Abstract
Osteochondral tissue-engineered grafts are proposed to hold greater potential to repair/regenerate damaged cartilage through enhanced biochemical and mechanical interactions with underlying subchondral bone as compared to simple engineered cartilage. Additionally, biomechanical stimulation of articular chondrocytes (ACs) or osteoblasts (OBs) was shown to induce greater morphogenesis of the engineered tissues composed of these cells. In this report, to define the advantages of biomechanical stimulation to osteochondral grafts for tissue engineering, we examined whether (1) ACs and OBs in three-dimensional (3D) osteochondral constructs support functional development of each other at the molecular level, and (2) biomechanical stimulation of osteochondral constructs further promotes the regenerative potential of such grafts. Various configurations of cell/scaffold assemblies, including chondral, osseous, and osteochondral constructs, were engineered with mechano-responsive electrospun poly(ɛ-caprolactone) scaffolds. These constructs were subjected to either static or dynamic (10% cyclic compressive strain at 1 Hz for 3 h/day) culture conditions for 2 weeks. The expression of bone morphogenetic proteins (BMPs) was examined to assess the regenerative potential of each treatment on the cells. Biomechanical stimulation augmented a marked upregulation of Bmp2, Bmp6, and Bmp7 as well as downregulation of BMP antagonist, Bmp3, in a time-specific manner in the ACs and OBs of 3D osteochondral constructs. More importantly, the presence of biomechanically stimulated OBs was especially crucial for the induction of Bmp6 in ACs, a BMP required for chondrocytic growth and differentiation. Biomechanical stimulation led to enhanced tissue morphogenesis possibly through this BMP regulation, evident by the improved effective compressive modulus of the osteochondral constructs (710 kPa of dynamic culture vs. 280 kPa of static culture). Similar BMP regulation was observed in the femoral cartilages of the rats subjected to gentle exercise, demonstrating the physiological relevance of in vitro biomechanical stimulation of osteochondral constructs. Overall, our findings show that biomechanical stimulation may be critical for cross signaling between ACs and OBs to support chondrocytic growth in 3D osteochondral tissues.
Introduction
Osteoarthritis (OA), a degenerative joint disorder, is one of the most prevalent diseases in elderly. In the United States alone, an estimated 27 million adults suffer from OA causing 18.9 million with limitations in physical activity.1,2 With the expected increase in longevity and thus susceptibility to OA, development of more efficacious strategies to treat OA is essential. In this context, cartilage tissue engineering has attracted much attention as an approach to reconstruct damaged joints. Attempts to fabricate engineered cartilage by combining articular chondrocytes (ACs) and various scaffolds have produced constructs having morphology similar to the native tissue.3–5 However, the efficacy of such engineered cartilage tissues appears to be limited in dynamic environments of the joints in vivo, largely due to the difficulties encountered in the integration of engineered cartilage tissue to native avascular cartilage.6 To develop cartilage grafts that could be successfully incorporated in the native tissues, a hybrid approach combining cartilage with subchondral bone has been recently proposed.7–9
Cartilage is a unique tissue; it is physically in contact with the underlying subchondral bone while not being integrated with it via connective, vascular, or nervous tissues. This suggests potential roles of factors produced by the subchondral bone that may directly support the homeostasis of cartilage. In fact, subchondral bone is also suggested to play an integral role in the cartilage degeneration and the pathogenesis of OA.10 Based on these observations, osteochondral tissue engineering approaches have attempted to use bi-layered structures that accommodate ACs or osteoblasts (OBs) in separate phases.11 By providing integrated bone layer, the osteochondral constructs may enhance neo-tissue integration through relatively routine bone remodeling, leading to better mechanical stability.12 Furthermore, biochemical interactions between ACs and OBs may further augment the regenerative potential of engineered osteochondral constructs. The osteochondral tissue engineering may also be beneficial in repairing the damaged bones underneath the cartilage lesion, typical of OA.
Both cartilage and bone are mechano-sensitive tissues.13,14 Their cellular components, ACs and OBs, change their metabolic activities according to surrounding mechanical environments, that is, compressive, tensile, shear and hydrostatic forces. We earlier reported that physiological magnitudes of applied mechanical stimulation are regenerative signals to ACs and OBs inducing key anabolic transcription factors Sox9 and Runx2, respectively.15,16 The activation of these transcription factors result in the upregulation of essential extracellular matrix (ECM)-associated genes such as collagen type II and aggrecan in ACs, and collagen type I, osteocalcin and osteonectin in OBs.17–21 More importantly, the biomechanical signals induce expression of bone morphogenetic proteins (BMPs), critical growth factors for skeletal development.22 The anabolic/regenerative effects of biomechanical stimulation were further confirmed in our previous animal studies, in which exercise/biomechanical stimulation significantly suppressed an experimentally induced arthritis of the knees.23 These observations strongly suggest that biomechanical stimulation can be utilized as a biological cue to facilitate the maturation of engineered osteochondral tissues, thus enhancing the mechanical functionality of such tissues.
Considering the significant potential of osteochondral tissue grafts in clinical applications, and biomechanical stimulation as a promoter for functionalization of the engineered tissues, it is important to know how biomechanical stimulation regulates each cellular component of the osteochondral constructs. In this report, we examined the molecular basis for the advantages of biomechanical stimulation of AC/OB co-culture, grown as adjacent layers. We utilized mechano-responsive microfibrous electrospun scaffolds, which we have previously shown to induce uniform cellular distribution and proliferation of both ACs and OBs while mechanically resilient to withstand the compression regimen used in this study.15,16 We show that three-dimensional (3D) AC/OB co-culture exhibits discrete BMP induction to promote maturation of engineered tissues. More importantly, biomechanical stimulation induces further upregulation of selective BMPs to increase their regenerative potential in a synergistic manner with 3D co-culture.
Materials and Methods
Scaffolds
A solution of 15% poly(ɛ-caprolactone) (PCL, 60,000 Mw; Sigma-Aldrich) dissolved in dichloromethane (Sigma-Aldrich) was electrospun to produce a microfibrous mesh composed of fibers having 10 μm average diameter with nano-sized surface pores as described previously.15 Briefly, 15 mL/h flow rate and 30-cm needle tip-to-collector distance with a varying electric field of 20 ∼ 25 kV were used to synthesize scaffolds. Cylindrical scaffolds having approximately 2 mm thickness and 6 mm diameter were cut from as-spun mesh using a biopsy punch (Miltex). To improve hydrophilicity, the scaffolds were first treated with plasma (Harrick Plasma) at 30 W for 15 min to polarize the surface, followed by overnight surface coating of collagen type I (1 mg/mL in 0.01 M hydrochloric acid).24 The coated collagen was then crosslinked to PCL by N-Ethyl-N′-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDAC, Sigma-Aldrich).25 After collagen coating and crosslinking, the scaffolds were sterilized by immersing in 70% ethanol overnight.
Cell culture
All protocols involving animals were approved by the Institutional Animal Care and Use Committee at the Ohio State University. Femurs from Sprague Dawley rats (10–12-week-old females) were used to harvest ACs.15 OBs were isolated from 3–4-day-old rat calvaria to allow collection of greater number of cells with comparable phenotypic cellular behavior to mature OBs.16,26 ACs and OBs with initial seeding densities of 20,000 and 5,000 cells/cm2, respectively, were separately cultured in the tissue culture medium (TCM) containing DMEM (Gibco, NY), 10% FBS (Atlanta Biologicals), 10 μg/mL streptomycin, 100 U/mL penicillin (Gibco), 1% L-glutamine (Gibco), supplemented with 50 μg/mL ascorbic acid (Sigma-Aldrich), 10 mM β-glycerophosphate (Sigma-Aldrich), and 10 nM dexamethasone, to maintain both mature chondrocyte and OB phenotypes.7 When the cells reached 90% confluency in T75 flasks, they were trypsinized and subcultured. After cell expansion and phenotype characterization as previously described,15,16 ACs (passage 2, 4×105) or OBs (passages 2∼4, 2×105) suspended in 45 μL of the TCM were statically seeded in separate PCL microfiber scaffolds (2 mm height×6 mm diameter) placed in 24-well plates, to form constructs with a relatively uniform cellular distribution throughout the thickness of the scaffolds.15 The as-seeded constructs were incubated for 2 h for cell attachment before replenishing with additional media. The cell/scaffold constructs were statically precultured in the TCM for 3 days. The separately cultured AC and OB constructs were then sutured together with a sterile braided silk suture (Ethicon) and fixed by a surgical knot in three different configurations, AC with AC (AC/AC), OB with OB (OB/OB), or AC with OB (AC/OB) constructs, as shown in Figure 1. The assembled constructs were further cultured for 3 days in the TCM before the exposure to biomechanical stimulation as described below.
FIG. 1.
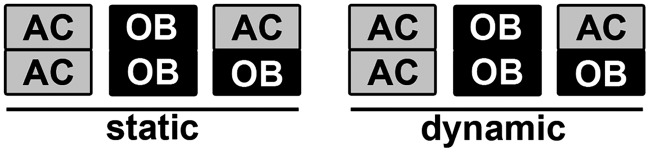
Schematic diagram of experimental configurations of the articular chondrocyte (AC) and osteoblast (OB) constructs, grown under static conditions (static) or exposed to unconfined 10% cyclic compressive strain at 1 Hz for 3 h/day (dynamic).
Application of dynamic compressive forces
The AC/OB, OB/OB, and AC/AC constructs with 1 mL of the TCM were subjected to 10% unconfined dynamic compressive strain at 1 Hz with a saw-tooth profile for 3 h/day for 2 weeks using a custom-designed compression device as described earlier.15 This regimen was shown to induce anabolic responses from both ACs and OBs in our previous studies.15,16,27 The constructs were in contact with the compression rams at all times in the compression regimen during the whole culture period in this study. The constructs without the treatment of biomechanical stimulation were used as static controls. Additionally, the constructs of both dynamic and static culture conditions were placed on an orbital shaker at 120 rpm for 30 min daily to completely exchange spent cell culture media with fresh TCM.
To investigate noncontact biochemical effects of co-culture, AC constructs were cultured in the conditioned media from statically cultured AC/AC or OB/OB, or dynamically compressed AC/AC or OB/OB constructs (n=6/condition). In these experiments, the dynamically cultured constructs were subjected to biomechanical stimulation for 3 h/day for 1 week. The spent media were collected during the daily media exchange as described earlier. Separate AC constructs (n=6/condition) were subsequently exposed to the spent media daily.
Gene expression analysis of ACs and OBs exposed to biomechanical stimulation in vitro
Each phase (i.e., either AC or OB phase) was separated from the construct assemblies shown in Figure 1 and total RNA was separately extracted using the RNeasy RNA extraction kit (Qiagen). The extracted mRNA was subjected to first strand cDNA synthesis and real-time polymerase chain reaction (rt-PCR) using custom-designed primers for Bmp2, -3, -4, -6, and -7 with ribosomal protein S18 (Rps18) expression as an endogenous control.15 The sequences of the custom primer pairs are described in Table 1.
Table 1.
Primer Sequences
| Gene | Sequence |
|---|---|
| Rps18 | Forward: 5′-GCGGCGGAAAATAGCCTTCG-3′ |
| Reverse: 5′-CCAGTGGTCTTGGTGTGCTG-3′ | |
| Bmp2 | Forward: 5′-AACACCGTGCTCAGCTTCCAT-3′ |
| Reverse: 5′-TTCGGGAACAAATGCAGGAA-3′ | |
| Bmp3 | Forward: 5′-CCCCAAGTCATTTGATGCCTA-3′ |
| Reverse: 5′-TGGCGTGATTTGATGGCTT-3′ | |
| Bmp4 | Forward: 5′-GGAAGAAGAGCAGAGCCAGGGAA-3′ |
| Reverse: 5′-CATTCTCTGGGATGCTGCTGAGGT-3′ | |
| Bmp6 | Forward: 5′-GCTACGCTGCCAACTATTGTGACG-3′ |
| Reverse: 5′-GAGATGGCATTCAGTTTGGTTGGTG-3′ | |
| Bmp7 | Forward: 5′-GACTGGATCATCGCACCTGAA-3′ |
| Reverse: 5′-ATAGCATGGTTGGTGGCGTTC-3′ |
Morphological characterization of cell/scaffold constructs
The cell/scaffold constructs were formalin-fixed overnight and embedded in optimal cutting temperature (OCT) compound (Sakura Finetek). The constructs were vertically sectioned at 20-μm thickness. The samples were double-stained with Alcian blue to stain glycosaminoglycan (GAG) and Alizarin red to stain calcium using a modified acid-free double-staining protocol.28 Briefly, the sectioned constructs were washed to remove OCT compounds, and incubated in a solution of 0.2% Alcian blue (Sigma-Aldrich), 0.05% Alizarin red (Sigma-Aldrich), and 60 mM MgCl2 (Sigma-Aldrich) in 70% ethanol for 2 h. The stained samples were cleared with 20% glycerol (Sigma-Aldrich) in 0.25% KOH (Sigma-Aldrich), washed with DI water, mounted, and examined under a microscope (Zeiss).
The overall morphology of osteochondral constructs in the cartilage and the bone phases was observed by scanning electron microscopy (SEM; FEI XL-30 Sirion).15 Briefly, the formalin-fixed samples were subjected to dehydration in graded DI water–ethanol and ethanol–hexamethyldisilazane (Electron Microscopy Sciences, PA) series. The dehydrated samples were osmium-coated before observation.
Mechanical characterization of cell/scaffold constructs
The mechanical properties of osteochondral (AC/OB) constructs that had been subjected to biomechanical stimulation for 2 weeks were measured as a whole using a 9.8-N load cell (Honeywell Sensotec) on a load frame (TestResources Inc.) at 10% unconfined dynamic compressive strain with a saw-tooth profile at a frequency of 1 Hz with a 10-mm-diameter ram. The specimens were submerged in the TCM at 37°C during the mechanical testing. After 50 preloading and unloading cycles for stabilization, stress–strain curves were generated to calculate the compressive modulus; the modulus was determined from the slope of the best linear regression fit during loading regimen. Acellular scaffolds and statically cultured osteochondral constructs were used as controls (n=3/condition).
Effects of biomechanical stimulation on cartilage and bone in vivo
To examine the effects of biomechanical stimulation in vivo, 12–14-week-old Sprague-Dawley rats were subjected to treadmill walking at a speed of 12 m/min for 45 min daily for 2, 5, or 15 days (5 rats/condition).23 This anabolic exercise regimen was based on our earlier studies.23 Nonexercised animals were used as controls. Animals had free access to food and water ad libitum and were housed in controlled environments (12-h light–dark cycle, 50% humidity, and 21°C). The rats were killed 2 h after the last exercise regimen for each time point with CO2 inhalation, and the distal ends of femurs were harvested and snap-frozen in liquid nitrogen for gene expression analysis, or formalin-fixed for immunohistochemistry. For gene expression analysis, cartilage or subchondral bone of each individual femur was pulverized under liquid nitrogen in a Micro-Dismembrator (Sartorius and Stedim Biotech.) and RNA extracted with Trizol (Invitrogen) as described earlier.23 The extracted mRNA was subjected to cDNA synthesis and rt-PCR as previously described above. For immunohistochemistry, the fixed femurs were decalcified, paraffin-embedded, and sectioned. The sections were stained with rabbit anti-BMP6 (Abcam) as primary antibody and CY3-conjugated donkey anti-rabbit IgG (Jackson Immunoresearch) as secondary antibody, and observed under an epifluorescence microscope (Zeiss). The samples stained with the secondary antibody alone were used as negative controls.
Statistical analysis
Unless otherwise noted, all experiments were conducted at least in triplicate and the number of samples used is indicated in each figure. Data are represented as means±standard deviation. Data were analyzed using SPSS (v.17.0) by t-test or one-way analysis of variance with Tukey's HSD post-hoc to test significances. p<0.05 was regarded as statistically significant.
Results
To examine the effects of (1) 3D co-culture of ACs and OBs, (2) dynamic biomechanical stimulation, or (3) the combination of both, various configurations of cell/scaffold constructs were fabricated and cultured with or without daily biomechanical stimulation (Fig. 1). Physically distinct scaffolds of ACs and OBs were used to precisely control spatial distribution of the cells. To examine possible inter-layer migration of ACs or OBs during the culture period, the cross sections of the AC/OB constructs cultured under dynamic biomechanical stimulation for 2 weeks were examined (Fig. 2A). Phenotype-specific double staining, that is, Alcian blue detecting GAG and Alizarin red detecting calcium as ECM markers for ACs and OBs, respectively, suggested minimal invasion of each cellular component into the counter-layer at the interface even after the extended culture period. These observations were further confirmed by SEM, which demonstrated the phenotypic morphologies of round ACs in the cartilage phase (Fig. 2B) and spread out OBs in the bone phase (Fig. 2C).
FIG. 2.
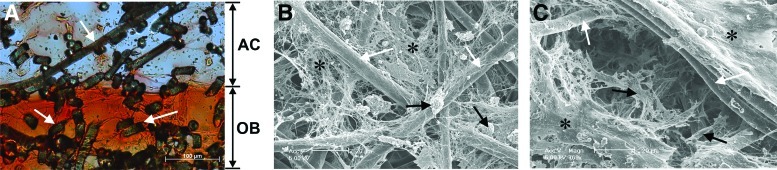
(A) A representative vertical cross section of an osteochondral construct following exposure to dynamic compression at 1 Hz for 3 h/day for 2 weeks showing minimal invasion of AC or OB into its counterpart, as shown by the distinct layers of Alcian blue-positive glycosaminoglycan in the AC layer and Alizarin red-positive calcium in the OB layer. White arrows indicate scaffold fibers. Representative SEM images of the cartilage (B) and the bone (C) layers of osteochondral cell/scaffold constructs after 2 weeks of dynamic culture. The images demonstrate phenotype-characteristic morphologies of round chondrocytes [black arrows in (B)] and spread out OBs [black arrows in (C)]. White arrows and asterisks represent poly(ɛ-caprolactone) fibers and extracellular matrix secreted by the cells, respectively. Color images available online at www.liebertpub.com/tea
The presence of OBs is essential for biomechanical signals to induce Bmp6 in ACs
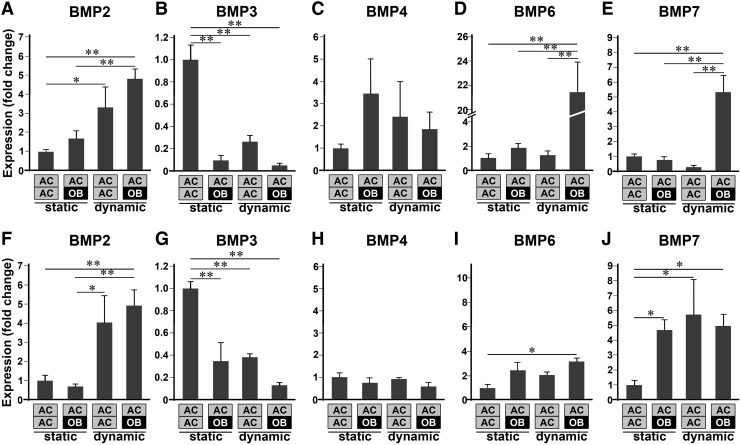
Since BMPs are known to be essential growth factors for skeletal development, the expression levels of Bmp2, -3, -4, -6, and -7 in the ACs exposed to various conditions were examined at week 1 or week 2 of culture (Fig. 3). At week 1, static 3D co-culture of ACs with OBs (i.e., from static AC/OB) resulted in a slight increase in the expression of Bmp2, -4, and -6 while ACs exposed to biomechanical stimulation (i.e., from dynamic AC/AC) exhibited significant upregulation of Bmp2. More importantly, the presence of OBs was critical for the upregulation of Bmp6 and Bmp7 in ACs by biomechanical stimulation (i.e., from dynamic AC/OB); Bmp6 expression of ACs in the dynamically cultured osteochondral constructs showed more than 21-fold increase over that in the statically cultured AC/AC constructs. In addition, the expression of Bmp3, a known antagonist of chondrogenic and osteogenic BMPs,29 was significantly downregulated by both co-culture and biomechanical stimulation.
FIG. 3.
Bmp2 (A, F), Bmp3 (B, G), Bmp4 (C, H), Bmp6 (D, I), and Bmp7 (E, J) mRNA expression of ACs in various configurations of cell/scaffold constructs at week 1 (A–E) and week 2 (F–J), demonstrating temporal and configurational regulation of BMPs in ACs by both co-culture and biomechanical stimulation (n=6, *p<0.05, **p<0.01). Static and dynamic represent static culture and dynamic culture subjected to 10% cyclic compressive strain at 1 Hz for 3 h/day, respectively. Each gene expression was normalized to that of ACs in the statically cultured AC/AC constructs.
At week 2, biomechanical stimulation only in the presence of OBs significantly upregulated Bmp6, while both 3D co-culture and biomechanical stimulation upregulated Bmp7 expression in ACs. Bmp2 was upregulated by biomechanical stimulation alone. As observed at week 1, Bmp3 was significantly downregulated by both co-culture and biomechanical stimulation, while there was minimal effect of co-culture, biomechanical stimulation, or the combination of both on the expression of Bmp4 in ACs.
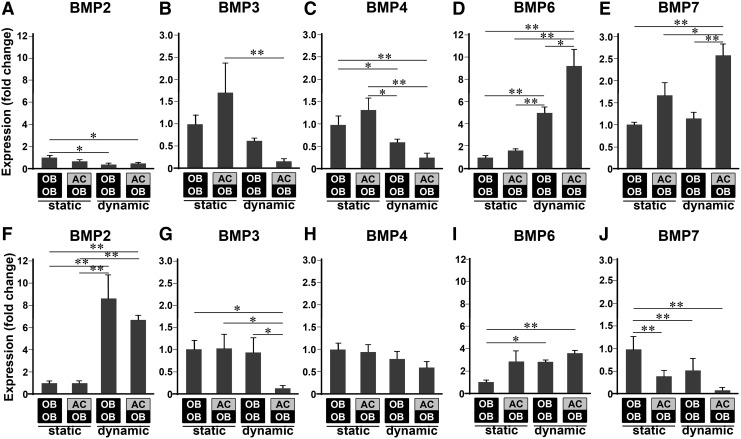
Biomechanical signals regulate expression of BMPs in OBs
BMP mRNA expression analysis in OBs revealed that biomechanical stimulation upregulates Bmp6 and Bmp7 at week 1 while suppressing Bmp2, Bmp3, and Bmp4 expression in both OB/OB and AC/OB constructs (Fig. 4). Interestingly, Bmp6 expression was further intensified by the presence of ACs in co-culture, possibly demonstrating synergistic effects of 3D co-culture and biomechanical stimulation in the regulation of Bmp6. Similarly, the upregulation of Bmp7 by biomechanical stimulation in OBs required the presence of ACs. In contrast to week 1, biomechanical stimulation resulted in significant upregulation of Bmp2 and Bmp6, and downregulation of Bmp7 at week 2. These observations along with little effects of static 3D co-culture may demonstrate the dominant control of BMP regulation in OBs by biomechanical stimulation.
FIG. 4.
Bmp2 (A, F), Bmp3 (B, G), Bmp4 (C, H), Bmp6 (D, I), and Bmp7 (E, J) mRNA expression of OBs in various configurations of cell/scaffold constructs at week 1 (A–E) and week 2 (F–J) demonstrating temporal and configurational regulation of bone morphogenetic proteins (BMPs) in OBs by both co-culture and biomechanical stimulation (n=6, *p<0.05, **p<0.01). Static and dynamic represent static culture and dynamic culture subjected to 10% cyclic compressive strain at 1 Hz for 3 h/day, respectively. Each gene expression was normalized to that of OBs in the statically cultured OB/OB constructs.
Biomechanical stimulation enhances the mechanical properties of osteochondral constructs
To examine how this regulation of various BMPs affected the maturation of engineered osteochondral constructs, the mechanical properties of the constructs cultured with or without biomechanical stimulation were compared (Fig. 5). After 2 weeks of either dynamic or static culture, stress–strain curves of the osteochondral constructs in both the conditions show hysteresis loops as compared to acellular scaffolds, probably indicating the viscoelastic nature of the deposited ECM within the constructs. However, compressive modulus of the constructs cultured under dynamic and static conditions exhibited considerable differences; that is, the average compressive modulus of dynamically cultured constructs was approximately 2.5 times greater than that of statically cultured ones (0.71±0.05 MPa for dynamic culture versus 0.28±0.03 MPa for static culture, n=3, p<0.01).
FIG. 5.
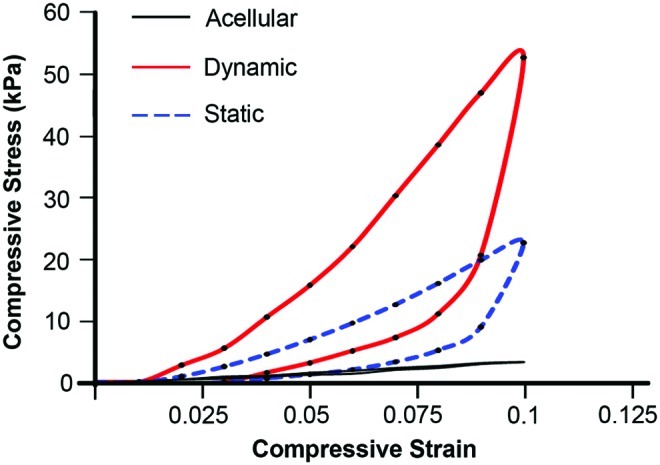
Representative compressive stress–strain curves of the AC/OB constructs that had been subjected to dynamic culture (red solid line) or static culture (blue dotted line) for 2 weeks demonstrating improved mechanical properties of engineered osteochondral constructs by biomechanical stimulation. Black solid line represents a stress–strain curve of acellular scaffolds. Static and dynamic represent static culture and dynamic culture subjected to 10% cyclic compressive strain at 1 Hz for 3 h/day, respectively. Color images available online at www.liebertpub.com/tea
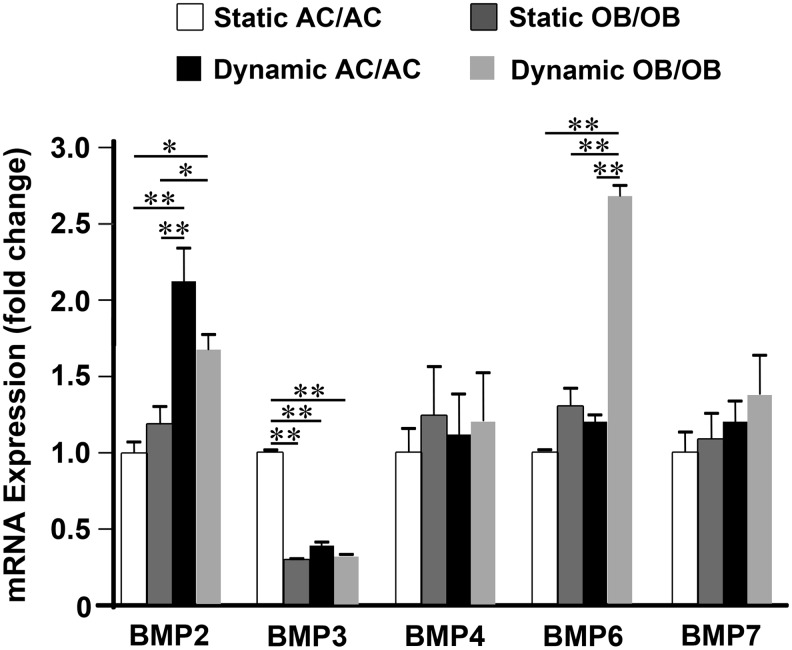
Biomechanically activated OBs regulate BMP expression in ACs via paracrine mechanisms
The presence of OBs in the culture significantly affected BMP expression in ACs as compared to contrariwise (Figs. 3 and 4). For example, the upregulation of Bmp6 and Bmp7 in ACs by biomechanical stimulation only occurred in the osteochondral constructs. In addition, the upregulation of Bmp2 and the downregulation of Bmp3 by biomechanical stimulation were further intensified in the presence of OBs. Therefore, we next determined whether the regulation of these BMPs in ACs was through the paracrine effects from mechano-activated OBs. To examine how the soluble factors secreted by biomechanically stimulated OBs affect BMP regulation in ACs, AC constructs were cultured with the conditioned media of (1) statically cultured AC/AC; (2) statically cultured OB/OB; (3) dynamically cultured AC/AC; or (4) dynamically cultured OB/OB for 1 week. As shown in Figure 6, Bmp6 expression in ACs was significantly upregulated only when the cells were cultured in the conditioned medium of dynamically activated OBs. This may demonstrate the critical importance of the presence of biomechanically activated OBs in the regulation of Bmp6 in ACs. On the other hand, both conditioned media of ACs and OBs subjected to biomechanical stimulation significantly upregulated Bmp2 expression while downregulating Bmp3 expression in ACs. In addition, static co-culture with OBs also significantly downregulated Bmp3 expression in ACs in the absence of biomechanical stimulation (p<0.01).
FIG. 6.
Paracrine effects of soluble factors secreted by biomechanically stimulated OBs on the BMP regulation of ACs. Regulation of Bmp2, -3, -4, -6, and -7 was analyzed in the ACs cultured in the conditioned media of statically cultured AC/AC, statically cultured OB/OB, dynamically cultured AC/AC, or dynamically cultured OB/OB constructs for 1 week (n=6, *p<0.05, **p<0.01). Static and dynamic represent static culture and dynamic culture subjected to 10% cyclic compressive strain at 1 Hz for 3 h/day, respectively.
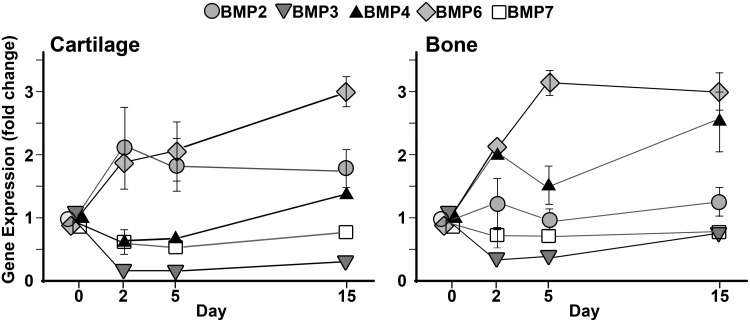
Biomechanical signals stimulate BMP6 induction in cartilage and subchondral bone in vivo
In order to examine the physiological relevance of the observed regulation of BMPs by biomechanical stimulation, BMP gene expression in the articular cartilage and subchondral bone of the rats subjected to exercise was investigated. The temporal changes of the BMP expression in ACs in vivo, including Bmp2, -3, -4, -6, and -7, are shown in Figure 7. Similar to the observations made in ACs in the osteochondral constructs in vitro at week 1, the biomechanical signals generated by exercise in vivo significantly upregulated the expression of Bmp2 (p<0.05) and Bmp6 (p<0.01) in ACs. In contrast, exercise significantly downregulated the expression of Bmp3 in ACs (p<0.01). Interestingly, the expression profile of Bmp7 in vivo did not agree with the in vitro results; that is, no significant changes in Bmp7 expression were observed after 2, 5, or 15 days of exercise (Fig. 7). As observed in the in vitro experiments, the expression profile of Bmp4 by exercise was steady.
FIG. 7.
Temporal regulation of Bmp2, −3, −4,−6, and -7 in the cartilage or subchondral bone of rats subjected to exercise/biomechanical stimulation for 2, 5, or 15 days (n=3). The rats were subjected to gentle treadmill walking at 12 m/min for 45 min/per day for the indicated durations. The data were normalized to those from nonexercised controls. Error bars indicate standard deviations. The error bars smaller than the data point markers are omitted.
Similar to cartilage, Bmp6 was the most significantly upregulated by exercise in subchondral bone (p<0.01, Fig. 7). The upregulation of the gene was maximal on day 5, agreeing with the upregulation of Bmp6 in the OBs of osteochondral constructs following biomechanical stimulation for 1 week. The downregulation of Bmp3 on day 5 (p<0.01) was also consistent with the observation from the in vitro study. However, contrary to the in vitro findings, a significant upregulation of Bmp4 was observed in response to 15 days of exercise.
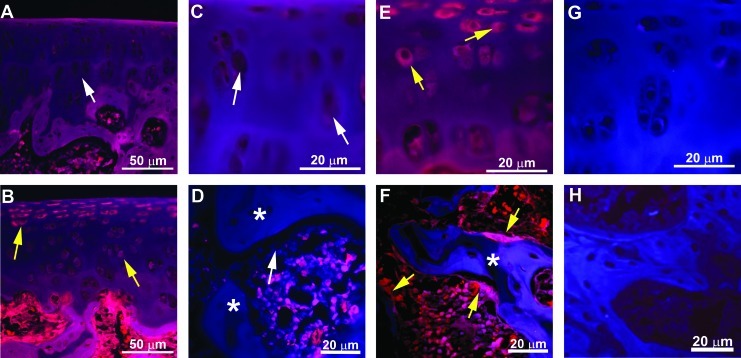
Based on the above finding, the upregulation of BMP6 was further confirmed by immunohistochemistry on the osteochondral tissues from exercised rats as compared to those from nonexercised rats. As evident in Figure 8, both chondrocytes and bone-lining cells expressed BMP6 in response to exercise, whereas nonexercised controls did not show such an expression of BMP6.
FIG. 8.
Regulation of BMP6 by exercise in vivo. Vertical sections of the distal end of rat femur through osteochondral zone (A, B), cartilage (C, E), and subchondral bone (D, F) from nonexercised control (A, C, D) or those exercised for 15 days (B, E, F) were stained for BMP6 (red). The images demonstrate the upregulation of BMP6 protein synthesis in chondrocytes and bone lining OBs by biomechanical stimulation (yellow arrows: BMP6-positive cells; white arrow: BMP6-negative cells). Auto-fluorescent background (blue) was used to visualize overall tissue morphology. Asterisks represent subchondral bone. Cartilage (G) and subchondral bone (H) sections stained with secondary antibody alone demonstrate the specificity of the primary antibody. Color images available online at www.liebertpub.com/tea
Discussion
The regeneration of cartilage by tissue engineering approaches is influenced by many factors, including composition and structure of biomaterials, different cell sources, and biological cues. Here, based on the molecular analysis of ACs and OBs in the osteochondral constructs along with their mechanical characterization, we have demonstrated that biomechanical signals are another important cue that facilitates the maturation of engineered osteochondral tissues for successful cartilage regeneration. In this study, we demonstrated that the maturation of osteochondral constructs during in vitro culture is significantly enhanced by biomechanical stimulation, as reflected by improved mechanical properties possibly through the gene regulation of BMPs that are required for enhancing phenotypical ECM synthesis to repair damaged cartilage.30–32 Similar to this observation, Kon et al. recently showed a faster recovery in “active” patients when osteochondral lesions were treated with acellular scaffolds, demonstrating the importance of biomechanical stimulation for osteochondral tissue morphogenesis.33
BMPs are potent growth factors required for the growth, differentiation, and homeostasis of both ACs and OBs.30,31,34–38 For example, induction of stem cell chondrogenic or osteogenic differentiation, and synthesis of phenotype-specific ECM (i.e., collagen type II and aggrecan in ACs and collagen type I, alkaline phosphatase, osteonectin, and osteocalcin in OBs) are upregulated by BMPs.35–38 Specifically, BMP2, -4, -6, and -7 have been shown to enhance chondrogenic differentiation of progenitor cells and cartilage-specific matrix production when supplemented to chondrogenic media.30 In fact, removal of Bmp2 and Bmp4 from developing limb bud mesenchyme results in the impairment of cartilage formation.34 More importantly, supplementary BMP6 has been shown to induce greater chondrogenesis of stem cells and significantly enhance matrix synthesis of ACs.31,32 Similarly, supplementation of BMP2, -4, -6, and -7 accelerates osteogenesis through the upregulation of alkaline phosphatase activity in vitro. Among these BMPs, BMP6 appears to be one of the most potent osteogenic proteins in a rat model of ectopic bone formation.39 Therefore, the substantial increase in Bmp6 expression by biomechanical stimulation in the osteochondral constructs observed in this study may be indicative of enhanced phenotype-specific regenerative activities of the ACs and OBs. In contrast, BMP3, an inhibitor of chondrogenesis and osteogenesis, was downregulated by biomechanical stimulation. BMP3 has been shown to suppress chondrocytic differentiation and bone formation in vivo through activating the TGF-β signaling pathway that competes with the BMP pathway.29,40 Therefore, the downregulation of Bmp3 under the dynamic culture condition may further support pro-chondrogenic and pro-osteogenic functions of biomechanical stimulation.
The anabolic gene upregulation in the engineered tissues with close spatial apposition of ACs and OBs in our results suggests that they may regulate each other for growth and differentiation. Indeed, Nakaoka et al.41 showed that both the co-cultures of these two cell types in contact-mediated monolayer two-dimensional (2D) culture and in noncontact Transwell-plate 2D culture enhanced proliferation and production of phenotype-specific ECM in ACs via direct contact as well as indirect paracrine signaling of secreted soluble factors. More importantly, we have shown that biomechanical stimulation together with co-culture appears to act in a synergistic manner for inducing BMPs required for skeletal tissue morphogenesis. We observed a significant upregulation of Bmp2, -6, and -7, and downregulation of Bmp3 in the ACs of the osteochondral constructs exposed to biomechanical stimulation as compared to the statically cultured ACs in a time-dependent manner. Similar BMP regulation, especially Bmp6, by mechano-stimulation generated by exercise in vivo (Figs. 7 and 8) that prevented the disease progression of OA in our earlier study23 suggests the physiological relevancy of the in vitro biomechanical stimulation in enhancing cartilage tissue regeneration. Although we observed high degree of upregulation of BMP6 secreted from osteochondral constructs under biomechanical stimulation (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/tea), the molecules responsible for the AC/OB interaction are still elusive and further investigations are required.
Our observations provide evidence that biomechanical stimulation exerts dominant effects on the BMP regulation in ACs and OBs over 3D co-culture. For example, Bmp2 in ACs and Bmp6 in OBs were significantly upregulated by biomechanical stimulation alone throughout the culture period. In addition, biomechanical stimulation exhibited synergistic effects with 3D co-culture, as evidenced by (1) upregulation of several BMP genes by biomechanical stimulation only in co-cultures, for example, upregulation of Bmp6 and Bmp7 in ACs, downregulation of Bmp3 in OBs, and (2) intensified gene regulation by biomechanical stimulation in co-cultures, for example, up- and downregulation of Bmp2 and Bmp3, respectively, in ACs, and upregulation of Bmp6 and Bmp7 in OBs.
The presence of mechano-activated OBs appears to play a significant role in the BMP regulation in the ACs of osteochondral constructs. This was evident by the fact that the biomechanically stimulated OBs affected the expression of Bmp2, -3, and -6 in the co-cultured ACs in a noncontact manner (Fig. 5). These observations are further supported by our in vivo findings that exercise-generated biomechanical signals induce upregulation of Bmp2 and Bmp6, and downregulation of Bmp3 in the cartilage (Fig. 7). However, the upregulation of Bmp7 shown in the in vitro experiments was not observed in vivo, likely due to the differences in the degree of tissue maturity in the developing engineered osteochondral constructs as compared to the fully developed in vivo tissues. Since there is continuous deposition of ECM within the scaffolds during the in vitro culture periods, the regulation of this particular gene by biomechanical stimulation may be affected by the extent of culture periods. Indeed, the regulation of Bmp7 by biomechanical stimulation in vitro appeared to converge to a trend observed in the in vivo experiments as the engineered tissue matured over the culture time (week 2).
In summary, we have demonstrated the molecular basis for the superiority of osteochondral grafts over those made of ACs alone. More importantly, we have shown that biomechanical stimulation dynamically regulates BMPs in the engineered osteochondral constructs. Biomechanical stimulation not only induces BMPs that are required for the synthesis of cartilage-associated ECM, but also inhibits the BMP that antagonizes activity of anabolic BMPs to further drive cartilage formation. Together, this regulation of BMPs by biomechanical stimulation in co-cultures may be important for greater deposition of phenotype-specific ECM, hence enhancing maturation and functionalization of the engineered osteochondral constructs.
Supplementary Material
Acknowledgments
This work was supported with funds from the National Institute of Health (Grant No. AR048781, DE015399, and DE014320) and initial complementary fund from University of California, Riverside.
Disclosure Statement
No competing financial interests exist.
References
- 1.Lawrence R.C. Felson D.T. Helmick C.G. Arnold L.M. Choi H. Deyo R.A., et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II Arthritis Rheum. 2008;58:26. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pleis J. Lucas J. Ward B. Summary health statistics for U.S. adults: National Health Interview Survey, 2008. Vital Health Stat. 2009;10:6. [PubMed] [Google Scholar]
- 3.Ng K.W. Ateshian G.A. Hung C.T. Zonal chondrocytes seeded in a layered agarose hydrogel create engineered cartilage with depth-dependent cellular and mechanical inhomogeneity. Tissue Eng Part A. 2009;15:2315. doi: 10.1089/ten.tea.2008.0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang Y.H. Barabino G.A. Requirement for serum in medium supplemented with insulin-transferrin-selenium for hydrodynamic cultivation of engineered cartilage. Tissue Eng Part A. 2011;17:2025. doi: 10.1089/ten.TEA.2010.0415. [DOI] [PubMed] [Google Scholar]
- 5.Neves S.C. Moreira Teixeira L.S. Moroni L. Reis R.L. Van Blitterswijk C.A. Alves N.M., et al. Chitosan/poly(epsilon-caprolactone) blend scaffolds for cartilage repair. Biomaterials. 2011;32:1068. doi: 10.1016/j.biomaterials.2010.09.073. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Z. McCaffery J.M. Spencer R.G. Francomano C.A. Growth and integration of neocartilage with native cartilage in vitro. J Orthop Res. 2005;23:433. doi: 10.1016/j.orthres.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 7.Schaefer D. Martin I. Shastri P. Padera R.F. Langer R. Freed L.E., et al. In vitro generation of osteochondral composites. Biomaterials. 2000;21:2599. doi: 10.1016/s0142-9612(00)00127-7. [DOI] [PubMed] [Google Scholar]
- 8.Grayson W.L. Chao P.H. Marolt D. Kaplan D.L. Vunjak-Novakovic G. Engineering custom-designed osteochondral tissue grafts. Trends Biotechnol. 2008;26:181. doi: 10.1016/j.tibtech.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mano J.F. Reis R.L. Osteochondral defects: present situation and tissue engineering approaches. J Tissue Eng Regen Med. 2007;1:261. doi: 10.1002/term.37. [DOI] [PubMed] [Google Scholar]
- 10.Prasadam I. Crawford R. Xiao Y. Aggravation of ADAMTS and matrix metalloproteinase production and role of ERK1/2 pathway in the interaction of osteoarthritic subchondral bone osteoblasts and articular cartilage chondrocytes—possible pathogenic role in osteoarthritis. J Rheumatol. 2012;39:621. doi: 10.3899/jrheum.110777. [DOI] [PubMed] [Google Scholar]
- 11.O'Shea T.M. Miao X.G. Bilayered scaffolds for osteochondral tissue engineering. Tissue Eng Part B Rev. 2008;14:447. doi: 10.1089/ten.teb.2008.0327. [DOI] [PubMed] [Google Scholar]
- 12.Shapiro F. Koide S. Glimcher M.J. Cell origin and differentiation in the repair of full-thickness defects of articular cartilage. J Bone Joint Surg Am. 1993;75:532. doi: 10.2106/00004623-199304000-00009. [DOI] [PubMed] [Google Scholar]
- 13.Allori A.C. Sailon A.M. Pan J.H. Warren S.M. Biological basis of bone formation, remodeling, and repair-part III: biomechanical forces. Tissue Eng Part B Rev. 2008;14:285. doi: 10.1089/ten.teb.2008.0084. [DOI] [PubMed] [Google Scholar]
- 14.Lee C. Grad S. Wimmer M. Alini M. The influence of mechanical stimuli on articular cartilage tissue engineering. In: Ashammakhi N., editor; Reis R.L., editor. Topics in Tissue Engineering. Expertissues; 2006. pp. 1–32. [Google Scholar]
- 15.Nam J. Rath B. Knobloch T.J. Lannutti J.J. Agarwal S. Novel electrospun scaffolds for the molecular analysis of chondrocytes under dynamic compression. Tissue Eng Part A. 2009;15:513. doi: 10.1089/ten.tea.2007.0353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rath B. Nam J. Knobloch T.J. Lannutti J.J. Agarwal S. Compressive forces induce osteogenic gene expression in calvarial osteoblasts. J Biomech. 2008;41:1095. doi: 10.1016/j.jbiomech.2007.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bell D.M. Leung K.K. Wheatley S.C. Ng L.J. Zhou S. Ling K.W., et al. SOX9 directly regulates the type-II collagen gene. Nat Genet. 1997;16:174. doi: 10.1038/ng0697-174. [DOI] [PubMed] [Google Scholar]
- 18.Gordeladze J.O. Noel D. Bony C. Apparailly F. Louis-Plence P. Jorgensen C. Transient down-regulation of cbfa1/Runx2 by RNA interference in murine C3H10T1/2 mesenchymal stromal cells delays in vitro and in vivo osteogenesis, but does not overtly affect chondrogenesis. Exp Cell Res. 2008;314:1495. doi: 10.1016/j.yexcr.2007.12.023. [DOI] [PubMed] [Google Scholar]
- 19.Takigawa Y. Hata K. Muramatsu S. Amano K. Ono K. Wakabayashi M., et al. The transcription factor Znf219 regulates chondrocyte differentiation by assembling a transcription factory with Sox9. J Cell Sci. 2010;123:3780. doi: 10.1242/jcs.071373. [DOI] [PubMed] [Google Scholar]
- 20.Yousfi M. Lasmoles F. Marie P.J. TWIST inactivation reduces CBFA1/RUNX2 expression and DNA binding to the osteocalcin promoter in osteoblasts. Biochem Biophys Res Commun. 2002;297:641. doi: 10.1016/s0006-291x(02)02260-x. [DOI] [PubMed] [Google Scholar]
- 21.Kern B. Shen J. Starbuck M. Karsenty G. Cbfa1 contributes to the osteoblast-specific expression of type I collagen genes. J Biol Chem. 2001;276:7101. doi: 10.1074/jbc.M006215200. [DOI] [PubMed] [Google Scholar]
- 22.Wan M. Cao X. BMP signaling in skeletal development. Biochem Biophys Res Commun. 2005;328:651. doi: 10.1016/j.bbrc.2004.11.067. [DOI] [PubMed] [Google Scholar]
- 23.Nam J. Perera P. Liu J. Wu L.C. Rath B. Butterfield T.A., et al. Transcriptome-wide gene regulation by gentle treadmill walking during the progression of monoiodoacetate induced arthritis. Arthritis Rheum. 2011;63:1613. doi: 10.1002/art.30311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chan C.K. Liao S. Li B. Lareu R.R. Larrick J.W. Ramakrishna S., et al. Early adhesive behavior of bone-marrow-derived mesenchymal stem cells on collagen electrospun fibers. Biomed Mater. 2009;4:035006. doi: 10.1088/1748-6041/4/3/035006. [DOI] [PubMed] [Google Scholar]
- 25.Barnes C.P. Pemble C.W. Brand D.D. Simpson D.G. Bowlin G.L. Cross-linking electrospun type II collagen tissue engineering scaffolds with carbodiimide in ethanol. Tissue Eng. 2007;13:1593. doi: 10.1089/ten.2006.0292. [DOI] [PubMed] [Google Scholar]
- 26.Declercq H. Van den Vreken N. De Maeyer E. Verbeeck R. Schacht E. De Ridder L., et al. Isolation, proliferation and differentiation of osteoblastic cells to study cell/biomaterial interactions: comparison of different isolation techniques and source. Biomaterials. 2004;25:757. doi: 10.1016/s0142-9612(03)00580-5. [DOI] [PubMed] [Google Scholar]
- 27.Rath B. Nam J. Deschner J. Schaumburger J. Tingart M. Grässel S., et al. Biomechanical forces exert anabolic effects on osteoblasts by activation of SMAD 1/5/8 through type 1 BMP receptor. Biorheology. 2011;48:37. doi: 10.3233/BIR-2011-0580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walker M.B. Kimmel C.B. A two-color acid-free cartilage and bone stain for zebrafish larvae. Biotech Histochem. 2007;82:23. doi: 10.1080/10520290701333558. [DOI] [PubMed] [Google Scholar]
- 29.Bahamonde M.E. Lyons K.M. BMP3: to be or not to be a BMP. J Bone Joint Surg Am. 2001;83A:S56. [PubMed] [Google Scholar]
- 30.Chubinskaya S. Segalite D. Pikovsky D. Hakimiyan A.A. Rueger D.C. Effects induced by BMPS in cultures of human articular chondrocytes: comparative studies. Growth Factors. 2008;26:275. doi: 10.1080/08977190802291733. [DOI] [PubMed] [Google Scholar]
- 31.Bobacz K. Gruber R. Soleiman A. Erlacher L. Smolen J.S. Graninger W.B. Expression of bone morphogenetic protein 6 in healthy and osteoarthritic human articular chondrocytes and stimulation of matrix synthesis in vitro. Arthritis Rheum. 2003;48:2501. doi: 10.1002/art.11248. [DOI] [PubMed] [Google Scholar]
- 32.Hennig T. Lorenz H. Thiel A. Goetzke K. Dickhut A. Geiger F., et al. Reduced chondrogenic potential of adipose tissue derived stromal cells correlates with an altered TGFbeta receptor and BMP profile and is overcome by BMP-6. J Cell Physiol. 2007;211:682. doi: 10.1002/jcp.20977. [DOI] [PubMed] [Google Scholar]
- 33.Kon E. Delcogliano M. Filardo G. Busacca M. Di Martino A. Marcacci M. Novel nano-composite multilayered biomaterial for osteochondral regeneration: a pilot clinical trial. Am J Sports Med. 2011;39:1180. doi: 10.1177/0363546510392711. [DOI] [PubMed] [Google Scholar]
- 34.Bandyopadhyay A. Tsuji K. Cox K. Harfe B.D. Rosen V. Tabin C.J. Genetic analysis of the roles of BMP2, BMP4, and BMP7 in limb patterning and skeletogenesis. Plos Genet. 2006;2:2116. doi: 10.1371/journal.pgen.0020216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin E.A. Kong L. Bai X.H. Luan Y. Liu C.J. miR-199a, a bone morphogenic protein 2-responsive MicroRNA, regulates chondrogenesis via direct targeting to Smad1. J Biol Chem. 2009;284:11326. doi: 10.1074/jbc.M807709200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Majumdar M.K. Wang E. Morris E.A. BMP-2 and BMP-9 promotes chondrogenic differentiation of human multipotential mesenchymal cells and overcomes the inhibitory effect of IL-1. J Cell Physiol. 2001;189:275. doi: 10.1002/jcp.10025. [DOI] [PubMed] [Google Scholar]
- 37.Matsubara T. Kida K. Yamaguchi A. Hata K. Ichida F. Meguro H., et al. BMP2 regulates Osterix through Msx2 and Runx2 during osteoblast differentiation. J Biol Chem. 2008;283:29119. doi: 10.1074/jbc.M801774200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yeh L.C. Tsai A.D. Lee J.C. Osteogenic protein-1 (OP-1, BMP-7) induces osteoblastic cell differentiation of the pluripotent mesenchymal cell line C2C12. J Cell Biochem. 2002;87:292. doi: 10.1002/jcb.10315. [DOI] [PubMed] [Google Scholar]
- 39.Li J.Z. Li H. Sasaki T. Holman D. Beres B. Dumont R.J., et al. Osteogenic potential of five different recombinant human bone morphogenetic protein adenoviral vectors in the rat. Gene Ther. 2003;10:1735. doi: 10.1038/sj.gt.3302075. [DOI] [PubMed] [Google Scholar]
- 40.Gamer L.W. Ho V. Cox K. Rosen V. Expression and function of BMP3 during chick limb development. Dev Dyn. 2008;237:1691. doi: 10.1002/dvdy.21561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nakaoka R. Hsiong S.X. Mooney D.J. Regulation of chondrocyte differentiation level via co-culture with osteoblasts. Tissue Eng. 2006;12:2425. doi: 10.1089/ten.2006.12.2425. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.