Abstract
BACKGROUND
Red blood cell (RBC) alloimmunization can be a serious complication of blood transfusion, but factors influencing the development of alloantibodies are only partially understood. Within FDA-approved time limits, RBCs are generally transfused without regard to length of storage. However, recent studies have raised concerns that RBCs stored for more than 14 days have altered biologic properties that may affect medical outcomes. To test the hypothesis that storage time alters RBC immunogenicity, we utilized a murine model of RBC storage and alloimmunization.
STUDY DESIGN AND METHODS
Blood from transgenic HOD donor mice, which express a model antigen (hen egg lysozyme [HEL]) specifically on RBCs, was filter leukoreduced and stored for 14 days under conditions similar to those used for human RBCs. Fresh or 14-day-stored RBCs were transfused into wild-type recipients. The stability of the HOD antigen and post-transfusion RBC survival were analyzed by flow cytometry. RBC alloimmunization was monitored by measuring circulating anti-HEL immunoglobulin levels.
RESULTS
Transfusion of 14-day-stored, leukoreduced HOD RBCs resulted in 10- to 100-fold higher levels of anti-HEL alloantibodies as detected by enzyme-linked immunosorbent assay than transfusion of freshly collected, leukoreduced RBCs. RBC expression of the HOD antigen was stable during storage.
CONCLUSIONS
These findings demonstrate that HOD murine RBCs become more immunogenic with storage and generate the rationale for clinical trials to test if the same phenomenon is observed in humans. Length of storage of RBCs may represent a previously unappreciated variable in whether or not a transfusion recipient becomes alloimmunized.
Alloantibody formation to foreign antigens on transfused red blood cells (RBCs) can be a serious development leading to adverse outcomes, including immediate and delayed hemolytic transfusion reactions as well as the inability to provide transfusion support due to difficulties in locating compatible RBC units.1 Thus, developing strategies to decrease rates of RBC alloimmunization is of medical importance. However, the rational development of such strategies requires detailed understanding of the biology of RBC alloimmunization. Only a fraction of patients become RBC alloimmunized, despite multiple transfusions with allogeneic RBCs.2–4 Genetics plays a role in this process, both in the degree of antigenic difference between donor(s) and recipient and also in background recipient immune response genes.5,6 However, environmental factors are also likely to be involved, because the same variable response that is seen in humans is also observed in age-matched, sex-matched, genetically identical mice.7
One potential variable that may regulate RBC alloimmunization is the storage conditions of the transfused RBCs. Anticoagulant preservative solutions allow storage of human RBCs for up to 42 days.1 Recent studies have raised concerns that RBCs stored for more than 14 days have altered biologic properties that may affect medical outcomes.8,9 In this context, we hypothesized that RBC alloimmunization is regulated by biologic changes in RBC units that accumulate as a function of storage time. Currently, within the approved 42-day time frame, most RBC units are transfused without regard to length of storage.
It is challenging to isolate factors in humans that regulate RBC alloimmunization by juxtaposing alloimmunized versus nonalloimmunized transfusion recipients, due to the large number of simultaneous independent variables, including antigenic differences between donor and recipient, recipient HLA type, dose and duration of antigen exposure, and clinical condition of the recipient at the time of transfusion.5,6,10–12 Additionally, the inflammatory status of the recipient at the time of transfusion may further complicate such studies.7,13,14 Although potentially variant from human biology, animal models circumvent the above difficulties by allowing for the independent isolation of variables.
Herein, we utilize a murine model of RBC alloimmunization to test the hypothesis that RBC immunogenicity is altered by storage in vitro. We previously optimized conditions for storing murine RBCs to closely mimic those in human blood banking15 and use those conditions in the present studies. Through the use of a tractable animal model and isolation of a single variable, we now report that RBCs become progressively more immunogenic as a function of storage time, with a 10- to 100-fold increase in immunogenicity after 14 days of storage.
MATERIALS AND METHODS
Mice
C57BL/6 mice were purchased from The Jackson Laboratory (Bar Harbor, ME); HOD and FVB mice were bred by the Emory University Department of Animal Resources. HOD mice have RBC-specific expression of the transgenic model antigen hen egg lysozyme (HEL) fused to a multipass human Duffy antigen (Fyb).16,17 Recipient mice were 8- to 12-week-old females, and all protocols were approved by Emory University Institutional Animal Care and Use Committee.
Murine blood collection, storage, and transfusion
Blood collection, leukoreduction, storage, and transfusion were performed as described.15 Briefly, HOD blood was collected into CPDA-1 by retro-orbital bleeding or cardiac puncture. The CPDA-1 was obtained directly from di(2-ethylhexyl)phthalate-polyvinyl chloride human blood storage bags, and a final CPDA-1 concentration of 14% was used. Blood was leukoreduced through a neonatal leukoreduction filter (Pall Biomedical Products Co., East Hills, NY). Efficiency of leukoreduction was monitored for each unit by propidium iodide (PI) staining as previously described.7 Blood was centrifuged for 10 minutes at 324 ×g, adjusted to a hematocrit (Hct) level of 75%, and stored in 500-μL Eppendorf tubes at 4°C for 14 days.
Recipients received 75 μL of RBCs in a total volume of 500 μL of phosphate-buffered saline via the lateral tail vein. At the indicated time points, peripheral blood (3 μL) was obtained by retro-orbital bleed for determination of posttransfusion RBC survival by flow cytometry.
Flow cytometry
The integrity of the HOD protein on stored RBCs and post-transfusion survival were monitored by flow cytometry. Before transfusion and at 10 minutes, 30 minutes, 2 hours, and 24 hours posttransfusion, the presence of HEL and Duffy epitopes was measured by staining with polyclonal anti-HEL antisera or monoclonal anti-Fy3 (MIMA 29 antibody, a gift from M. Reid and G. Halverson), under conditions previously described.17 Goat anti-mouse immunoglobulins conjugated to allophycocyanin (Becton-Dickinson, San Jose, CA) was used as a secondary antibody. Fresh HOD RBCs and wild-type FVB mice were utilized as positive and negative controls, respectively. Twenty-four–hour posttransfusion survival was determined by the percentage of HOD-positive RBCs at 24 hours divided by the estimated percentage at Time 0 (determined by logistic regression analysis using Prism, GraphPad Software, San Diego, CA).
Detection of IgG anti-HEL
Enzyme-linked immunosorbent assays (ELISAs) and flow cytometry–based crossmatches for anti-HEL IgG were performed 2 weeks posttransfusion, as described.7 Briefly, wells were coated in triplicate with HEL or borate-buffered saline, and immune sera was plated at dilutions of 1:50 to 1:500,000. The secondary antibody was horseradish peroxidase–conjugated goat anti-mouse IgG Fcγ fragment specific (Jackson ImmunoResearch, West Grove, PA). Absorbance of the converted horseradish peroxidase substrate was read at an optical density of 415 nm. For crossmatching, sera was used at a 1:5 dilution; HOD RBCs and control FVB RBCs were used at a concentration of 3%.
Statistical analysis
Two-way analysis of variance with a Bonferroni posttest was performed on ELISA data, utilizing computer software (Prism, GraphPad Software). A significant result was determined by a p value of 0.05 or less.
RESULTS
HOD RBCs become more immunogenic during storage
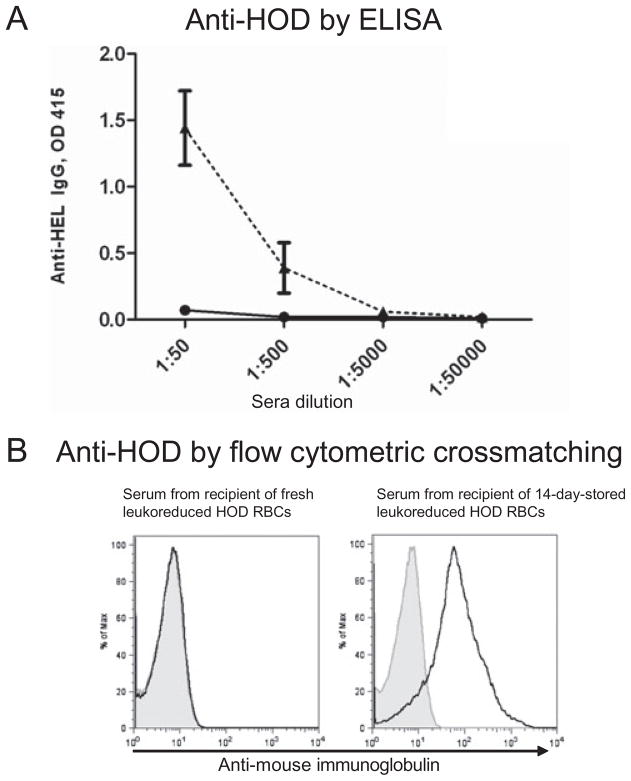
To determine the effect of RBC storage on alloimmunogenicity, HOD RBCs were stored under conditions that mimic human RBC storage;15 HOD RBCs were collected in a final CPDA-1 concentration of 14%, filter leukoreduced, Hct adjusted to 75%, and stored at 4°C in plastic tubes. After 14 days, the stored RBCs were transfused into C57BL/6 recipients; fresh HOD RBCs collected under the same conditions were transfused into parallel recipients. Two weeks after transfusion, alloantibody responses were determined by anti-HEL ELISA. In five of five independent experiments (total n = 52 mice [five to six mice per group per experiment]), transfusion of 14-day-stored HOD RBCs led to 10- to 100-fold higher levels of anti-HEL compared to transfusion of fresh HOD RBCs (Fig. 1A shows a representative experiment). Significance was reached in four of five experiments, with a p value of less than 0.05 comparing the alloimmunogenicity of stored versus fresh HOD RBCs. A low but detectable anti-HEL response was observed in 25 of 27 recipients of fresh HOD RBCs, compared with 25 of 25 recipients of stored RBCs (with a response defined as exceeding 2 standard deviations [SD] of historic naive controls).
Fig. 1.
HOD RBCs stored for 14 days result in higher levels of alloantibodies after transfusion. HOD RBCs were filter leukoreduced and transfused fresh or stored for 14 days and then transfused. Alloantibody response was assessed 14 days after transfusion. (A) Anti-HEL response as determined by ELISA in a representative experiment with five mice per group, transfused with fresh leukoreduced HOD RBCs (–●–) or 14-day-stored leukoreduced HOD RBCs (--▲--); mean and SD are shown with sera dilutions of 1:50–1:500,000. This experiment was repeated five times (five to six mice/group/experiment) with similar results. (B) Anti-HOD response as determined by flow cytometric crossmatch. Representative flow histograms are shown, with serum at a 1:5 dilution from a recipient of fresh HOD RBCs and from a recipient of 14-day-stored HOD RBCs crossmatched with control FVB RBCs (shaded) or HOD RBCs (solid).
Although ELISA provides a highly sensitive and quantitative measure of anti-HEL levels, the target antigen in the ELISA may differ slightly from the immunogen (i.e., native HEL is used in the ELISA); moreover, there are additional epitopes on the HOD antigen other than HEL. To control for these differences, flow cytometric–based crossmatching was also performed using HOD RBCs as the antigen source. Similar to the ELISA findings, the flow-based crossmatch showed increased anti-HOD RBC antibodies in recipients transfused with stored compared to fresh HOD RBCs (Fig. 1B). By this less-sensitive technique, 2 of 27 recipients of fresh HOD RBCs had a detectable anti-HOD response, compared with 23 of 25 recipients of 14-day-stored HOD RBCs (with a response defined as a mean fluorescence intensity of sera crossmatched with HOD RBCs exceeding 2 SDs of the mean per experiment of the mean fluorescence intensities of sera crossmatched with control FVB RBCs).
Pretransfusion evaluation of HOD RBCs
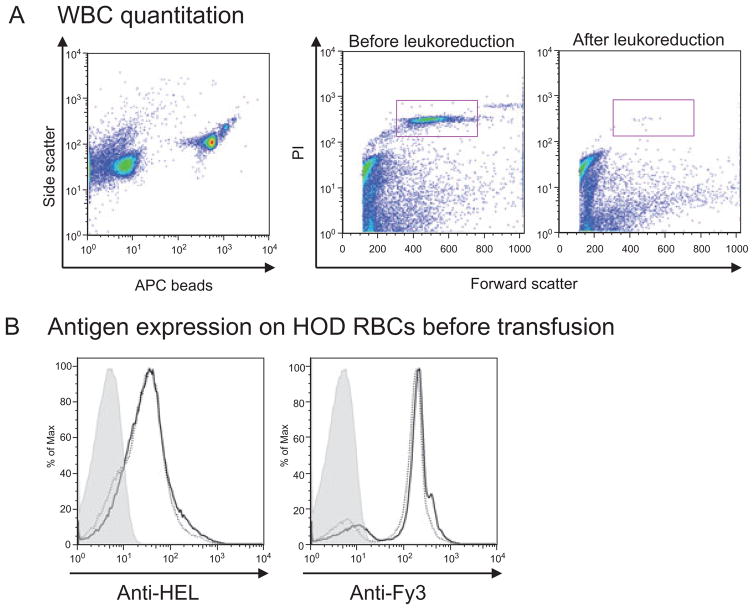
Before transfusion, units of RBCs were evaluated for residual white blood cells (WBCs), bacterial contamination, and HOD antigen integrity. Filter leukoreduction resulted in a 3 log or more decrease in WBCs in each case, as determined by PI staining (Fig. 2A). All transfused units tested for bacteria by direct Gram stain were negative. Additionally, no bacteria were detected by thiol glycolate culture on the transfused units tested. To increase sensitivity as much as possible, some RBC units were cultured past the time the transfusion was performed. A single RBC unit cultured after 21 days of storage grew a Gram-positive rod; all remaining units were negative.
Fig. 2.
Analysis of HOD RBCs. (A) After filter leukoreduction, WBCs were enumerated using PI and Trucount beads; a 3-log or greater WBC reduction seen. (B) Before transfusion, HEL and Fy3 expression were evaluated by flow cytometry, using polyclonal sera from HEL-immunized mice and the MIMA 29 anti-Fy3 monoclonal antibody, respectively, with control FVB RBCs (shaded), fresh HOD RBCs (solid), and 14-day-stored HOD RBCs (dotted). This experiment was repeated five times, with similar results.
To test the stability of the HOD antigen during storage, HOD RBCs were stained with antibodies to either HEL or to the Duffy anchor present in the HOD antigen. HEL and Fy3 antigen expression was similar on stored and freshly collected blood in four of the five experiments (Fig. 2B shows a representative experiment). In a single experiment, a small population of RBCs had decreased HEL expression on Storage Day 14 (data not shown); the significance of this negative population is unclear, and the increased alloimmune response induced by transfusion of these RBCs was similar to those in other experiments.
Posttransfusion survival of HOD RBCs
To determine the survival in vivo of transfused HOD RBCs, mice were bled at 10 minutes, 30 minutes, 2 hours, and 24 hours posttransfusion. Transfused RBCs were visualized with antibodies specific for epitopes on the HOD protein (anti-HEL and anti-Fy3), and expression levels of both antigens were similar in all samples evaluated. Representative flow plots for anti-HEL are presented for control and transfused mice (Figs. 3A and 3B). Transfused RBCs were enumerated by the percentage of HOD RBCs to total RBCs at the indicated time points. Compared to fresh RBCs, which had a survival of 97.9% (95% confidence interval [CI], 95.4%–100%), 14-day-stored RBCs had a 24-hour survival of 43.9% (95% CI, 35.9%–51.9%); Fig. 3C shows a representative experiment.
Fig. 3.
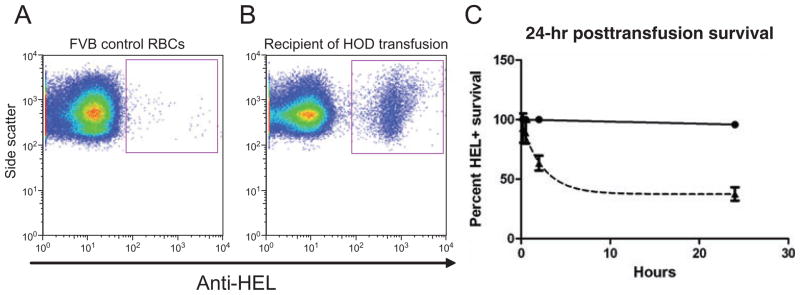
Posttransfusion HOD RBC survival. To determine 24-hour posttransfusion survival, recipients were bled at 10 minutes, 30 minutes, 2 hours, and 24 hours posttransfusion, and the percentage of transfused HEL-positive RBCs circulating in the recipient was assessed by flow cytometry. (A) HEL expression in control FVB RBCs. (B) Representative plot of HEL-positive RBCs in a recipient after transfusion. (C) Representative plot of posttransfusion HEL-positive RBC survival (mean and SD), with three mice per group. (▲) 14-day-stored leukoreduced HOD; (●) fresh leukoreduced HOD.
DISCUSSION
The data presented herein demonstrate that leukoreduced murine HOD RBCs, stored for 14 days under conditions similar but not identical to those used in human blood banking, induce a significantly stronger alloantibody response than freshly collected, leukoreduced HOD RBCs. This strong alloimmune response to stored RBCs was detectable by both anti-HEL ELISA and flow cytometric crossmatching against HOD RBCs. In contrast, a weak response to fresh RBCs was detected only by sensitive ELISA. In aggregate, these data introduce length of in vitro storage as a factor influencing alloimmunization to an erythroid-specific antigen in a reductionist murine model and suggest length of storage may be a previously unrecognized factor influencing alloimmunization to other RBC antigens.
The ability to extrapolate findings in the murine storage model to human biology is untested, and there are notable differences between murine and human RBCs as well as the murine and human immune systems.18 One relevant difference between murine and human RBCs is life span: murine RBCs have a 55- to 60-day lifespan versus 120 days in humans. It is for this reason that mouse RBCs were used after 14 days of storage, a time point that approximates the 35-day storage limit of human RBCs in CPDA-1 when corrected for the normal murine RBC life span. We recently reported that under the storage conditions utilized herein, 14-day-stored mouse RBCs (C57BL/6 background) have a 24-hour posttransfusion survival similar to 35-day-stored human RBCs.15
In the current studies, HOD RBCs (FVB background) had only a 44% 24-hour posttransfusion survival after 14 days of storage. It is currently unclear if this is due to differences between the FVB and C57BL/6 backgrounds and/or a function of expression of the HOD antigen on RBCs. Furthermore, the impact of posttransfusion survival on alloimmunogenicity remains to be determined. However, this finding is not inconsistent with what is observed in humans; that is, the posttransfusion survival of human RBCs is often well below the 75% guideline.19–21 Substantially decreased survival of stored human RBCs, due to donor factors, has been well documented; RBCs collected from certain healthy human donors consistently have 24-hour posttransfusion survivals as low as 40%.20 The Biomedical Excellence for Safer Transfusion (BEST) Collaborative evaluated 941 RBC recoveries and determined that the probability of passing the current FDA approval guidelines was poor.21 Luten and colleagues19 recently demonstrated that 6 of 10 units stored for 25 to 35 days had a posttransfusion survival less than 75%. Thus, while the 24-hour posttransfusion survival of HOD RBCs is well below the FDA guidelines, it is within the range of stored human RBCs and serves as a model to test the effects of biologic changes of stored RBCs on immunogenicity.
The mechanisms by which stored RBCs induce a stronger antibody response are unclear, and biochemical changes associated with stored murine blood have not been fully analyzed. However, there are several potential hypotheses consistent with the current paradigms of immunology that may explain these findings. First, antigenic dose is a well-known determinant in immune responses. The more rapidly RBCs are cleared, the larger the dose of antigen delivered to antigen-presenting cells (i.e., macrophages and to a lesser extent, dendritic cells22). At first consideration, these data may seem contradictory, given that mothers are less likely to develop anti-D after exposure to fetal D+ RBCs in the presence of ABO incompatibility leading to rapid clearance.23 However, the mechanisms of clearance of senescent RBCs are distinct from those of IgM-mediated complement lysis.
Another possible hypothesis is that storage of RBCs results in activation of inflammatory or innate immune pathways that enhance subsequent adaptive immunity.24,25 In fact, we and others have reported that the inflammatory status of transfusion recipients can regulate rates of RBC alloimmunization.7,13,26 It is unlikely that ligation of toll-like receptors is involved, because microbial contamination was undetectable in experiments that showed increased immunogenicity with stored RBCs. However, the receptor for advanced glycation end products (RAGE) is now understood to respond to damaged self-molecules in a fashion analogous to toll-like receptors.27 Thus, degeneration of RBC molecules, with subsequent activation of receptors that recognize damaged self (e.g., RAGE), may be involved. An alternative hypothesis is that the accumulation of cytokines may play a role. It is unlikely that such cytokines would come from WBCs, because filter leukoreduction substantially decreases WBC contamination (Fig. 2). However, as in human RBC units, platelets persist at some level in the murine storage conditions used in this study and are capable of releasing immune-regulatory molecules.28–30
In summary, the data presented herein demonstrate that storage of murine HOD RBCs in vitro significantly increases humoral immune response after transfusion. The generalizability of these findings to other murine RBC antigens, as well as to human transfusion biology, remains unclear. However, these studies provide proof of principle that RBC storage length is a factor influencing alloimmunization in one model system and thereby provide a rational basis to test the hypothesis that a similar effect is present in human transfusion therapy.
Acknowledgments
This work was supported in part by grants from the National Institutes of Health (K08HL092959), the American Society of Hematology, and the Emory Egleston Children’s Research Center (to JEH).
ABBREVIATION
- HEL
hen egg lysozyme
Footnotes
JEH and JCZ designed the research, performed the research, analyzed the data, and wrote the manuscript; EAH and SLS designed the research, analyzed the data, and wrote the manuscript; and CDH analyzed the data and wrote the manuscript.
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest relevant to the manuscript submitted to TRANSFUSION.
References
- 1.Hillyer CD, Silberstein LE, Ness PM, Anderson KC, Roback JD, editors. Blood banking and transfusion medicine. 2. Philadelphia (PA): Churchill Livingstone, Elsevier; 2007. [Google Scholar]
- 2.Heddle NM, Soutar RL, O’Hoski PL, Singer J, McBride JA, Ali MA, Kelton JG. A prospective study to determine the frequency and clinical significance of alloimmunization post-transfusion. Br J Haematol. 1995;91:1000–5. doi: 10.1111/j.1365-2141.1995.tb05425.x. [DOI] [PubMed] [Google Scholar]
- 3.Hoeltge GA, Domen RE, Rybicki LA, Schaffer PA. Multiple red cell transfusions and alloimmunization. Experience with 6996 antibodies detected in a total of 159,262 patients from 1985 to 1993. Arch Pathol Lab Med. 1995;119:42–5. [PubMed] [Google Scholar]
- 4.Seyfried H, Walewska I. Analysis of immune response to red blood cell antigens in multitransfused patients with different diseases. Mater Med Pol. 1990;22:21–5. [PubMed] [Google Scholar]
- 5.Noizat-Pirenne F, Tournamille C, Bierling P, Roudot-Thoraval F, LePennec PY, Rouger P, Ansart-Pirenne H. Relative immunogenicity of Fya and K antigens in a Caucasian population, based on HLA class II restriction analysis. Transfusion. 2006;46:1328–33. doi: 10.1111/j.1537-2995.2006.00900.x. [DOI] [PubMed] [Google Scholar]
- 6.Reviron D, Dettori I, Ferrera V, Legrand D, Touinssi M, Mercier P, deMicco P, Chiaroni J. HLA-DRB1 alleles and Jk(a) immunization. Transfusion. 2005;45:956–9. doi: 10.1111/j.1537-2995.2005.04366.x. [DOI] [PubMed] [Google Scholar]
- 7.Hendrickson JE, Desmarets M, Deshpande SS, Chadwick TE, Hillyer CD, Roback JD, Zimring JC. Recipient inflammation affects the frequency and magnitude of immunization to transfused red blood cells. Transfusion. 2006;46:1526–36. doi: 10.1111/j.1537-2995.2006.00946.x. [DOI] [PubMed] [Google Scholar]
- 8.Koch CG, Li L, Sessler DI, Figueroa P, Hoeltge GA, Mihaljevic T, Blackstone EH. Duration of red-cell storage and complications after cardiac surgery. N Engl J Med. 2008;358:1229–39. doi: 10.1056/NEJMoa070403. [DOI] [PubMed] [Google Scholar]
- 9.Tinmouth A, Fergusson D, Yee IC, Hebert PC ABLE Investigators; Canadian Critical Care Trials Group. Clinical consequences of red cell storage in the critically ill. Transfusion. 2006;46:2014–27. doi: 10.1111/j.1537-2995.2006.01026.x. [DOI] [PubMed] [Google Scholar]
- 10.Brantley SG, Ramsey G. Red cell alloimmunization in multitransfused HLA-typed patients. Transfusion. 1988;28:463–6. doi: 10.1046/j.1537-2995.1988.28588337338.x. [DOI] [PubMed] [Google Scholar]
- 11.Higgins JM, Sloan SR. Stochastic modeling of human RBC alloimmunization: evidence for a distinct population of immunologic responders. Blood. 2008;112:2546–53. doi: 10.1182/blood-2008-03-146415. [DOI] [PubMed] [Google Scholar]
- 12.Singer ST, Wu V, Mignacca R, Kuypers FA, Morel P, Vichinsky EP. Alloimmunization and erythrocyte autoimmunization in transfusion-dependent thalassemia patients of predominantly Asian descent. Blood. 2000;96:3369–73. [PubMed] [Google Scholar]
- 13.Hendrickson J, Roback JD, Hillyer CD, Easley KA, Zimring JC. Discrete toll like receptor agonists have differential effects on alloimmunization to red blood cells. Transfusion. 2008;48:1869–77. doi: 10.1111/j.1537-2995.2008.01801.x. [DOI] [PubMed] [Google Scholar]
- 14.Zimring JC, Hendrickson JE. The role of inflammation in alloimmunization to antigens on transfused red blood cells. Curr Opin Hematol. 2008;15:631–5. doi: 10.1097/MOH.0b013e328313695e. [DOI] [PubMed] [Google Scholar]
- 15.Gilson CR, Kraus T, Hod EA, Hendrickson JE, Spitalnik SL, Hillyer CD, Shaz BH, Zimring JC. A novel mouse model of red blood cells storage and post-transfusion in vivo survival. Transfusion. 2009;48:1456–553. doi: 10.1111/j.1537-2995.2009.02173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zimring JC, Cadwell CM, Hendrickson JE, Desmarets D, Neades R, Patel S, Peterson K. Generation of a novel murine model of RBC immunology: the HEL-OVA-DUFFY mouse. Transfusion. 2008;48:186A. [Google Scholar]
- 17.Desmarets M, Cadwell CM, Peterson K, Neades R, Zimring JC. Minor histocompatibility antigens on transfused leuko-reduced units of red blood cells induce bone marrow transplant rejection in a mouse model. Blood. 2009;114:2315–22. doi: 10.1182/blood-2009-04-214387. epub ahead of print Jun 12, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mestas J, Hughes CC. Of mice and not men: differences between mouse and human immunology. J Immunol. 2004;172:2731–8. doi: 10.4049/jimmunol.172.5.2731. [DOI] [PubMed] [Google Scholar]
- 19.Luten M, Roerdinkholder-Stoelwinder B, Schaap NP, de Grip WJ, Bos HJ, Bosman GJ. Survival of red blood cells after transfusion: a comparison between red cells concentrates of different storage periods. Transfusion. 2008;48:1478–85. doi: 10.1111/j.1537-2995.2008.01734.x. [DOI] [PubMed] [Google Scholar]
- 20.Mishler JM, Darley JH, Haworth C, Mollison PL. Viability of red cells stored in diminished concentration of citrate. Br J Haematol. 1979;43:63–7. doi: 10.1111/j.1365-2141.1979.tb03720.x. [DOI] [PubMed] [Google Scholar]
- 21.Dumont LJ, AuBuchon JP. Evaluation of proposed FDA criteria for the evaluation of radiolabeled red cell recovery trials. Transfusion. 2008;48:1053–60. doi: 10.1111/j.1537-2995.2008.01642.x. [DOI] [PubMed] [Google Scholar]
- 22.Hendrickson JE, Chadwick TE, Roback JD, Hillyer CD, Zimring JC. Inflammation enhances consumption and presentation of transfused RBC antigens by dendritic cells. Blood. 2007;110:2736–43. doi: 10.1182/blood-2007-03-083105. [DOI] [PubMed] [Google Scholar]
- 23.Bowman JM, Chown B, Lewis M, Pollock JM. Rh isoimmunization during pregnancy: antenatal prophylaxis. Can Med Assoc J. 1978;118:623–7. [PMC free article] [PubMed] [Google Scholar]
- 24.Mangalmurti NS, Xiong Z, Hulver M, Ranganathan M, Liu SH, Oriss T, Fitzpatrick M, Rubin M, Triulzi D, Choi A, Lee JS. Loss of red cell chemokine scavenging promotes transfusion-related lung inflammation. Blood. 2009;113:1158–66. doi: 10.1182/blood-2008-07-166264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McFaul S, Corely J, Mester C, Nath J. Packed blood cells stored in AS-5 become proinflammatory during storage. Transfusion. 2009;49:1451–60. doi: 10.1111/j.1537-2995.2009.02158.x. epub ahead of print April 3, 2009. [DOI] [PubMed] [Google Scholar]
- 26.Yu J, Heck S, Yazdanbakhsh K. Prevention of red cell alloimmunization by CD25 regulatory T cells in mouse models. Am J Hematol. 2007;82:691–6. doi: 10.1002/ajh.20959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gebhardt C, Riehl A, Durchdewald M, Nemeth J, Furstenberger G, Muller-Decker K, Enk A, Arnold B, Bierhaus A, Nawroth PP, Hess J, Angel P. RAGE signaling sustains inflammation and promotes tumor development. J Exp Med. 2008;205:275–85. doi: 10.1084/jem.20070679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gleissner CA, von Hundelshausen P, Ley K. Platelet chemokines in vascular disease. Arterioscler Thromb Vasc Biol. 2008;28:1920–7. doi: 10.1161/ATVBAHA.108.169417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morrell CN, Sun H, Swaim AM, Baldwin WM., 3rd Platelets an inflammatory force in transplantation. Am J Transplant. 2007;7:2447–54. doi: 10.1111/j.1600-6143.2007.01958.x. [DOI] [PubMed] [Google Scholar]
- 30.Sprague DL, Sowa JM, Elzey BD, Ratliff TL. The role of platelet CD154 in the modulation in adaptive immunity. Immunol Res. 2007;39:185–93. doi: 10.1007/s12026-007-0074-3. [DOI] [PubMed] [Google Scholar]