Abstract
BACKGROUND
Several differences exist between antigens on transfused red blood cells (RBCs) and other immunogens, including anatomical compartmentalization. Whereas antigens from microbial pathogens and solid organ transplants drain into local lymph nodes, circulating RBCs remain segregated in the peripheral circulation, where they are consumed by antigen-presenting cells (APCs) in the spleen and liver. Accordingly, it was hypothesized that the splenic APCs play a central role in primary alloimmunization to transfused RBCs.
STUDY DESIGN AND METHODS
Recipient mice were splenectomized and transfused with transgenic RBCs expressing the membrane-bound hen egg lysozyme (mHEL) model RBC antigen. In some experiments, mHEL-specific CD4+ T cells were adoptively transferred into recipient mice to allow investigation of helper T-cell responses. Unmanipulated or sham-splenectomized mice served as controls. Recombinant murine cytomegalovirus expressing mHEL (mHEL-MCMV) was used as a control non-RBC immunogen. Humoral responses were measured by mHEL-specific enzyme-linked immunosorbent assay and flow cytometric–based RBC cross-match.
RESULTS
Control animals synthesized detectable anti-HEL immunoglobulin (Ig)G after a single mHEL RBC transfusion. mHEL-specific CD4+ T cells underwent robust expansion, and adoptive transfer of CD4+ T cells resulted in a 1000-fold increase in anti-HEL IgG. In contrast, minimal anti-HEL IgG was detectable in splenectomized mice, mHEL-specific CD4+ T cells did not proliferate, and adoptive transfer did not increase anti-HEL IgG. However, anti-HEL IgG response after exposure to mHEL-MCMV was equivalent in control and splenectomized mice.
DISCUSSION
Together, these findings illustrate the distinct properties of transfused RBCs as immunologic stimuli, with the spleen playing a critical role in primary RBC alloimmunization at the level of CD4+ T-cell activation.
There are substantial differences in immune responses to alloantigens on transfused red blood cells (RBCs) compared to other alloantigenic exposures (e.g., solid organ transplant). In particular, although exposure to non-RBC alloantigens frequently leads to an antibody response (e.g., anti-HLA), exposure to RBC alloantigens typically does not.1 With the exception of Rh(D), RBC antigens are considered weak antigenic stimuli, and less than 5% to 10% of chronically transfused patients make detectable RBC alloantibodies despite repeated exposure to hundreds of different foreign RBC antigens.2–4
There are several differences between antigens on transfused RBCs compared to other immunogens, including dose of antigen, duration of antigen exposure, and lack of an obvious source of inflammation. Transfused RBCs are anatomically sequestered in the vasculature and are consumed primarily by antigen-presenting cells (APCs) in the spleen and the liver, but not the lymph nodes.5 In contrast, alloantigens from transplanted solid tissues or antigens from microbial infections frequently drain into local lymph nodes. Because RBCs do not enter the lymphatics, we tested the hypothesis that the spleen plays a central role in primary RBC alloimmunization.
Herein, we report that splenectomized mice make very little antibody in response to transfused RBCs expressing a model RBC antigen (membrane-bound hen egg lysozyme [mHEL]). In contrast, splenectomized mice have normal anti-HEL IgG responses to infection with murine cytomegalovirus containing mHEL in its genome (mHEL-MCMV). We also demonstrate the essential role of the spleen in mHEL specific CD4+ T-cell activation and expansion. Taken together, these findings underscore the distinctive nature of RBCs as immunogens and advance the understanding of immune responses to transfused RBCs.
MATERIALS AND METHODS
Mice
C57BL/6 (H-2b), B10.BR (H-2k), and B6.PL-Thy1.1(H-2b) mice were purchased from the Jackson Laboratories (Bar Harbor, ME), and mHEL (H-2b), C57BL/6 × B10.BR (H-2b × H-2k), and 3A9 × B6.PL-Thy1.1 (H-2k × H-2b) were bred by the Emory University Department of Animal Resources. Recipient mice were used at 8 to 12 weeks of age, and all protocols were approved by Emory University Institutional Animal Care and Use Committee.
Manipulation of mice: surgical splenectomy and adoptive transfer
Splenectomies were carried out using standard murine surgical techniques; control mice either were unmanipulated or underwent sham operations. In adoptive transfer experiments, recipient mice were injected with 1.5 × 107 3A9 × B6.PL-Thy1.1 whole splenocytes (containing 1.5 × 106 CD4+ T cells) via lateral tail vein 24 hours before transfusion, as previously described.5 In experiments examining cell division, adoptively transferred CD4+ T cells were isolated via immunodensity separation using the Spin Sep system (Stem Cell Technologies, Vancouver, British Columbia, Canada) and labeled with 5 μmol/L CFSE (Invitrogen, Eugene, OR) before transfer.
Exposure of mice to foreign antigen: transfusion of blood and inoculation with CMV
Blood was collected, filter leukoreduced, and transfused as previously described.6 Briefly, mHEL blood was collected in anticoagulant citrate dextrose solution-A (ACD-A, BD Biosciences, Franklin Lakes, NJ), passed through a neonatal leukoreduction filter (Purecell, Pall Biomedical Products, East Hills, NY), and resuspended in phosphate-buffered saline (PBS). A total of 500 μL of a 20% solution of leukoreduced mHEL RBCs in PBS was transfused through the tail vein or injected intraperitoneally (IP). Mice were inoculated IP with 1 × 106 pfu of mHEL-MCMV.
Generation of mHEL-MCMV
The human cytomegalovirus IE1/IE2 promoter7 was cloned upstream of the mHEL cDNA (pON4029) and the resulting expression cassette was then cloned between two HpaI sites to replace the ie2 promoter of plasmid pON4007 to create pON4451. mHEL-MCMV was generated by cotransfection of RM4278 genomic DNA with EcoRI linearized pON4451 followed by limiting dilution for loss of β-galactosidase activity and, finally, triple plaque purification. Insertion of mHEL and overall integrity were confirmed by DNA blot hydribidization of isolated viral DNA restriction digests. Recombinant virus in which the sgg1 mutation of RM4278 had been repaired was selected and designated RM427+.
Detection of anti-HEL IgG humoral response
Enzyme-linked immunosorbent assays (ELISAs) and flow cytometry–based cross-match for anti-HEL IgG were performed 2 weeks after transfusion, as previously described.6 Sera samples were titrated from 1:50 to 1:100,000 for the ELISAs, with the secondary antibody being specific for murine IgG Fcγ and peroxidase conjugated (Jackson Immunoresearch Laboratory, West Grove, PA).
Sera samples at a 1:5 dilution were mixed with a 4% solution of mHEL or control C57BL/6 RBCs for the flow cytometric cross-match, with goat anti-mouse immunoglobulin conjugated to allophycocyanin being used as the secondary antibody (BD Pharmingen, San Diego, CA).
Isolation of spleen, liver, and lymph nodes
Spleen, liver, and lymph nodes were harvested on Days 1 to 5 after transfusion. Briefly, spleens and lymph nodes were processed in Hanks’ balanced salt solution (HBSS; Cellgro, Mediatech, Herdnon, VA) and RBCs were lysed with ammonium chloride (Sigma, St Louis, MO). In adoptive transfer experiments, CD4+ T cells from 3A9 × B6-PL.Thy1.1 spleens were enriched as described above.
Livers were processed in HBSS with 5% fetal bovine serum, incubated with collagenase (Worthington, Lakewood, NJ), and mechanically disrupted using the flat portion of a plunger from a 10-mL syringe. The cell suspension was passed through a 100-μm-pore-size cell strainer (Falcon, Franklin Lakes, NJ) and centrifuged at 873 × g for 10 minutes at 4°C. The pellet was resuspended in 44% Percoll, and a gradient was developed with 66% Percoll. White blood cells were isolated after centrifuging at 873 × g for 20 minutes at room temperature (without brake), and RBCs were lysed with ammonium chloride.
Flow cytometry
Allophycocyanin-conjugated anti-CD4 was purchased from BD Biosciences. Adoptively transferred CD4+ T cells from 3A9 × B6-PL.Thy1.1 donors were tracked utilizing allophycocyanin-conjugated anti-CD4 and anti-Thy1.1 (BD Biosciences), with division assessed by CFSE. One million events from spleen, liver, and lymph nodes were collected on a flow cytometer (FACSCalibur, BD Biosciences).
RESULTS
Splenectomy decreases immunization to an alloantigen (mHEL) on transfused RBCs
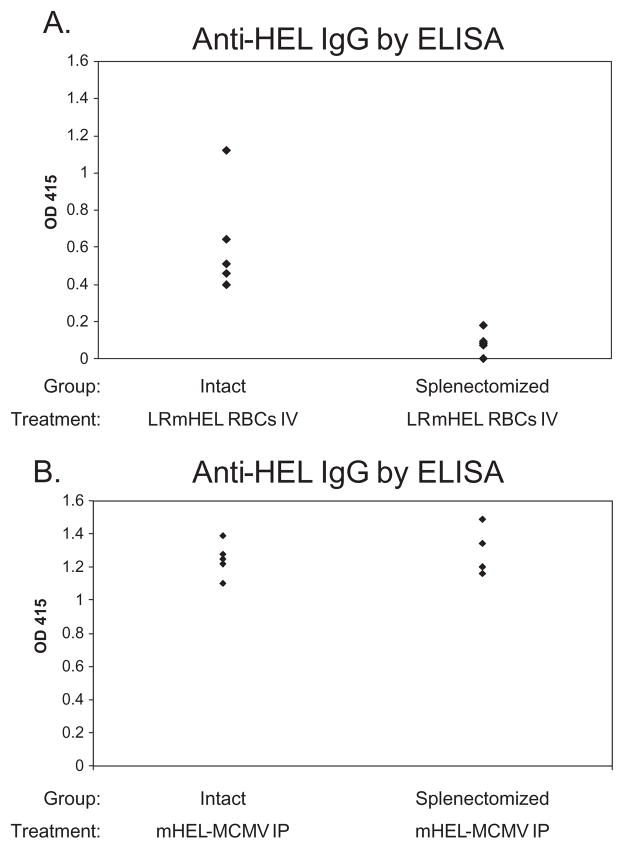
To assess the role of the spleen in primary alloimmunization to antigens on transfused RBCs, mHEL RBCs were transfused into intact or splenectomized mice. Analysis of a compilation of four experiments (38 mice total) demonstrated that splenectomized mice had substantially lower antibody responses than intact (not splenectomized) recipients. Figure 1A shows a representative experiment. The mean anti-HEL IgG response of intact mice was compared to the mean anti-HEL IgG response of splenectomized mice, with a fold difference reported. In aggregate, 19 of 19 splenectomized mice synthesized very low levels of anti-HEL IgG, while 19 intact (not splenectomized) recipients made sevenfold greater levels of anti-HEL IgG (95% confidence interval, 3.5–10.5; SD, 3.6). The low response in splenectomized mice was not due to trauma from surgery, as additional control mice that underwent sham surgery responded the same as unmanipulated animals (data not shown).
Fig. 1.
Splenectomy decreases immunization to an alloantigen on RBCs but not to the same antigen on a viral pathogen. Intact and splenectomized mice were transfused IV with 500 μL of a 20% solution of leukoreduced mHEL RBCs in PBS (A) or infected IP with 106 pfu of mHEL-MCMV (B). Anti-HEL IgG response was measured by ELISA 2 weeks later. Representative experiments with four to five mice/group are shown, with sera at a 1:50 dilution. These experiments have been repeated three to four times with similar results (38 mice total in [A], 25 mice total in [B]).
Splenectomy does not alter immunization to the same antigen (mHEL) on a viral pathogen (MCMV)
To test if the requirement for a spleen was a general property of humoral responses to HEL or a function of RBCs as an immunogen, intact and splenectomized mice were inoculated IP with 1 × 106 pfu of mHEL-MCMV. Two weeks after infection, anti-HEL IgG was assessed by ELISA. In a compilation of three experiments (25 mice total), splenectomized mice mounted an anti-HEL IgG response equivalent to intact mice; Fig. 1B shows a representative experiment.
Providing high frequencies of mHEL-specific CD4+ T cells does not compensate for the defect resulting from splenectomy
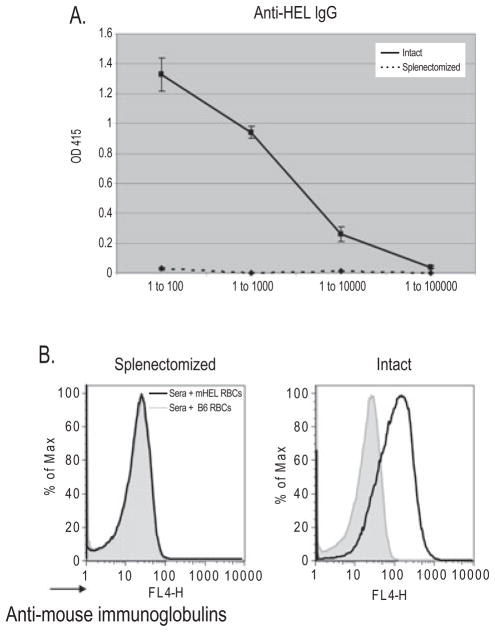
We have previously reported that mHEL-specific CD4+ T-cell help is rate limiting in alloimmunization to transfused leukoreduced mHEL RBCs. This raised the possibility that the splenic environment was not involved in antigen presentation per se, but that removal of the spleen simply reduced the levels of CD4+ T cells capable of recognizing RBC antigens. To test this possibility, we increased the precursor frequency of mHEL-specific CD4+ T cells using adoptive transfer from the 3A9 mouse, which expresses a transgenic T-cell receptor that recognizes a HEL-derived peptide presented by MHC II (I-Ak).9 C57BL/6 × B10.BR mice were either splenectomized, sham splenectomized, or unmanipulated. A total of 1.5 × 106 CD4+ T cells from 3A9 × B6.PL-Thy1.1 mice were adoptively transferred into recipient animals; 24 hours later these recipients were transfused with leukoreduced mHEL or control C57BL/6 RBCs. As previously described, the adoptive transfer of 3A9 CD4+ T cells substantially enhanced humoral immunity; intact mice and sham-splenectomized mice made a robust anti-HEL IgG response after transfusion of mHEL RBCs (Fig. 2A). No anti-HEL was detected in control mice transfused with wild-type C57BL/6 RBCs (data not shown). In stark contrast to intact mice, in a compilation of four experiments, 19 of 20 splenectomized mice failed to make detectable anti-HEL IgG by ELISA (Fig. 2A). In addition to ELISA, flow cytometric cross-matching was performed to detect anti-HEL immunoglobulins bound to mHEL RBCs (Fig. 2B). Although an anti-HEL response was detected by flow cytometric cross-matching in intact mice, anti-HEL was not detected in splenectomized mice.
Fig. 2.
Increasing the precursor frequency of mHEL-specific CD4+ T cells in splenectomized mice does not increase alloimmunization after RBC transfusion. (A) Intact (—) and splenectomized (···) mice were adoptively transferred with 1.5 × 106 mHEL-specific CD4+ T cells and transfused with leukoreduced mHEL RBCs. Anti-HEL IgG response was measured by ELISA 2 weeks later. A representative experiment with five mice/group is shown with sera titrated from 1:100 to 1:100,000; mean and SDs are depicted. This experiment has been performed four times (41 mice total) with similar results. (B) Flow cytometric cross-match of a representative mouse from each group, with sera at a dilution of 1 to 5, mixed with control C57BL/6 RBCs (gray line) and mHEL RBCs (black line).
The spleen is required for mHEL CD4+ T-cell expansion and division after mHEL RBC transfusion
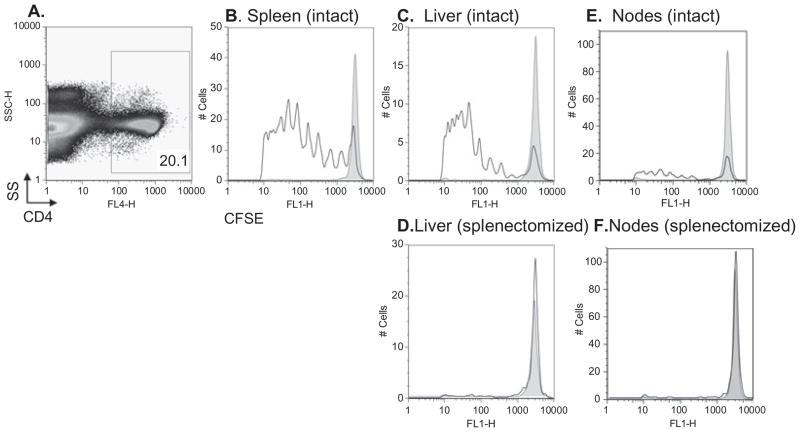
To test the hypothesis that the splenic microenvironment is required for CD4+ T-cell expansion in response to RBC transfusion, CSFE labeled 3A9 × B6.PL-Thy1.1 CD4+ T cells were adoptively transferred into splenectomized or intact mice followed by transfusion with leukoreduced mHEL RBCs. Flow cytometry was utilized to visualize the adoptively transferred cells in spleen (if present), liver, and lymph nodes, with division being assessed by CFSE dilution. Whereas robust division was observed in the spleen and liver of intact animals (Fig. 3B and 3C), division was noted in only a trace number of cells in the liver of splenectomized mice (Fig. 3D). Division but minimal expansion was noted in the lymph nodes of intact animals (Fig. 3E), whereas only minimal division was observed in the nodes of splenectomized mice (Fig. 3F).
Fig. 3.
Antigen-specific CD4+ T cells fail to undergo division in splenectomized mice despite exposure to their antigen on transfused RBCs. Intact and splenectomized recipients were adoptively transferred with 1.5 × 106 CFSE-labeled antigen-specific 3A9 × B6.PL-Thy1.1 CD4+ T cells, followed by transfusion with leukoreduced mHEL RBCs or no RBCs. Five days later division was assessed by gating on adoptively transferred CD4+ T cells (A = gate) and measuring CFSE dilution. The CFSE-positive gate was determined by gating on unlabeled adoptively transferred CD4+ T cells in control mice. Division was assessed by dilution of CFSE signal in the spleen, liver, or lymph nodes of an intact mouse (B, C, and E) or in the liver and lymph nodes of a splenectomized mouse (D and F). In all panels, gray shaded histograms represent control mice not transfused with mHEL RBCs. Staining from representative mice is shown; the y-axes represent cell number. This experiment was performed three times (15 mice total) with similar results.
The requirement for a spleen is not due to lack of RBC antigen access to peripheral lymphatics
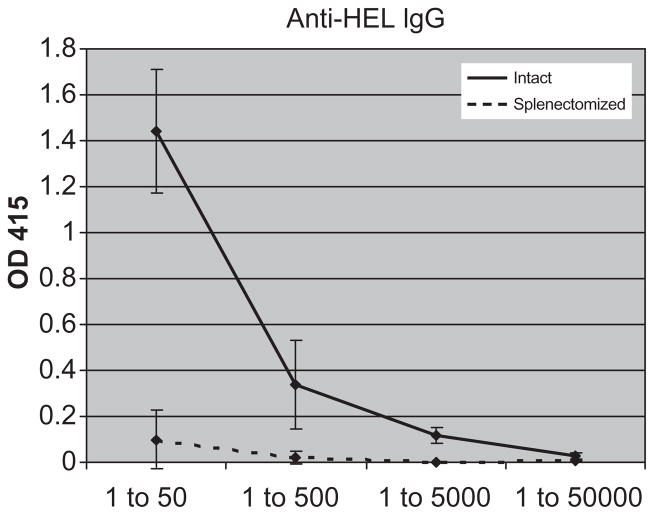
We hypothesized that the strong humoral response to mHEL-MCMV but not mHEL RBCs in splenectomized mice was because unlike mHEL-MCMV, transfused RBCs are sequestered to the peripheral circulation and do not have access to lymphatic tissues other than the spleen. To test this hypothesis, mHEL RBCs were injected IP into intact or splenectomized mice. As the cells in the peritoneum drain through peripheral lymphatics,10,11 this experiment tests the hypothesis by exposing RBC antigens to extrasplenic lymph nodes. As above, to compensate for any loss of antigen-specific CD4+ T cells by splenectomy, recipients were adoptively transferred with mHEL-specific CD4+ T cells 24 hours before mHEL injection. In a compilation of two experiments (20 mice total), 10 of 10 splenectomized mice synthesized very low levels of anti-HEL IgG. In comparison, 10 of 10 intact mice made a robust anti-HEL IgG response. Figure 4 shows a representative experiment.
Fig. 4.
The requirement for a spleen is not due to lack of RBC antigen access to peripheral lymphatics. Intact (—) and splenectomized (- - -) mice were adoptively transferred with 1.5 × 106 mHEL-specific CD4+ T cells, and injected IP with 500 μL of a 20% solution of leukoreduced mHEL RBCs in PBS. Anti-HEL IgG response was measured by ELISA 2 weeks later. A representative experiment with five mice/group is shown, with sera titrated from 1:50 to 1:50,000; mean and SDs are depicted. This experiment has been repeated twice, with similar results (20 mice total).
DISCUSSION
In the current report, we test the role of the spleen in primary alloimmunization to antigens on transfused RBCs. Compared to intact mice that made a modest anti-HEL IgG response after transfusion with mHEL RBCs, minimal anti-HEL IgG was observed in splenectomized recipients. This difference was not due to properties of HEL as an immunogen, as splenectomized and intact mice had equivalent responses to HEL delivered by a non-RBC vector (mHEL-MCMV). Furthermore, the minimal anti-HEL response in splenectomized mice after transfusion was not solely due to the inability of transfused RBCs to access lymphatic tissues, as splenectomized mice also made minimal anti-HEL IgG after IP injection of mHEL RBCs. These data underscore the distinct properties of RBCs as immunogens, compared to other means of exposure to foreign antigen (including microbial pathogens and solid organ transplants).12
RBCs differ from MCMV by the relative lack of an activator of innate immunity (danger signal).13 In a previous report, we demonstrated enhancement of RBC alloimmunization in recipients pretreated with the inflammatory agent poly(I:C) before transfusion.6 We hypothesized that the spleen plays a role in the mechanism by which poly (I:C) enhances mHEL RBC alloimmunization, as no enhancement of anti-HEL by poly(I:C) was observed in splenectomized mice. However, decreased anti-HEL responses were observed in control splenectomized mice not treated with poly(I:C).5 This observation served as the nidus for the current investigations.
Neither proliferation of CD4+ T cells specific for mHEL nor a significant anti-HEL IgG response was detected in splenectomized mice after transfusion of leukoreduced mHEL RBCs. In stark contrast, robust expansion of CD4+ T cells and synthesis of anti-HEL IgG were observed in intact mice. These data indicate that a spleen is necessary for CD4+ T-cell priming and proliferation. A spleen may also be required for priming of antigen-specific B cells. However, based on the current data, we cannot rule out the possibility that B cells are also activated in extrasplenic compartments but do not differentiate into plasma cells in splenectomized mice due to a lack of CD4+ T-cell help. Of interest, it has recently been reported that the presence of a spleen alters the composition of extrasplenic B cells, with the spleen being important for the survival of B-1a cells.14 Thus, we cannot rule out the possibility that depleted B-1a cells contribute to the lack of immune response in splenectomized recipients, particularly if CD4+ T-cell activation were to occur in the lymph nodes after IP injection of mHEL RBCs.
In the absence of a spleen, it is probable that the APCs in the liver consume the vast majority of RBCs after intravenous (IV) transfusion. Given that the liver has been described as an organ that can promote a tolerogenic state when provided with foreign antigen,15,16 it is possible that the conditions of hepatic mHEL presentation not only lead to a lack of immunity, but also to tolerance to the transfused antigen. Ongoing studies are investigating both patterns of consumption by liver APCs in splenectomized mice, as well as the tolerogenic potential of the liver in this setting.
It is currently not possible to assess whether our observations can be extended to RBC antigens in general, as to the best of our knowledge, there are currently no other models of RBC alloimmunization that contain the ability to measure antigen specific CD4+ T cells in vivo. Moreover, whether one can extrapolate the findings described herein to the human setting remains to be determined; observational reports of patients who have undergone either functional or surgical splenectomy have generated conflicting data regarding the role of a spleen in human RBC alloimmunization.17–19
In summary, the data presented herein demonstrate that the murine spleen plays a central role in primary humoral alloimmunization to transfused mHEL RBCs as well as to mHEL RBCs injected IP. One mechanism by which a spleen is required appears to involve initial activation of antigen-specific CD4+ T cells, as these cells do not proliferate in the liver or lymph nodes of splenectomized mice after transfusion of mHEL RBCs. Studies aimed at determining which splenic components are responsible for this initial CD4+ T-cell activation are ongoing.
Acknowledgments
This work was supported in part by grants from the National Institutes of Health (HL069769), the National Blood Foundation, and the American Society of Hematology (to JEH).
The authors would like to thank Yu-Chun Lin for his work on the construction and characterization of mHEL-MCMV.
ABBREVIATIONS
- APC(s)
antigen-presenting cell(s)
- mHEL
membrane-bound hen egg lysozyme
- mHEL-MCMV
murine cytomegalovirus containing mHEL
Footnotes
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.
References
- 1.Blood banking and transfusion medicine. 2. Philadelphia (PA): Churchill Livingstone, Elsevier; 2007. [Google Scholar]
- 2.Heddle NM, Soutar RL, O’Hoski PL, Singer J, McBride JA, Ali MA, Kelton JG. A prospective study to determine the frequency and clinical significance of alloimmunization post-transfusion. Br J Haematol. 1995;91:1000–5. doi: 10.1111/j.1365-2141.1995.tb05425.x. [DOI] [PubMed] [Google Scholar]
- 3.Hoeltge GA, Domen RE, Rybicki LA, Schaffer PA. Multiple red cell transfusions and alloimmunization. Experience with 6996 antibodies detected in a total of 159,262 patients from 1985 to 1993. Arch Pathol Lab Med. 1995;119:42–5. [PubMed] [Google Scholar]
- 4.Seyfried H, Walewska I. Analysis of immune response to red blood cell antigens in multitransfused patients with different diseases. Mat Med Pol. 1990;22:21–5. [PubMed] [Google Scholar]
- 5.Hendrickson JE, Chadwick TE, Roback JD, Hillyer CD, Zimring JC. Inflammation enhances consumption and presentation of transfused RBC antigens by dendritic cells. Blood. 2007;110:2736–43. doi: 10.1182/blood-2007-03-083105. [DOI] [PubMed] [Google Scholar]
- 6.Hendrickson JE, Desmarets M, Deshpande SS, Chadwick TE, Hillyer CD, Roback JD, Zimring JC. Recipient inflammation affects the frequency and magnitude of immunization to transfused red blood cells. Transfusion. 2006;46:1526–36. doi: 10.1111/j.1537-2995.2006.00946.x. [DOI] [PubMed] [Google Scholar]
- 7.Manning WC, Mocarski ES. Insertional mutagenesis of the murine cytomegalovirus genome: one prominent alpha gene (ie2) is dispensable for growth. Virology. 1988;167:477–84. [PubMed] [Google Scholar]
- 8.Manning WC, Stoddart CA, Lagenaur LA, Abenes GB, Mocarski ES. Cytomegalovirus determinant of replication in salivary glands. J Virol. 1992;66:3794–802. doi: 10.1128/jvi.66.6.3794-3802.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ho WY, Cooke MP, Goodnow CC, Davis MM. Resting and anergic B cells are defective in CD28-dependent costimulation of naive CD4+ T cells. J Exp Med. 1994;179:1539–49. doi: 10.1084/jem.179.5.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Batista FD, Harwood NE. The who, how and where of antigen presentation to B cells. Nat Rev Immunol. 2009;9:15–27. doi: 10.1038/nri2454. [DOI] [PubMed] [Google Scholar]
- 11.Parungo CP, Soybel DI, Colson YL, Kim SW, Ohnishi S, DeGrand AM, Laurence RG, Soltesz EG, Chen FY, Cohn LH, Bawendi MG, Frangioni JV. Lymphatic drainage of the peritoneal space: a pattern dependent on bowel lymphatics. Ann Surg Oncol. 2007;14:286–98. doi: 10.1245/s10434-006-9044-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Starzl TE, Zinkernagel RM. Antigen localization and migration in immunity and tolerance. N Engl J Med. 1998;339:1905–13. doi: 10.1056/NEJM199812243392607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matzinger P. The danger model: a renewed sense of self. Science. 2002;296:301–5. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- 14.Wardemann H, Boehm T, Dear N, Carsetti R. B-1a B cells that link the innate and adaptive immune responses are lacking in the absence of the spleen. J Exp Med. 2002;195:771–80. doi: 10.1084/jem.20011140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bowen DG, McCaughan GW, Bertolino P. Intrahepatic immunity: a tale of two sites? Trends Immunol. 2005;26:512–7. doi: 10.1016/j.it.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 16.You Q, Cheng L, Kedl RM, Ju C. Mechanism of T cell tolerance induction by murine hepatic Kupffer cells. Hepatology. 2008;48:978–90. doi: 10.1002/hep.22395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cserti C, Braunstein J, Cunningham MJ. Red cell alloantibodies and autoantibodies in thalassemia patients at Children’s Hospital Boston: incidence and influential factors. Blood. 2006;108:287a. [Google Scholar]
- 18.Ho HK, Ha SY, Lam CK, Chan GC, Lee TL, Chiang AK, Lau YL. Alloimmunization in Hong Kong southern Chinese transfusion-dependent thalassemia patients. Blood. 2001;97:3999–4000. doi: 10.1182/blood.v97.12.3999. [DOI] [PubMed] [Google Scholar]
- 19.Singer ST, Wu V, Mignacca R, Kuypers FA, Morel P, Vichinsky EP. Alloimmunization and erythrocyte autoimmunization in transfusion-dependent thalassemia patients of predominantly Asian descent. Blood. 2000;96:3369–73. [PubMed] [Google Scholar]