Abstract
Toll like receptors (TLRs) activate multiple steps in inflammatory reactions in innate immune responses. They also activate signals that are critically involved in the initiation of adaptive immune responses. Many tumorigenic chemicals have been associated with endotoxin hypersensitivity mediated through TLR4. To determine the role of TLR4 in cutaneous skin carcinogenesis, we treated TLR4 deficient C3H/HeJ mice and their TLR4 normal C3H/HeN mice with the carcinogenic polyaromatic hydrocarbon 7,12-dimethylbenz(a)anthracene (DMBA). TLR4 deficient C3H/HeJ mice developed more tumors relative to the TLR4 normal C3H/HeN mice. Both C3H/HeN and C3H/HeJ mice developed a T-cell-mediated immune response to topically applied DMBA. Interestingly, the cell-mediated immune response was mediated by interferon-γ in C3H/HeN mice and by IL-17 in C3H/HeJ mice. Moreover, C3H/HeN mice had elevated circulating levels of interferon-γ following topical application of DMBA whereas IL-17 was elevated in C3H/HeJ mice. The results of this study indicate that TLR4 plays an important role in the prevention of DMBA skin tumorigenesis and that this is associated with differences in the T-cell subtype activated. Efforts to divert the cell-mediated immune response from one that is IL-17 mediated to one that is interferon-γ mediated may prove to be beneficial in the prevention of DMBA-induced cutaneous tumors.
Keywords: Toll-like receptor 4 (TLR4); Skin Carcinogenesis; 7,12-dimethylbenz(a)anthracene (DMBA); Contact Hypersensitivity; Angiogenesis
INTRODUCTION
Toll like receptors (TLRs), a family of molecules that recognize conserved pathogen-associated molecular patterns (PAMPs), have emerged as a key component of the innate immune system. Their ligation leads to the induction of inflammatory reponses by transcriptional activation of cytokine, chemokine and adhesion molecule genes. Mammalian TLRs consist of at least 13 different membrane proteins collectively known as pattern recognition receptors (PRRs). Some of them are expressed at the cell surface, whereas others are located on the membrane of endocytic vesicles or other intracellular organelles. Individual TLRs differ in their ligand specificity, expression patterns and signal transduction pathways that they activate (1–3).
Recognition of endogenous ligands by TLRs is now thought to have an important role in the regulation of inflammation, both in infectious and non-infectious diseases (4). This is due in part to the fact that a subset of TLR-induced signals is critically involved in the control of adaptive immune responses (5). There is experimental evidence to suggest that, in some instances, neoplasms may also exploit TLR signaling pathways to advance cancer progression. It has been demonstrated that inflammatory processes promote (rather than initiate) carcinogenesis in a non-cell-autonomous fashion (6). At sites of inflammation, cytokines, chemokines and matrix degrading enzymes are released in a persistent unregulated manner predominantly by cells of the innate immune system and these molecules enhance angiogenesis, invasiveness and survival of pre-malignant and neoplastic cells (7).
Polyaromatic hydrocarbons (PAHs), such as 7,12-dimethylbenz(a)anthracene (DMBA), have been employed extensively to investigate the mechanisms by which chemicals cause cancer. While the immune mediated destruction of the malignant cells is well established, the role of the innate immune system in the development of epithelial malignancies and its potential for immunoprevention has received much less attention (8). The presence of TLRs on resident skin cells may assist in mounting a rapid and efficient host defense against early events in the development of these neoplasms. TLRs are expressed on epidermal cells (ECs), including keratinocytes (9,10), Langerhans cells (LCs) (11) and dermal mast cells (12). TLRs expressed on human and murine epidermal cells are functional and play an important role in innate immunity in the skin (11,13).
We have previously reported that application of DMBA to the skin of selected strains of mice results in the development of antigen specific allergic contact hypersensitivity and that mice in which such a response is present are resistant to tumor development when subjected to a chemical skin carcinogenesis protocol (14). This has lead to the hypothesis that in addition to the host immune response against chemically induced tumors, there are immunological influences that target the chemicals that cause the tumors. In other words, we have proposed that contact hypersensitivity to DMBA has a protective effect on skin carcinogenesis, by reducing the number of mutant cells that can go on to become tumors. Since TLRs have an influence on adaptive immune responses and many tumorigenic chemicals have been associated with endotoxin hypersensitivity mediated through TLR4, the purpose of this study was to determine whether TLR4 was involved in DMBA cutaneous carcinogenesis.
Materials & Methods
Animals and Reagents
Female C3H/HeN mice were purchased from Charles River Laboratories (Boston, MA). Female C3H/HeJ mice were obtained from the Jackson Laboratory (Bar Harbor, Maine). Mice used for experiments were 6–8 weeks of age. All animal procedures were performed according to NIH guidelines under protocols approved by the Institute Animal Care and Use Committee of the University of Alabama at Birmingham.
Hybridoma line XMG1.2 (anti-IFN-γ) was from the ATCC (Manassas, VA) and antibody was purified from culture supernatants by affinity chromatography. Normal rat IgG was purchased from Rockland Immunochemicals (Gilbertsville, PA). Mouse rGM-CSF, anti-CD3e, Streptavidin PE-Cy5 and anti-CD31 (platelet/endothelial cell adhesion molecule) antibodies were purchased from Pharmingen (San Diego, CA) and monoclonal rat anti-mouse IL-17 antibody (TC11-18H10) was acquired from Southern Biotechnology (Birmingham, AL). 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), 7,12-dimethylbenz(a)anthracene (DMBA), and IL-4 were purchased from Sigma Chemical Co. (St. Louis, MO).
Sensitization and elicitation of CHS
The induction and elicitation of CHS in mice were carried out as described (15). Briefly, C3H/HeN and C3H/HeJ mice were sensitized with 100μl of 0.1% DMBA (w/v in acetone) on their shaved and depilated abdomen on day 0. The mice were challenged on the ear five days later. CHS was measured daily for 5 days beginning 24 hours after challenge. To detect the roles of IL-17 and IFN-γ in the elicitation of CHS, mice were injected intraperitoneally with either anti- IL-17 antibody (200μg) or anti-IFN-γ antibody (400μg) on days +4 and +5 as described earlier (17). In order to assess the cytokine profile, mice were sensitized with DMBA (0.1% w/v in acetone) on day 0. On day +5 mice were sacrificed and lymph node cell suspensions were prepared by gentle pressure through a wire mesh screen. Cells were then washed and placed in 96-well culture plates at a concentration of 2 × 105 cells/200μl in complete medium. Prior to addition of cells, the plates were coated with anti-CD3 antibody as described previously (16). After an incubation period of 48 hrs, media were harvested and assayed for cytokine concentrations.
ELISA for cytokine production
Cytokine concentrations in culture media from bone marrow derived dendritic cells, skin and tumor lysates and serum were measured using cytokine-specific enzyme linked immunosorbent assays (ELISA), using commercial kits from Invitrogen (Carlsbad, CA) according to the manufacturer's instructions.
Skin tumorigenesis
We used a standard DMBA complete carcinogenesis protocol for this study. DMBA (400 nmol, w/v in acetone) was painted weekly on the shaved and depilated dorsal skin of C3H/HeN and C3H/HeJ mice (15 mice/panel). Mice were evaluated weekly for tumors. Only tumors that had attained a size of one mm or greater and were present for two weeks or longer were counted.
Generation of Bone marrow-derived dendritic cells (BMDC)
Bone marrow-derived DC were prepared from C3H/HeN and C3H/HeJ mice as described previously (18) with some modifications. Briefly, bone marrow cells were isolated from femurs and tibias. The cells were incubated in RPMI 1640 medium with a cocktail of antibodies against Iak, CD45R/B220, Lyt-2 and GK1.5 (2 μg/106 cells) on ice for 1 h and washed once with HBSS after lysis of RBCs. Different cellular populations were removed from suspension by antibody mediated depletion using sheep anti-rat IgG dynabeads according to the manufacturer's instructions. Cells were washed once with HBSS and cultured in 10% FCS RPMI 1640 medium supplemented with recombinant mouse GM-CSF (10 ng/ml) and IL-4 (10 ng/ml) in 6-well plates (5×105 cells/well). On day 6, half of the medium was replaced with fresh medium and cells were incubated in culture with Polymyxin B (10μg/ml) to prevent LPS contamination. After 24h of culture at 37°C in the presence of 5% CO2, culture media were collected and evaluated for cytokines.
Preparation of tissue lysates
The back skin samples were scraped of subcutaneous fat and washed with PBS. Both skin and tumors were collected from at least 4 mice and homogenized in ice-cold lysis buffer (50 mM Tris-HCl, 150 mM NaCl, 1 mM EGTA, 1 mM EDTA, 20 mM NaF, 100 mM Na3VO4, 0.5% NP-40, 1% Triton X-100, 1 mM PMSF, pH 7.4) with freshly added protease inhibitor cocktail (Protease Inhibitor Cocktail, Sigma). The homogenate was then centrifuged at 14,000g for 25 min at 4°C and the supernatant (total cell lysate) was collected, aliquoted and stored at −80°C. The protein content in the lysates was measured with a DC Bio-Rad assay according to the manufacturer's protocol.
Proliferation assay in tumor cells
The proliferative potential of tumor cells obtained from TLR deficient C3H/HeJ and C3H/HeN mice was assessed using the MTT assay as described previously (19). Tumor cells were cultured as described by Schwarz et al. (20) with some modifications. Briefly, tumor pieces of <2 mm3 size were seeded into culture plates and cultured in RPMI 1640 supplemented with 10% fetal bovine serum, 1% L-glutamine, 1% MEM nonessential amino acid, and 1% antibiotic/penicillin-streptomycin solution. When the cultures had reached ~80% confluence, the adherent cells were detached with 0.1% trypsin/0.05% EDTA (Life Technologies) and used for subsequent passage. Cells were maintained at 37°C in a humidified atmosphere containing 5% CO2. Cells were harvested and seeded in triplicate into 96-well plates at a density of 2 × 104 per well. At 0, 1, 2, or 3 days after seeding, MTT solution was added to each well followed by incubation for 2 hours at 37°C. After incubation, DMSO was added to each well. Spectrophotometric absorbance of each sample was measured at 540 nm using a microplate reader (Bio-Rad, Hercules, CA).
Assessment of expression of vascular endothelial cell antigen, CD31
Immunostaining for CD31 was done on frozen tumor sections from C3H/HeJ and C3H/HeN mice. Briefly, frozen sections (5 μm thick) were fixed in cold acetone and nonspecific binding sites were blocked by immersing the sections in Tris-HCl buffer containing 5% goat serum and bovine serum albumin (0.5% w/v). The sections were then incubated with monoclonal antibodies specific for CD31 for 1 h. Antibody binding was detected by subsequent incubation of sections with streptavidin-phycoerythrin-Cy5 secondary antibody for 1 h. After washing, the sections were counterstained with Hoechst 33342, which stains nuclei. The intensity of the staining was evaluated using a microscope equipped for immunofluorescence analysis.
Statistical analysis
The differences between experimental groups for CHS and cytokine ELISAs were analyzed using the Student's t-test. The Fisher exact test was employed for analysis of the tumors per group and the percentage of mice with tumors. In all cases, a p<0.05 was considered significant.
Results
Contact hypersensitivity in TLR4 deficient mice is mediated mainly by IL-17
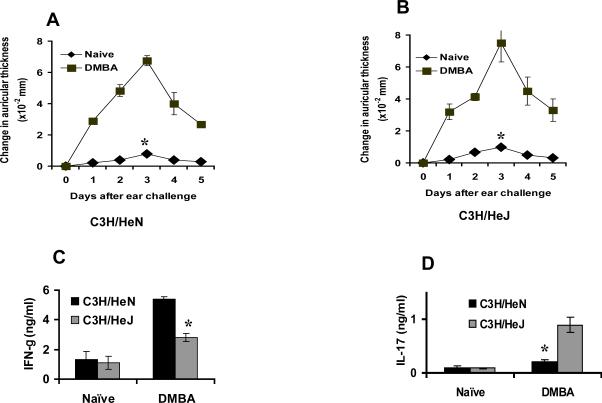
Topical application of the carcinogenic polyaromatic hydrocarbon dimethylbenz(a)anthracene (DMBA) results in the development of antigen specific contact hypersensitivity (15). To determine whether TLR4 influences contact hypersensitivity to DMBA, panels of C3H/HeN and C3H/HeJ mice were sensitized to that agent and the contact hypersensitivity response in the two strains was compared. Both strains developed contact hypersensitivity to DMBA and the magnitude of the ear swelling response in the two strains was comparable (p>0.05) (Fig. 1A&B).
Figure 1.
DMBA contact hypersensitivity (CHS) and cytokine profile in TLR4 deficient C3H/HeJ and C3H/HeN mice (A and B, respectively). C3H/HeN and C3H/HeJ mice were sensitized with DMBA and ear challenged five days later as described in the Methods section. The magnitude of CHS response was comparable in both groups of mice (p>0.05). (C) The draining lymph nodes were harvested 5 days after sensitization and lymph node cell suspension from C3H/HeJ and C3H/HeN mice was prepared as described in the Methods section. Results are the mean ± SEM with 3 mice per group and each experiment was repeated twice. Cells were then stimulated with anti-CD3 antibody. Cytokine levels were measured by ELISA. Lymph node cells from DMBA sensitized C3H/HeN mice secreted significantly higher levels (*p<0.05) of IFN-γ (C) whereas C3H/HeJ mice secreted significantly higher levels (*p<0.05) of IL-17 (D). Results are the mean ± SD with 3 mice per group and each experiment was repeated twice.
Effector T-cells for contact hypersensitivity to other haptens have been shown to produce IFN-γ and IL-17 (16, 17). To determine the influence of TLR4 on the cytokine pattern of the effector T-cells, DMBA sensitized lymph node cells were isolated, placed in culture in anti-CD3 coated plates. Media were removed 48 hours later and were examined for secreted IFN-γ and IL-17 by ELISA. Cells from C3H/HeN sensitized mice produced significantly greater amounts of IFN-γ (p<0.05) than C3H/HeJ mice (Fig.1C). In contrast, the amount of secreted IL-17 produced by lymph node cells from DMBA sensitized C3H/HeN mice was not significantly greater than that produced by cells from naïve mice. In contrast there was a marked increase in IL-17 (p<0.05) secretion by lymph node cells from DMBA sensitized C3H/HeJ mice (Fig.1D). These results indicated that inactivation of TLR4 shifts that balance from IFN-γ producing cells to those that produce IL-17.
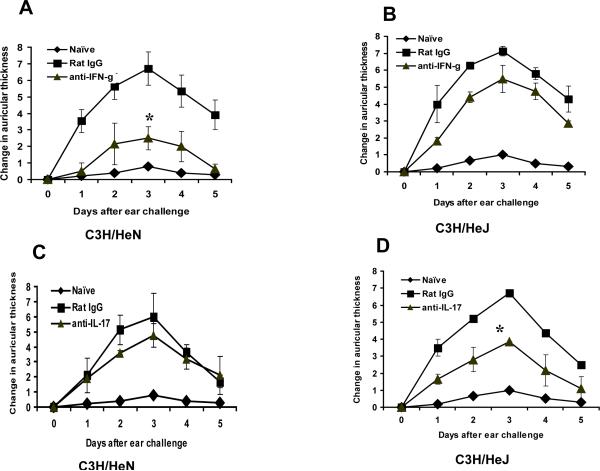
To examine the importance of IL-17 or IFN-γ in vivo in DMBA contact hypersensitivity, C3H/HeN and C3H/HeJ mice were sensitized to DMBA and were then treated with either anti-IL-17, anti-IFN-γ antibodies or rat IgG (as a control) prior to challenge. The CHS response was significantly inhibited in C3H/HeN mice (p<0.05) when treated with anti-IFN-γ antibody (Fig. 2A). In contrast, treatment of C3H/HeJ mice, which are deficient in TLR4, with neutralizing anti-IFN-γ antibody (400 μg/mouse) did not show a significant inhibitory effect (Fig. 2B). On the other hand, treatment with anti-IL-17 (200 μg/mouse) had no effect on DMBA contact hypersensitivity in C3H/HeN mice (Fig. 2C) whereas, in C3H/HeJ mice the CHS response was significantly inhibited (p<0.05) compared to controls treated with normal rat IgG at the same dose (Fig 2D)
Figure 2.
TLR4 deficient C3H/HeJ mice and TLR4 normal C3H/HeN mice mediate the CHS response to DMBA by different effector cells. Panels of mice (4 mice per panel) were sensitized to DMBA after treatment with either anti-IFN-γ or anti-IL-17 antibodies and were then ear challenged five days later as described in the Methods section. In vivo treatment with IFN-γ antibodies produced significantly greater inhibition of CHS in C3H/HeN mice (A) than in C3H/HeJ mice (B). In contrast, in vivo treatment with anti-IL-17 antibodies produced significantly greater inhibition of CHS in C3H/HeJ mice (D) as compared to C3H/HeN mice (C). Results are the mean ± SEM with 3 mice per group and each experiment was repeated twice with similar results.
TLR4 deficient mice are more susceptible to cutaneous DMBA carcinogenesis
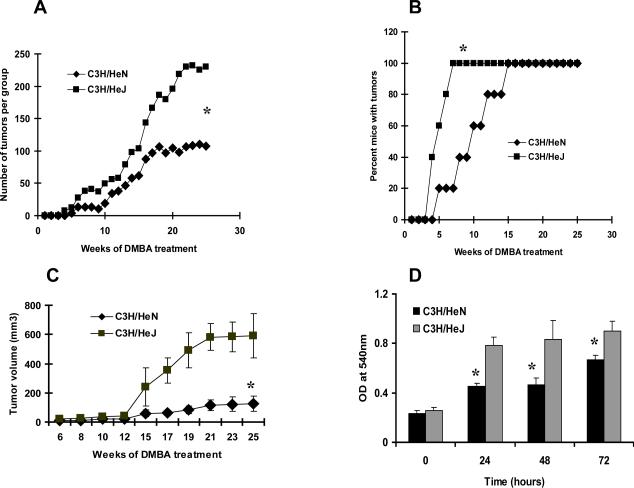
The role of TLR4 in DMBA cutaneous carcinogenesis was next investigated by comparing C3H/HeN and C3H/HeJ mice for tumor development. The two strains were subjected to a complete carcinogenesis protocol in which DMBA was applied weekly to the skin. The number of tumors was significantly greater (p<0.05) in C3H/HeJ mice compared to C3H/HeN mice (Fig. 3A). Similar results were obtained when the data were evaluated as the cumulative number of tumors and the number of tumors per tumor bearing mouse (data not shown). Although all animals in both strains eventually developed tumors with this protocol, they arose more rapidly in TLR4 deficient C3H/HeJ mice. For example, by week 7, 100% of C3H/HeJ mice had developed tumors whereas only 20 % C3H/HeN mice had tumors, and these differences were significant (p<0.05) (Fig. 3B). The tumors from C3H/HeJ mice grew progressively and showed a significant (p<0.05) increase in volume compared to tumors from C3H/HeN mice (Fig. 3C).
Figure 3.
TLR4 deficient C3H/HeJ mice are more susceptible to DMBA induced carcinogenesis than TLR normal C3H/HeN mice. Mice were subjected to a complete cutaneous carcinogenesis protocol (15 mice/panel) using DMBA as described in the Methods section. (A) The number of tumors per group was plotted as a function of the number of weeks on the test. There were significantly more tumors in C3H/HeJ mice (*p<0.05) than in C3H/HeN mice. (B) The percentage of mice with tumors was plotted as a function of the number of weeks of the test. By the seventh week, 100% of C3H/HeJ mice had tumors compared to C3H/HeN mice that had 20%. (C) Tumors from C3H/HeJ mice were significantly larger (*p<0.05) in volume than tumors from C3H/HeN mice. (D) Proliferative capacity of tumor cells from DMBA-induced skin tumors. Tumor cells were obtained from DMBA-induced tumors from C3H/HeN or C3H/HeJ mice and were subjected to an MTT assay immediately, and 1, 2 or 3 days after having been placed in culture as described in Methods section. Proliferative potential of tumor cells is expressed in terms of absorbance of each sample. The proliferative capacity was higher (*p<0.05) in tumor cells obtained from C3H/HeJ mice when compared with tumor cells derived from C3H/HeN mice. Results are the mean ± SD of triplicate cultures and each experiment was repeated three times.
Tumor cell lines from TLR4 deficient mice show an increase in proliferative capacity
To study the growth behavior of the DMBA-induced tumors, tumor specimens were obtained from both C3H/HeJ and C3H/HeN mice. Tumor pieces of <2 mm3 were seeded into culture plates and cultured as described in the Methods section. When the cultures had reached ~80% confluence, the adherent cells were detached with 0.1% trypsin/0.05% EDTA and used for subsequent passage. To determine their capacity to proliferate, cell lines were subjected to a MTT proliferation assay, which was done immediately or at 1, 2, and 3 days after seeding. In all samples tested, the proliferative capacity was higher (p<0.05) in cell lines obtained from DMBA treated C3H/HeJ mice compared with cell lines developed in C3H/HeN mice (Fig. 3D).
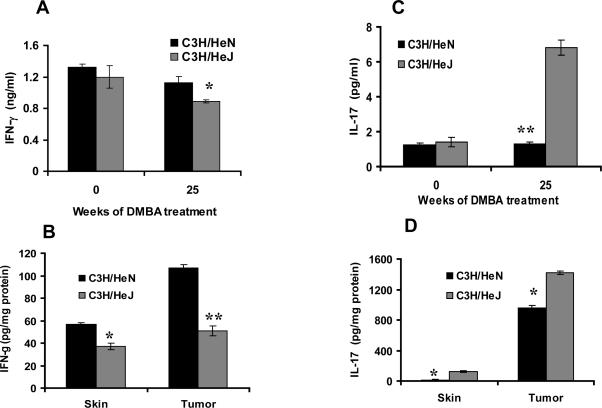
TLR4 deficient mice express higher levels of IL-17 and lower levels of IFN-γ after DMBA carcinogenesis
Since IFN-γ and IL-17 have an influence on development of the DMBA CHS response, we also assessed their role during DMBA-induced tumor development. Tumor, skin, and blood samples were collected from C3H/HeJ and C3H/HeN mice after 25 weeks on the DMBA skin tumorigenesis protocol. At 25 weeks, serum from these mice showed a slight, but significant increase in IFN-γ (p<0.05) in C3H/HeN mice compared to C3H/HeJ mice. Conversely, there was a significant decrease in IL-17 (p<0.001) production in C3H/HeN mice compared to C3H/HeJ mice (Fig 4A&C). These differences in cytokine levels were accentuated in tumor lysates. C3H/HeN mice showed a much greater increase in IFN-γ (p<0.001) and a greater reduction in IL-17 (p<0.05) than in C3H/HeJ mice (Fig 4B&D). The greater differences in tumor cytokine levels likely reflects the selective accumulation of T-cells producing IFN-γ and IL-17 in tumors from C3H/HeN and C3H/HeJ mice respectively.
Figure 4.
Tumor bearing TLR4 deficient C3H/HeJ mice produce less IFN-γ and more IL-17 than TLR4 normal C3H/HeN tumor bearing mice. Cytokines were measured in serum, tumor and skin lysates from mice after having been subjected to a DMBA cutaneous carcinogenesis protocol for 25 weeks. C3H/HeN mice showed higher levels of IFN-γ in their serum (A), skin (B) and tumor lysates (B) compared to C3H/HeJ mice. C3H/HeJ mice showed higher levels of IL-17 in their serum (C), skin (D) and tumor lysates (D) (*p<0.05) as compared to C3H/HeJ mice. Results are the mean ± SD of panels containing 4 mice per group. Each experiment was repeated twice with similar results. * = p<0.05; ** = p<0.001
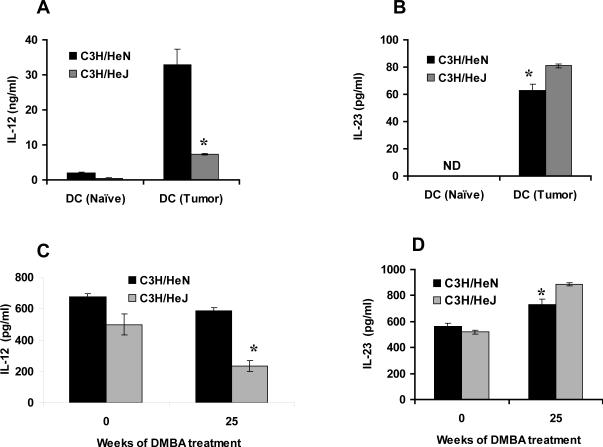
TLR4 deficient mice express higher levels of IL-23 and lower levels of IL-12 after DMBA carcinogenesis and are more prone to angiogenesis
Bone marrow derived dendritic cells from DMBA treated C3H/HeJ mice secreted smaller amounts of IL-12 (p<0.05) and more IL-23 (p<0.05) than C3H/HeN mice (Fig 5A&B). Similar patterns of circulating cytokines, IL-12 (p<0.05) and IL-23 (p<0.05) were found in the serum of DMBA treated C3H/HeN and C3H/HeJ mice (Fig. 5C&D).
Figure 5.
Tumor bearing TLR4 deficient C3H/HeJ mice secrete less IL-12 and more IL-23 than TLR4 normal C3H/HeN tumor bearing mice. Cytokines were measured in media from cultured BMDCs and serum from mice after having been subjected to a DMBA cutaneous carcinogenesis protocol for 25 weeks. (A) BMDCs from C3H/HeN mice secrete higher levels of IL-12 as compared to C3H/HeJ mice. (B) C3H/HeJ mice showed higher levels of IL-23 compared to C3H/HeN mice. (C) C3H/HeN mice showed higher levels of IL-12 in their serum compared to C3H/HeJ mice. (D) C3H/HeJ mice showed higher levels of IL-23 in their serum compared to C3H/HeJ mice. Results are the mean ± SD with 4 mice per group and each experiment was repeated three times. * = p<0.05)
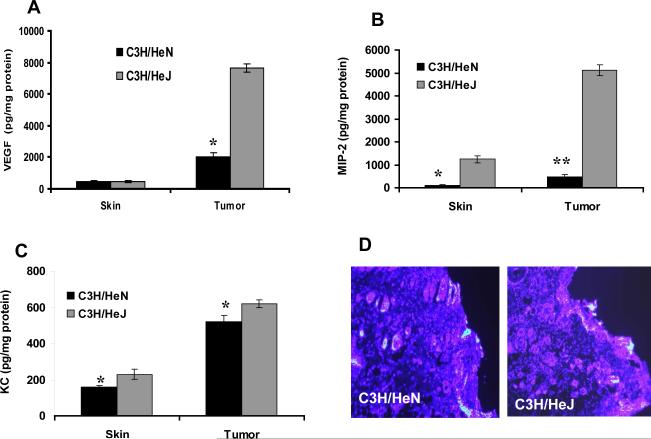
Since TLR4 deficient C3H/HeJ mice had higher levels of IL-17 in their serum and tumors and C3H/HeN mice had greater amounts of IL-12 and IFN-γ, we were interested in determining whether angiogenic growth factors vascular endothelial growth factor (VEGF), macrophage inflammatory protein-2 (MIP-2) and keratinocyte chemoattractant (KC) levels lead to an increase in tumor angiogenesis in C3H/HeJ mice than in C3H/HeN mice. We observed that this was the case; following treatment with DMBA, VEGF (p<0.05), MIP-2 (p<0.001), and KC (p<0.05) levels in tumor lysates were significantly higher in C3H/HeJ mice than in C3H/HeN mice (Fig. 6A, B&C).
Figure 6.
TLR4 deficiency promotes angiogenesis. Angiogenic factors VEGF, MIP-2 and KC were measured in tumor lysates after 25 weeks from mice placed on a DMBA cutaneous carcinogenesis protocol. TLR4 deficient C3H/HeJ mice showed significantly higher levels of (A) VEGF; (B) MIP-2 and (C) KC compared to TLR4 normal C3H/HeN mice. Results are the mean ± SD with 4 mice per group and each experiment was repeated three times. In panel D, the expression level of CD31 in DMBA-induced tumors was greater in C3H/HeJ mice compared to C3H/HeN mice (D). Samples of tumors were examined for CD31 fluorescence staining. Representative photomicrographs are shown from experiments conducted in tumor samples from at least five mice in each group. Identical patterns were observed in all of the tumors examined. CD31-positive staining is indicated by bright pink fluorescence (Bar = 50 μm). * = p<0.05; ** = p<0.001.
We next assessed the extent of neovascularization of the tumors by examination of the expression of CD31, which is known to contribute to the formation of new vasculature and is used as a biomarker of angiogenesis (21). The intensity of the CD31 immunofluorescent staining was increased in the DMBA-induced tumors of C3H/HeJ mice tumors as compared to C3H/HeN mice (Fig. 6D).
Discussion
Positional cloning of the locus responsible for LPS hyporesponsiveness in C3H/HeJ mice and generation of TLR4 knockout mice have demonstrated that TLR4 is essential for LPS signaling (22). In addition to activation by LPS signaling, TLR4 is also associated with signaling by many environmental chemicals, many of which are tumorigenic (23). Stimulation of TLRs by various ligands leads to signaling pathways that activate not only innate, but also adaptive immunity by antigen presenting cell (APC) dependent or independent mechanisms (5).
In previous studies from our laboratory, it has been shown that topical application of the carcinogenic polyaromatic hydrocarbon dimethylbenz(a)anthracene (DMBA) results in the development of antigen specific contact hypersensitivity (15). We had also proposed that the allergic contact hypersensitivity response to DMBA that occurs in some strains of mice, serves to protect those animals against the carcinogenic activities of that compound. This hypothesis is based in part on observations that there is an inverse relationship between cell-mediated immunity to DMBA and the number of tumors that develop in C3H/HeN and C3H.SW mice, MHC congenic mice (14). We have also observed that mice deficient in CD4+ T-cells are resistant to the development of DMBA-induced skin tumors than normal mice, whereas mice deficient in CD8+ T-cells are more likely to develop them (Yusuf et al. unpublished data). The results of these studies support that hypothesis with modifications. Although the magnitude of the contact hypersensitivity response in both C3H/HeN and C3H/HeJ mice was equivalent, C3H/HeJ were more susceptible to DMBA skin tumorigenesis while C3H/HeN were not. However, the DMBA contact hypersensitivity response in C3H/HeN mice was mediated primarily by IFN-γ producing T-cells and in C3H/HeJ mice by IL-17 producing T-cells.
Based on these findings, a more appropriate hypothesis for the relationship between T-cell immunity to DMBA and the initiation of DMBA skin carcinogenesis is that mice that develop a cell-mediated immune response to DMBA in which the effector cells are IFN-γ producing T-cells will be resistant to DMBA skin tumorigenesis, but those that have IL-17 effector T-cells or do not develop a contact hypersensitivity response at all will be susceptible to the carcinogenic activities of that agent.
Our results extend those observations by providing evidence that TLR4 determines the type of effector T-cell that is generated following topical application of hapten. An intact TLR4 signaling pathway results in IFN-γ-producing effector T-cells whereas a defect in the TLR4 signaling pathway leads to the generation of IL-17 producing effector T-cells. These two types of effector T-cells have distinct activities. Although both are effective at producing DMBA contact hypersensitivity, only the IFN-γ producing T-cells confer resistance to DMBA tumorigenesis. An obvious question is whether the controlling role that TLR4 has in determining the types of effector T-cells that develop applies to other contact allergens. Our preliminary studies indicate that it does. Contact hypersensitivity to DNFB also occurs in both C3H/HeN and C3H/HeJ mice, and is mediated primarily by IL-17 cells in C3H/HeJ mice but by cells that produce IFN-γ in C3H/HeN mice (data not shown). Effector T-cells for contact hypersensitivity to other haptens have been shown to produce IFN-γ and IL-17 (16, 17).
The process by which TLR4 leads to the preferential activation of IFN-γ-producing T-cells is apt to be a multi-step process. We observed that IL-12 levels were much greater (p<0.05) in serum of C3H/HeN than in C3H/HeJ mice. IL-12 has been shown to encourage the development of IFN-γ T-cells. On the other hand, IL-23 is required for IL-17 producing T-cells (24, 29). Thus, TLR4-induced suppression of IL-23 and the unimpeded production of IL-12 is likely to be a step in this process. IL-17 has been shown to increase the growth of several different types of tumors (24–27). It has been proposed that this is due at least in part to its pro-angiogenic effects (24,26). On the other hand, IL-12 and IFN-γ, which were produced in greater amounts in C3H/HeN mice, have anti-angiogenic properties. IL-17 up-regulates elaboration of various proangiogenic factors and modulates production of KC, MIP-2, and VEGF by fibroblasts. IL-17 might be a potential contributor to the inflammatory angiogenesis via induction of proangiogenic factors by stromal fibroblasts (28).
If it is true that IFN-γ producing T-cells are important effector cells for DMBA contact hypersensitivity and those cells are involved in reducing the development of DMBA-induced tumors, then it may be possible to use this information to develop methods that augment the induction of IFN-γ-producing T-cells at the expense of IL-17 producing effector T-cells and regulatory T-cells that have a permissive role of chemical carcinogenesis.
Acknowledgments
Grant Support: NIH Grants P30 (CAE) AR050948, P30 AR050948, Veterans Administration Merit Review Award (CAE) 18-103-02 and a grant from the Department of Defense.
Abbreviations
- BMDC
bone marrow dendritic cells
- CHS
contact hypersensitivity
- DMBA
7,12-dimethylbenz(a)anthracene
- KC
keratinocyte chemoattractant
- LCs
Langerhans cells
- MIP-2
macrophage-inflammatory protein-2
- MTT
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- TLR4
toll-like receptor-4
- VEGF
vascular endothelial growth factor
References
- 1.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 2.Akira S. TLR signaling. Curr Top Microbiol Immunol. 2006;311:1–16. doi: 10.1007/3-540-32636-7_1. [DOI] [PubMed] [Google Scholar]
- 3.Trinchieri G, Sher A. Cooperation of Toll-like receptor signals in innate immune defence. Nat Rev Immunol. 2007;7:179–90. doi: 10.1038/nri2038. [DOI] [PubMed] [Google Scholar]
- 4.Rifkin IR, Leadbetter EA, Busconi L, et al. Toll-like receptors, endogenous ligands, and systemic autoimmune disease. Immunol Rev. 2005;204:27–42. doi: 10.1111/j.0105-2896.2005.00239.x. [DOI] [PubMed] [Google Scholar]
- 5.Iwasaki A, Medzhitov R. Toll like receptor control of the adaptive immune response. Nat Immunol. 2004;5:987–95. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- 6.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Killeen SD, Wang JH, Andrews EJ, et al. Exploitation of the Toll-like receptor system in cancer: a double edged sword? British J Cancer. 2006;95:247–52. doi: 10.1038/sj.bjc.6603275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Woods GM, Malley RC, Muller HK. The skin immune system and the challenge of tumour immunosurveillance. Eur J Dermatol. 2005;15:63–9. [PubMed] [Google Scholar]
- 9.Miller LS, Modlin R. Human keratinocyte Toll-like receptors promote distinct immune responses. J Invest Dermatol. 2007;127:262–3. doi: 10.1038/sj.jid.5700559. [DOI] [PubMed] [Google Scholar]
- 10.Lebre MC, Angelic MG, van der Aar L, et al. Human keratinocytes express functional Toll-like receptor 3, 4, 5, and 9. J Invest Dermatol. 2007;127:331–41. doi: 10.1038/sj.jid.5700530. [DOI] [PubMed] [Google Scholar]
- 11.Mitsui H, Watanabe T, Saeki H, et al. Differential function and expression of Toll-like receptors in langerhan cells: comparison with splenic dendritic cells. J Invest Dermatol. 2004;122:95–102. doi: 10.1046/j.0022-202X.2003.22116.x. [DOI] [PubMed] [Google Scholar]
- 12.Matsushima H, Yamada N, Matsue H, et al. TLR3-, TLR7-, and TLR9-mediated production of proinflammatory cytokines and chemokines from murine connective tissue type skin-derived mast cells but not from bone marrow-marrow derived mast cells. J Immunol. 2004;173:531–41. doi: 10.4049/jimmunol.173.1.531. [DOI] [PubMed] [Google Scholar]
- 13.Kawai K, Shimura H, Minagawa M, et al. Expression of functional Toll-like receptor 2 on human epidermal keratinocytes. J Dermatol Sci. 2002;30:185–94. doi: 10.1016/s0923-1811(02)00105-6. [DOI] [PubMed] [Google Scholar]
- 14.Yusuf N, Timares L, Seibert MD, et al. Acquired and innate immunity to polyaromatic hydrocarbons. Toxicol Appl Pharmacol. 2006 doi: 10.1016/j.taap.2006.12.009. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klemme JC, Mukhtar H, Elmets CA. Induction of contact hypersensitivity to dimethylbenz(a)anthracene and benzo(a)pyrene in C3H/HeN mice. Cancer Res. 1987;47:6074–8. [PubMed] [Google Scholar]
- 16.Xu H, DiIulio N, Fairchild R. T cell populations primed by hapten sensitization in contact sensitivity are distinguished by polarized patterns of cytokine production: interferon gamma-producing (Tc1) effector CD8+ T cells and interleukin (IL) 4/IL-10-producing (Th2) negative regulatory CD4+ T cells. J Exp Med. 1996;183:1001–12. doi: 10.1084/jem.183.3.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.He D, Wu L, Kim HK, et al. CD8+ IL-17-Producing T Cells Are Important in Effector Functions for the Elicitation of Contact Hypersensitivity Responses. J Immunol. 2006;177:6852–8. doi: 10.4049/jimmunol.177.10.6852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Inaba K, Inaba M, Romani N, et al. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992;176:1693–1702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 20.Maeda A, Schneider SW, Kojima M, et al. Enhanced photocarcinogenesis in interleukin-12-deficient mice. Cancer Res. 2006;66:2962–9. doi: 10.1158/0008-5472.CAN-05-3614. [DOI] [PubMed] [Google Scholar]
- 21.Risau W. Differentiation of endothelium. FASEB J. 1995;9:926–933. [PubMed] [Google Scholar]
- 22.Akira S, Sato S. Toll-like receptors and their signaling mechanisms. Scand J Infect Dis. 2003;35:555–62. doi: 10.1080/00365540310015683. [DOI] [PubMed] [Google Scholar]
- 23.Elmets CA, Athar M, Tubesing KA, et al. Susceptibility to the biological effects of polyaromatic hydrocarbons is influenced by genes of the major histocompatibility complex. Proc Natl Acad Sci U S A. 1998;95:14915–9. doi: 10.1073/pnas.95.25.14915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Numasaki M, Watanabe M, Suzuki T, et al. IL-17 Enhances the Net Angiogenic Activity and In Vivo Growth of Human Non-Small Cell Lung Cancer in SCID Mice through Promoting CXCR-2-Dependent Angiogenesis. J Immunol. 2005;175:6177–89. doi: 10.4049/jimmunol.175.9.6177. [DOI] [PubMed] [Google Scholar]
- 25.Honorati MC, Neri S, Cattini L, et al. IL-17 enhances the susceptibility of U-2 OS osteosarcoma cells to NK cell lysis. Clin Exp Immunol. 2003;133:344–9. doi: 10.1046/j.1365-2249.2003.02234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Numasaki M, Fukushi J, Ono M, et al. Interleukin-17 promotes angiogenesis and tumor growth. Blood. 2003;101:2620–7. doi: 10.1182/blood-2002-05-1461. [DOI] [PubMed] [Google Scholar]
- 27.Benchetrit F, Ciree A, Vives V, et al. Interleukin-17 inhibits tumor cell growth by means of a T-cell-dependent mechanism. Blood. 2002;99:2114–21. doi: 10.1182/blood.v99.6.2114. [DOI] [PubMed] [Google Scholar]
- 28.Numasaki M, Lotze MT, Sasaki H. Interleukin-17 augments tumor necrosis factor-alpha-induced elaboration of proangiogenic factors from fibroblasts. Immunol Lett. 2004;93:39–43. doi: 10.1016/j.imlet.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 29.Langowski JL, Zhang X, Wu L, et al. IL-23 promotes tumour incidence and growth. Nature. 2006;442:461–465. doi: 10.1038/nature04808. [DOI] [PubMed] [Google Scholar]