDNF signaling rescues synaptic plasticity in aging. J Neurosci. 2011;31:17800–17810. doi: 10.1523/JNEUROSCI.3878-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]