Abstract
The increasing clinical demand for bone substitutes has driven significant progress in cell-based therapies for bone tissue engineering. The underpinning goals for success are to identify the most appropriate cell source and to provide three-dimensional (3D) scaffolds that support cell growth and enhance osteogenic potential. In this study, human dental pulp stromal cells (HDPSCs) were cultured under basal or osteogenic conditions either in monolayers or on 3D Bioglass® scaffolds in vitro for 2 or 4 weeks. Cell–scaffold constructs were also implanted intraperitoneally in nude mice for 8 weeks. Osteogenic potential was assessed using quantitative real-time polymerase chain reaction and histological/immunohistochemical assays. In monolayer culture, osteoinductive conditions enhanced HDPSC expression of osteogenic gene markers (COL1A1, RUNX2, OC, and/or OCN) compared with basal conditions while culture of HDPSCs on 3D scaffolds promoted osteogenic gene expression compared with monolayer culture under both basal and osteogenic conditions. These results were confirmed using histological and immunohistochemical analyses. In vivo implantation of the HDPSC 3D Bioglass constructs showed evidence of sporadic woven bone-like spicules and calcified tissue. In conclusion, this study has demonstrated the potential of using a combination of HDPSCs with 3D 45S5 Bioglass scaffolds to promote bone-like tissue formation in vitro and in vivo, offering a promising approach for clinical bone repair and regeneration.
Introduction
Restoration of bone or complex tissue loss due to trauma, tumors, or degenerative disease is still a major medical challenge.1–4 To date, autologous bone grafts are considered to be the optimum choice for fracture repair and bone restoration. However, the major disadvantages of these include limited bone stock, the requirement for additional surgical intervention, and complications at the donor site.5–10 Other types of bone grafts, for example, allograft or xenograft, carry risks of immunogenicity, poor bio-incompatibility, and the potential transmission of communicable viral infections.5–8 Stem-cell-based bone tissue engineering may provide an alternative approach to address these problems.1,5–7,9,11,12
Bone tissue engineering requires cells that are capable of differentiating down the osteogenic lineage to produce bone-relevant extracellular matrix (ECM).13,14 However, a three-dimensional (3D) scaffold framework is essential to support cell growth and allow vascular invasion and ECM deposition. Scaffolds can be also osteoinductive to accelerate osteogenic differentiation.15–17 Human dental pulp stromal cells (HDPSCs) are known to be highly proliferative, multipotent, and capable of producing mineralized nodules in monolayer culture18,19 as well as producing dentine-like regenerative tissue or mineralized tissue similar to bone.20–22
Silicate bioactive glasses, first investigated by Hench et al. (1971),23 have been well researched as 3D bone tissue scaffolds. The application of bioactive glasses and glass–ceramics in bone tissue engineering is expanding.24–26 Further, bioactive glasses can also serve as carriers for the local delivery of metal ions to control cellular functions.27 The dissolution products from such glasses can upregulate expression of genes that control osteogenesis.28,29 In addition, there is increasing evidence for the positive effects of bioactive glass on vascularization of tissue engineering constructs.29–31
The aim of this study was to investigate the potential use of HDPSCs in combination with 45S5 Bioglass® scaffolds for bone tissue engineering.
Materials and Methods
Cell culture plastics were purchased from Corning. Alpha-modified minimum essential medium (α-MEM), phosphate-buffered saline solution, and fetal bovine serum (FBS) were obtained from Lonza. Antibiotics, growth factors, enzymes, and other reagents were purchased from Sigma unless stated otherwise.
The starting bioactive glass powder selected for this work was 45S5 Bioglass with the standard composition of 45 wt% silicon dioxide, 24.5 wt% sodium oxide, 24.5 wt% calcium oxide, and 6 wt% phosphorus pentoxide.32 Scaffolds were produced by the foam replication technique developed earlier and the foams exhibited porosity of ∼90%.33 Briefly, a polyurethane (PU) foam, which serves as a sacrificial template, is immersed in a Bioglass slurry leading to a homogeneous glass particle coating adhering on the PU foam surfaces. The slurry contains polyvinyl alcohol (PVA) (Sigma Aldrich) as binder. After air drying, the foams are sintered at 1100°C with a presintering step to burn off the binder and the PU template. During this process, the foam struts densify and the glass crystallizes, leading to a Bioglass-based scaffold of suitable structural integrity for further investigation.
Cell isolation and in vitro expansion
Three wisdom teeth were obtained from one male (age 19 years) and two female (age 20 years and 37 years) donors with full patient consent and ethical approval (LREC 07/H1306/93). HDPSCs were isolated using the collagenase digestion method previously described by Ricordi et al. (1992)34 and Gronthos et al. (2000).18 The cells were maintained in basal media (α-MEM supplemented with 20% FBS, 200 mM L-glutamine, and 100 unit/mL penicillin–streptomycin) at 37°C and 5% CO2 until 80% confluent. Passage 4 cells (P4) were used for this study.
HDPSC culture as monolayers in vitro
HDPSCs were seeded in six-well plates (5×105 cells per well, n=3) and cultured under basal or osteogenic (basal medium supplemented with 0.1 nM dexamethasone and 100 μM of L-ascorbic-2-acid) conditions for up to 2 and 4 weeks. The samples were then collected for quantitative real-time polymerase chain reaction (qRT-PCR).
HDPSC seeding and growth on 3D scaffolds
The 3D Bioglass scaffolds were cut into standardized cubes of 5×5×5 mm3 and sterilized for 45 min under UV light (253.7 nm wavelength) using a reflective surface to allow simultaneous sterilization of all scaffold surfaces.
HDPSCs (5×105 cells per scaffold) were seeded dynamically on 3D Bioglass scaffolds for 5 days before being statically cultured in 12-well plates (n=3). The cell–scaffold constructs were cultured under basal or osteogenic conditions for 2 and 4 weeks. Monolayer cultures were used as controls.
Determination of osteogenic gene expression using qRT-PCR
Expression of osteogenic marker genes (COL1A1, ALP, RUNX2, and OC) was assessed using qRT-PCR. GAPDH was used as housekeeping gene. RNA was extracted using the trizol reagent kit (Invitrogen) according to the manufacturer's instructions. One microgram of RNA from each sample was used for reverse transcription using the ABI high-capacity RNA to cDNA kit (Applied Bioscience) according to the supplier's instructions. cDNA was then amplified using ABI TaqMan primers (Table 1) in a 20 μL reaction mix in 96-well plates (Roche). Amplification was performed using a Roche LC480 cycler. The results were analyzed using the 2−ΔΔct method35 where ct values at each time point were normalized to the house keeping gene in the same sample and further normalized to ct values of control samples (monolayers or constructs cultured under basal conditions) at the corresponding time points. Results were then expressed as mean±SD.
Table 1.
Details of Primers Used for Taqman® Gene Expression Assays
| Gene name | Gene group | Taqman gene primer |
|---|---|---|
| Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) | Dehydrogenase (housekeeping gene/control) | Hs99999905-m1 |
| Collagen type I, alpha 1 (COL1A1) | Extracellular matrix structural protein (gene of interest) | Hs00164004-m1 |
| Alkaline phosphatase (ALP) | Phosphatase (bone marker, gene of interest) | Hs01029144-m1 |
| Runt related transcription factor 2 (RUNX2) | Transcription factor (bone marker, gene of interest) | Hs00231692-m1 |
| Bone gamma- carboxyglutamate (gla)/Osteocalcin (OC) | Select calcium binding protein (bone marker, gene of interest) | Hs00609452-g1 |
Cell viability and growth on 3D Bioglass scaffolds
At 2 and 4 weeks, the constructs were labeled with Cell Tracker™ Green (CMFDA). Cell viability and growth on the scaffolds was visualized under the confocal laser scanning microscope (CLSM; LEICA TCS SP2). Cell morphology and tissue formation were observed under the scanning electron microscope (SEM; JEOLJSM35).
In vivo implantation of HDPSCs and 3D Bioglass scaffolds
After dynamic seeding for 5 days and in vitro culture under basal conditions for a further 2 days, the constructs were sealed in diffusion chambers (Millipore) prior to intraperitoneal implantation in male immunecompromised nude mice (MF1-Nu/Nu, 4–5 weeks old).36,37 After 8 weeks, the diffusion chambers were retrieved and samples were fixed in 10% NBF or 98% ethanol (for ALP staining) prior to being processed for histology and immunohistocytochemistry.
Histological and immunohistochemical examination of cell–scaffold constructs
To detect alkaline phosphatase (ALP) activity, HDPSC–Bioglass constructs were stained directly using the ALP staining kit (Sigma)38,39 and viewed using a 3D light microscope.
In addition, sections were prepared of the fixed and wax-embedded constructs and stained with either standard hematoxylin and eosin (H&E) or Alizarin red [2% aqueous alizarin red (pH 4.3) for 30 s] to detect calcium deposits.
Immunohistochemical staining of the sections was carried out using primary antibodies against collagen type I (Abcam; mouse monoclonal, ab6308) and osteocalcin (Abcam; mouse monoclonal, ab13420) (Table 2). The Envision kit (Dako) was used to provide secondary antibodies and substrate in each case.
Table 2.
Details of Antibodies/Counter Stains Used in Immunohistochemical Analyses
| Primary antibody name/number | Antigen retrieval | Primary antibody concentration and incubation time | Counter staining |
|---|---|---|---|
| Collagen type I (mouse monoclonal, ab6308) | Pressure cooker with vector unmasking solution for 5 min | 1/100 Overnight |
H&E |
| Osteocalcin (mouse monoclonal, ab13420) | 0.1% chymotrypsin (0.05 g of chymotrypsin) for 15 min | 1/100 Overnight |
H&E |
H&E, hematoxylin and eosin.
Statistical analysis
qRT-PCR data were statistically analyzed using an analysis of variance one-way test followed by Bonferroni multiple comparison tests. The statistical analyses were carried out using the GraphPad InStat software (version 3). Each experiment was repeated three times from three different donors for each cell type. Results shown are presented from one representative donor per cell type.
Results
HDPSC viability, growth, and osteoblastic differentiation on 3D Bioglass scaffolds under basal culture conditions in vitro
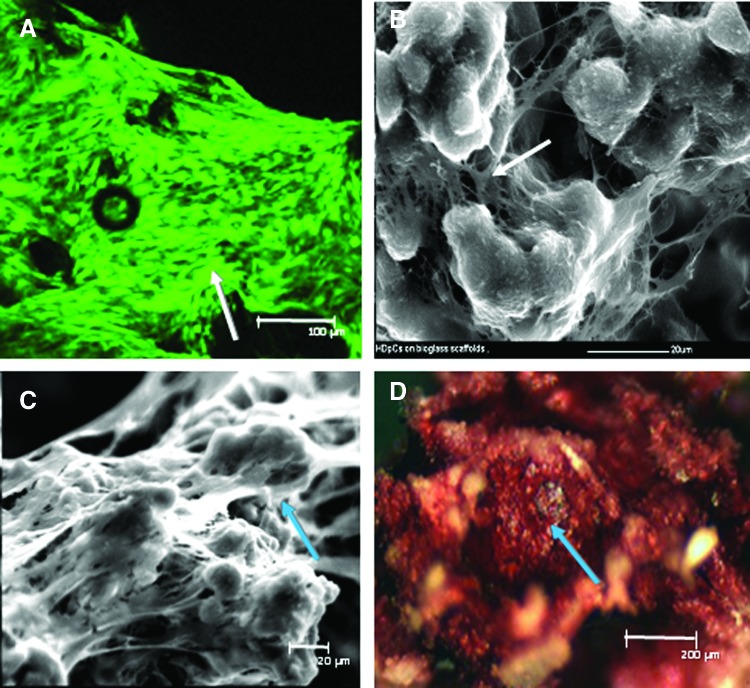
HDPSCs on 3D Bioglass scaffolds were cultured under basal conditions to determine any osteogenic effect of the scaffolds themselves. Live/dead fluorescent staining coupled with CLSM images showed that the majority of the attached HDPSCs had maintained viability following culture on the scaffolds. Cells appeared to have a fibroblastic morphology—indicative of cell spreading—and had covered the scaffolds after 2 weeks of culture, suggesting good proliferation (Fig. 1A). SEM images confirmed the presence of confluent cell layers and cell bridge formation after both 2 (Fig. 1B) and 4 weeks (Fig. 1C). Intense positive staining for ALP was seen within the constructs even under basal conditions, indicating osteoblastic differentiation after 4 weeks of culture in vitro (Fig. 1D).
FIG. 1.
Human dental pulp stromal cell (HDPSC) viability and growth on three-dimensional (3D) 45S5 Bioglass® scaffolds. (A) Confocal laser scanning microscope image showing HDPSC viability (arrow–CMFDA) on 45S5 Bioglass scaffolds after 2 weeks of in vitro culture under basal condition. (B, C) scanning electron microscope (SEM) images showing HDPSC spreading and cell bridge formation (arrows) on 45S5 Bioglass scaffolds after in vitro culture under basal conditions for 2 weeks (B) and 4 weeks (C). (D) Alkaline phosphatase (ALP) staining (arrow) indicative of osteogenic differentiation of HDPSC–45S5 Bioglass scaffolds after 4 weeks of culture in basal medium. Color images available online at www.liebertpub.com/tea
Effect of osteogenic culture conditions on gene expression of HDPSCs on 3D Bioglass scaffolds
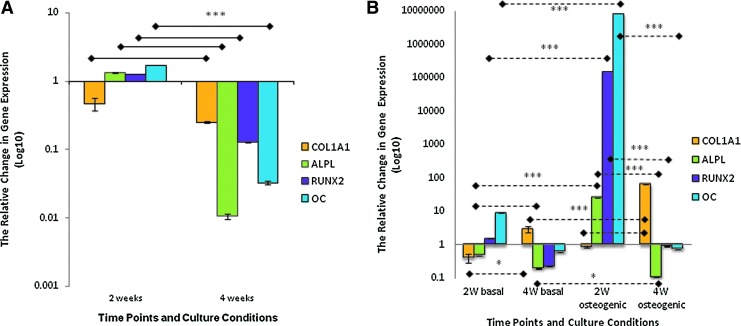
Under osteogenic culture conditions, the levels of COL1A1 gene expression in HDPSC–Bioglass scaffold constructs were lower at both 2 and 4 weeks compared with those of the basal culture group. In contrast, the levels of expression of ALP, RUNX2, and OC were significantly greater at 2 weeks (p<0.001) under osteogenic compared with basal conditions. However, there was a dramatic drop in the levels of expression of these genes at 4 weeks (Fig. 2A).
FIG. 2.
Relative changes in osteogenic marker gene expression for HDPSCs cultured on 45S5 Bioglass scaffolds in vitro. (A) The effect of osteogenic culture conditions on the expression of osteogenic marker genes by HDPSCs on 3D Bioglass scaffolds after 2 and 4 weeks (n=3). The data for osteogenic conditions were normalized to corresponding controls cultured under basal conditions using the ΔΔct method. (B) The effect of 3D Bioglass scaffolds on osteogenic marker gene expression by HDPSCs cultured under basal or osteogenic condition on 3D Bioglass scaffolds for 2 and 4 weeks (n=3). The data for 3D culture were normalized to corresponding controls in monolayers using the ΔΔct method. The data are presented as log10 of the mean 2−ΔΔct±SD (*p<0.05, ***p<0.001). Color images available online at www.liebertpub.com/tea
Effect of 3D Bioglass scaffolds on HDPSC osteogenic marker gene expression compared with monolayer culture
When comparing the levels of gene expression in 3D constructs with monolayer controls cultured under the same conditions, the levels of COL1A1 in the constructs under basal conditions were lower at 2 weeks but higher at 4 weeks compared with those of monolayer culture group (p≤0.001). The levels of ALP expression in 3D culture were lower than in monolayer culture at both time points. RUNX2 and OC showed a significantly higher expression at 2 weeks (p≤0.001) with a dramatically lower expression at 4 weeks compared with monolayer cultures (Fig. 2B). Under osteogenic culture conditions, COL1A1 levels were downregulated at 2 weeks in the 3D constructs compared with monolayer controls but were significantly upregulated at 4 weeks (p≤0.001). In contrast, ALP, RUNX2, and OC levels were raised significantly at 4 weeks (p≤0.001) compared with monolayer controls and then drastically decreased at 4 weeks.
Histological appearance of neotissue formation by HDPSCs on 3D Bioglass scaffold constructs in vitro
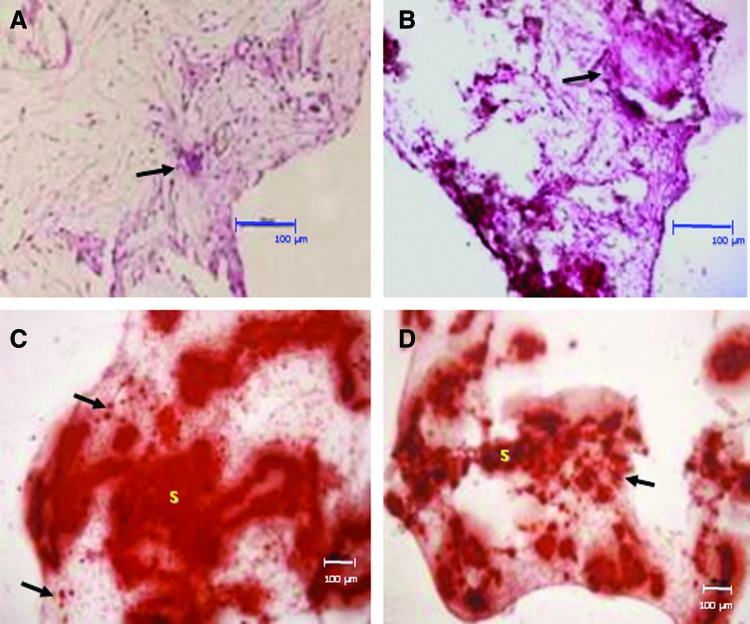
After 6 weeks of in vitro culture under basal and osteogenic conditions, H&E staining showed that all scaffold pores appeared to have been filled with cells and ECM. In many cases, the appearance was typical of mesenchymal tissue condensation, comprising of dense ECM. Cells in these regions sometimes acquired a more cuboidal, osteoblast-like morphology irrespective of the culture conditions used (Fig. 3A, B).
FIG. 3.
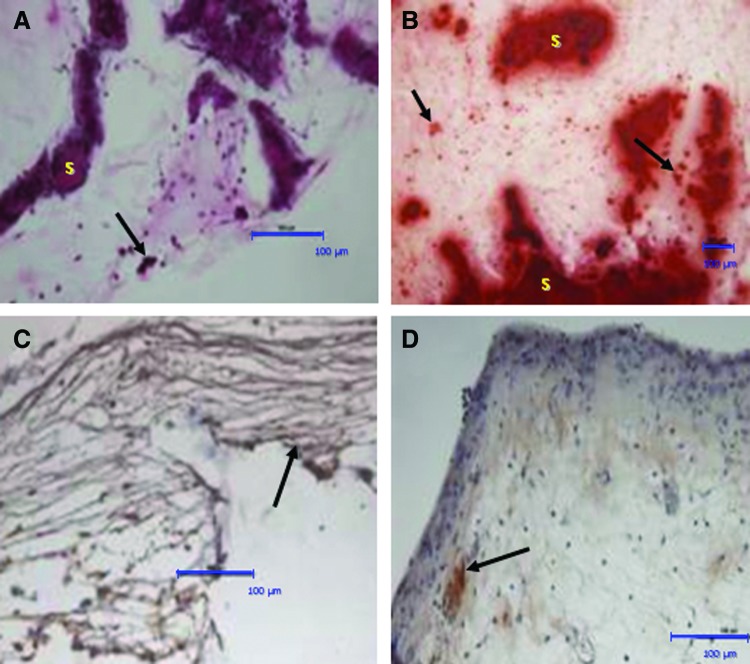
Hematoxylin and eosin (H&E) and Alizarin red staining of HDPSCs cultured on 3D Bioglass scaffolds in vitro. (A, B) H&E staining following 6 weeks of culture under basal conditions (A) and osteogenic conditions (B). Arrows point to regions of extracellular matrix condensation/woven bone-like spicules. (C, D) Alizarin red staining for calcium deposits in the cell–scaffold constructs cultured in vitro for 6 weeks under basal conditions (C) and osteogenic conditions (D). Despite the fact that the calcium-rich scaffolds themselves (“S”) are intensely stained with the Alizarin red, staining can also be seen in the “tissue” surrounding the scaffold (arrows). Scale bars=100 μm. Color images available online at www.liebertpub.com/tea
Alizarin red staining was used to indicate any calcium-rich deposits within the constructs. The Bioglass scaffolds themselves contained calcium and are known to be able to initiate hydroxyapatite deposition40–42 and this was reflected by the presence of high amounts of the red/orange Alizarin red staining associated with the scaffolds. However, it was still possible to discern small Alizarin-red-positive deposits within the surrounding cell layers and/or matrix for constructs cultured under both basal (Fig. 3C) and osteogenic cultures (Fig. 3D).
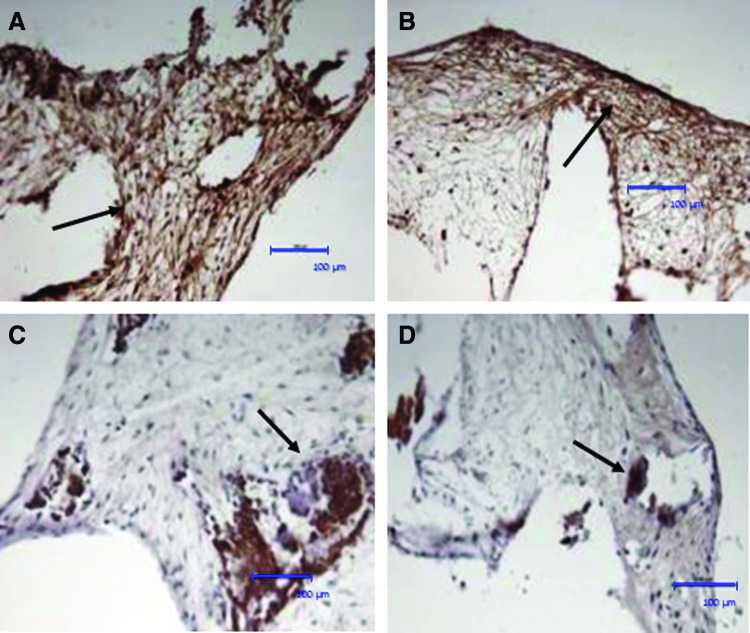
Immunohistochemistry revealed that the “tissue” formed within the constructs stained positively for collagen type I. Positively stained fibers showed a compact bundle arrangement and were oriented parallel to the inner or outer scaffold surface (Fig. 4A, B), which was comparable in the osteogenic culture group. Osteocalcin was also detected in the constructs under basal conditions (Fig. 4C) with higher staining intensity apparent under osteogenic conditions (Fig. 4D).
FIG. 4.
Immunohistochemical staining of HDPSC–scaffold constructs cultured in vitro. After 6 weeks of culture in basal condition in vitro, positive immunohistochemical staining was seen for collagen type I (A, B); arrows indicate collagen-I-positive fibers/bundles running parallel to the construct surface. The constructs were also positive for osteocalcin (C, D). All sections were counterstained with Harris hematoxylin. Scale bars=100 μm. Color images available online at www.liebertpub.com/tea
Osteogenic differentiation of HDPSCs in 3D Bioglass scaffolds in vivo
After 8 weeks of intraperitoneal implantation in nude mice, H&E staining revealed extensively condensed mesenchymal tissue formation within the constructs, including apparently polarized cuboidal/columnar cells with a parallel orientation adjacent to the scaffold surface, together with the presence of larger, woven bone-like spicules (Fig. 5A).
FIG. 5.
H&E and Alizarin red staining of HDPSC–3D Bioglass scaffold constructs following intraperitoneal implantation in vivo in diffusion chambers. After 8 weeks in vivo implantation, H&E staining (A) indicated extracellular matrix condensation/woven bone-like spicule formation (arrows). Alizarin red staining (B) for calcium deposits in HDPSC–scaffold constructs showed that despite the fact that the calcium-rich scaffolds themselves (“S”) were intensely stained with the Alizarin red, additional staining was also seen in the “tissue” surrounding the scaffold (arrows). (C) Immunohistochemical staining for collagen type I shows parallel collagen-I-positive fiber/bundle formation (arrows). (D) Immunohistochemical staining for osteocalcin was positive in all samples (sections counterstained with Harris hematoxylin). Scale bars=100 μm. Color images available online at www.liebertpub.com/tea
Alizarin red staining showed evidence of calcium deposition within the neotissue formed around the scaffolds. Further, the scaffolds themselves seemed to be “thickened” with Alizarin red deposits at the interface between the scaffolds and the ECM (Fig. 5B).
Immunohistochemistry confirmed that newly formed “tissue” in the in vivo constructs was positive for osteogenic markers, including collagen type I (Fig. 5C) and osteocalcin (Fig. 5D). In addition, the cell–scaffold constructs had evidence of condensed bundles of collagen type I–positive fibers parallel to the outer surface of the scaffold (Fig. 5C).
Discussion
The emerging field of cell-based tissue engineering currently offers great promise to meet the clinical need for bone substitutes. There has been growing interest in using HDPSCs for mineralized tissue regeneration.18,19 Despite the fact that we do not yet know enough in respect of the total stem cell number and type within dental pulp and how this compares with cells from other sources (e.g., bone marrow or adipose tissue), there is evidence to suggest that the stem cells from dental pulp are more “immature” and have a higher colony-forming unit-fibroblast (CFU-F) capacity compared with human bone marrow stem/stromal cells (HBMSCs) (Gronthos et al., 2000).18 In addition, deciduous teeth, permanent premolars, and wisdom teeth are readily accessible during everyone's life time. This has led to the establishment of many dental cell banks worldwide (e.g., Biobank, Tayside Tissue Bank, UK Stem Cell Bank, UK Biobank, Precious Cells, Store-a-Tooth Stem Cell Bank, BioEden Tooth Cell Bank, and Dhruv Dental Stem Cell Bank),43 emphasizing the need for more research on stem cells from this tissue source.
Various criteria have been set out for selection of appropriate 3D scaffolds for tissue engineering applications.13,14,16,44 Yang et al. (2006)37 showed that Bioglass-containing composite scaffolds supported HBMSC attachment, growth, and osteogenic differentiation in vitro and in vivo. Therefore, in the present study, a Bioglass-based scaffold was used due to its reported porosity, bioactivity, and osteoconductivity.42,45–47
This study has confirmed that the Bioglass scaffolds are biocompatible as evidenced by good cell attachment and spreading and maintenance of cell viability within the constructs. This agrees with our previous report for HBMSCs37 and extends it to HDPSCs. HDPSCs seeded on to Bioglass scaffolds were shown to form neotissue structures in vitro and in vivo with evidence of osteogenic differentiation as evidenced by the PCR data and immunohistochemistry.
Most previous publications related to HDPSC gene expression were carried out in monolayer culture.48–50 In this study, HDPSC expression of osteogenic marker genes was investigated in 3D culture using the Bioglass scaffolds to provide a construct. A number of studies have shown that Bioglass scaffolds stimulate osteoblastic differentiation37,51,52 and the ionic dissolution products of 45S5 Bioglass are known to affect the gene expression profile of human osteoblasts in monolayer cultures.29,53 However, these studies used relatively short culture periods of 48 h52 and/or 5 days compared with the longer periods used here.53
This study showed that in monolayer culture, COL1A was downregulated at both 2 and 4 weeks under osteoinductive conditions compared with controls in basal medium. Previous studies have suggested that COL1A1 is closely correlated with the osteoblast proliferation phase. It would therefore be expected to be expressed highly during proliferation and at the beginning of ECM production and to be downregulated in more advanced stages of the active osteoblasts, when mineralization starts.54,55 Alternatively or in addition, this may also due to the inhibitory effect of Dexamethasone (present in the osteoinductive medium) on proliferation of the cells under osteogenic culture conditions.56
ALP, RUNX2, and OC were all upregulated in the monolayer HDPSCs under osteogenic conditions at 2 weeks compared with controls in basal medium. This was followed by a dramatic downregulation at 4 weeks, commensurate with advancing osteoblast differentiation.54 The expression patterns seen in this monolayer study were very similar to those reported by Yamada (2010).57
In comparison with monolayers, HDPSCs cultured on 45S5 Bioglass scaffolds and in osteoinductive culture showed enhanced expression of osteogenic gene after 2 weeks. These findings confirmed that 45S5 Bioglass scaffolds are osteoinductive and able to promote mineralization as confirmed by the downregulation of ALP, upregulation of OC, and by the Alizarin red staining data. Osteogenic growth factors, such as BMPs, are known to stimulate inhibitory growth factors and antagonist binding proteins, leading to a feedback inhibitory loop58 that can explain the overall downregulation of osteogenic marker genes by the constructs after 4 weeks of culture.
The histological and immunohistochemical results suggested that constructs cultured under basal and osteogenic conditions in vitro showed a shift in cell morphology toward more plump and cuboidal cells, which produce an organized ECM with evidence of calcium-rich deposits. In vivo results showed a greater extent of organized ECM and more evidence of calcium-rich deposits within the constructs. These data were further supported by the immunohistochemistry findings, where in vivo constructs showed extensive staining for both collagen type I and OCN, characteristic of more mature osteoblasts.59–61 Further, bone-like spicules were seen only within in vivo–retrieved constructs. This might be attributed to the longer period of culture (8 weeks) or due to the provision of an appropriate microenvironment in vivo that enhances blood supply and exchange of fluids, together with cytokines and host diffusible products.22,62–64
Zhang et al. (2006) demonstrated the formation of thick capsules and ECM by HDPSCs under osteogenic conditions on three types of 3D scaffolds in vivo. However, mineralization was only observed for titanium scaffold constructs.48 The results presented here showed that constructs using 45S5 Bioglass formed tissue condensations with osteoblast-like cells (e.g., cuboidal morphology) and Alizarin red positive that are indicative of calcium-rich deposits. Although the scaffolds themselves picked up the Alizarin red stain due to their intrinsic calcium content, constructs seeded with HDPSCs showed more staining between and/or surrounding the scaffolds compared with scaffolds alone, an indication of the well-documented bioactivity of Bioglass.45
Mineralized tissue formation has been observed previously when HDPSCs have been implanted in vivo. Otaki et al. (2007) implanted HDPSCs with hydroxyapatite/tricalcium phosphate (HA/TCP) powder subcutaneously in immunecompromised mice for 7 and 15 weeks and demonstrated lamellae bone formation.49 Graziano et al. (2008) showed formation of bone nodules by combining human dental pulp tissue with poly(lactic-co-glycolic acid) scaffolds in a subcutaneous implantation module in immunecompromised rats.65 Other studies demonstrated that HDPSCs formed dentine-like tissue in vivo while HBMSCs formed bone and these results may be attributed to the use of the subcutaneous implantation model where epithelial tissue from the host can contribute to reciprocally inducing the HDPSCs into odontoblasts rather than osteoblasts.18,66–68 This is in contrast to the present study, where there was no evidence of odontoblastic differentiation in the intraperitoneal diffusion chamber that does not permit any cellular contribution to the constructs from the host cells. The diffusion chamber model provided a simple in vivo bioreactor for this work. However, a bone defect model may provide a better microenvironment for angiogenesis and bone remodeling for future studies.69,70
Conclusion
45S5 Bioglass scaffolds can enhance osteogenic differentiation of HDPSCs both in vitro and in vivo, indicating the potential use of this combination for bone tissue engineering for clinical applications.
Acknowledgments
This work was partially funded through NIHR and WELMEC, a Centre of Excellence in Medical Engineering funded by the Wellcome Trust and EPSRC, under grant number WT 088908/Z/09/Z. R.E. was funded by the University of Suez Canal through the Egyptian Ministry of Higher Education.
Disclosure Statement
No competing financial interests exist.
References
- 1.Arrington E.D. Smith W.J. Chambers H.G. Bucknell A.L. Davino N.A. Complications of iliac crest bone graft harvesting. Clin Orthop Relat Res. 1996;329:300. doi: 10.1097/00003086-199608000-00037. [DOI] [PubMed] [Google Scholar]
- 2.Laurencin C. Khan Y. El-Amin S.F. Bone graft substitutes. Expert Rev Med Devices. 2006;3:49. doi: 10.1586/17434440.3.1.49. [DOI] [PubMed] [Google Scholar]
- 3.Gu Y.D. Cheng D.S. Zhang G.M. Chen X.M. Xu J.G. Yang X.B. Long-term results of toe transfer: retrospective analysis. J Reconstr Microsurg. 1997;13:405. doi: 10.1055/s-2007-1006420. [DOI] [PubMed] [Google Scholar]
- 4.Yang X.B. Oreffo R. Bone tissue engineering. In: Hong-wen Deng Y.-z.L., editor; Guo C.-Y., editor. Current topics in bone biology. 1st. Singapore: World Scientific Publishing Ltd; 2005. p. 435. [Google Scholar]
- 5.Kroon F.H. Perren S.M. van den Hoof A. Behavior of cortical bone grafts under different types of fixation. Int J Oral Surg. 1977;6:131. doi: 10.1016/s0300-9785(77)80045-8. [DOI] [PubMed] [Google Scholar]
- 6.Prolo D.J. Rodrigo J.J. Contemporary bone graft physiology and surgery. Clin Orthop Relat Res. 1985;200:322. [PubMed] [Google Scholar]
- 7.Yaszemski M.J. Payne R.G. Hayes W.C. Langer R. Mikos A.G. Evolution of bone transplantation: molecular, cellular and tissue strategies to engineer human bone. Biomaterials. 1996;17:175. doi: 10.1016/0142-9612(96)85762-0. [DOI] [PubMed] [Google Scholar]
- 8.Blau H.M. Brazelton T.R. Weimann J.M. The evolving concept of a stem cell: entity or function? Cell. 2001;105:829. doi: 10.1016/s0092-8674(01)00409-3. [DOI] [PubMed] [Google Scholar]
- 9.Morishita T. Honoki K. Ohgushi H. Kotobuki N. Matsushima A. Takakura Y. Tissue engineering approach to the treatment of bone tumors: three cases of cultured bone grafts derived from patients' mesenchymal stem cells. Artif Organs. 2006;30:115. doi: 10.1111/j.1525-1594.2006.00190.x. [DOI] [PubMed] [Google Scholar]
- 10.Yang X.B. Gu Y.D. The donor foot in free toe or joint transfers. J Hand Surg Br. 2000;25:382. doi: 10.1054/jhsb.2000.0397. [DOI] [PubMed] [Google Scholar]
- 11.Attawia M.A. Herbert K.M. Uhrich K.E. Langer R. Laurencin C.T. Proliferation, morphology, and protein expression by osteoblasts cultured on poly(anhydride-co-imides) J Biomed Mater Res. 1999;48:322. doi: 10.1002/(sici)1097-4636(1999)48:3<322::aid-jbm17>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 12.Damien C.J. Parsons J.R. Bone graft and bone graft substitutes: a review of current technology and applications. J Appl Biomater. 1991;2:187. doi: 10.1002/jab.770020307. [DOI] [PubMed] [Google Scholar]
- 13.Rose F.R. Oreffo R.O. Bone tissue engineering: hope vs hype. Biochem Biophys Res Commun. 2002;292:1. doi: 10.1006/bbrc.2002.6519. [DOI] [PubMed] [Google Scholar]
- 14.Salgado A.J. Coutinho O.P. Reis R.L. Bone tissue engineering: state of the art and future trends. Macromol Biosci. 2004;4:743. doi: 10.1002/mabi.200400026. [DOI] [PubMed] [Google Scholar]
- 15.Yang X.B. Roach H.I. Clarke N.M. Howdle S.M. Quirk R. Shakesheff K.M. Oreffo R.O. Human osteoprogenitor growth and differentiation on synthetic biodegradable structures after surface modification. Bone. 2001;29:523. doi: 10.1016/s8756-3282(01)00617-2. [DOI] [PubMed] [Google Scholar]
- 16.Rezwan K. Chen Q.Z. Blaker J.J. Boccaccini A.R. Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials. 2006;27:3413. doi: 10.1016/j.biomaterials.2006.01.039. [DOI] [PubMed] [Google Scholar]
- 17.Graziano A. d'Aquino R. Cusella-De Angelis M.G. De Francesco F. Giordano A. Laino G. Piattelli A. Traini T. De Rosa A. Papaccio G. Scaffold's surface geometry significantly affects human stem cell bone tissue engineering. J Cell Physiol. 2008;214:166. doi: 10.1002/jcp.21175. [DOI] [PubMed] [Google Scholar]
- 18.Gronthos S. Mankani M. Brahim J. Robey P.G. Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A. 2000;97:13625. doi: 10.1073/pnas.240309797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gronthos S. Brahim J. Li W. Fisher L.W. Cherman N. Boyde A. DenBesten P. Robey P.G. Shi S. Stem cell properties of human dental pulp stem cells. J Dent Res. 2002;81:531. doi: 10.1177/154405910208100806. [DOI] [PubMed] [Google Scholar]
- 20.Batouli S. Miura M. Brahim J. Tsutsui T.W. Fisher L.W. Gronthos S. Robey P.G. Shi S. Comparison of stem-cell-mediated osteogenesis and dentinogenesis. J Dent Res. 2003;82:976. doi: 10.1177/154405910308201208. [DOI] [PubMed] [Google Scholar]
- 21.About I. Dentin Regeneration in vitro: the pivotal role of supportive cells. Adv Dent Res. 2011;23:320. doi: 10.1177/0022034511405324. [DOI] [PubMed] [Google Scholar]
- 22.Graziano A. d'Aquino R. Laino G. Papaccio G. Dental pulp stem cells: a promising tool for bone regeneration. Stem Cell Rev. 2008;4:21. doi: 10.1007/s12015-008-9013-5. [DOI] [PubMed] [Google Scholar]
- 23.Hench L.L. Splinter R.J. Allen W.C. Bonding mechanisms at interface of ceramic prosthetic materials. J Biomed Mater Res Symp. 1971;2:117. [Google Scholar]
- 24.Vitale-Brovarone C. Baino F. Three-dimensional glass-derived scaffolds for bone tissue engineering: Current trends and forecasts for the future. J Biomed Mater Res Part A. 2011;97A:514. doi: 10.1002/jbm.a.33072. [DOI] [PubMed] [Google Scholar]
- 25.Rahaman M.N. Day D.E. Bal B.S. Fu Q. Jung S.B. Bonewald L.F. Tomsia A.P. Bioactive glass in tissue engineering. Acta Biomaterialia. 2011;7:2355. doi: 10.1016/j.actbio.2011.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gerhardt L.C. Boccaccini A.R. Bioactive glass and glass-ceramic scaffolds for bone tissue engineering. Materials. 2010;3:3867. doi: 10.3390/ma3073867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boccaccini A.R. Hoppe A. Guldal N.S. A review of the biological response to ionic dissolution products from bioactive glasses and glass-ceramics. Biomaterials. 2011;32:2757. doi: 10.1016/j.biomaterials.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 28.Polak J.M. Xynos I.D. Hukkanen M.V.J. Batten J.J. Buttery L.D. Hench L.L. Bioglass (R) 45S5 stimulates osteoblast turnover and enhances bone formation in vitro: implications and applications for bone tissue engineering. Calcified Tissue Int. 2000;67:321. doi: 10.1007/s002230001134. [DOI] [PubMed] [Google Scholar]
- 29.Polak J.M. Xynos I.D. Edgar A.J. Buttery L.D.K. Hench L.L. Gene-expression profiling of human osteoblasts following treatment with the ionic products of Bioglass (R) 45S5 dissolution. J Biomed Mater Res. 2001;55:151. doi: 10.1002/1097-4636(200105)55:2<151::aid-jbm1001>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 30.Gorustovich A.A. Roether J.A. Boccaccini A.R. Effect of bioactive glasses on angiogenesis: a review of in vitro and in vivo evidences. Tissue Eng Part B Rev. 2010;16:199. doi: 10.1089/ten.TEB.2009.0416. [DOI] [PubMed] [Google Scholar]
- 31.Deb S. Mandegaran R. Di Silvio L. A porous scaffold for bone tissue engineering/45S5 Bioglass(A (R)) derived porous scaffolds for co-culturing osteoblasts and endothelial cells. J Mater Sci-Mater M. 2010;21:893. doi: 10.1007/s10856-009-3936-5. [DOI] [PubMed] [Google Scholar]
- 32.Hench L.L. Splinter R.J. Allen W.C. Greenlee T.K. Bonding mechanisms at the interface of ceramic prosthetic materials. J Biomed Mater Res. 1971;5:117. [Google Scholar]
- 33.Chen Q.Z. Thompson I.D. Boccaccini A.R. 45S5 Bioglass-derived glass-ceramic scaffolds for bone tissue engineering. Biomaterials. 2006;27:2414. doi: 10.1016/j.biomaterials.2005.11.025. [DOI] [PubMed] [Google Scholar]
- 34.Ricordi C. Tzakis A.G. Carroll P.B. Zeng Y.J. Rilo H.L. Alejandro R. Shapiro A. Fung J.J. Demetris A.J. Mintz D.H, et al. Human islet isolation and allotransplantation in 22 consecutive cases. Transplantation. 1992;53:407. doi: 10.1097/00007890-199202010-00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Livak K.J. Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(-Delta Delta C) method. Methods. 2001;25:402. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 36.Partridge K. Yang X. Clarke N.M. Okubo Y. Bessho K. Sebald W. Howdle S.M. Shakesheff K.M. Oreffo R.O. Adenoviral BMP-2 gene transfer in mesenchymal stem cells: in vitro and in vivo bone formation on biodegradable polymer scaffolds. Biochem Biophys Res Commun. 2002;292:144. doi: 10.1006/bbrc.2002.6623. [DOI] [PubMed] [Google Scholar]
- 37.Yang X.B. Webb D. Blaker J. Boccaccini A.R. Maquet V. Cooper C. Oreffo R.O. Evaluation of human bone marrow stromal cell growth on biodegradable polymer/bioglass composites. Biochem Biophys Res Commun. 2006;342:1098. doi: 10.1016/j.bbrc.2006.02.021. [DOI] [PubMed] [Google Scholar]
- 38.Green D. Walsh D. Yang X.B. Mann S. Oreffo R.O.C. Stimulation of human bone marrow stromal cells using growth factor encapsulated calcium carbonate porous microspheres. J Mater Chem. 2004;206:212. [Google Scholar]
- 39.Yang X.B. Green D.W. Roach H.I. Clarke N.M. Anderson H.C. Howdle S.M. Shakesheff K.M. Oreffo R.O. Novel osteoinductive biomimetic scaffolds stimulate human osteoprogenitor activity—implications for skeletal repair. Connect Tissue Res. 2003;44(Suppl 1):312. [PubMed] [Google Scholar]
- 40.Bil M. Ryszkowska J. Roether J.A. Bretcanu O. Boccaccini A.R. Bioactivity of polyurethane-based scaffolds coated with Bioglass. Biomed Mater. 2007;2:93. doi: 10.1088/1748-6041/2/2/006. [DOI] [PubMed] [Google Scholar]
- 41.Wirtz D.C. Wolff J.M. Ittel T.H. Jakse G. Niethard F.U. [Is skeletal alkaline phosphatase a valid staging marker in detection of osteoblastic skeletal metastases of prostate carcinoma?] Z Orthop Ihre Grenzgeb. 1998;136:255. doi: 10.1055/s-2008-1054232. [DOI] [PubMed] [Google Scholar]
- 42.Bretcanu O. Misra S. Roy I. Renghini C. Fiori F. Boccaccini A.R. Salih V. In vitro biocompatibility of 45S5 Bioglass-derived glass-ceramic scaffolds coated with poly(3-hydroxybutyrate) J Tissue Eng Regen Med. 2009;3:139. doi: 10.1002/term.150. [DOI] [PubMed] [Google Scholar]
- 43.Specimen Central LLC. Global Directory Of Biobanks, Tissue Banks And Biorepositories. www.specimencentral.com/biobank-directory.aspx. [Jul 24;2012 ]. www.specimencentral.com/biobank-directory.aspx
- 44.Hutmacher D.W. Scaffolds in tissue engineering bone and cartilage. Biomaterials. 2000;21:2529. doi: 10.1016/s0142-9612(00)00121-6. [DOI] [PubMed] [Google Scholar]
- 45.Boccaccini A.R. Blaker J.J. Bioactive composite materials for tissue engineering scaffolds. Expert Rev Med Devices. 2005;2:303. doi: 10.1586/17434440.2.3.303. [DOI] [PubMed] [Google Scholar]
- 46.Boccaccini A.R. Chen Q. Lefebvre L. Gremillard L. Chevalier J. Sintering, crystallisation and biodegradation behaviour of Bioglass-derived glass-ceramics. Faraday Discuss. 2007;136:27. doi: 10.1039/b616539g. discussion 107. [DOI] [PubMed] [Google Scholar]
- 47.Chen Q.Z. Rezwan K. Armitage D. Nazhat S.N. Boccaccini A.R. The surface functionalization of 45S5 Bioglass-based glass-ceramic scaffolds and its impact on bioactivity. J Mater Sci Mater Med. 2006;17:979. doi: 10.1007/s10856-006-0433-y. [DOI] [PubMed] [Google Scholar]
- 48.Zhang W. Walboomers X.F. van Kuppevelt T.H. Daamen W.F. Bian Z. Jansen J.A. The performance of human dental pulp stem cells on different three-dimensional scaffold materials. Biomaterials. 2006;27:5658. doi: 10.1016/j.biomaterials.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 49.Otaki S. Ueshima S. Shiraishi K. Sugiyama K. Hamada S. Yorimoto M. Matsuo O. Mesenchymal progenitor cells in adult human dental pulp and their ability to form bone when transplanted into immunocompromised mice. Cell Biol Int. 2007;31:1191. doi: 10.1016/j.cellbi.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 50.Zhang W. Walboomers X.F. van Osch G.J. van den Dolder J. Jansen J.A. Hard tissue formation in a porous HA/TCP ceramic scaffold loaded with stromal cells derived from dental pulp and bone marrow. Tissue Eng Part A. 2008;14:285. doi: 10.1089/tea.2007.0146. [DOI] [PubMed] [Google Scholar]
- 51.Xynos I.D. Hukkanen M.V. Batten J.J. Buttery L.D. Hench L.L. Polak J.M. Bioglass 45S5 stimulates osteoblast turnover and enhances bone formation In vitro: implications and applications for bone tissue engineering. Calcif Tissue Int. 2000;67:321. doi: 10.1007/s002230001134. [DOI] [PubMed] [Google Scholar]
- 52.Foppiano S. Marshall S.J. Marshall G.W. Saiz E. Tomsia A.P. Bioactive glass coatings affect the behavior of osteoblast-like cells. Acta Biomater. 2007;3:765. doi: 10.1016/j.actbio.2007.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xynos I.D. Edgar A.J. Buttery L.D. Hench L.L. Polak J.M. Gene-expression profiling of human osteoblasts following treatment with the ionic products of Bioglass 45S5 dissolution. J Biomed Mater Res. 2001;55:151. doi: 10.1002/1097-4636(200105)55:2<151::aid-jbm1001>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 54.Owen T.A. Aronow M. Shalhoub V. Barone L.M. Wilming L. Tassinari M.S. Kennedy M.B. Pockwinse S. Lian J.B. Stein G.S. Progressive development of the rat osteoblast phenotype in vitro: reciprocal relationships in expression of genes associated with osteoblast proliferation and differentiation during formation of the bone extracellular matrix. J Cell Physiol. 1990;143:420. doi: 10.1002/jcp.1041430304. [DOI] [PubMed] [Google Scholar]
- 55.Nakashima K. de Crombrugghe B. Transcriptional mechanisms in osteoblast differentiation and bone formation. Trends Genet. 2003;19:458. doi: 10.1016/S0168-9525(03)00176-8. [DOI] [PubMed] [Google Scholar]
- 56.Canalis E. Effect of glucocorticoids on type I collagen synthesis, alkaline phosphatase activity, and deoxyribonucleic acid content in cultured rat calvariae. Endocrinology. 1983;112:931. doi: 10.1210/endo-112-3-931. [DOI] [PubMed] [Google Scholar]
- 57.Yamada Y. Nakamura S. Ito K. Sugito T. Yoshimi R. Nagasaka T. Ueda M. A feasibility of useful cell-based therapy by bone regeneration with deciduous tooth stem cells, dental pulp stem cells, or bone-marrow-derived mesenchymal stem cells for clinical study using tissue engineering technology. Tissue Eng Part A. 2010;16:1891. doi: 10.1089/ten.TEA.2009.0732. [DOI] [PubMed] [Google Scholar]
- 58.Hughes F.J. Turner W. Belibasakis G. Martuscelli G. Effects of growth factors and cytokines on osteoblast differentiation. Periodontol 2000. 2006;41:48. doi: 10.1111/j.1600-0757.2006.00161.x. [DOI] [PubMed] [Google Scholar]
- 59.Yamaguchi A. Komori T. Suda T. Regulation of osteoblast differentiation mediated by bone morphogenetic proteins, hedgehogs, and Cbfa1. Endocr Rev. 2000;21:393. doi: 10.1210/edrv.21.4.0403. [DOI] [PubMed] [Google Scholar]
- 60.Liggett W.H., Jr. Lian J.B. Greenberger J.S. Glowacki J. Osteocalcin promotes differentiation of osteoclast progenitors from murine long-term bone marrow cultures. J Cell Biochem. 1994;55:190. doi: 10.1002/jcb.240550206. [DOI] [PubMed] [Google Scholar]
- 61.Liu F. Malaval L. Aubin J.E. The mature osteoblast phenotype is characterized by extensive plasticity. Exp Cell Res. 1997;232:97. doi: 10.1006/excr.1997.3501. [DOI] [PubMed] [Google Scholar]
- 62.Laino G. d'Aquino R. Graziano A. Lanza V. Carinci F. Naro F. Pirozzi G. Papaccio G. A new population of human adult dental pulp stem cells: a useful source of living autologous fibrous bone tissue (LAB) J Bone Miner Res. 2005;20:1394. doi: 10.1359/JBMR.050325. [DOI] [PubMed] [Google Scholar]
- 63.d'Aquino R. De Rosa A. Laino G. Caruso F. Guida L. Rullo R. Checchi V. Laino L. Tirino V. Papaccio G. Human dental pulp stem cells: from biology to clinical applications. J Exp Zool B Mol Dev Evol. 2009;312B:408. doi: 10.1002/jez.b.21263. [DOI] [PubMed] [Google Scholar]
- 64.d'Aquino R. Graziano A. Sampaolesi M. Laino G. Pirozzi G. De Rosa A. Papaccio G. Human postnatal dental pulp cells co-differentiate into osteoblasts and endotheliocytes: a pivotal synergy leading to adult bone tissue formation. Cell Death Differ. 2007;14:1162. doi: 10.1038/sj.cdd.4402121. [DOI] [PubMed] [Google Scholar]
- 65.Graziano A. d'Aquino R. Laino G. Proto A. Giuliano M.T. Pirozzi G. De Rosa A. Di Napoli D. Papaccio G. Human CD34+ stem cells produce bone nodules in vivo. Cell Prolif. 2008;41:1. doi: 10.1111/j.1365-2184.2007.00497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Huang G.T. Pulp and dentin tissue engineering and regeneration: current progress. Regen Med. 2009;4:697. doi: 10.2217/rme.09.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ohazama A. Modino S.A. Miletich I. Sharpe P.T. Stem-cell-based tissue engineering of murine teeth. J Dent Res. 2004;83:518. doi: 10.1177/154405910408300702. [DOI] [PubMed] [Google Scholar]
- 68.Yu J.H. Shi J.N. Deng Z.H. Zhuang H. Nie X. Wang R.N. Jin Y. Cell pellets from dental papillae can reexhibit dental morphogenesis and dentinogenesis. Biochem Biophys Res Commun. 2006;346:116. doi: 10.1016/j.bbrc.2006.05.096. [DOI] [PubMed] [Google Scholar]
- 69.Horner E. Kirkham J. Wood D. Curran S. Smith M. Thomson B. Yang X. Long bone defect models for tissue engineering applications: criteria for choice. Tissue Eng Part B. 2010;16:263. doi: 10.1089/ten.TEB.2009.0224. [DOI] [PubMed] [Google Scholar]
- 70.Horner E. Kirkham J. Yang X. Polak J. Mantalaris S. Harding S.E. Advances in tissue engineering. 1st. London: Imperial College Press; 2008. Animal Models; p. 763. [Google Scholar]