Abstract
Keratinocytes migrating from a wound edge or initiating malignant invasion greatly increase their expression of the basement membrane protein Laminin-322 (Lam332). In culture, keratinocytes initiate sustained directional hypermotility when plated onto an incompletely processed form of Lam332 (Lam332′) or when treated with TGFβ, an inducer of Lam332 expression. The development and tissue architecture of stratified squamous and prostate epithelia are very different, yet the basal cells of both express p63, α6β4 integrin, and Lam332. Keratinocytes and prostate epithelial cells grow well in nutritionally-optimized culture media with pituitary extract and certain mitogens. We report that prostate epithelial cells display hypermotility responses indistinguishable from those of keratinocytes. Several culture medium variables attenuated TGFβ-induced hypermotility, including Ca++, serum, and some pituitary extract preparations, without impairing growth, TGFβ growth-inhibition, or hypermotility on Lam322′. Distinct from its role as a mitogen, EGF proved to be a required cofactor for TGFβ-induced hypermotility and could not be replaced by HGF or KGF. Prostate epithelial cells have a short replicative lifespan, restricted both by p16INK4A and telomere-related mechanisms. We immortalized the normal prostate epithelial cell line HPrE-1 by transduction to express bmi1 and TERT. Prostate epithelial cells lose expression of p63, β4 integrin, and Lam332 when they transform to invasive carcinoma. In contrast, HPrE-1/bmi1/TERT cells retained expression of these proteins and normal TGFβ signaling and hypermotility for >100 doublings. Thus, keratinocytes and prostate epithelial cells possess common hypermotility and senescence mechanisms and immortalized prostate cell lines can be engineered using defined methods to yield cells retaining normal properties.
Keywords: prostate, epithelial, keratinocyte, motility, TGFbeta, laminin, EGF
Introduction
Somatic cells possess tissue-specific mechanisms for regulating the three major behaviors they display: cell division, differentiated function, and motility. How cells convert from their normally sessile state to become motile and which features of this are cell type-specific remain incompletely understood. During embryogenesis, epithelial progenitors migrate on surfaces and invade subjacent mesenchyme to form glands and other structures. In the adult, keratinocyte motility in the context of wound healing has received much attention because of the frequency and morbidity of skin injury and its accessibility to observation. Another area of active inquiry is how carcinoma cells use cell migration mechanisms to invade the underlying stroma.
The basal cells of the epidermis and many other epithelia normally form stable adhesion complexes and hemidesmosome anchoring complexes, nucleated by the transmembrane protein α6β4, to Laminin-332 (Lam332), a heterotrimeric basement membrane protein that they synthesize and secrete beneath themselves [Carter et al., 1990; Langhofer et al., 1993; Litjens et al., 2006; Rousselle et al., 1991; Tzu and Marinkovich, 2008]. Keratinocytes at the edge of a skin wound convert from this sessile state to initiate sustained directional hypermotility until they have reepithelialized the exposed dermal surface [Goldfinger et al., 1999; Nguyen et al., 2000]. Wound edge keratinocytes and invasive squamous cell carcinoma (SCC) cells have been found to greatly increase Lam332 synthesis [Kainulainen et al., 1997; Kainulainen et al., 1998; Larjava et al., 1993; Skyldberg et al., 1999; Stoltzfus et al., 2004; Thorup et al., 1998]. We and others have found that Lam332 overexpression begins in premalignant dysplasias of the cervix, oral mucosa, and epidermis at the final stage before progression to invasive SCC [Natarajan et al., 2003; Noel et al., 2005; Svensson-Mansson et al., 2007] and that this is correlated with induced expression of the cell cycle inhibitor p16INK4A [Natarajan et al., 2005; Natarajan et al., 2003; Svensson-Mansson et al., 2007].
As a cell culture model of the behavior of wound and early invasive keratinocytes, normal human keratinocytes immediately display sustained directional hypermotility when plated at low cell density on a surface precoated with a form of Lam332 in which the γ2 chain remains unprocessed (Lam332′) [Natarajan et al., 2006]. Keratinocytes plated on uncoated surfaces in medium containing TGFβ become hypermotile after about a one day lag [Natarajan et al., 2006], consistent with the induction by TGFβ of increased Lam332 synthesis [Decline et al., 2003; Kainulainen et al., 1998; Korang et al., 1995; Zapatka et al., 2007], much of which remains after secretion in the Lam332′ form [Natarajan et al., 2006]. Lam332 overexpression had been noted for carcinomas arising from other epithelial cell types that form a Lam332 basement membrane [Pyke et al., 1994] and we reported recently that normal urothelial cells and tracheobronchial epithelial cells display hypermotility responses to Lam332′ and TGFβ in culture [Dabelsteen et al., 2009]. These two stratified epithelial cell types share with keratinocytes expression of the developmentally important transcriptional regulator p63 [Wang et al., 2002; Yang et al., 1998].
Prostatic basal epithelial cells share with the basal cells of stratified epithelia expression of p63, Lam332, and α6β4 integrin [Nagle et al., 1995; Yang et al., 1998] and they depend upon p63 for development and, in the adult, for differentiation [Signoretti et al., 2000]. The prostate epithelium is developmentally and structurally very different from stratified surface epithelia, however. Dependent upon androgen, the prostate develops from the endoderm-derived urogenital sinus to form a branched tubuloalveolar gland comprised of a basal cell layer and suprabasal, post-mitotic secretory cells whose products empty into ducts connecting to the urethra. Suprabasal prostatic cells cease expression of p63, Lam332, and α6β4 integrin and begin to express androgen receptor (AR), which mediates androgen-dependent expression of differentiation-related genes. In marked contrast to the development of SCC in stratified epithelia, neoplastic transformation in the prostate does not include a Lam332high/p16INK4A+ preneoplastic stage and invasive prostatic carcinomas do not retain markers of basal cells, instead resembling, by loss of p63, Lam332, and β4 integrin expression, a suprabasal cell that retains division potential [Davis et al., 2001; Weinstein et al., 2002]. Thus prostate carcinoma cells become invasive by mechanisms that do not employ Lam332 or α6β4 integrin. Whether the motility characteristics of normal prostate epithelial cells are the same as or different from those of other p63+ epithelial cell types is therefore of interest.
Normal human prostate epithelial cells can be grown in culture, expressing proteins characteristic of basal cells [Peehl, 2005]. A broad range of culture medium formulations have been used by various investigators [Berger et al., 2004; Jarrard et al., 1999; Kogan et al., 2006; Lechner et al., 1978; Peehl and Stamey, 1986; Peehl et al., 1988; Sandhu et al., 2000]. In all of these media, normal adult prostate epithelial cells have a very short replicative lifespan, restricted, as is the case for other p63+ cell types, by a p16INK4A-enforced senescence mechanism [Jarrard et al., 1999; Schwarze et al., 2001]. Attempts at generating immortalized lines by TERT expression typically do not result in immortalization and in some cases have not yielded lines that stably retain properties of normal prostate basal cells [Gu et al., 2006; Ke et al., 2008; Kogan et al., 2006]. In light of the differences in tissue structure, differentiation, and transformation between prostate epithelial cells and stratified, surface epithelial cells, we have asked whether normal prostate epithelial cells display a hypermotility response, whether the same factors modulate this response in keratinocytes and prostate epithelial cells, and whether an immortalized prostate epithelial cell line could be engineered that stably retains normal characteristics during serial culture.
Materials and Methods
Cell lines and culture methods
The normal human newborn foreskin epidermal keratinocyte primary line G5Ep, which we initiated, was cultured in keratinocyte serum-free medium (Ksfm) (GIBCO/Invitrogen, Carlsbad, CA), supplemented as described previously [Dickson et al., 2000; Rheinwald et al., 2002] with 25 μg/ml bovine pituitary extract (BPE) (GIBCO), 0.2 ng/ml epidermal growth factor (EGF) (R&D Systems, Minneapolis, MN), 0.3 mM CaCl2 (to bring the [Ca++] to 0.4 mM), and penicillin-streptomycin (GIBCO). G5Ep has a total replicative lifespan of ~70 population doublings (PD) and was used for experiments at 30–50 PD. The normal primary epidermal keratinocyte line strain N [Dabelsteen et al., 2009; Dickson et al., 2000] and its immortalized derivative N/bmi1/TERT [Dabelsteen et al., 2009; Dickson et al., 2000] were cultured in Ksfm. The normal primary human prostate epithelial line HPrE-1 was obtained from Clonetics/Lonza (Walkersville, MD) and was grown in PrEGM medium (Clonetics) or in Ksfm.
Human prostate carcinoma cell lines PC3 [Kaighn et al., 1979], MDAPCa-2b [Rajagopal et al., 1998], and LNCaP [Horoszewicz et al., 1983], obtained from the American Type Culture Collection by the Harvard Skin Disease Research Center, were grown in DMEM/F12 medium (GIBCO) supplemented with 10% newborn calf serum (Hyclone, Logan, UT). The rat bladder carcinoma cell line 804G [Izumi et al., 1981], provided by Jonathan Jones, Northwestern Univ., was cultured in DMEM +10% newborn calf serum. One day conditioned medium (CM) harvested from confluent 804G cultures was used as a source of the γ2-unprocessed form of Lam332 (Lam332′), as confirmed previously by the Jones and Rheinwald labs [Langhofer et al., 1993; Natarajan et al., 2006]. All cell lines were confirmed to be free of mycoplasma using the Hoechst 33258 staining method.
TGFβ1 (R&D Systems), EGF (R&D systems), HGF (GIBCO), and KGF (GIBCO) were prepared as 500X or 1,000X concentrated stock solutions dissolved in 4 mM HCl + 0.1% BSA (for TGFβ) or in PBS +0.1% BSA (for EGF, KGF, and HGF) and were stored frozen between uses.
For replicative lifespan determination, cells were plated at 104 cells/p60 dish, refed every 2–3 days, and subcultured 5–9 days after each plating, before growth was slowed by density. Population doublings (PD) was calculated as log2(# cells at subculture /# cells plated). Cumulative PD was plotted against total time in culture to determine onset of senescence or immortalization [Dabelsteen et al., 2009; Dickson et al., 2000].
Hypermotility and growth inhibition assays
As described [Natarajan et al., 2006], cells were plated in six-well plates (9 cm2/well) (Costar, Acton, MA) in Ksfm + or − various polypeptide factors. For some experiments wells were precoated for 30 min at 37°C with 804G CM as a source of Lam332′ and then rinsed twice with PBS and once with Ksfm before plating cells. For some experiments wells were precoated for 30 min at 37°C with DMEM +10% serum, then rinsed twice with PBS and once with Ksfm before plating cells
To assess directional hypermotility, 700 cells/well were plated and then fixed in −20°C methanol ~18 hr later to assess Lam322′-induced motility or ~48 hr later to assess TGFβ-induced motility. Fixed cultures were immunostained with a mouse monoclonal antibody specific for the γ2 chain of Lam332 (D4B5 [Mizushima et al., 1998], Chemicon/Millipore, Temecula, CA) using the ABC peroxidase method with Nova Red substrate (Vector Labs, Burlingame, CA). Immunostained cultures were mounted with coverslips using GEL/MOUNT (Biomeda Corp., Foster City, CA). Images were captured on a NIKON E600 Microscope with a SPOT2 digital camera using SPOTcam v.3.5.5 software (Digital Instruments, Tonowanda, NY). About fifteen 20X total magnification fields were photographed and 100 to 200 individual cells for each experimental condition were scored for hypermotility based on the length of the Lam322 tracks they deposited on the culture dish surface as described [Natarajan et al., 2006]: cells were scored as non-motile (track <500 μm), or hypermotile (track ≥500 μm).
To assess mitogenicity of growth factors, wells were plated at 2000 or 3000 cells in Ksfm preparerd with half the normal concentration of BPE, which we found reduces background growth in the absence of added mitogen. To assess Lam332′ or TGFβ growth inhibition, wells plated with 2000 cells in regular Ksfm. For both assays, cells were grown for 6–8 days with refeeding on days 3 and 5, then trypsinized, counted with a hemacytometer, and ave. growth rate calculated as log2 [# cells counted/#cells plated]/#days = PD/day.
All growth and motility experiments were performed three different times and results are shown as averages with error bars indicating standard error of the mean.
Antibodies and immunochemical analysis
For immunofluorescence staining, cultures were fixed with cold 100% methanol and air-dried. The primary antibodies mouse anti-Laminin γ2 (D4B5 [Mizushima et al., 1998], Chemicon/Millipore, Temecula, CA), anti-β4 integrin (clone M126, Abcam, Cambridge, MA), and rabbit anti-p63 (#4892, Cell Signaling Technology, Beverly, MA) were applied at 1:100 dilution for 30 minutes and then rinsed in PBS. Alexafour 568 (red fluorescence) and Alexafluor 488 (green fluorescence) secondary antibodies (Molecular Probes/Invitrogen, Eugene, OR) were applied for 30 minutes and then rinsed. Cultures were coverslip-mounted with Prolong Gold antifade reagent with DAPI (Molecular Probes/Invitrogen). Cells were photographed using a Nikon Eclipse Ti microscope and a SPOTCam digital camera.
For Western blotting, rabbit anti-β-actin antibody A-2066 (SIGMA, St. Louis, MO) was used at 1:2000 dilution. Mouse anti-smad2/3 (clone 18, BD Biosciences, San Jose, CA) and rabbit anti-phospho-smad2(Ser465/467) antibody (#3108, Cell Signaling Technologies) were used at 1:100 dilution. The D4B5 mouse anti-laminin γ2, 4A4 anti-p63 ([Yang et al., 1998], provided by F. McKeon, Harvard Medical School or obtained from Thermo Scientific, Fremont, CA), and clone M126 anti-β4 integrin antibodies were used at 1:500 dilution.
For flow cytometric analysis of p16INK4A expression, cells were suspended from growing cultures with trypsin/EDTA, resuspended in PBS, fixed by addition of paraformaldehyde to a final concentration of 1% for 20 min at 4°C, permeabilized by resuspending in PBS + 0.2% Triton X100 for 15 min, rinsed twice and resuspended in PBS +2% calf serum, and then resuspended for 30 min each in PBS +0.1% BSA + mouse monoclonal anti-p16INK4A antibody (G175-405; Pharmingen/BD Biosciences, San Diego, CA) and phycoerythrin-conjugated, goat anti-mouse IgG secondary antibody (SouthernBiotech, Birmingham, AL). At least 10,000 cells of each cell line, gated by forward and side scatter to subtract fluorescent cell debris, were analyzed for fluorescence intensity using a BD FACS Canto flow cytometer (Becton-Dickinson). The data were analyzed using the FlowJo 7.6 program (Tree Star, Inc., Ashland, OR).
Western blotting
Cultures were lysed in 20 mM Tris buffer (pH 7.3) + 2% SDS + EDTA-free protease inhibitor cocktail (Roche, Indianapolis, IN) + phosphatase inhibitors (Halt, Thermo Scientific), and then were sonically disrupted and stored at −80°C until use. Protein concentrations were determined using the BCA assay (Pierce, Rockford, IL) with human IgG as standards. 20 μg protein extract diluted in Laemmli sample buffer was loaded per lane, separated by SDS-PAGE under reducing conditions using precast 4–20% gradient gels (Bio-Rad, Hercules, CA), and blotted to polyvinylidene difluoride membranes (Millipore Corp., Billerica, MA). Blots were blocked for 1 hour with 10% non-fat milk in Tris-buffered saline + 0.05% Tween 20 and then incubated with primary and secondary antibodies in 5% non-fat milk in Tween buffer. Blots were incubated with primary antibody for 2 hours or overnight at 4°C and with peroxidase-conjugated anti-rabbit or anti-mouse Ig (Dako, Glostruo, Denmark), for 1 hour at room temperature. Blots were then incubated with Lumi-light western blotting substrate (Roche, Indianapolis, IN) or SuperSignal West Femto Substrate (Thermo Scientific, Rockford, IL) and exposed to BioMaz XAR Film (Kodak, Rochester, NY). For some experiments, membranes were stripped of antibodies using BlotFresh (SignaGen Labs, Gaithersburg, MD) and reprobed.
Immunocytochemistry
Cultures were quickly rinsed with water, fixed for ≥10 min in −20°C 100% methanol, air-dried. They then were incubated with D4B5 anti Laminin γ2 antibody for 30 min at room temperature, rinsed, incubated with biotinylated secondary antibody for 30 min, rinsed, and incubated with avidin/biotin/peroxidase complex (ABC) reagent (Vectastain ABC kit, Vector Laboratories) for 45 min. After rinsing with PBS, cultures were incubated with NovaRED (Vector Laboratories) for ~3 min, rinsed in water, and mounted with coverslips using GEL/MOUNT (Biomeda Corp., Foster City, CA). Images were captured on a NIKON E600 Microscope with a SPOT2 digital camera using SPOTcam v.3.5.5 software (Digital Instruments, Tonowanda, NY).
Retroviral transduction
The amphotropic retroviral packaging cell line Phoenix [Swift et al., 2001] was transfected with pBABE/bmi1.puro [Dimri et al., 2002] or pWzl.Bsd/TERT [Wei and Sedivy, 1999] plasmid DNA using TransIT-LT1 transfection reagent (Mirus Bio LCC, Madison, WI). Keratinocytes and prostate epithelial cells are severely growth inhibited by exposure to serum, so retroviral supernatants were obtained in serum-free “1:1 medium”, which is a 1:1 (v:v) mixture of Ksfm and DF-K medium. DF-K medium is a 1:1 (v:v) mixture of calcium-free, glutamine-free DMEM (GIBCO) and Ham’s F12 (GIBCO) +0.2 ng/ml EGF +25 μg/ml BPE +1.5 mM L-glutamine +pen/strep. Beginning 1 day after transfection, Phoenix cultures were refed alternately with DMEM+10% calf serum and 1:1 medium twice daily for two days. The conditioned medium containing shed viral particles (the retroviral supernatant) was collected, filtered through a 0.45 μm pore-size filter (Nalgene/Thermo Scientific), aliquoted, and stored at −80°C until use.
HPrE-1 cells were transduced and drug-selected as described [Dabelsteen et al., 2009; Rheinwald et al., 2002]. One day after plating 105 cells per 9 cm2 well, cells were transduced by incubating with retroviral supernatants for 6–7 hours in 1:1 medium in the presence of 2 μg/ml polybrene (Sigma-Aldrich, St. Louis, MO). The transduced cells were subcultured the next day into p100 dishes in Ksfm + 2 μg/ml puromycin or 5 μg/ml blastocidin (Sigma-Aldrich) for 5 days to kill untransduced cells.
Results
Effects on growth and motility of culture medium formulation and pituitary extract
In order to determine whether prostate epithelial cells display hypermotility responses similar to those we had found to be attributes of keratinocytes and other stratified epithelial cell types [Dabelsteen et al., 2009; Natarajan et al., 2006], we first sought to identify culture conditions in which both keratinocytes and prostate epithelial cells would grow well from low plating densities. Employing a common medium would ensure that any observed differences could not be caused by a difference in some medium component. We compared two serum-free, bovine pituitary extract (BPE)-supplemented, nutritionally optimized medium formulations: Ksfm, optimized originally for keratinocyte growth [Pirisi et al., 1987] and PrEGM, a proprietary formulation claimed by the manufacturer to be optimized for prostate epithelial cell growth. Both media stimulated growth of early-to-mid lifespan cultures of G5Ep at a rate of ~0.8 PD/day and HPrE-1 at ~0.6 PD/day and both cell types displayed similar dose-responses for growth-inhibition by TGFβ, as discussed below. G5Ep keratinocytes formed more cohesive colonies than did HPrE-1 prostate epithelial cells and both cell types formed less dispersed colonies in the 0.4 mM Ca++ Ksfm medium than in the 0.1 mM Ca++ PrEGM medium (Fig. 1a). As expected from earlier studies, prostate epithelial cells shared with keratinocytes expression of the nuclear transcription factor p63 [Signoretti et al., 2000], β4 integrin [Nagle et al., 1995], and Laminin-332 (Lam332) [Hao et al., 1996], the latter confirmed by immunostaining for the Laminin γ2 chain, which is unique to this member of the family of Laminins (Fig. 1b).
Figure 1. Colony morphology and marker expression by cultured human keratinocytes and prostate epithelial cells.
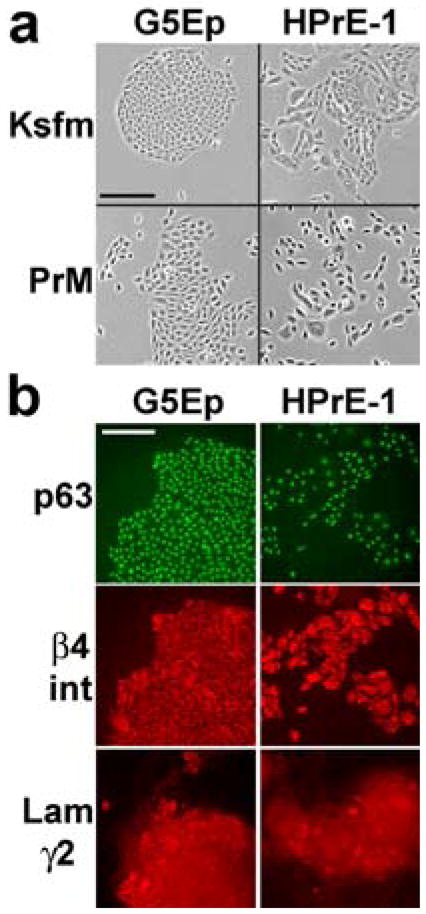
a. Phase contrast images of typical colonies formed by G5Ep keratinocytes and HPrE-1 prostate epithelial cells one week after plating in Ksfm or PrEGM medium. Note the more stable cell-cell association by both cell types in the 0.4 mM Ca++ Ksfm than in the 0.1 mM Ca++ PrEGM medium and the less scattered morphology of G5Ep compared to HPrE-1 cells in both media. Bar in upper left panel: 200 microns.
b. G5Ep and HPrE-1 cultures immunostained for p63, β4 integrin, and the γ2 chain of Lam332. Note expression of these proteins by all cells of both types and that Lam332 is detectable in the cytoplasm and deposited by cells onto the dish surface. Bar in upper left panel: 200 microns.
In Ksfm, both HPrE-1 and G5Ep cells responded with sustained directional hypermotility to the addition of TGFβ to the medium or when plated on culture dishes precoated with the γ2 chain-unprocessed form of Laminin-332 (Lam332′) (Fig. 2a). In contrast, in this first experiment neither cell type was induced to hypermotility by TGFβ in PrEGM (Fig. 2a). Subsequent experiments revealed that the BPE supplied with the first batch of PrEGM we had obtained was responsible for this result. As shown in Fig. 2b, both cell types became hypermotile in response to TGFβ if plated in either medium prepared with BPE that had been supplied with the Ksfm (BPE1) but not in medium prepared with BPE that had been supplied with the PrEGM (BPE2). These two lots of BPE did not differentially affect cell growth or TGFβ signaling in cells because control growth rates and the dose-response of TGFβ growth inhibition were indistinguishable in medium prepared with BPE1 or BPE2 (Fig. 2c). We found that eight different lots of BPE provided by the manufacturer of Ksfm were indistinguishable with respect to growth stimulation and dose-response of TGFβ growth inhibition, but the TGFβ-induced hypermotility response in medium supplemented with these BPE lots varied greatly (data not shown). For all subsequent experiments we used either of two BPE lots that were permissive for TGFβ-induced hypermotility.
Figure 2. Ability of epithelial cells to become hypermotile in response to TGFβ varies among bovine pituitary extract (BPE) lots used as medium supplement.
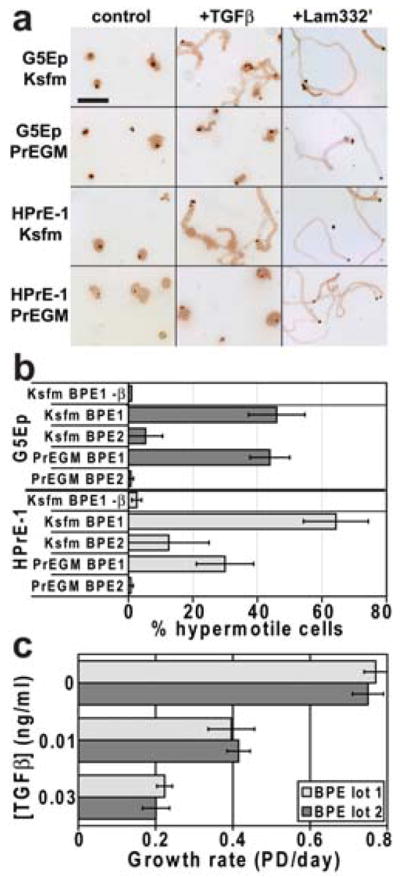
a. Typical fields motility assay cultures of G5Ep and HPrE-1 immunostained for Laminin γ2 to reveal migration tracks. Cells were plated in Ksfm medium made using the BPE supplement provided by GIBCO/Invitrogen or in PrEGM medium made using the BPE supplement provided by Clonetics/Lonza. Some wells received 0.1 ng/ml TGFβ and some wells had been precoated with Lam332′. Note that G5Ep and HPrE-1 cells were sessile under control conditions, that Lam332′ induced hypermotility in both media, and that TGFβ induced hypermotility only in Ksfm. Bar in upper left panel: 500 microns.
b. G5Ep and HPrE-1 cells assayed for TGFβ-induced hypermotility when plated in Ksfm or PrEGM prepared with two different lots of BPE obtained from the same supplier. The top bar for each cell line shows the result with no TGFβ. All other bars show results with 0.1 ng/ml TGFβ added at plating. The average of replicate experiments is shown with standard error of the mean (s.e.m.) indicated by error bars. Note that TGFβ induced hypermotility in both cell types in both media when supplemented with BPE lot 1 but not BPE lot 2.
c. G5Ep cells assayed for TGFβ growth inhibition in Ksfm medium prepared with the same two lots of BPE used in panel b above. Note the same dose-response for growth inhibition in both medium preparations.
Effects of calcium and serum on growth and motility
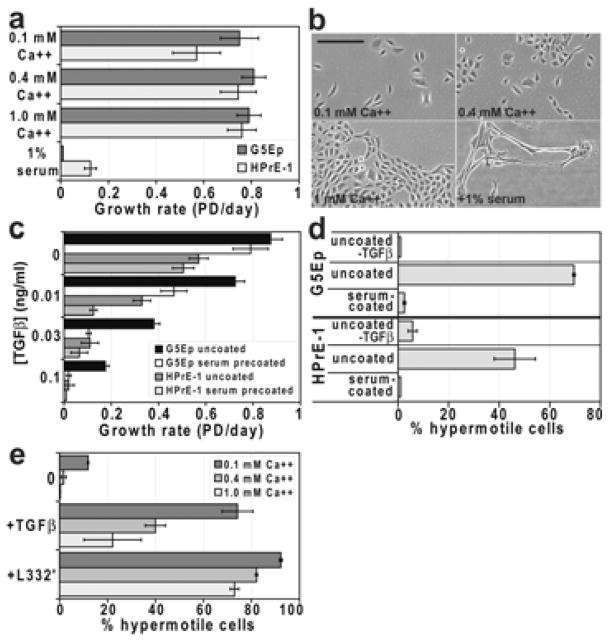
Human keratinocytes cultured without fibroblast feeder cells in semi-defined media grow well in [Ca++] up to 1.0 mM [Wille et al., 1984], unlike murine keratinocytes which are irreversibly growth-inhibited by [Ca++] >0.1 mM [Hennings et al., 1980]. We have long cultured primary human keratinocytes in Ksfm medium that we supplement with additional CaCl2 to bring the [Ca++] to 0.4 mM [Dabelsteen et al., 2009; Dickson et al., 2000; Schon and Rheinwald, 1996]. PrEGM has 0.1 mM Ca++ and PFMR-4a, a published medium formulation optimized for prostate epithelial cells [Peehl, 2003; Peehl et al., 1988], has 0.9 mM Ca++. As expected, keratinocytes and prostate epithelial cells grew well [Ca++] ranging from 0.1–1 mM (Fig. 3a). Prostate epithelial cells benefited more from higher [Ca++] than did keratinocytes, perhaps because they required higher Ca++ to be able to grow as more tightly packed colonies (Fig. 3b). The dose-response for TGFβ growth inhibition of both cell types was indistinguishable at all [Ca++] (data not shown). However, the hypermotility response to TGFβ was markedly impaired, and to Lam332′ very modestly impaired, by increased [Ca++] (Fig. 3c). Of note, more than 10% of cells were hypermotile when plated in 0.1 mM Ca++ medium under control conditions, without either TGFβ or Lam332′ (Fig. 3c). Cells displayed very little constitutive, but robust TGFβ- and Lam332′-induced, hypermotility in medium containing 0.2 mM Ca++ (data not shown), so 0.2 mM Ca++ was used for all subsequent motility experiments.
Figure 3. Serum and Ca++ affect growth and TGFβ-induced hypermotility differently.
a,b. Effects of [Ca++] and serum on keratinocyte and prostate epithelial cell growth. Panel a: G5Ep and HPrE-1 cells plated at low density (~250 cells/cm2) in Ksfm supplemented with 0.1, 0.4, or 1.0 mM Ca++ or with 0.4 mM Ca++ and 1% calf serum. Note that both cell types grew as well or better in higher [Ca++] but were strongly inhibited by addition of 1% serum to the medium. Panel b: typical fields of HPrE-1 cells in the various conditions. Note inability to form closely packed colonies in low and medium [Ca++] and abnormal elongation associated with growth inhibition in presence of serum.
c. Increased sensitivity to TGFβ growth inhibition of keratinocyte and prostate epithelial cells when plated on serum-precoated dishes. Cells were plated in Ksfm in wells that were not pretreated or that were precoated with 10% serum. Note little change in growth rate −TGFβ and a significant dose-response shift toward increased TGFβ-induced growth inhibition for both cell types on serum-precoated dishes.
d. A substratum-bound component of serum blocks TGFβ-induced hypermotility. G5Ep and HPrE-1 cells were plated + or −TGFβ in Ksfm with 0.2 mM Ca++ in untreated or serum-precoated wells. Note lack of hypermotility response to TGFβ of cells in serum-precoated wells.
e. TGFβ-induced hypermotility is inversely related to [Ca++] of the medium. G5Ep cells were plated + or −TGFβ or on Lam332′-precoated surfaces in Ksfm with [Ca++] = 0.1 mM, 0.4 mM, or 1.0 mM. Note that hypermotility induced by TGFβ was greatly reduced, and that by Lam332′ slightly attenuated, by increasing [Ca++].
Some medium formulations used for prostate epithelial cell culture contain 1% serum (e.g., refs. [Jarrard et al., 1999; Kogan et al., 2006]). The addition of even as little as 1% serum to the medium proved to severely growth-inhibit both cell types when they were plated at low density, associated with abnormal cell elongation (Fig. 3a,b). The presence of ≥1% serum in the medium substantially reduced TGFβ-induced motility of both types (data not shown), possibly due all or in part to the deleterious effects of serum on the health of the cells. In light of our earlier finding that a substratum-bound component of serum acts as a cofactor to enhance Lam332′-induced hypermotility of keratinocytes [Natarajan et al., 2006], we tested the effect of precoating dishes with 10% serum on growth and motility. In the absence of TGFβ, cells grew nearly as rapidly on serum-precoated as on uncoated dishes (Fig. 3d). However, cells plated on serum-precoated dishes did not become hypermotile in response to TGFβ (Fig. 3e). This was not the result of inactivation of TGFβ, as cells were even more sensitive to the growth inhibitory effects of TGFβ when plated on serum-precoated dishes (Fig. 3d).
Effects of mitogens on motility induction
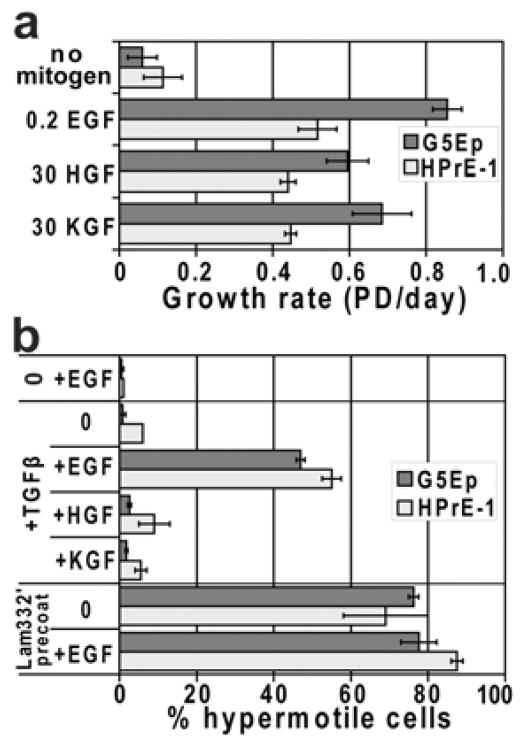
As is true for other p63+ epithelial cell types, prostate epithelial cells express Lam332 and α6β4 integrin [Nagle et al., 1995]. On tissue culture plastic, keratinocytes form rudimentary hemidesmosome-like structures called the stable adhesion complexes (SAC), marked by focal concentrations of α6β4 integrin [Carter et al., 1990; Langhofer et al., 1993]. Phosphorylation of the cytoplasmic domain of β4 integrin as a downstream effect of EGF receptor stimulation has been found to induce SAC disassembly [Mariotti et al., 2001]. We asked whether in our experimental system cells require EGF in order to be induced to hypermotility and, if so, whether this can be distinguished from a general requirement for mitogen stimulation. As expected from earlier studies [Marchese et al., 1990; Peehl et al., 1996; Wilson et al., 1994], KGF was mitogenic for both cell types and we found that HGF also could substitute for EGF in stimulating growth (Fig. 4a). TGFβ failed to induce hypermotility in the absence of added EGF and neither KGF or HGF could substitute for EGF to make cells permissive for TGFβ-induced hypermotility (Fig. 4b). In contrast, Lam332′-induced hypermotility was independent of EGF (Fig. 4b).
Figure 4. Specificity for EGF as a cofactor to permit TGFβ-induced hypermotility.
a. G5Ep and HPrE-1 cells were assessed for responsiveness to various mitogens by plating at low density in Ksfm containing half the usual [BPE] and no EGF or supplemented with 0.2 ng/ml EGF, 30 ng/ml HGF, or 30 ng/ml KGF and grown for 7 days. Note that EGF was the most potent mitogen for both cell types but that at high concentrations HGF and KGF were also good mitogens, stimulating progressive growth compared to no added mitogen controls.
b. G5Ep and HPrE-1 were assessed for hypermotility induced by TGFβ or by plating on Lam332′ in the same medium + or −mitogens as in panel a. The top pair of bars in the graph shows the result −TGFβ in medium containing EGF. The middle bars show the effect of TGFβ in medium with no mitogen vs. with EGF, HGF, or KGF. The bottom bars show the result of plating cells on uncoated or Lam332′-precoated dishes in medium without mitogen or +EGF. Note that TGFβ-induced hypermotility specifically required EGF, whereas Lam332′-induced hypermotility was independent of added mitogen.
Engineering and characterization of an immortalized normal prostate epithelial cell line
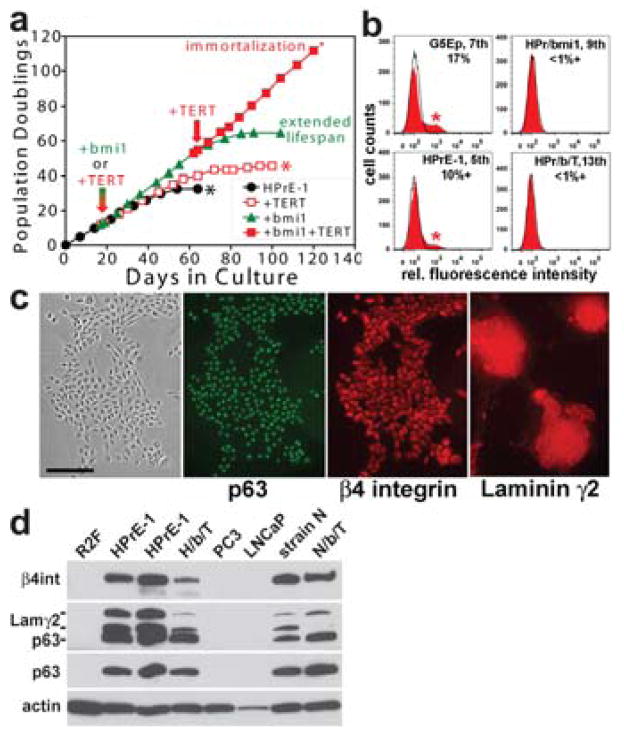
HPrE-1 proved to have a rather short replicative lifespan (~33 PD) in PrEGM or Ksfm (Fig. 5a). This is substantially less total proliferative potential than that of newborn foreskin epidermal keratinocyte primary lines such as G5Ep but similar to that of many adult primary lines of keratinocytes and other p63+ epithelial cell types we have studied [Dabelsteen et al., 2009]. Experimentation with normal prostate epithelial cells would be facilitated by extending their replicative lifespan or immortalizing them by methods that do not alter growth and motility regulatory mechanisms. Previous studies had found an increase of p16INK4A in senescent primary prostate epithelial cell cultures [Sandhu et al., 2000] and genetic loss or epigenetic silencing of the CDKN2A gene, which encodes p16INK4A and p14ARF, in HPVE6-immortalized prostate epithelial cell lines [Jarrard et al., 1999]. Engineering keratinocytes to express TERT proved insufficient to immortalize them [Dickson et al., 2000; Kiyono et al., 1998], requiring additional spontaneous heritable events that prevent or greatly reduce the frequency of p16 expression to occur in the TERT-expressing cells before they would grow as immortalized lines. Alternatively, keratinocytes can be immortalized by engineering them in a multistep process, first expressing proteins that permit cells to evade p16INK4A- and p14ARF-mediated controls and then expressing TERT [Dabelsteen et al., 2009; Rheinwald et al., 2002]. A previous report [Kogan et al., 2006] found that 3 of 4 primary prostate epithelial cell lines tested failed to become immortalized following TERT expression and that the one line that became immortalized had lost p16INK4A expression. We found that TERT expression was insufficient to immortalize HPrE-1 (Fig. 5a). The polycomb protein bmi1, a normal repressor of the CDKN2A locus, ceases to be expressed by late passage cells, associated with p16 induction [Jacobs et al., 1999]. Engineering cells by retroviral vector transduction to constitutively express bmi1 has been found to substantially extend the replicative lifespan of primary human mammary epithelial cells [Dimri et al., 2002], keratinocytes [Dabelsteen et al., 2009; Kim et al., 2007], and urothelial and tracheobronchial epithelial cells [Dabelsteen et al., 2009]. HPrE-1, like G5Ep, continuously generated p16INK4A-expressing, senescent cells during serial passage (Fig. 5b). Engineering HPrE-1 to express bmi1 prevented p16 expression (Fig. 5b) and conferred an extended lifespan of ~25 additional PD (Fig. 5a). HPrE-1/bmi1 cells became immortalized when subsequently engineered to express TERT (Fig. 5a) and p16 remained repressed in the HPrE-1/bmi1/TERT line (Fig. 5b).
Figure 5. Characteristics of experimentally immortalized human prostate epithelial cells.
a. HPrE-1 was transduced early in its lifespan (indicated by the red/green arrow) to express either bmi1 or TERT. Note that untransduced HPrE-1 cells senesced after 33 PD, HPrE-1/TERT cells did not become immortalized and senesced after 43 PD, but that HPrE-1/bmi1 cells displayed a long extended lifespan totaling 63 PD. HPrE-1/bmi1 cells subsequently transduced to express TERT (at the point in their lifespan indicated by the red arrow) grew progressively and at a constant growth rate for >115 PD, indicating immortalization.
b. Flow cytometric analysis of p16INK4A expression in normal primary and immortalized prostate epithelial cells. G5Ep keratinocytes at 7th passage (at ~50 PD of their 70 PD lifespan), HPrE-1 cells at 5th passage (at ~22 PD of their 33 PD lifespan), HPrE-1/bmi1 cells at 9th passage (at ~45 PD of their 63 PD lifespan), and immortalized HPrE-1/bmi1/TERT cells at 13th passage (~65 PD) were analyzed for p16INK4A expression. Fluorescence intensity of cells incubated with non-immune IgG isotype control is shown by the black line and p16INK4A immunofluorescence intensity of the population shown by the solid red curve. Note p16+ cells indicated by asterisk in the G5Ep and HPrE-1 panels, representing 17% and 10% of these populations, respectively, and absence of p16+ cells in the HPrE-1/bmi1 and HPrE-1/bmi1/TERT cultures.
c. Immunofluorescence staining of HPrE-1/bmi1/TERT cells after 90 PD. Note p63, β4 integrin, and Lam332 expression in all cells. Bar in left panel: 200 microns.
d. Western blot analysis of p63, Laminin γ2, and β4 integrin expression. Total protein extracts of preconfluent cultures were analyzed. Early- and mid-lifespan cultures of HPrE-1 are shown in the two lanes labeled HPrE-1. H/b/T: HPrE-1/bmi1/TERT. N/b/T: N/bmi1/TERT. The two bands of γ2 are the 155kd unprocessed form and the 105 kd processed, mature form of this subunit of Lam332. Note expression of p63, Laminin γ2, and β4 integrin by HPrE-1/bmi1/TERT cells (albeit with lower levels of Laminin γ2 and β4 integrin than the HPrE-1 parent line), in contrast to undetectable expression by PC3 and LNCaP prostate carcinoma cells. The strain N epidermal keratinocyte line and its bmi1/TERT-immortalized derivative displayed similar levels of p63, β4 integrin, and γ2 expression.
Prostate carcinoma differs from carcinomas that arise from most other p63+ epithelial cell types by the consistent loss of expression of p63, β4 integrin, and the γ2 chain of Lam332 during neoplastic progression [Davis et al., 2001; Hao et al., 1996; Nagle et al., 1995; Weinstein et al., 2002]. We therefore asked whether experimental immortalization and prolonged serial cultivation of normal prostate epithelial cells would result in loss of these normal markers. We examined HPrE-1/bmi1/TERT cells at 90–110 PDs (60–80 PD beyond the lifespan limit of the HPrE-1 parent primary line (see Fig. 5a)). HPrE-1/bmi1/TERT cells continued to express p63, β4 integrin, and Lam332 (Figs. 5c,d), in contrast to the prostate carcinoma cell lines PC3 and LNCaP.
HPrE-1/bmi1/TERT cells remained sensitive to TGFβ-induced growth inhibition, albeit with a 2- to 3-fold shift in dose-response relative to the parent HPrE-1 line, in contrast to the prostate carcinoma cell lines PC3 (Fig. 6a) and LNCaP (data not shown) which were completely resistant to TGFβ. HPrE-1/bmi1/TERT cells remained inducible to hypermotility by TGFβ and by Lam332′ (Fig. 6b) and displayed a normal smad2 phosphorylation response to TGFβ (Fig. 6c). PC3 responded to TGFβ with modest smad2 phosphorylation and very low levels of Laminin γ2 expression. MDAPCa-2b and LNCaP had no detectable constitutive or TGFβ-inducible smad2 phosphorylation or γ2 expression. We conclude that human prostate epithelial cells immortalized by bmi1+TERT expression stably maintain expression of normal prostate basal epithelial cell markers and hypermotility and growth arrest responses and do not acquire features of transformation during long-term serial culture.
Figure 6. TGFβ-induced responses and TGFβ receptor-dependent signaling in normal primary and immortalized prostate epithelial cells.
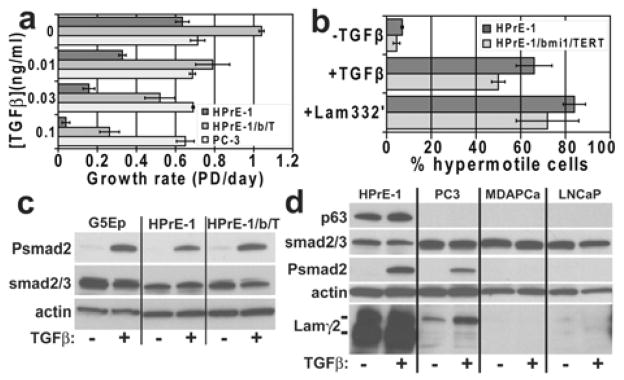
a. TGFβ-induced growth inhibition. Note that HPrE-1/bmi1/TERT cells (HPrE-1/b/T) remained sensitive to TGFβ growth inhibition, although their dose-response was shifted about 3-fold toward reduced sensitivity compared with HPrE-1 cells. In contrast, PC3 cells were completely resistant to TGFβ.
b. TGFβ and Lam332′-induced hypermotility. Note that HPrE-1/bmi1/TERT cells remained sensitive to TGFβ- and Lam332′-induced hypermotility.
c. Smad2 phosphorylation induced by TGFβ in normal primary keratinocytes and prostate epithelial cells and in experimentally immortalized prostate epithelial cells. Preconfluent cultures of G5Ep, HPrE-1, and HPrE-1/bmi1/TERT growing in Ksfm were untreated or treated with 0.1 ng/ml TGFβ for the final 30 min before lysis and analysis by Western blotting. Note very low or undetectable constitutive, and TGFβ-activated, smad2 phosphorylation in all three cell lines.
d. Defects in TGFβ-induced smad signaling and p63 and Lam332 expression by prostate carcinoma cell lines. Preconfluent cultures were untreated or treated with 0.1 ng/ml TGFβ for the final 24 hr before lysis and analysis by Western blotting. Note absence of p63 expression by the prostate carcinoma lines. The Laminin γ2 blot was overexposed for HPrE1 to permit detection of very low levels of γ2 expression and its induction by TGFβ in PC3, consistent with smad2 phosphorylation stimulated by TGFβ in this line. In contrast, MDAPCa-2b and LNCaP did not phosphorylate smad2 in response to TGFβ and did not synthesize detectable γ2.
Discussion
Despite the marked differences between the prostate epithelium and stratified surface epithelia with respect to embryonic development, tissue structure, pathway to neoplasia, and properties of invasive carcinomas that arise from them, prostate epithelial cells proved to display inducible hypermotility responses identical to those of keratinocytes and other stratified epithelial cell types. For several reasons it would not have been predicted that normal prostate basal cells would display such hypermotility responses. The prostate is a gland that does not appear to require the ability to rapidly repair surfaces denuded by wounding and, in contrast to stratified surface epithelia, malignant invasion in the prostate is accomplished by cells that no longer express Lam332. Prostate epithelial cells may use this hypermotility response mechanism during embryonic development of the gland and in the adult it may remain as a vestigial ability, associated with its expression of Lam332 and cognate integrins.
A consensus has not yet been reached about optimal culture conditions for human prostate epithelial cells, in contrast to the field of keratinocyte culture which typically uses published, commercially available medium formulations (eg., Ksfm [Pirisi et al., 1987], KGM/MCDB154 [Shipley and Pittelkow, 1987], or the fibroblast feeder layer system [Allen-Hoffmann and Rheinwald, 1984; Rheinwald and Green, 1975]. Our experiments comparing keratinocytes and prostate epithelial cells found no advantage for growth rate or replicative potential of growing either cell type in the proprietary PrEGM formulation over Ksfm, consistent with the use of Ksfm as a prostate cell medium by a number of investigators (e.g., refs. [Berger et al., 2004; Gu et al., 2006]). Importantly, studies by others have shown that normal prostate epithelial cells cultured in Ksfm retain the ability to undergo prostate-specific luminal differentiation [Lamb et al., 2010] and if engineered to express SV40(LT+st), HrasV12, TERT and AR they form tumors of histology similar to that of prostate carcinoma if inoculated into the prostate of immune-deficient mice [Berger et al., 2004]). We did not compare Ksfm with the non-commercially available PFMR-4a prostate epithelial cell medium [Peehl, 2003; Peehl et al., 1988] for growth rate or replicative lifespan, but short lifespans have been noted for adult-derived normal prostate epithelial cells in this and other medium formulations [Peehl, 2005].
Our results did not support the practice of some investigators of culturing prostate epithelial cells in 1% serum-supplemented medium (e.g. refs. [Jarrard et al., 1999; Kogan et al., 2006]. We found strong growth inhibitory effects of serum at concentrations as low as 0.3–1% for both keratinocytes and prostate epithelial cells when cultured from low density platings, which we use for motility, growth, and replicative lifespan determinations. Our results were at odds with a report that normal prostate epithelial cells remain proliferative only if maintained in medium of [Ca++] <0.4 mM [Dalrymple et al., 2005] We found that the growth of neither prostate epithelial cells or keratinocytes was inhibited by [Ca++] between 0.1 and 1 mM and both cell types grew better with moderate to high [Ca++] than in 0.1 mM Ca++. We conclude from our studies that culture conditions optimal for prostate epithelial cells are the same as those for other p63+ epithelial cell types we have studied (e.g., ref. [Dabelsteen et al., 2009]).
We unexpectedly found that some culture variables that have little or no effect on cell proliferation have major inhibiting or attenuating effects on motility induction by TGFβ. We detected great differences in TGFβ-induced motility, but not TGFβ-induced growth arrest, in medium prepared with different production lots of bovine pituitary extract (BPE). BPE, a crude extract of total pituitary gland prepared in large batches from pools of glands obtained from slaughterhouses, is provided by culture medium suppliers as a supplement to the nutrient medium, used in addition to defined mitogens and other growth-promoting molecules. We found similar growth promoting activities of dozens of BPE lots over a period of many years, but very large differences among these lots in the ability of epithelial cells to be induced to hypermotility by TGFβ. We found that serum, and even a subfraction of serum that binds to the culture dish surface, markedly inhibits TGFβ-induced motility. It is possible that fluctuation from batch to batch in the amount of residual blood and, therefore, serum protein, in the pools of pituitary glands used to make the BPE supplement is too small to make a detectable difference in cell growth rate but large enough for some batches to impair motility. We do not know the mechanism by which increased [Ca++] attenuates TGFβ-induced motility. Ca++ bridges cell-cell adhesion complexes containing proteins such as E-cadherin but our assays employ very low density platings and visual inspection of the cultures when immunostained to measure track lengths reveals no increase in cell-cell aggregation in high [Ca++] medium that might impair single cell migration.
This study has revealed an essential and specific role for EGF as a permissive factor for epithelial cell migration in response to TGFβ. Our use of an assay that detects and quantitates sustained directional hypermotility, also termed persistent or processive migration[Frank and Carter, 2004], permits a clear distinction to made between factors that induce hypermotility and those that play a permissive role. The default situation for a normal basal epithelial cell is to be proliferative, or potentially proliferative, and sessile. Cells require the continual presence of certain polypeptide survival factors such as Insulin or IGF, common requirements of all somatic cells, and mitogenic factors such as EGF, which often are specific for certain subsets of somatic cell types. EGF, HGF, and KGF all proved to be good mitogens for keratinocytes and prostate epithelial cells, stimulating progressive growth from low density platings. Our earlier study [Natarajan et al., 2006] detected no ability of either EGF or HGF to stimulate directional hypermotility and the present study adds KGF to the list of epithelial mitogens that do not, by themselves, stimulate hypermotility.
It was noted long ago that EGF promotes progressive, long-term proliferation of human keratinocytes in culture in part by permitting cells in large colonies to move and spread out so as to permit continued colony expansion [Barrandon and Green, 1987; Rheinwald and Green, 1977]. In contrast to KGF, both EGF and HGF cause increased random cell movement resulting in colony scattering in low [Ca++] medium [Hudson and McCawley, 1998; McCawley et al., 1998; Peehl et al., 1996] and, compared to serum- or BPE-starved cells, their addition to the medium increases general cell movement detected in phagokinetic assays [Cha et al., 1996; Li W. et al., 2004; Li Y. et al., 2006]. These cited studies have likely detected an important effect of EGF and HGF receptor activation besides mitogenic signaling, characterized in other studies as stimulating fyn/src-mediated phosphorylation of the cytoplasmic tail of β4 integrin to cause disassembly of hemidesmosomes and stable adhesion complexes [Litjens et al., 2006; Mariotti et al., 2001; Trusolino et al., 2001]. Interpreting our results in light of such a mechanism, cells plated on a Lam332′-coated surface, which immediately stimulates them to become directionally hypermotile, may not form stable adhesion complexes at all, consistent with our result that such migration is EGF-independent. In contrast, cells plated onto an uncoated substratum and exposed to TGFβ at the time of plating become hypermotile after a one day lag, temporally associated with induction of increased Lam332 synthesis and secretion of unprocessed Lam332′ [Natarajan et al., 2006], (Bouez, Hercule, Barron, and Rheinwald, unpublished). During the time before the cells respond to TGFβ by changing mRNA and protein expression and initiating hypermotility, in the absence of EGF they may form stable adhesion complexes that prevent them from becoming migratory. We found no ability of HGF to replace EGF in rendering cells permissive for TGFβ-induced hypermotility. The report that concluded that the HGF receptor (c-met) can accomplish β4 integrin phosphorylation studied tumor-derived cell lines that naturally, or that were engineered to, overexpress c-met [Trusolino et al., 2001], so this may not be a function of HGF in normal epithelial cells.
Coordinate Lam332 and p16INK4A expression is found in late stages of neoplastic progression in the epidermis and oral mucosa in vivo [Natarajan et al., 2003], identifying the stage during neoplastic progression at which p16 acts as a tumor suppressor during development of SCC. Coexpression also is found in normal keratinocytes at the leading edge of wounds and in cultured keratinocytes as they become senescent, the latter frequently displaying a burst of hypermotility [Natarajan et al., 2006; Natarajan et al., 2005; Natarajan et al., 2003]. We therefore have speculated that keratinocytes respond to contact with an abnormal substratum--presumably the absence of mature Lam332, by sequentially inducing Lam332 overexpression and p16 expression. No preneoplastic state associated with Lam332 overexpression has been identified in the prostate and invasive prostatic carcinomas invariably are found to have lost Lam332 and β4 integrin expression, so this cancer must invade by interacting with matrix proteins other than Lam332. One might predict, therefore, that induction of p16INK4A would not occur during the process of prostatic neoplastic progression. However, p16INK4A deletions/mutations are found in 20–50% of prostate carcinomas [Jarrard et al., 1997; Perinchery et al., 1999], indicating that an as yet to be determined step during neoplastic progression to prostate carcinoma is blocked by p16 induction.
Induction of p16 expression was identified early on as being associated with prostate epithelial cell senescence in culture and to act as a barrier to immortalization [Jarrard et al., 1999; Sandhu et al., 2000]. Transduction of normal prostate epithelial cells to express TERT alone has been found to occasionally yield immortalized lines, associated with loss of p16 expression [Kogan et al., 2006]. We used a defined method to maintain repression of the CDKN2A locus---transduction to constitutively express bmi1, a polycomb protein naturally expressed by early passage cells that helps to keep the CDKN2A locus in a transcriptionally inaccessible state [Jacobs et al., 1999]. We and others have used bmi1 to prevent CDKN2A derepression during serial culture as part of a multistep method for immortalizing keratinocytes and other p63+ epithelial cell types [Dabelsteen et al., 2009; Kim et al., 2007]. The HPrE-1/bmi1/TERT line we generated proved to retain normal prostate basal epithelial characteristics during serial culture, including marker expression and TGFβ pathway signaling and growth inhibition and hypermotility responses, clearly distinct from prostate carcinoma cell lines which were deficient in all these characteristics. This experimentally immortalized line will be useful for future studies of normal prostate epithelial growth, differentiation, and motility mechanisms.
Acknowledgments
We thank Charbel Bouez for advice on Western blotting and other helpful discussions, Paula Hercule for assistance with cell culture and retroviral transduction, and Frank McKeon for the 4A4 p63 antibody. We thank G. Dimri, North Shore University Health System Research Institute, Evanston, IL, and John Sedivy, Brown University, Providence, RI for providing the bmi1 and TERT retroviral vectors. This research was supported by NIH grant R01-DE13178 and a grant from Organogenesis, Inc., to JGR, by an NIH Skin Disease Research Center grant P30-AR42689 to T. S. Kupper, and by a sabbatical stipend from the Chinese government to WW.
References
- Allen-Hoffmann BL, Rheinwald JG. Polycyclic aromatic hydrocarbon mutagenesis of human epidermal keratinocytes in culture. Proc Natl Acad Sci U S A. 1984;81:7802–7806. doi: 10.1073/pnas.81.24.7802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrandon Y, Green H. Cell migration is essential for sustained growth of keratinocyte colonies: the roles of transforming growth factor-alpha and epidermal growth factor. Cell. 1987;50:1131–1137. doi: 10.1016/0092-8674(87)90179-6. [DOI] [PubMed] [Google Scholar]
- Berger R, Febbo PG, Majumder PK, Zhao JJ, Mukherjee S, Signoretti S, Campbell KT, Sellers WR, Roberts TM, Loda M, Golub TR, Hahn WC. Androgen-induced differentiation and tumorigenicity of human prostate epithelial cells. Cancer Res. 2004;64:8867–8875. doi: 10.1158/0008-5472.CAN-04-2938. [DOI] [PubMed] [Google Scholar]
- Carter WG, Kaur P, Gil SG, Gahr PJ, Wayner EA. Distinct functions for integrins α3β1 in focal adhesions and α6β4/bullous pemphigoid antigen in a new stable anchoring contact (SAC) of keratinocytes: relation to hemidesmosomes. J Cell Biol. 1990;111:3141–3154. doi: 10.1083/jcb.111.6.3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha D, O’Brien P, O’Toole EA, Woodley DT, Hudson LG. Enhanced modulation of keratinocyte motility by transforming growth factor-alpha (TGFα) relative to epidermal growth factor (EGF) J Invest Dermatol. 1996;106:590–597. doi: 10.1111/1523-1747.ep12345083. [DOI] [PubMed] [Google Scholar]
- Dabelsteen S, Hercule P, Barron P, Rice M, Dorsainville G, Rheinwald JG. Epithelial cells derived from human embryonic stem cells display p16INK4A senescence, hypermotility, and differentiation properties shared by many p63+ somatic cell types. Stem Cells. 2009;27:1388–1399. doi: 10.1002/stem.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalrymple S, Antony L, Xu Y, Uzgare AR, Arnold JT, Savaugeot J, Sokoll LJ, De Marzo AM, Isaacs JT. Role of notch-1 and E-cadherin in the differential response to calcium in culturing normal versus malignant prostate cells. Cancer Res. 2005;65:9269–9279. doi: 10.1158/0008-5472.CAN-04-3989. [DOI] [PubMed] [Google Scholar]
- Davis TL, Cress AE, Dalkin BL, Nagle RB. Unique expression pattern of the α6β4 integrin and laminin-5 in human prostate carcinoma. Prostate. 2001;46:240–248. doi: 10.1002/1097-0045(20010215)46:3<240::aid-pros1029>3.0.co;2-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decline F, Okamoto O, Mallein-Gerin F, Helbert B, Bernaud J, Rigal D, Rousselle P. Keratinocyte motility induced by TGFβ1 is accompanied by dramatic changes in cellular interactions with laminin 5. Cell Motil Cytoskeleton. 2003;54:64–80. doi: 10.1002/cm.10086. [DOI] [PubMed] [Google Scholar]
- Dickson MA, Hahn WC, Ino Y, Ronfard V, Wu JY, Weinberg RA, Louis DN, Li FP, Rheinwald JG. Human keratinocytes that express hTERT and also bypass a p16INK4A- enforced mechanism that limits lifespan become immortal yet retain normal growth and differentiation characteristics. Mol Cell Biol. 2000;20:1436–1447. doi: 10.1128/mcb.20.4.1436-1447.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimri GP, Martinez JL, Jacobs JJ, Keblusek P, Itahana K, Van Lohuizen M, Campisi J, Wazer DE, Band V. The Bmi-1 oncogene induces telomerase activity and immortalizes human mammary epithelial cells. Cancer Res. 2002;62:4736–4745. [PubMed] [Google Scholar]
- Frank DE, Carter WG. Laminin 5 deposition regulates keratinocyte polarization and persistent migration. J Cell Sci. 2004;117:1351–1363. doi: 10.1242/jcs.01003. [DOI] [PubMed] [Google Scholar]
- Goldfinger LE, Hopkinson SB, deHart GW, Collawn S, Couchman JR, Jones JC. The α3 laminin subunit, α6β4 and α3β1 integrin coordinately regulate wound healing in cultured epithelial cells and in the skin. J Cell Sci. 1999;112 ( Pt 16):2615–2629. doi: 10.1242/jcs.112.16.2615. [DOI] [PubMed] [Google Scholar]
- Gu Y, Li H, Miki J, Kim KH, Furusato B, Sesterhenn IA, Chu WS, McLeod DG, Srivastava S, Ewing CM, Isaacs WB, Rhim JS. Phenotypic characterization of telomerase-immortalized primary non-malignant and malignant tumor-derived human prostate epithelial cell lines. Exp Cell Res. 2006;312:831–843. doi: 10.1016/j.yexcr.2005.11.029. [DOI] [PubMed] [Google Scholar]
- Hao J, Yang Y, McDaniel KM, Dalkin BL, Cress AE, Nagle RB. Differential expression of laminin 5 (α3β3γ2) by human malignant and normal prostate. Am J Pathol. 1996;149:1341–1349. [PMC free article] [PubMed] [Google Scholar]
- Hennings H, Michael D, Cheng C, Steinert P, Holbrook K, Yuspa SH. Calcium regulation of growth and differentiation of mouse epidermal cells in culture. Cell. 1980;19:245–254. doi: 10.1016/0092-8674(80)90406-7. [DOI] [PubMed] [Google Scholar]
- Horoszewicz JS, Leong SS, Kawinski E, Karr JP, Rosenthal H, Chu TM, Mirand EA, Murphy GP. LNCaP model of human prostatic carcinoma. Cancer Res. 1983;43:1809–1818. [PubMed] [Google Scholar]
- Hudson LG, McCawley LJ. Contributions of the epidermal growth factor receptor to keratinocyte motility. Microsc Res Tech. 1998;43:444–455. doi: 10.1002/(SICI)1097-0029(19981201)43:5<444::AID-JEMT10>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Izumi K, Hirao Y, Hopp L, Oyasu R. In vitro induction of ornithine decarboxylase in urinary bladder carcinoma cells. Cancer Res. 1981;41:405–409. [PubMed] [Google Scholar]
- Jacobs JJ, Kieboom K, Marino S, DePinho RA, van Lohuizen M. The oncogene and Polycomb-group gene bmi-1 regulates cell proliferation and senescence through the INK4A locus. Nature. 1999;397:164–168. doi: 10.1038/16476. [DOI] [PubMed] [Google Scholar]
- Jarrard DF, Bova GS, Ewing CM, Pin SS, Nguyen SH, Baylin SB, Cairns P, Sidransky D, Herman JG, Isaacs WB. Deletional, mutational, and methylation analyses of CDKN2 (p16/MTS1) in primary and metastatic prostate cancer. Genes Chromosomes Cancer. 1997;19:90–96. [PubMed] [Google Scholar]
- Jarrard DF, Sarkar S, Shi Y, Yeager TR, Magrane G, Kinoshita H, Nassif N, Meisner L, Newton MA, Waldman FM, Reznikoff CA. p16/pRb pathway alterations are required for bypassing senescence in human prostate epithelial cells. Cancer Res. 1999;59:2957–2964. [PubMed] [Google Scholar]
- Kaighn ME, Narayan KS, Ohnuki Y, Lechner JF, Jones LW. Establishment and characterization of a human prostatic carcinoma cell line (PC-3) Invest Urol. 1979;17:16–23. [PubMed] [Google Scholar]
- Kainulainen T, Autio-Harmainen H, Oikarinen A, Salo S, Tryggvason K, Salo T. Altered distribution and synthesis of laminin-5 (kalinin) in oral lichen planus, epithelial dysplasias and squamous cell carcinomas. Br J Dermatol. 1997;136:331–336. [PubMed] [Google Scholar]
- Kainulainen T, Hakkinen L, Hamidi S, Larjava K, Kallioinen M, Peltonen J, Salo T, Larjava H, Oikarinen A. Laminin-5 expression is independent of the injury and the microenvironment during reepithelialization of wounds. J Histochem Cytochem. 1998;46:353–360. doi: 10.1177/002215549804600309. [DOI] [PubMed] [Google Scholar]
- Ke XS, Qu Y, Goldfinger N, Rostad K, Hovland R, Akslen LA, Rotter V, Oyan AM, Kalland KH. Epithelial to mesenchymal transition of a primary prostate cell line with switches of cell adhesion modules but without malignant transformation. PLoS One. 2008;3:e3368. doi: 10.1371/journal.pone.0003368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim RH, Kang MK, Shin KH, Oo ZM, Han T, Baluda MA, Park NH. Bmi-1 cooperates with human papillomavirus type 16 E6 to immortalize normal human oral keratinocytes. Exp Cell Res. 2007;313:462–472. doi: 10.1016/j.yexcr.2006.10.025. [DOI] [PubMed] [Google Scholar]
- Kiyono T, Foster SA, Koop JI, McDougall JK, Galloway DA, Klingelhutz AJ. Both Rb/p16INK4a inactivation and telomerase activity are required to immortalize human epithelial cells. Nature. 1998;396:84–88. doi: 10.1038/23962. [DOI] [PubMed] [Google Scholar]
- Kogan I, Goldfinger N, Milyavsky M, Cohen M, Shats I, Dobler G, Klocker H, Wasylyk B, Voller M, Aalders T, Schalken JA, Oren M, Rotter V. hTERT-immortalized prostate epithelial and stromal-derived cells: an authentic in vitro model for differentiation and carcinogenesis. Cancer Res. 2006;66:3531–3540. doi: 10.1158/0008-5472.CAN-05-2183. [DOI] [PubMed] [Google Scholar]
- Korang K, Christiano AM, Uitto J, Mauviel A. Differential cytokine modulation of the genes LAMA3, LAMB3, and LAMC2, encoding the constitutive polypeptides, α3, β3, and γ2, of human laminin 5 in epidermal keratinocytes. FEBS Lett. 1995;368:556–558. doi: 10.1016/0014-5793(95)00740-z. [DOI] [PubMed] [Google Scholar]
- Lamb LE, Knudsen BS, Miranti CK. E-cadherin-mediated survival of androgen-receptor-expressing secretory prostate epithelial cells derived from a stratified in vitro differentiation model. J Cell Sci. 2010;123:266–276. doi: 10.1242/jcs.054502. [DOI] [PubMed] [Google Scholar]
- Langhofer M, Hopkinson SB, Jones JC. The matrix secreted by 804G cells contains laminin-related components that participate in hemidesmosome assembly in vitro. J Cell Sci. 1993;105 ( Pt 3):753–764. doi: 10.1242/jcs.105.3.753. [DOI] [PubMed] [Google Scholar]
- Larjava H, Salo T, Haapasalmi K, Kramer RH, Heino J. Expression of integrins and basement membrane components by wound keratinocytes. J Clin Invest. 1993;92:1425–1435. doi: 10.1172/JCI116719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechner JF, Narayan KS, Ohnuki Y, Babcock MS, Jones LW, Kaighn ME. Replicative epithelial cell cultures from normal human prostate gland. J Natl Cancer Inst. 1978;60:797–801. doi: 10.1093/jnci/60.4.797. [DOI] [PubMed] [Google Scholar]
- Li W, Henry G, Fan J, Bandyopadhyay B, Pang K, Garner W, Chen M, Woodley DT. Signals that initiate, augment, and provide directionality for human keratinocyte motility. J Invest Dermatol. 2004;123:622–633. doi: 10.1111/j.0022-202X.2004.23416.x. [DOI] [PubMed] [Google Scholar]
- Li Y, Fan J, Chen M, Li W, Woodley DT. Transforming growth factor-α: a major human serum factor that promotes human keratinocyte migration. J Invest Dermatol. 2006;126:2096–2105. doi: 10.1038/sj.jid.5700350. [DOI] [PubMed] [Google Scholar]
- Litjens SH, de Pereda JM, Sonnenberg A. Current insights into the formation and breakdown of hemidesmosomes. Trends Cell Biol. 2006;16:376–383. doi: 10.1016/j.tcb.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Marchese C, Rubin J, Ron D, Faggioni A, Torrisi MR, Messina A, Frati L, Aaronson SA. Human keratinocyte growth factor activity on proliferation and differentiation of human keratinocytes: differentiation response distinguishes KGF from EGF family. J Cell Physiol. 1990;144:326–332. doi: 10.1002/jcp.1041440219. [DOI] [PubMed] [Google Scholar]
- Mariotti A, Kedeshian PA, Dans M, Curatola AM, Gagnoux-Palacios L, Giancotti FG. EGF-R signaling through Fyn kinase disrupts the function of integrin α6β4 at hemidesmosomes: role in epithelial cell migration and carcinoma invasion. J Cell Biol. 2001;155:447–458. doi: 10.1083/jcb.200105017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCawley LJ, O’Brien P, Hudson LG. Epidermal growth factor (EGF)- and scatter factor/hepatocyte growth factor (SF/HGF)- mediated keratinocyte migration is coincident with induction of matrix metalloproteinase (MMP)-9. J Cell Physiol. 1998;176:255–265. doi: 10.1002/(SICI)1097-4652(199808)176:2<255::AID-JCP4>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Mizushima H, Koshikawa N, Moriyama K, Takamura H, Nagashima Y, Hirahara F, Miyazaki K. Wide distribution of laminin-5 γ2 chain in basement membranes of various human tissues. Horm Res. 1998;50(Suppl 2):7–14. doi: 10.1159/000053118. [DOI] [PubMed] [Google Scholar]
- Nagle RB, Hao J, Knox JD, Dalkin BL, Clark V, Cress AE. Expression of hemidesmosomal and extracellular matrix proteins by normal and malignant human prostate tissue. Am J Pathol. 1995;146:1498–1507. [PMC free article] [PubMed] [Google Scholar]
- Natarajan E, Omobono JD, 2nd, Guo Z, Hopkinson S, Lazar AJ, Brenn T, Jones JC, Rheinwald JG. A keratinocyte hypermotility/growth-arrest response involving Laminin 5 and p16INK4A activated in wound healing and senescence. Am J Pathol. 2006;168:1821–1837. doi: 10.2353/ajpath.2006.051027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natarajan E, Omobono JD, 2nd, Jones JC, Rheinwald JG. Co-expression of p16INK4A and laminin 5 by keratinocytes: a wound-healing response coupling hypermotility with growth arrest that goes awry during epithelial neoplastic progression. J Investig Dermatol Symp Proc. 2005;10:72–85. doi: 10.1111/j.1087-0024.2005.200415.x. [DOI] [PubMed] [Google Scholar]
- Natarajan E, Saeb M, Crum CP, Woo SB, McKee PH, Rheinwald JG. Co-expression of p16(INK4A) and laminin 5 γ2 by microinvasive and superficial squamous cell carcinomas in vivo and by migrating wound and senescent keratinocytes in culture. Am J Pathol. 2003;163:477–491. doi: 10.1016/s0002-9440(10)63677-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen BP, Ryan MC, Gil SG, Carter WG. Deposition of laminin 5 in epidermal wounds regulates integrin signaling and adhesion. Curr Opin Cell Biol. 2000;12:554–562. doi: 10.1016/s0955-0674(00)00131-9. [DOI] [PubMed] [Google Scholar]
- Noel JC, Fernandez-Aguilar S, Fayt I, Buxant F, Ansion MH, Simon P, Anaf V. Laminin-5 γ2 chain expression in cervical intraepithelial neoplasia and invasive cervical carcinoma. Acta Obstet Gynecol Scand. 2005;84:1119–1123. doi: 10.1111/j.0001-6349.2005.00879.x. [DOI] [PubMed] [Google Scholar]
- Peehl DM. Growth of prostatic epithelial and stromal cells in vitro. Methods Mol Med. 2003;81:41–57. doi: 10.1385/1-59259-372-0:41. [DOI] [PubMed] [Google Scholar]
- Peehl DM. Primary cell cultures as models of prostate cancer development. Endocr Relat Cancer. 2005;12:19–47. doi: 10.1677/erc.1.00795. [DOI] [PubMed] [Google Scholar]
- Peehl DM, Stamey TA. Serum-free growth of adult human prostatic epithelial cells. In Vitro Cell Dev Biol. 1986;22:82–90. doi: 10.1007/BF02623537. [DOI] [PubMed] [Google Scholar]
- Peehl DM, Wong ST, Rubin JS. KGF and EGF differentially regulate the phenotype of prostatic epithelial cells. Growth Regul. 1996;6:22–31. [PubMed] [Google Scholar]
- Peehl DM, Wong ST, Stamey TA. Clonal growth characteristics of adult human prostatic epithelial cells. In Vitro Cell Dev Biol. 1988;24:530–536. doi: 10.1007/BF02629087. [DOI] [PubMed] [Google Scholar]
- Perinchery G, Bukurov N, Nakajima K, Chang J, Li LC, Dahiya R. High frequency of deletion on chromosome 9p21 may harbor several tumor-suppressor genes in human prostate cancer. Int J Cancer. 1999;83:610–614. doi: 10.1002/(sici)1097-0215(19991126)83:5<610::aid-ijc7>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Pirisi L, Yasumoto S, Feller M, Doniger J, DiPaolo JA. Transformation of human fibroblasts and keratinocytes with human papillomavirus type 16 DNA. J Virol. 1987;61:1061–1066. doi: 10.1128/jvi.61.4.1061-1066.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyke C, Romer J, Pekka K, Leif R, Elisabeth R, Keld D, Karl T. The γ2 chain of Kalinin/Laminin is preferentially expressed in Invading Malignant Cells in Human Cancers. Am J Pathol. 1994;145:782–791. [PMC free article] [PubMed] [Google Scholar]
- Rajagopal S, Navone NM, Troncoso P, Fritsche HA, Chakrabarty S. Modulation of cellular proliferation and production of prostate-specific antigen and matrix adhesion molecules in human prostate carcinoma cells by polypeptide growth factors: comparative analyses of MDA PCa2a with established cell lines. Int J Oncol. 1998;12:589–595. doi: 10.3892/ijo.12.3.589. [DOI] [PubMed] [Google Scholar]
- Rheinwald JG, Green H. Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell. 1975;6:331–343. doi: 10.1016/s0092-8674(75)80001-8. [DOI] [PubMed] [Google Scholar]
- Rheinwald JG, Green H. Epidermal growth factor and the multiplication of cultured human epidermal keratinocytes. Nature. 1977;265:421–424. doi: 10.1038/265421a0. [DOI] [PubMed] [Google Scholar]
- Rheinwald JG, Hahn WC, Ramsey MR, Wu JY, Guo Z, Tsao H, De Luca M, Catricala C, O’Toole KM. A two-stage, p16INK4A- and p53-dependent keratinocyte senescence mechanism that limits replicative potential independent of telomere status. Mol Cell Biol. 2002;22:5157–5172. doi: 10.1128/MCB.22.14.5157-5172.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousselle P, Lunstrum GP, Keene DR, Burgeson RE. Kalinin: an epithelium-specific basement membrane adhesion molecule that is a component of anchoring filaments. J Cell Biol. 1991;114:567–576. doi: 10.1083/jcb.114.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandhu C, Peehl DM, Slingerland J. p16INK4A mediates cyclin dependent kinase 4 and 6 inhibition in senescent prostatic epithelial cells. Cancer Res. 2000;60:2616–2622. [PubMed] [Google Scholar]
- Schon M, Rheinwald JG. A limited role for retinoic acid and retinoic acid receptors RARα and RARβ in regulating keratin 19 expression and keratinization in oral and epidermal keratinocytes. J Invest Dermatol. 1996;107:428–438. doi: 10.1111/1523-1747.ep12363411. [DOI] [PubMed] [Google Scholar]
- Schwarze SR, Shi Y, Fu VX, Watson PA, Jarrard DF. Role of cyclin-dependent kinase inhibitors in the growth arrest at senescence in human prostate epithelial and uroepithelial cells. Oncogene. 2001;20:8184–8192. doi: 10.1038/sj.onc.1205049. [DOI] [PubMed] [Google Scholar]
- Shipley GD, Pittelkow MR. Control of growth and differentiation in vitro of human keratinocytes cultured in serum-free medium. Arch Dermatol. 1987;123:1541a–1544a. [PubMed] [Google Scholar]
- Signoretti S, Waltregny D, Dilks J, Isaac B, Lin D, Garraway L, Yang A, Montironi R, McKeon F, Loda M. p63 is a prostate basal cell marker and is required for prostate development. Am J Pathol. 2000;157:1769–1775. doi: 10.1016/S0002-9440(10)64814-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skyldberg B, Salo S, Eriksson E, Aspenblad U, Moberger B, Tryggvason K, Auer G. Laminin-5 as a marker of invasiveness in cervical lesions. J Natl Cancer Inst. 1999;91:1882–1887. doi: 10.1093/jnci/91.21.1882. [DOI] [PubMed] [Google Scholar]
- Stoltzfus P, Salo S, Eriksson E, Aspenblad U, Tryggvason K, Auer G, Avall-Lundqvist E. Laminin-5 γ2 chain expression facilitates detection of invasive squamous cell carcinoma of the uterine cervix. Int J Gynecol Pathol. 2004;23:215–222. doi: 10.1097/01.pgp.0000130107.95607.f6. [DOI] [PubMed] [Google Scholar]
- Svensson-Mansson S, Reis-Filho J, Landberg G. Transcriptional upregulation and unmethylation of the promoter region of p16 in invasive basal cell carcinoma cells and partial co-localization with the γ2 chain of laminin-332. J Pathol. 2007;212:102–111. doi: 10.1002/path.2152. [DOI] [PubMed] [Google Scholar]
- Swift S, Lorens J, Achacoso P, Nolan GP. Rapid production of retroviruses for efficient gene delivery to mammalian cells using 293T cell-based systems. Curr Protoc Immunol. 2001;Chapter 10(Unit 10):17C. doi: 10.1002/0471142735.im1017cs31. [DOI] [PubMed] [Google Scholar]
- Thorup AK, Reibel J, Schiodt M, Stenersen TC, Therkildsen MH, Carter WG, Dabelsteen E. Can alterations in integrin and laminin-5 expression be used as markers of malignancy? Apmis. 1998;106:1170–1180. doi: 10.1111/j.1699-0463.1998.tb00274.x. [DOI] [PubMed] [Google Scholar]
- Trusolino L, Bertotti A, Comoglio PM. A signaling adapter function for α6β4 integrin in the control of HGF-dependent invasive growth. Cell. 2001;107:643–654. doi: 10.1016/s0092-8674(01)00567-0. [DOI] [PubMed] [Google Scholar]
- Tzu J, Marinkovich MP. Bridging structure with function: structural, regulatory, and developmental role of laminins. Int J Biochem Cell Biol. 2008;40:199–214. doi: 10.1016/j.biocel.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang BY, Gil J, Kaufman D, Gan L, Kohtz DS, Burstein DE. P63 in pulmonary epithelium, pulmonary squamous neoplasms, and other pulmonary tumors. Hum Pathol. 2002;33:921–926. doi: 10.1053/hupa.2002.126878. [DOI] [PubMed] [Google Scholar]
- Wei S, Sedivy JM. Expression of catalytically active telomerase does not prevent premature senescence caused by overexpression of oncogenic Ha-Ras in normal human fibroblasts. Cancer Res. 1999;59:1539–1543. [PubMed] [Google Scholar]
- Weinstein MH, Signoretti S, Loda M. Diagnostic utility of immunohistochemical staining for p63, a sensitive marker of prostatic basal cells. Mod Pathol. 2002;15:1302–1308. doi: 10.1097/01.MP.0000038460.95912.6E. [DOI] [PubMed] [Google Scholar]
- Wille JJ, Jr, Pittelkow MR, Shipley GD, Scott RE. Integrated control of growth and differentiation of normal human prokeratinocytes cultured in serum-free medium: clonal analyses, growth kinetics, and cell cycle studies. J Cell Physiol. 1984;121:31–44. doi: 10.1002/jcp.1041210106. [DOI] [PubMed] [Google Scholar]
- Wilson SE, He YG, Weng J, Zieske JD, Jester JV, Schultz GS. Effect of epidermal growth factor, hepatocyte growth factor, and keratinocyte growth factor, on proliferation, motility and differentiation of human corneal epithelial cells. Exp Eye Res. 1994;59:665–678. doi: 10.1006/exer.1994.1152. [DOI] [PubMed] [Google Scholar]
- Yang A, Kaghad M, Wang Y, Gillett E, Fleming MD, Dotsch V, Andrews NC, Caput D, McKeon F. p63, a p53 homolog at 3q27–29, encodes multiple products with transactivating, death-inducing, and dominant-negative activities. Mol Cell. 1998;2:305–316. doi: 10.1016/s1097-2765(00)80275-0. [DOI] [PubMed] [Google Scholar]
- Zapatka M, Zboralski D, Radacz Y, Bockmann M, Arnold C, Schoneck A, Hoppe S, Tannapfel A, Schmiegel W, Simon-Assmann P, Schwarte-Waldhoff I. Basement membrane component laminin-5 is a target of the tumor suppressor Smad4. Oncogene. 2007;26:1417–1427. doi: 10.1038/sj.onc.1209918. [DOI] [PubMed] [Google Scholar]