Abstract
Irreversible damage to the nervous system can result from many causes including trauma, disruption of blood supply, pathogen infection or neurodegenerative disease. Common features following CNS injury include a disruption of axons, neuron death and injury, local B-cell and microglial activation, and the synthesis of pathogenic autoantibodies. CNS injury results in a pervasive inhibitory microenvironment that hinders regeneration. Current approaches to eliminate the inhibitory environment have met with limited success. These results argue for a paradigm shift in therapeutic approaches to CNS injury. Targeting CNS cells (neurons, oligodendrocytes and astrocytes) themselves may drive CNS repair. For example, our group and others have demonstrated that autoreactive antibodies can participate in aspects of CNS regeneration, including remyelination. We have developed recombinant autoreactive natural human IgM antibodies with the therapeutic potential for CNS repair in several neurologic diseases.
Keywords: Alzheimer’s disease, antibodies, brain injury, catalytic antibodies, CNS injury, ischemia, multiple sclerosis, natural autoantibodies, spinal cord injury, stroke, therapeutic strategies
Injury to the CNS results in a cascade of molecular and cellular events that affect the functional recovery of the organism. Following injury, in most cases, the blood–brain barrier (BBB) is breached through damage to blood vessels that results in the leakage of proteins and factors from the blood to the lesion. As a result, platelets, neutrophils, monocytes and macrophages are carried into the lesion. A classical response to either systemic or local brain injury is inflammation. This is exhibited by edema, circulating immune cells, complement, cytokines and glial activation. Until recently, this phenomenon was considered nonexistent in many degenerative CNS disorders [1]. Over evolution, mammals have developed a complex innate immune system where immune cells and natural antibodies play a major role in limiting damage. Inflammation and innate immune system responses are important mechanisms that allow organisms to limit the damage caused by injury. Most mechanisms of CNS injury ultimately lead to disruption of axons and neurons, and oxidative damage to nerves. Following injury, the adult mammalian CNS is not capable of spontaneous regeneration owing to the presence of an inhibitory extrinsic environment and a diminished intrinsic regenerative capacity. Therapeutic approaches to modulate this hostile environment usually focus on eliminating the inhibitory factors, albeit with partial success, in experimental CNS injury models. Far more necessary than once thought is a unified approach to also target CNS cells that participate in repair, thus arguing in favor of a paradigm shift in therapy for CNS injury and disease.
CNS injury
Importance of microglial cells in CNS injury
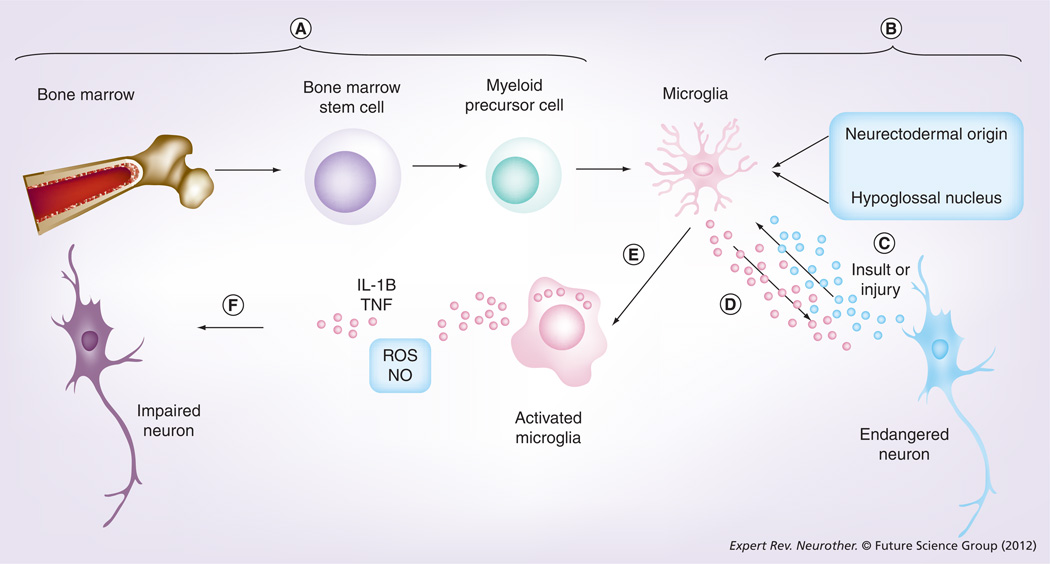
Infection, trauma, malignancy, ischemia or idiopathic degeneration often results in CNS injury. Following CNS injury, microglial cells primarily mediate the local innate immune response. Microglia comprise approximately 12% of glial cells, the other components being astrocytes and oligodendrocytes [2]. Microglia are derived from circulating monocytes or precursor cells in the monocyte–macrophage lineage that originates in bone marrow (Figure 1) [3]. Others have proposed microglia of non-monocyte–macrophage lineage origin [4,5]. In response to any injurious event, microglia respond by a reaction, termed microglial activation [6]. Activated microglia accumulate at sites of tissue damage and express genes related to enzymes, adhesion molecules, proinflammatory cytokines and free radicals (reviewed in [7]). Studies by Butovsky et al. suggested that the function of microglia is modulated by the microenvironment. Accordingly, activation of microglia by aggregated β-amyloid (Aβ) or lipopolysaccharide impairs MHC-II expression and renders the microglia cytotoxic, whereas IFN-γ and IL-4 renders them protective [8]. Although once considered harmful, a recent perspective shift has hypothesized microglia to stabilize the CNS [9]. The role of microglia in neurological disease is currently a matter of much intrigue and intense debate, and unfortunately is still an understudied area of research [10].
Figure 1. Origin and function of microglia.
(A) Microglia are derived from circulating monocytes or precursor cells that originate in bone marrow. (B) A non-monocyte–macrophage lineage origin for microglia was also proposed. (C) Disruption of the homeostatic mileu due to an insult or injury will enable neurons to seek the help of microglia. (D) Microglia can in turn produce neurotrophic factors to maintain failing or endangered neurons. (E) Constant presence of danger signals will result in the activation of microglia at sites of tissue damage and expression of genes related to enzymes, adhesion molecules, proinflammatory cytokines and free radicals. (F) Persistent or excessive microglial activation may lead to impairment of neurons.
NO: Nitric oxide; ROS: Reactive oxygen species.
In this article, we use the following three categories of lesions as examples for CNS injury (described below): traumatic spinal cord injury (SCI); focal ischemic stroke; and degenerative disorders exemplified by Alzheimer’s disease (AD), amyotrophic lateral sclerosis (ALS) and multiple sclerosis (MS).
Spinal cord injury
Many thousands of Americans are affected by SCI each year. The causes can include violence, motor vehicle accidents, recreational activities or falls. The pathophysiology of SCI progresses in two phases. Primary injury: the initial mechanical trauma comprises of compression and traction forces. The compression by bone fragments of the neural elements leads to injuries of both the CNS and the peripheral nervous system (PNS). Damaged blood vessels result in microhemorrhages in the central gray matter. Secondary injury sets in as a result of systemic hypotension that leads to ischemia. Ischemia and the release of toxic substances from damaged neural membranes then triggers an injury cascade that substantially damages neighboring cells, exacerbating the injury [11]. Despite many years of research, the pathophysiology of SCI remains poorly defined.
Focal ischemic stroke & cerebral ischemia
Arterial occlusion triggers stroke and results in diminished blood flow to portions of the brain, resulting in ischemia that leads to dysfunction of neurons [12]. Stroke often results in permanent damage to brain cells. There are two common ways by which a stroke can occur: when a clot forms in a narrow artery in the brain (thrombotic stroke); or when a preformed clot elsewhere travels up to the brain (embolic stroke). When a blood vessel in the brain ruptures and leaks blood, a hemorrhagic stroke occurs. Stroke is among the three major causes of death worldwide and is also the most frequent cause of permanent disability. Spontaneous cerebral ischemia affects more than 750,000 patients in the USA every year.
Degenerative disorders
Alzheimer’s disease
AD is the most common form of dementia among people over 65 years of age. The majority of patients with AD have a sporadic disease, whereas patients with a familial form of AD have earlier onset and more severe disease [13]. This progressive neurodegenerative disorder may feature with different symptoms, such as cognitive impairment, confusion, mood swings, aggression, irritability and, finally, loss of memory. Some investigators consider accumulation of abnormally folded Aβ as a key early event in the pathogenesis of AD. The increased deposition of this small peptide in the brains of patients is thought to contribute to neurodegenerative symptoms. A second pathological phenomenon of the disease is based on the appearance of insoluble neurofibrillary tangles formed by hyperphosphorylated τ protein, the so-called ‘tauopathy’. It is still debated whether the τ tangle is the primary cause of cell degeneration in AD [14]. It is still not fully understood how accumulation of these misfolded proteins gives rise to AD – that is, whether it is through a gain-of-function or a loss-of-function mechanism.
Amyotrophic lateral sclerosis
ALS is a devastating neurological illness that primarily affects anterior horn cells and cortico-spinal tract neurons. Patients often die within 2–3 years from symptom onset owing to respiratory failure. Despite extensive research, the etiology of this disorder is predominantly unknown and there are no effective treatments. A potential clue to this disorder has been the identification of genes implicated in a minority of patients with a rare genetic form of ALS [15]. One identified gene mutation is the copper/zinc superoxide dismutase (Cu/Zn SOD) mutation, which is present in a small percentage of patients with the genetic form of ALS [16]. Patients carrying the SOD mutations have very similar neurological outcomes compared with patients who develop the disease spontaneously without an associated genetic mutation [17]. This enzyme catalyzes superoxide to oxygen and hydrogen peroxide. SOD1 is the form of the enzyme associated with ALS. This is a gain of function of SOD1, which affects fast axonal transport [18]. The frequency of the SOD mutation in familial ALS varies from 12 to 23%. The gene is inherited as an autosomal dominant. Until recently, SOD1 mutation was the only know genetic cause of familial ALS. A recent article provided evidence that TDP-43 was present in neuronal inclusions in ALS patients, and therefore proposed that TDP-43 may represent a new specific marker for ALS [19]. Several other genes involved with autosomal dominant ALS have also been identified, such as angiogenin (ANG), FUS, SETX and VAPB [20]. Finally, a most recent article provided evidence that expansion of a hexanucleotide repeat (GGGGCC) in intron 1 of C9ORF72 represents a major cause of genetic forms of ALS [21].
Multiple sclerosis
Studies using imaging, serology, pathology and genetics, and patient response to anti-inflammatory treatments suggests that MS is primarily an inflammatory demyelinating disease of the CNS with varied clinical presentations and heterogeneous histopathological features. The disease has a peak onset between the ages of 20 and 40 years [22]; however, it may develop in children and has been reported in individuals over 60 years of age. MS affects women approximately twice as often as men [23,24]. MS usually begins in early adulthood and presents with a plethora of neurological manifestations with variable prognosis [25]. It has been thought that within 15 years from the onset of MS, approximately half of the patients will need assistance with walking [26]. According to the National MS Society, in the USA alone, approximately 400,000 people have MS; with 200 more patients added each week. To date, the pathogenesis of MS still remains elusive and there is no definitive cause and no effective cure. Therefore, MS can be classified as an episodic inflammatory demyelinating disease of the CNS. Disease pathophysiology is complex and involves genetic susceptibility, environmental factors, and development of pathologic immune-mediated responses leading to focal myelin destruction, axonal loss and local inflammatory infiltrates.
Dynamics of damage in CNS injury
Trauma to the spinal cord induces direct damage to the neural cells and vasculature followed by hemorrhage and secondary damage to previously unaffected neural cells [27]. Subsequently, a reactive glial scar obstructs regenerating axons from rebuilding neural circuitry [28]. One of the most studied, but inadequately understood obstacles to regeneration of axons is the glial scar. The glial scar is histologically apparent, and is composed of elements of connective tissue and astrocytes. Many studies in the last decade demonstrate that production of inhibitory molecules and molecular composition of the scar as factors that contribute to failure of regeneration [29–31].
In many cases of cerebral ischemia following occlusion, a necrotic center is observed. The center is surrounded by a penumbra containing partially injured brain tissue [32]. Progressive CNS atrophy and neural cell death are often associated with neurodegenerative disorders, in which loss of neural cells and disruption of neural transmission result in disease symptoms [33]. The remarkable property of any organ lies in its regenerative capacity; however, the nervous system appears to be privy to this capacity. Adult neurons lose the embryonic intrinsic growth capacity in order to accommodate proper synaptic development [34]. Spontaneous repair is almost nonexistent owing to persistent neuronal barriers to axonal regeneration [35]. A few identified inhibitory molecules have been associated either with myelin debris (myelin-associated glycoprotein, Nogo, Omgp and Ephrin-B3) or glial scar (tenasin and chondroitin sulfate proteoglycan) [36–38]. However, genetic ablation of identified major myelin inhibitors [39] or receptors for myelin or chondroitin sulfate proteoglycans [40,41] have failed to induce long distance axon regeneration. Ramón y Cajal demonstrated the formation of dystrophic growth cones in the late 1920s and suggested that cones were no longer capable of regeneration (reviewed in [42]). The neuronal growth cone is a sensory–motile structure located at the tip of an axon. Recent evidence indicates otherwise, as Tom et al. described that the cones are in fact very active structures that have been repressed in the extrinsic harmful environment [43]. Accordingly, Hur et al. reported on engineering neuronal growth cones to promote axon regeneration over inhibitory molecules [44]. The authors showed that pharmacological inhibition or genetic silencing of nonmuscle myosin II, which powers retrograde actin flow in the growth cone, markedly enhances axon growth over potent CNS inhibitory substrates via reorganization of the growth cone cytoskeleton.
A useful model to understand mechanisms of neural regeneration is the use of dorsal root ganglia (DRG). Irrefutable evidence that the environment within the lesion underlies the failure of CNS regeneration came from studies in which DRG neurons were introduced into prelesioned or undamaged white matter tracts [45]. These neurons were able to regenerate rapidly and put out processes within the white matter tracts of spinal cord and brain. However, when the neurons reached an area of CNS damage, axon growth stalled [46]. Both the CNS and PNS contain axons from DRG neurons; however, when compared with the axons of the central branch that ascend to the dorsal column in the spinal cord, only the axons of the peripheral branch projecting to the peripheral nerve are capable of regeneration. Subsequent studies showed that the regeneration of the peripheral branch positively influenced repair of the central branch in the CNS [47], suggesting that the local microenvironment plays a major role in regeneration and repair.
Presence of autoantibodies following CNS injury
Numerous autoantigens have been identified as playing a role in the exacerbation of human neurological conditions. Studies demonstrated the serological and/or cerebrospinal fluid presence of antibodies directed against MBP and/or myelin/oligodendrocyte glycoprotein in patients with MS [48]. However, the presence of myelin-specific antibodies are not limited to MS. Using an ELISA, Karni et al. compared the levels and frequencies of anti-myelin/oligodendrocyte glycoprotein antibodies in patients with MS, patients with other neurological disorders and healthy control subjects and found only minor differences [49]. In a parallel line of research, other reports suggested lipids or carbohydrates as possible candidate antigens for a humoral immune response [50]. An interesting report identified anti-α-glucose-based glycan IgM antibodies as predictors of relapse activity in MS after the first neurological event [51]. Others studies suggest that serum anti-Glc(α1,4)Glc(α) antibodies may serve as biomarkers for relapsing–remitting MS [52]. Autoantibodies to myelin proteins, lipids and carbohydrates can be found in tissue and sera of some MS patients.
The presence of autoantibodies to neurofilament has been described after neurologic injury. Following an acute first-ever stroke, the levels of anti-neurofilament antibodies are elevated above baseline for several months [53]. Autoantibodies against the NR2A/2B subunits of N-methyl-d-aspartate are reported to be similarly elevated in patients suffering from acute ischemic stroke and transient ischemic attacks [54]. Antibodies to myelin-associated glycoprotein, gangliosides, nuclear antigens, glutamate receptors and β-III tubulin have also been reported in a majority of patients who suffered traumatic brain injury and SCI [55–58]. In Table 1, we summarized a few examples of neurological disorders where autoantibodies play a pathological role.
Table 1.
Examples of diseases where antibodies play a pathological role.
| Disease | Antigens | Antibody | Ref. |
|---|---|---|---|
| Myasthenia gravis | Acetylcholine receptor | Anti-Ach receptor | [114] |
| Neuromyelitis optica | Aquaporin-4 | Anti-aquaporin-4 antibody | [115] |
| Lambert–Eaton myasthenic syndrome | Presynaptic voltage-gated calcium channel | Anti-voltage-gated calcium channel | [116] |
| Sox-1 | Anti-Sox-1 | [117] | |
| Guillain–Barré syndrome | Gangliosides | Anti-GM1 | [118] |
| Paraneoplastic syndromes | Neuronal nuclei, 55 and 80 kDa | Anti-Ri | [119] |
| Neuronal nuclei and cytoplasm, 40 kDa | Anti-Ta | [120] | |
| Neuronal nuclei and cytoplasm, 37 and 40 kDa | Anti-Ma | [121] | |
| Amphyphysin | Anti-amphyphysin | [122] | |
| Cytoplasm neurons, Purkinje cells | Anti-Tr | [123] | |
| Cytoplasm, Purkinje cells | Anti-Yo | [124] | |
| All neuronal nuclei, 35–40 kDa | Anti-Hu | [125] | |
| Retinal photoreceptor, 23 kDa | Anti-recoverin | [126,127] | |
| Subset of glia, 66 kDa | Anti-CV2 | [128] | |
Antibodies with hydrolytic properties towards self-antigens
Antibodies are versatile proteins and express activities such as neutralization, agglutination, fixation with activation of complement and activation of effector cells. In addition to this plethora of functions, some antibodies express enzymatic activity and are known as antibody enzymes, abzymes or catalytic antibodies. Abzymes have been reported in patients with neurodegenerative disorders. Anti-MBP antibodies with hydrolytic properties were isolated from patients with MS [59]. Interestingly, the expanded disability status showed correlations with the hydrolytic activity of the anti-MBP antibodies [60]. Hydrolytic IgMs were isolated from sera of patients with AD. The IgMs hydrolyzed Aβ at rates superior to IgMs that were isolated from age-matched humans without dementia [61]. IgMs from nonelderly humans that were used as controls expressed the least catalytic activity. The presence of abzymes in patients with ischemic stroke, ALS and SCI, with underlying vascular complications, has not yet been reported. Nevertheless, coagulation factor hydrolyzing antibodies have been reported in hemophilia A [62], acquired hemophilia [63,64], sepsis [65], multiple myeloma [66] and in patients with renal graft transplant [67]. Some of the reported target antigens for disease-associated hydrolytic antibodies include prothrombin, vasoactive intestinal peptide, thyroglobulin, DNA, RNA, the antiplatelet integrin GPIIIa (β3), factor VIII and factor IX [68]. Given that coagulation factor hydrolyzing antibodies exist normally in humans and under pathological conditions, we hypothesize that similar antibodies could exist in patients suffering from SCIs with associated vascular complications, and/or in patients with ischemic stroke. It is certainly not clear to date whether the autoantibodies reported in neurological disorders play a pathological or a beneficial role [69].
Natural autoreactive antibodies with therapeutic potential
We demonstrated that immunization with CNS homogenates or transfer of antiserum to CNS homogenates promoted repair of demyelinated CNS lesions in a mouse model of MS [70]. Based on this observation, we hypothesized that it might be possible to promote CNS repair through regulation of the immune system. As a proof-of-concept, myelin-binding antibodies in the sera of immunized mice preserved oligodendrocytes [71] and promoted remyelination [72]. Following the success of preliminary studies, to tempt the shift in therapeutics, our laboratory employed a novel strategy to identify human monoclonal antibodies that promote CNS protection and repair. These repair-promoting natural human antibodies are of IgM subtype and have characteristics of classic natural autoreactive antibodies. We isolated antibodies from the sera of patients with monoclonal gammopathies, using selection criteria of monoclonal immunoglobulin concentration of 3 g/dl or greater and a lack of neurologic or antibody-associated pathologies. The screening strategy we employed involved binding to live CNS tissue (cerebellar slices) [73]. We identified two IgM antibodies (sHIgM22 and sHIgM46) that promoted significant remyelination in vivo. We then constructed a recombinant version of sHIgM22, rHIgM22, by cloning the antibody variable region DNA sequence into an expression vector [74] providing the heavy and light chain framework. GMP-grade rHIgM22 antibody was purified in gram quantities for formal toxicology studies prior to Phase I clinical trials. Using a similar strategy we identified other monoclonal IgM antibodies as candidates to test in different models of neurologic injury and disease. We described two neuron-binding antibodies (sHIgM12 and sHIgM42) that stimulated neurite extension [75]. A recombinant version of sHIgM12, rHIgM12, was constructed and synthesized [76]. We recently reported that the binding of rHIgM12 to the neuronal surface reorganized the membrane and signaled the promotion of axonal outgrowth [77]. A single peripheral dose of rHIgM12 also improved motor function in a virus-induced demyelinating disease mouse model of MS [78]. This neuron-binding antibody represents a promising candidate for the treatment of not only MS, but also a number of other CNS disorders, such as ALS, stroke and SCI. Neuroprotection is of particular interest in ALS because of two reasons: a clear genetic cause is known only in a minority of patients; and currently only one therapeutic agent has some effect in slowing the disease. On the other hand, accumulated evidence to date, unless proven otherwise, points that the remyelination-promoting antibody may basically be limited to the treatment of only demyelinating disorders. In Table 2, we summarize the antigen specificity, function and results obtained with beneficial natural autoantibodies in animal models.
Table 2.
Beneficial antibodies, antigen specificity and results obtained in animal models.
| Target | Function | Antibody | Result | Ref. |
|---|---|---|---|---|
| LINGO-1 | Enhance axon myelination | Anti-LINGO-1 | Promote spinal cord remyelination and axonal integrity in MOG-induced experimental autoimmune encephalomyelitis | [95] |
| Sphingomyelin | Promote oligodendrocyte progenitors, reduce caspase levels | rHIgM22 | CNS remyelination in TMEV and lysolecithin models | [96] |
| Neurons | Promote neurite extension | rHIgM12 | Improve function in chronic axonal phase of TMEV infection | [78] |
| Gangliosides GQ1c, GT3, and Neu5Acα2–8Neu5Acα2–8Neu5Acα structure on both glycolipids and glycoproteins | Promote axon growth | A2B5 | Not treated in animals | [129,130] |
| Galactocerbrocide, sulfatide | Promote oligodendrocyte proliferation, Ca2+ entry into cells | O1, O4 | Improve remyelination in TMEV model | [131] |
MOG: Myelin oligodendrocyte glycoprotein; TMEV: Theiler’s murine encephalomyelitis virus.
Natural autoantibodies are polyspecific, react with self-molecules and are produced in healthy individuals without any deliberate antigenic stimuli [79]. B-1 cells synthesize natural IgM autoantibodies that help clear aging and damaged cells and have inflammation-modulatory functions [80]. During development, the B-1 cells are positively selected and secrete a wide repertoire of polyreactive natural antibodies. We have demonstrated that therapeutic antibodies can also be isolated from Epstein–Barr virus-immortalized human B cell lines obtained from normal adults, fetal umbilical cord blood, and rheumatoid arthritis and MS patients [81]. This leads us to believe that therapeutic antibodies are present and common in normal circulation. Mechanistic studies performed in our laboratory indicate that natural autoantibodies function via intracellular signaling pathways that promote protection and repair [82]. The use of rHIgM22 to enhance remyelination in humans would be the first attempt that is entirely in contrast to the present therapeutic approaches (summarized in the next section). A similar approach to treat CNS degenerative diseases or stroke with human antibodies would also target intrinsic cells of the CNS (neurons). Most of the US FDA-approved drugs currently in market for treatment of neurologic diseases target immune cells.
Expert commentary
Many of the current therapeutic strategies rely on maneuvers to deplete components of the immune system [83]. The main mechanisms of therapeutic strategies to treat SCI target phagocytes, T and B lymphocytes and cytokines to suppress lymphocyte activation and macrophage infiltration. Other approaches act on cytokine production, genetic manipulation, blocking adhesion molecules, neutralizing cytokines, promoting phagocytosis by transplanting activated macrophages, or inducing protective autoimmunity. A recent article demonstrated recovery in rats with SCI when transplants of oligodendrocyte precursor cells were given to the animals [84]. Treating SCI still represents a challenge with abysmal prognosis. Therapeutic targets in stroke and cerebral ischemia are phagocytes, T and B lymphocytes, and cytokines. Accordingly, the treatment mechanisms rely on the use of antibodies directed against chemokines or adhesion molecules to block leukocyte adhesion and migration into brain, leukocyte depletion via antibodies or genetic manipulation, vaccination to induce protective autoimmunity or bystander suppression, inhibition of inflammatory cytokines, and/or impairing macrophage/microglial activation. Therapeutic management of cerebral ischemia relies on prevention and reduction of risk factors, such as elevated blood pressure, thrombosis and carotid artery thickening. Interventional therapy of cerebral ischemia remains limited to enzymatic thrombolysis by use of recombinant tissue plasminogen activator, or by interventional removal of the blood clot. However, recombinant tissue plasminogen activator has a short 3-h therapeutic window and increased incidence of hemorrhagic cerebral ischemia if infused beyond the 3-h time frame [85]. The target molecules in AD are Aβ and senile plaques. Proposed therapeutic strategies aimed to vaccinate patients to increase Aβ antibodies, activate T-cell populations, plaque clearance and passive immunization to promote Aβ efflux from the brain. There are polyclonal and monoclonal antibodies that are currently under clinical trials for AD. These include octagam, γ-guard, BAN2401, gantenerumab, ponezumab, solanezumab and bapineuzumab. The epitope specificities of each antibody are different and it is, at present, difficult to predict which of the antibody preparations can be used as a successful therapy to treat AD.
Unfavorable prospects hinder the treatment of ALS. Numerous therapeutic approaches have been tested in animal models and despite further Phase II and Phase III clinical trials [86], only one drug, riluzole [87], is in use and has shown only mild efficacy in slowing disease progression [88]. Strategies to treat MS rely on immunosuppression to suppress lymphocyte and macrophage proliferation, antigen presentation, proinflammatory cytokine production and antibody production. In the recent years there has been an increasing use of several monoclonal antibodies as promising agents in the treatment of MS. The purpose of these antibodies is to deplete specific subsets of T cells, B lymphocytes and cytokines which are implicated in disease pathogenesis, in order to decrease inflammatory influx and reduce disease burden. Examples of such approaches in treatments for MS are alemtuzumab (anti-CD52 antibody) [89,90], daclizumab (anti-CD25 antibody) [91] and rituximab (anti-CD20 antibody) [92]. It is clear that in addition to immunosuppressive/immunomodulatory approaches there is also a need to begin promoting neuroprotection. The works of Mi et al. highlighted the importance of regulation of oligodendrocyte differentiation and myelination through the use of an antagonist to LINGO-1 [93,94]. Studies from this group reported that anti-LINGO-1 antibody resulted in spinal cord remyelination and improved axonal integrity [95]. Our work represented proof-of-principle that autoreactive human monoclonal antibodies, despite binding different cell membrane antigens, activate target cells in a conserved manner leading to beneficial biological effect [70,73,77,82,96]. Therefore, we believe there is a need towards a paradigm shift, and to start directing treatments towards intrinsic CNS cells that are affected in neurological diseases (neurons and oligodendrocytes). Moreover, a combination of treatments can be envisaged for beneficial synergistic effect.
It is essential to discuss the importance of the BBB breakdown in the context of therapeutic approaches. There are several anatomical features between the vascular and the nervous systems that have been recognized. The interface between the two systems is known as the neurovascular unit, comprised of endothelial cells, astrocytes, neurons and pericytes. A major component of the neurovascular unit is the BBB, which is formed by endothelial cells and isolates the CNS from the blood circulation. Almost every aspect of CNS function is affected due to this interface. The term ‘barrier’ gives the notion of a rigid, inaccessible obstruction between the vascular and nervous systems. However, the barrier is in fact a region of dynamic molecular transport to constantly regulate the flow of essential components, such as amino acids, across endothelial walls and maintain the homeostasis of the microenvironment [97]. Following CNS injury, the BBB is compromised and promotes accumulation of proinflammatory cytokines and cell lytic products. The resulting inflammation may attenuate the efflux activity of the BBB, but also ameliorate the permeability to bring in adaptive immune cells. Under these conditions, breakdown of the BBB may in fact be beneficial, as this will result in an increased influx of natural autoantibodies or drugs with reparative properties to target and protect intrinsic cells of the CNS.
Alternative approaches to treat CNS injury may target sugar epitopes, as they seem to play an important role in inhibition of regeneration. Steinmetz et al. have demonstrated that in vivo digestion of proteoglycan side chains through enzymatic treatment resulted in remarkable long-distance regeneration of adult axons through CNS white matter tracts [98]. Uninjured serotonergic fibers and injured corticospinal fibers increased sprouting after enzymatic treatment [99]. A contemporary introduction to the therapeutic arm of CNS injury is the deoxyribozymes. DNA enzymes, or DNAzymes, are single stranded and have been reported not to exist in nature. Instead, they have been developed in the laboratory using a technique called systematic evolution of ligands by exponential enrichment [100]. Following injury, inhibitory components probably exert their inhibitory effects through glycosaminoglycan side chains. Their synthesis is mediated by enzyme XT-1. Application of DNAzyme to XT-1 avoided glycosylation and assembly of proteoglycan (a major group of molecules that participate in axon growth inhibition) core protein into the extracellular matrix. This led to the successful demonstration of microtransplanted and endogenous severed sensory axons to grow beyond the lesion site owing to reduction of the inhibitory environment [101]. Recently, Park et al. reported on the important role of the mTOR pathway regulation and protein translation to determine the intrinsic axon regrowth responsiveness of injured CNS neurons [102]. Axotomized adult neurons had a suppression of this pathway, thus limiting new protein synthesis required for sustained axon regeneration. When either PTEN or TSC1 were silenced in order to reactivate the mTOR pathway, it led to induction of extensive axon regeneration in adult neurons. This supports the idea that initiation of the neuronal regenerative program for axon regrowth can be achieved by simply retaining active protein synthesis in axotomized mature neurons.
Alternate CNS repair strategies rely on cell transplantation in order to rewire neural network connections and restore functional activity. Embryonic stem cells [103], adult neural [104] and non-neural stem cells [105–107], and endogenous neural stem cells [108,109] have been used previously to repair damaged neural networks. Accordingly, transplantation of olfactory ensheathing cells (specialized glia of the olfactory system) improved functional outcome and showed neuroprotective effects [110]. Sykova et al. reported on autologous bone marrow transplantation in patients with subacute and chronic SCI [107]. Interestingly, five out of seven acute patients demonstrated improvement of motor and/or sensory functions. Accumulated evidence indicates that transplanted cells do not reconstruct the neural circuits but only modulate immune responses and produce neurotrophic factors. This is in contrast to what was expected to occur – that is, replacement of damaged neural cells. The transplanted cells may require additional signals in the form of hematopoietic cytokines. Kawada et al. documented that the administration of granulocyte colony-stimulating factor and stem cell factor in the subacute phase after focal cerebral ischemia is effective for functional recovery. The reason was an enhanced cytokine-induced generation of neuronal cells from both bone marrow-derived cells and intrinsic neural stem/progenitor cells [111].
However, neural stem cells pose ethical problems for acquiring and possess a potential for tumorigenesis. Accordingly, undifferentiated embryonic stem cells transplanted into experimental stroke animal models resulted in tumorigenesis [112]. The injected cells may not be able to migrate to the site of lesion, and even if this was not the case, the injured CNS microenvironment may not favor the differentiation and survival of newly formed neural cells [113].
A final but nevertheless most important problem of all CNS repair strategies is the inability to recover complex activities, developed on the basis of synaptic circuitry over decades of experience, such as learning and memory. Hence, protecting neurons and axons already existent from further damage and to coax them to repair seems to be a more potent therapeutic approach. Accordingly, the use of natural autoantibodies or other approaches that stimulate CNS cells are potentially important therapeutic techniques in combating a wide spectrum of neurologic diseases. Finally, monoclonal antibodies that bind to the surface of neurons to enhance neuronal outgrowth and prevent axon death could provide a new approach to treatment of traumatic SCI, ALS, primary motor peripheral neuropathies or even stroke.
Five-year view
Future prospects for CNS repair are encouraging with several promising therapeutic approaches close to clinical trials. These approaches include cell transplantation, remyelination-promoting and neuron-protecting antibodies and hormonal therapy. A crucial step to repair involves the prevention of neural degeneration. There is a need towards identifying definite risk factors causing these diseases and to investigate additional inhibitory molecules responsible for the hostile, nonpermissive environment that prevents regeneration. Knowledge obtained from such work should help in designing prevention therapies for neurodegenerative disorders. Within the next 5 years, we believe that effective treatments to stop and/or reverse neurodegeneration may be available on the market. A combinatorial approach seems like a relevant option to bring about CNS repair. Potential combinatorial therapeutic approaches could include neurotrophic factors that potentially protect neural cells; ion channel blockers that inhibit neurotransmitter-induced excitatory toxicity; neutralizers to reactive oxygen species; and agents that target the immune system to eliminate deleterious immune system-mediated injury and perhaps natural autoantibodies that replace deleterious micro-environment with beneficial outcomes. Indeed, human monoclonal IgMs that target the CNS may enhance the permissive environment for regeneration of neural cells or damaged neural networks, and thus regulate homeostasis.
Key issues.
CNS injury results in permanent neurological damage.
Definite risk factors for neurological disorders are unknown.
Spontaneous CNS repair is limited because of the persistent hostile microenvironment.
Transplantation of neural cells into chronic CNS injury lesions may not induce repair in diffuse diseases.
CNS injury results in activation of microglial cells and production of autoreactive antibodies.
Current therapeutic strategies aim primarily at eliminating the inhibitory environment.
Natural autoreactive antibodies may be reparative by stimulating neurons and oligodendrocytes.
Effective treatment strategy should be a combinatorial approach to inhibit the hostile microenvironment and stimulate intrinsic CNS cells for repair.
Acknowledgments
This work was supported by grants from the NIH (R01s – GM092993, NS024180, NS032129, NS048357, R21 – NS073684), the National Multiple Sclerosis Society (CA1060A11), the Applebaum Foundation, the Hilton Foundation, the Peterson Foundation, Minnesota Partnership for Biotechnology and Medical Genomics and the European Regional Development Fund – Project FNUSA-ICRC (No. CZ.1.05/1.1.00/02.0123). The authors also thank the McNeilus Family and a High-Impact Pilot and Feasibility Award (HIPFA) from the Mayo Clinic Center for Translational Science Activities (CTSA) for financial support in this project. B Wootla is the recipient of a fellowship from the Mayo-Applebaum funds. The technology for remyelination-promoting antibody rHIgM22 has been licensed to Acorda Therapeutics, Inc. No royalties have accrued to M Rodriguez or Mayo Clinic to date, but both have rights to receive future royalties.
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.Kelley KW, Johnson RW, Dantzer R. Immunology discovers physiology. Vet Immunol. Immunopathol. 1994;43(1–3):157–165. doi: 10.1016/0165-2427(94)90132-5. [DOI] [PubMed] [Google Scholar]
- 2.Lawson LJ, Perry VH, Dri P, Gordon S. Heterogeneity in the distribution and morphology of microglia in the normal adult mouse brain. Neuroscience. 1990;39(1):151–170. doi: 10.1016/0306-4522(90)90229-w. [DOI] [PubMed] [Google Scholar]
- 3. Sievers J, Parwaresch R, Wottge HU. Blood monocytes and spleen macrophages differentiate into microglia-like cells on monolayers of astrocytes: morphology. Glia. 1994;12(4):245–258. doi: 10.1002/glia.440120402. • Provided evidence that blood monocytes and spleen macrophages differentiate into microglia.
- 4.Kitamura T, Miyake T, Fujita S. Genesis of resting microglia in the gray matter of mouse hippocampus. J. Comp. Neurol. 1984;226(3):421–433. doi: 10.1002/cne.902260310. [DOI] [PubMed] [Google Scholar]
- 5.Schelper RL, Adrian EK., Jr Non-specific esterase activity in reactive cells in injured nervous tissue labeled with 3H-thymidine or 125iododeoxyuridine injected before injury. J. Comp. Neurol. 1980;194(4):829–844. doi: 10.1002/cne.901940408. [DOI] [PubMed] [Google Scholar]
- 6. Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308(5726):1314–1318. doi: 10.1126/science.1110647. •• Showed that blood–brain barrier disruption provoked immediate and focal activation of microglia, switching their behavior from patroling to shielding of the injured site.
- 7.Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat. Rev. Neurosci. 2007;8(1):57–69. doi: 10.1038/nrn2038. [DOI] [PubMed] [Google Scholar]
- 8.Butovsky O, Talpalar AE, Ben-Yaakov K, Schwartz M. Activation of microglia by aggregated β-amyloid or lipopolysaccharide impairs MHC-II expression and renders them cytotoxic whereas IFN-γ and IL-4 render them protective. Mol. Cell Neurosci. 2005;29(3):381–393. doi: 10.1016/j.mcn.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 9.Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat. Neurosci. 2007;10(11):1387–1394. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- 10.Barres BA. The mystery and magic of glia: a perspective on their roles in health and disease. Neuron. 2008;60(3):430–440. doi: 10.1016/j.neuron.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 11.McDonald JW, Sadowsky C. Spinal-cord injury. Lancet. 2002;359(9304):417–425. doi: 10.1016/S0140-6736(02)07603-1. [DOI] [PubMed] [Google Scholar]
- 12.Iadecola C, Anrather J. The immunology of stroke: from mechanisms to translation. Nat. Med. 2011;17(7):796–808. doi: 10.1038/nm.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Larner AJ, Doran M. Clinical phenotypic heterogeneity of Alzheimer’s disease associated with mutations of the presenilin-1 gene. J. Neurol. 2006;253(2):139–158. doi: 10.1007/s00415-005-0019-5. [DOI] [PubMed] [Google Scholar]
- 14.Obulesu M, Venu R, Somashekhar R. Tau mediated neurodegeneration: an insight into Alzheimer’s disease pathology. Neurochem. Res. 2011;36(8):1329–1335. doi: 10.1007/s11064-011-0475-5. [DOI] [PubMed] [Google Scholar]
- 15.Gurney ME, Liu R, Althaus JS, Hall ED, Becker DA. Mutant CuZn superoxide dismutase in motor neuron disease. J. Inherit. Metab. Dis. 1998;21(5):587–597. doi: 10.1023/a:1005475206997. [DOI] [PubMed] [Google Scholar]
- 16.Morrison BM, Morrison JH. Amyotrophic lateral sclerosis associated with mutations in superoxide dismutase: a putative mechanism of degeneration. Brain Res. Rev. 1999;29(1):121–135. doi: 10.1016/s0165-0173(98)00049-6. [DOI] [PubMed] [Google Scholar]
- 17.Siddique T, Nijhawan D, Hentati A. Familial amyotrophic lateral sclerosis. J. Neural. Transm. Suppl. 1997;49:219–233. doi: 10.1007/978-3-7091-6844-8_23. [DOI] [PubMed] [Google Scholar]
- 18.Cluskey S, Ramsden DB. Mechanisms of neurodegeneration in amyotrophic lateral sclerosis. Mol. Pathol. 2001;54(6):386–392. [PMC free article] [PubMed] [Google Scholar]
- 19.Neumann M, Sampathu DM, Kwong LK, et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314(5796):130–133. doi: 10.1126/science.1134108. [DOI] [PubMed] [Google Scholar]
- 20.Valdmanis PN, Daoud H, Dion PA, Rouleau GA. Recent advances in the genetics of amyotrophic lateral sclerosis. Curr. Neurol. Neurosci. Rep. 2009;9(3):198–205. doi: 10.1007/s11910-009-0030-9. [DOI] [PubMed] [Google Scholar]
- 21.Stewart H, Rutherford NJ, Briemberg H, et al. Clinical and pathological features of amyotrophic lateral sclerosis caused by mutation in the C9ORF72 gene on chromosome 9p. Acta Neuropathol. 2012;123(3):409–417. doi: 10.1007/s00401-011-0937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kurtzke JF, Hyllested K. Multiple sclerosis in the Faroe Islands and the lack of protection by exposure in infancy. Neuroepidemiology. 1992;11(2):90–99. doi: 10.1159/000110917. [DOI] [PubMed] [Google Scholar]
- 23.Orton SM, Herrera BM, Yee IM, et al. Sex ratio of multiple sclerosis in Canada: a longitudinal study. Lancet Neurol. 2006;5(11):932–936. doi: 10.1016/S1474-4422(06)70581-6. [DOI] [PubMed] [Google Scholar]
- 24.Wallin MT, Page WF, Kurtzke JF. Multiple sclerosis in US veterans of the Vietnam era and later military service: race, sex, and geography. Ann. Neurol. 2004;55(1):65–71. doi: 10.1002/ana.10788. [DOI] [PubMed] [Google Scholar]
- 25.Noseworthy JH, Lucchinetti C, Rodriguez M, Weinshenker BG. Multiple sclerosis. N. Engl. J. Med. 2000;343(13):938–952. doi: 10.1056/NEJM200009283431307. [DOI] [PubMed] [Google Scholar]
- 26.Weinshenker BG, Bass B, Rice GP, et al. The natural history of multiple sclerosis: a geographically based study. I. Clinical course and disability. Brain. 1989;112(Pt 1):133–146. doi: 10.1093/brain/112.1.133. [DOI] [PubMed] [Google Scholar]
- 27.Hagg T, Oudega M. Degenerative and spontaneous regenerative processes after spinal cord injury. J. Neurotrauma. 2006;23(3–4):264–280. doi: 10.1089/neu.2006.23.263. [DOI] [PubMed] [Google Scholar]
- 28.Silver J, Miller JH. Regeneration beyond the glial scar. Nat. Rev. Neurosci. 2004;5(2):146–156. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- 29.Fitch MT, Doller C, Combs CK, Landreth GE, Silver J. Cellular and molecular mechanisms of glial scarring and progressive cavitation: in vivo and in vitro analysis of inflammation-induced secondary injury after CNS trauma. J. Neurosci. 1999;19(19):8182–8198. doi: 10.1523/JNEUROSCI.19-19-08182.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leal-Filho MB. Spinal cord injury: from inflammation to glial scar. Surg. Neurol. Int. 2011;2:112. doi: 10.4103/2152-7806.83732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang H, Muramatsu T, Murase A, Yuasa S, Uchimura K, Kadomatsu K. N-acetylglucosamine 6-O-sulfotransferase-1 is required for brain keratan sulfate biosynthesis and glial scar formation after brain injury. Glycobiology. 2006;16(8):702–710. doi: 10.1093/glycob/cwj115. [DOI] [PubMed] [Google Scholar]
- 32.Lo EH. A new penumbra: transitioning from injury into repair after stroke. Nat. Med. 2008;14(5):497–500. doi: 10.1038/nm1735. [DOI] [PubMed] [Google Scholar]
- 33.LaFerla FM, Green KN, Oddo S. Intracellular amyloid-β in Alzheimer’s disease. Nat. Rev. Neurosci. 2007;8(7):499–509. doi: 10.1038/nrn2168. [DOI] [PubMed] [Google Scholar]
- 34.Abe N, Cavalli V. Nerve injury signaling. Curr. Opin. Neurobiol. 2008;18(3):276–283. doi: 10.1016/j.conb.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. David S, Aguayo AJ. Axonal elongation into peripheral nervous system “bridges” after central nervous system injury in adult rats. Science. 1981;214(4523):931–933. doi: 10.1126/science.6171034. •• Demonstrated that the regenerative potential of central neurons is expressed when the CNS glial environment is changed to that of the peripheral nervous system.
- 36.Fitch MT, Silver J. CNS injury, glial scars, and inflammation: Inhibitory extracellular matrices and regeneration failure. Exp. Neurol. 2008;209(2):294–301. doi: 10.1016/j.expneurol.2007.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yiu G, He Z. Glial inhibition of CNS axon regeneration. Nat. Rev. Neurosci. 2006;7(8):617–627. doi: 10.1038/nrn1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Benson MD, Romero MI, Lush ME, Lu QR, Henkemeyer M, Parada LF. Ephrin-B3 is a myelin-based inhibitor of neurite outgrowth. Proc. Natl Acad. Sci. USA. 2005;102(30):10694–10699. doi: 10.1073/pnas.0504021102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee JK, Geoffroy CG, Chan AF, et al. Assessing spinal axon regeneration and sprouting in Nogo-, MAG-, and OMgp-deficient mice. Neuron. 2010;66(5):663–670. doi: 10.1016/j.neuron.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shen Y, Tenney AP, Busch SA, et al. PTPσ is a receptor for chondroitin sulfate proteoglycan, an inhibitor of neural regeneration. Science. 2009;326(5952):592–596. doi: 10.1126/science.1178310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zheng B, Atwal J, Ho C, et al. Genetic deletion of the Nogo receptor does not reduce neurite inhibition in vitro or promote corticospinal tract regeneration in vivo. Proc. Natl Acad. Sci. USA. 2005;102(4):1205–1210. doi: 10.1073/pnas.0409026102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lobato RD. Historical vignette of Cajal’s work ‘Degeneration and regeneration of the nervous system’ with a reflection of the author. Neurocirugia (Astur.) 2008;19(5):456–468. doi: 10.1016/s1130-1473(08)70215-x. [DOI] [PubMed] [Google Scholar]
- 43.Tom VJ, Steinmetz MP, Miller JH, Doller CM, Silver J. Studies on the development and behavior of the dystrophic growth cone, the hallmark of regeneration failure, in an in vitro model of the glial scar and after spinal cord injury. J. Neurosci. 2004;24(29):6531–6539. doi: 10.1523/JNEUROSCI.0994-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hur EM, Yang IH, Kim DH, et al. Engineering neuronal growth cones to promote axon regeneration over inhibitory molecules. Proc. Natl Acad. Sci. USA. 2011;108(12):5057–5062. doi: 10.1073/pnas.1011258108. • Proposed nonmuscle myosin II and growth cone cytoskeletal components as effective targets for promoting axon regeneration.
- 45.Davies SJ, Fitch MT, Memberg SP, Hall AK, Raisman G, Silver J. Regeneration of adult axons in white matter tracts of the central nervous system. Nature. 1997;390(6661):680–683. doi: 10.1038/37776. [DOI] [PubMed] [Google Scholar]
- 46.Davies SJ, Goucher DR, Doller C, Silver J. Robust regeneration of adult sensory axons in degenerating white matter of the adult rat spinal cord. J. Neurosci. 1999;19(14):5810–5822. doi: 10.1523/JNEUROSCI.19-14-05810.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cai D, Deng K, Mellado W, Lee J, Ratan RR, Filbin MT. Arginase I and polyamines act downstream from cyclic AMP in overcoming inhibition of axonal growth MAG and myelin in vitro. Neuron. 2002;35(4):711–719. doi: 10.1016/s0896-6273(02)00826-7. [DOI] [PubMed] [Google Scholar]
- 48.Schmidt S, Haase CG, Bezman L, et al. Serum autoantibody responses to myelin oligodendrocyte glycoprotein and myelin basic protein in X-linked adrenoleukodystrophy and multiple sclerosis. J. Neuroimmunol. 2001;119(1):88–94. doi: 10.1016/s0165-5728(01)00345-9. [DOI] [PubMed] [Google Scholar]
- 49. Karni A, Bakimer-Kleiner R, Abramsky O, Ben-Nun A. Elevated levels of antibody to myelin oligodendrocyte glycoprotein is not specific for patients with multiple sclerosis. Arch. Neurol. 1999;56(3):311–315. doi: 10.1001/archneur.56.3.311. •• Important study that demonstrated that elevated presence of antimyelin oligodendrocyte glycoprotein antibody is not specific for multiple sclerosis because a similar appearance was also demonstrated in patients with other neurologic diseases of the CNS.
- 50.Endo T, Scott DD, Stewart SS, Kundu SK, Marcus DM. Antibodies to glycosphingolipids in patients with multiple sclerosis and SLE. J. Immunol. 1984;132(4):1793–1797. [PubMed] [Google Scholar]
- 51.Freedman MS, Laks J, Dotan N, Altstock RT, Dukler A, Sindic CJ. Anti-α-glucose-based glycan IgM antibodies predict relapse activity in multiple sclerosis after the first neurological event. Mult. Scler. 2009;15(4):422–430. doi: 10.1177/1352458508101944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schwarz M, Spector L, Gortler M, et al. Serum anti-Glc(α1,4)Glc(α) antibodies as a biomarker for relapsing-remitting multiple sclerosis. J. Neurol. Sci. 2006;244(1–2):59–68. doi: 10.1016/j.jns.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 53.Bornstein NM, Aronovich B, Korczyn AD, Shavit S, Michaelson DM, Chapman J. Antibodies to brain antigens following stroke. Neurology. 2001;56(4):529–530. doi: 10.1212/wnl.56.4.529. [DOI] [PubMed] [Google Scholar]
- 54.Dambinova SA, Khounteev GA, Izykenova GA, Zavolokov IG, Ilyukhina AY, Skoromets AA. Blood test detecting autoantibodies to N-methyl-d-aspartate neuroreceptors for evaluation of patients with transient ischemic attack and stroke. Clin. Chem. 2003;49(10):1752–1762. doi: 10.1373/49.10.1752. [DOI] [PubMed] [Google Scholar]
- 55.Davies AL, Hayes KC, Dekaban GA. Clinical correlates of elevated serum concentrations of cytokines and autoantibodies in patients with spinal cord injury. Arch. Phys. Med. Rehabil. 2007;88(11):1384–1393. doi: 10.1016/j.apmr.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 56.Hayes KC, Hull TC, Delaney GA, et al. Elevated serum titers of proinflammatory cytokines and CNS autoantibodies in patients with chronic spinal cord injury. J. Neurotrauma. 2002;19(6):753–761. doi: 10.1089/08977150260139129. [DOI] [PubMed] [Google Scholar]
- 57.Stefan J, Prochazka M, Voltnerova M. Studies of immunologic reactions after brain injury. I. Antibodies against brain tissue lipids after experimental injury of the brain in rabbits. Int. Surg. 1971;55(5):316–321. [PubMed] [Google Scholar]
- 58.Skoda D, Kranda K, Bojar M, et al. Antibody formation against β-tubulin Class 3 in response to brain trauma. Brain Res. Bull. 2006;68(4):213–216. doi: 10.1016/j.brainresbull.2005.05.032. [DOI] [PubMed] [Google Scholar]
- 59. Ponomarenko NA, Durova OM, Vorobiev II, et al. Autoantibodies to myelin basic protein catalyze site-specific degradation of their antigen. Proc. Natl Acad. Sci. USA. 2006;103(2):281–286. doi: 10.1073/pnas.0509849103. •• Identified antibodies with catalytic properties capable of hydrolyzing MBP, arguing in favor of a pathological role for anti-MBP antibodies in multiple sclerosis.
- 60.Ponomarenko NA, Durova OM, Vorobiev II, et al. Catalytic activity of autoantibodies toward myelin basic protein correlates with the scores on the multiple sclerosis expanded disability status scale. Immunol. Lett. 2006;103(1):45–50. doi: 10.1016/j.imlet.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 61.Taguchi H, Planque S, Nishiyama Y, et al. Autoantibody-catalyzed hydrolysis of amyloid β peptide. J. Biol. Chem. 2008;283(8):4714–4722. doi: 10.1074/jbc.M707983200. [DOI] [PubMed] [Google Scholar]
- 62.Lacroix-Desmazes S, Moreau A, Sooryanarayana, et al. Catalytic activity of antibodies against factor VIII in patients with hemophilia A. Nat. Med. 1999;5(9):1044–1047. doi: 10.1038/12483. [DOI] [PubMed] [Google Scholar]
- 63.Wootla B, Christophe OD, Mahendra A, et al. Proteolytic antibodies activate factor IX in patients with acquired hemophilia. Blood. 2011;117(7):2257–2264. doi: 10.1182/blood-2010-07-296103. [DOI] [PubMed] [Google Scholar]
- 64.Wootla B, Dasgupta S, Dimitrov JD, et al. Factor VIII hydrolysis mediated by anti-factor VIII autoantibodies in acquired hemophilia. J. Immunol. 2008;180(11):7714–7720. doi: 10.4049/jimmunol.180.11.7714. [DOI] [PubMed] [Google Scholar]
- 65. Lacroix-Desmazes S, Bayry J, Kaveri SV, et al. High levels of catalytic antibodies correlate with favorable outcome in sepsis. Proc. Natl Acad. Sci. USA. 2005;102(11):4109–4113. doi: 10.1073/pnas.0500586102. •• First study to demonstrate a beneficial effect of the presence of catalytic antibodies in patients with sepsis.
- 66.Thiagarajan P, Dannenbring R, Matssura K, Tramontano A, Gololobov G, Paul S. Monoclonal antibody light chain with prothrombinase activity. Biochemistry. 2000;39:6459–6465. doi: 10.1021/bi992588w. [DOI] [PubMed] [Google Scholar]
- 67.Wootla B, Nicoletti A, Patey N, et al. Hydrolysis of coagulation factors by circulating IgG is associated with a reduced risk for chronic allograft nephropathy in renal transplanted patients. J. Immunol. 2008;180(12):8455–8460. doi: 10.4049/jimmunol.180.12.8455. [DOI] [PubMed] [Google Scholar]
- 68.Wootla B, Lacroix-Desmazes S, Warrington AE, Bieber AJ, Kaveri SV, Rodriguez M. Autoantibodies with enzymatic properties in human autoimmune diseases. J. Autoimmun. 2011;37(2):144–150. doi: 10.1016/j.jaut.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wootla B, Denic A, Keegan BM, et al. Evidence for the role of B cells and immunoglobulins in the pathogenesis of multiple sclerosis. Neurol. Res. Int. 2011;2011 doi: 10.1155/2011/780712. 780712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rodriguez M, Lennon VA, Benveniste EN, Merrill JE. Remyelination by oligodendrocytes stimulated by antiserum to spinal cord. J. Neuropathol. Exp. Neurol. 1987;46(1):84–95. doi: 10.1097/00005072-198701000-00008. [DOI] [PubMed] [Google Scholar]
- 71.Howe CL, Bieber AJ, Warrington AE, Pease LR, Rodriguez M. Antiapoptotic signaling by a remyelination-promoting human antimyelin antibody. Neurobiol. Dis. 2004;15(1):120–131. doi: 10.1016/j.nbd.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 72.Miller DJ, Rodriguez M. A monoclonal autoantibody that promotes central nervous system remyelination in a model of multiple sclerosis is a natural autoantibody encoded by germline immunoglobulin genes. J. Immunol. 1995;154(5):2460–2469. [PubMed] [Google Scholar]
- 73.Warrington AE, Asakura K, Bieber AJ, et al. Human monoclonal antibodies reactive to oligodendrocytes promote remyelination in a model of multiple sclerosis. Proc. Natl Acad. Sci. USA. 2000;97(12):6820–6825. doi: 10.1073/pnas.97.12.6820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mitsunaga Y, Ciric B, Van Keulen V, et al. Direct evidence that a human antibody derived from patient serum can promote myelin repair in a mouse model of chronic-progressive demyelinating disease. FASEB J. 2002;16(10):1325–1327. doi: 10.1096/fj.01-0994fje. [DOI] [PubMed] [Google Scholar]
- 75.Warrington AE, Bieber AJ, Van Keulen V, Ciric B, Pease LR, Rodriguez M. Neuron-binding human monoclonal antibodies support central nervous system neurite extension. J. Neuropathol. Exp. Neurol. 2004;63(5):461–473. doi: 10.1093/jnen/63.5.461. [DOI] [PubMed] [Google Scholar]
- 76.Van Keulen VP, Ciric B, Radhakrishnan S, et al. Immunomodulation using the recombinant monoclonal human B7-DC cross-linking antibody rHIgM12. Clin. Exp. Immunol. 2006;143(2):314–321. doi: 10.1111/j.1365-2249.2005.02992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xu X, Warrington AE, Wright BR, et al. A human IgM signals axon outgrowth: coupling lipid raft to microtubules. J. Neurochem. 2011;119(1):100–112. doi: 10.1111/j.1471-4159.2011.07416.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Denic A, Macura SI, Warrington AE, et al. A single dose of neuron-binding human monoclonal antibody improves spontaneous activity in a murine model of demyelination. PLoS One. 2011;6(10) doi: 10.1371/journal.pone.0026001. e26001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Casali P, Schettino EW. Structure and function of natural antibodies. Curr. Top Microbiol. Immunol. 1996;210:167–179. doi: 10.1007/978-3-642-85226-8_17. [DOI] [PubMed] [Google Scholar]
- 80.Baumgarth N. The double life of a B-1 cell: self-reactivity selects for protective effector functions. Nat. Rev. Immunol. 2011;11(1):34–46. doi: 10.1038/nri2901. [DOI] [PubMed] [Google Scholar]
- 81.Warrington AE, Van Keulen V, Pease LR, Rodriguez M. Naturally occurring antibodies as therapeutics for neurologic disease: can human monoclonal IgMs replace the limited resource IVIg? In: Lutz HU, editor. Naturally Occurring Antibodies (NAbs) NY, USA: Landes Bioscience and Springer Science + Business Media; 2011. [DOI] [PubMed] [Google Scholar]
- 82.Watzlawik J, Holicky E, Edberg DD, et al. Human remyelination promoting antibody inhibits apoptotic signaling and differentiation through Lyn kinase in primary rat oligodendrocytes. Glia. 2010;58(15):1782–1793. doi: 10.1002/glia.21048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Popovich PG, Longbrake EE. Can the immune system be harnessed to repair the CNS? Nat. Rev. Neurosci. 2008;9(6):481–493. doi: 10.1038/nrn2398. [DOI] [PubMed] [Google Scholar]
- 84.Cao Q, He Q, Wang Y, et al. Transplantation of ciliary neurotrophic factor-expressing adult oligodendrocyte precursor cells promotes remyelination and functional recovery after spinal cord injury. J. Neurosci. 2010;30(8):2989–3001. doi: 10.1523/JNEUROSCI.3174-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Clark WM, Wissman S, Albers GW, Jhamandas JH, Madden KP, Hamilton S. Recombinant tissue-type plasminogen activator (Alteplase) for ischemic stroke 3 to 5 hours after symptom onset. The ATLANTIS study: a randomized controlled trial. Alteplase thrombolysis for acute noninterventional therapy in ischemic stroke. JAMA. 1999;282(21):2019–2026. doi: 10.1001/jama.282.21.2019. [DOI] [PubMed] [Google Scholar]
- 86.Cudkowicz ME, Katz J, Moore DH, et al. Toward more efficient clinical trials for amyotrophic lateral sclerosis. Amyotroph Lateral Scler. 2010;11(3):259–265. doi: 10.3109/17482960903358865. [DOI] [PubMed] [Google Scholar]
- 87.Gurney ME, Fleck TJ, Himes CS, Hall ED. Riluzole preserves motor function in a transgenic model of familial amyotrophic lateral sclerosis. Neurology. 1998;50(1):62–66. doi: 10.1212/wnl.50.1.62. [DOI] [PubMed] [Google Scholar]
- 88.Miller RG, Mitchell JD, Lyon M, Moore DH. Riluzole for amyotrophic lateral sclerosis (ALS)/motor neuron disease (MND) Cochrane Database Syst. Rev. 2007;1 doi: 10.1002/14651858.CD001447.pub2. CD001447. [DOI] [PubMed] [Google Scholar]
- 89.Coles AJ, Fox E, Vladic A, et al. Alemtuzumab versus interferon β-1a in early relapsing-remitting multiple sclerosis: post-hoc and subset analyses of clinical efficacy outcomes. Lancet Neurol. 2011;10(4):338–348. doi: 10.1016/S1474-4422(11)70020-5. [DOI] [PubMed] [Google Scholar]
- 90.Fox EJ, Sullivan HC, Gazda SK, et al. A single-arm, open-label study of alemtuzumab in treatment-refractory patients with multiple sclerosis. Eur. J. Neurol. 2012;19(2):307–311. doi: 10.1111/j.1468-1331.2011.03507.x. [DOI] [PubMed] [Google Scholar]
- 91.Bielekova B, Richert N, Herman ML, et al. Intrathecal effects of daclizumab treatment of multiple sclerosis. Neurology. 2011;77(21):1877–1886. doi: 10.1212/WNL.0b013e318239f7ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Brown BA, Torabi M. Incidence of infusion-associated reactions with rituximab for treating multiple sclerosis: a retrospective analysis of patients treated at a US centre. Drug Saf. 2011;34(2):117–123. doi: 10.2165/11585960-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 93.Mi S, Lee X, Shao Z, et al. LINGO-1 is a component of the Nogo-66 receptor/p75 signaling complex. Nat. Neurosci. 2004;7(3):221–228. doi: 10.1038/nn1188. [DOI] [PubMed] [Google Scholar]
- 94.Mi S, Miller RH, Lee X, et al. LINGO-1 negatively regulates myelination by oligodendrocytes. Nat. Neurosci. 2005;8(6):745–751. doi: 10.1038/nn1460. [DOI] [PubMed] [Google Scholar]
- 95.Mi S, Hu B, Hahm K, et al. LINGO-1 antagonist promotes spinal cord remyelination and axonal integrity in MOG-induced experimental autoimmune encephalomyelitis. Nat. Med. 2007;13(10):1228–1233. doi: 10.1038/nm1664. [DOI] [PubMed] [Google Scholar]
- 96.Warrington AE, Bieber AJ, Ciric B, Pease LR, Van Keulen V, Rodriguez M. A recombinant human IgM promotes myelin repair after a single, very low dose. J. Neurosci. Res. 2007;85(5):967–976. doi: 10.1002/jnr.21217. [DOI] [PubMed] [Google Scholar]
- 97.Banerjee S, Bhat MA. Neuron-glial interactions in blood-brain barrier formation. Annu. Rev. Neurosci. 2007;30:235–258. doi: 10.1146/annurev.neuro.30.051606.094345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Steinmetz MP, Horn KP, Tom VJ, et al. Chronic enhancement of the intrinsic growth capacity of sensory neurons combined with the degradation of inhibitory proteoglycans allows functional regeneration of sensory axons through the dorsal root entry zone in the mammalian spinal cord. J. Neurosci. 2005;25(35):8066–8076. doi: 10.1523/JNEUROSCI.2111-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Barritt AW, Davies M, Marchand F, et al. Chondroitinase ABC promotes sprouting of intact and injured spinal systems after spinal cord injury. J. Neurosci. 2006;26(42):10856–10867. doi: 10.1523/JNEUROSCI.2980-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Santoro SW, Joyce GF. A general purpose RNA-cleaving DNA enzyme. Proc. Natl Acad. Sci. USA. 1997;94(9):4262–4266. doi: 10.1073/pnas.94.9.4262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Grimpe B, Silver J. A novel DNA enzyme reduces glycosaminoglycan chains in the glial scar and allows microtransplanted dorsal root ganglia axons to regenerate beyond lesions in the spinal cord. J. Neurosci. 2004;24(6):1393–1397. doi: 10.1523/JNEUROSCI.4986-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Park KK, Liu K, Hu Y, et al. Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science. 2008;322(5903):963–966. doi: 10.1126/science.1161566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.McDonald JW, Liu XZ, Qu Y, et al. Transplanted embryonic stem cells survive, differentiate and promote recovery in injured rat spinal cord. Nat. Med. 1999;5(12):1410–1412. doi: 10.1038/70986. [DOI] [PubMed] [Google Scholar]
- 104.Toda H, Takahashi J, Iwakami N, et al. Grafting neural stem cells improved the impaired spatial recognition in ischemic rats. Neurosci. Lett. 2001;316(1):9–12. doi: 10.1016/s0304-3940(01)02331-x. [DOI] [PubMed] [Google Scholar]
- 105.Chen J, Li Y, Wang L, et al. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke. 2001;32(4):1005–1011. doi: 10.1161/01.str.32.4.1005. [DOI] [PubMed] [Google Scholar]
- 106.Munoz-Elias G, Marcus AJ, Coyne TM, Woodbury D, Black IB. Adult bone marrow stromal cells in the embryonic brain: engraftment, migration, differentiation, and long-term survival. J. Neurosci. 2004;24(19):4585–4595. doi: 10.1523/JNEUROSCI.5060-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sykova E, Homola A, Mazanec R, et al. Autologous bone marrow transplantation in patients with subacute and chronic spinal cord injury. Cell Transplant. 2006;15(8–9):675–687. doi: 10.3727/000000006783464381. [DOI] [PubMed] [Google Scholar]
- 108.Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat. Med. 2002;8(9):963–970. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- 109.Ming GL, Song H. Adult neurogenesis in the mammalian central nervous system. Annu. Rev. Neurosci. 2005;28:223–250. doi: 10.1146/annurev.neuro.28.051804.101459. [DOI] [PubMed] [Google Scholar]
- 110.Verdu E, Garcia-Alias G, Fores J, Lopez-Vales R, Navarro X. Olfactory ensheathing cells transplanted in lesioned spinal cord prevent loss of spinal cord parenchyma and promote functional recovery. Glia. 2003;42(3):275–286. doi: 10.1002/glia.10217. [DOI] [PubMed] [Google Scholar]
- 111.Kawada H, Takizawa S, Takanashi T, et al. Administration of hematopoietic cytokines in the subacute phase after cerebral infarction is effective for functional recovery facilitating proliferation of intrinsic neural stem/progenitor cells and transition of bone marrow-derived neuronal cells. Circulation. 2006;113(5):701–710. doi: 10.1161/CIRCULATIONAHA.105.563668. [DOI] [PubMed] [Google Scholar]
- 112.Erdo F, Buhrle C, Blunk J, et al. Host-dependent tumorigenesis of embryonic stem cell transplantation in experimental stroke. J. Cereb. Blood Flow Metab. 2003;23(7):780–785. doi: 10.1097/01.WCB.0000071886.63724.FB. [DOI] [PubMed] [Google Scholar]
- 113.Case LC, Tessier-Lavigne M. Regeneration of the adult central nervous system. Curr. Biol. 2005;15(18):R749–R753. doi: 10.1016/j.cub.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 114.Souan ML, Geffard M, Vieillemaringe J, Lebrun-Grandie P, Orgogozo JM. Anti-acetylcholine antibodies and the pathogenesis of myasthenia gravis. Ann. NY Acad. Sci. 1987;505:423–438. doi: 10.1111/j.1749-6632.1987.tb51313.x. [DOI] [PubMed] [Google Scholar]
- 115.Lennon VA, Kryzer TJ, Pittock SJ, Verkman AS, Hinson SR. IgG marker of optic-spinal multiple sclerosis binds to the aquaporin-4 water channel. J. Exp. Med. 2005;202(4):473–477. doi: 10.1084/jem.20050304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Motomura M, Lang B, Johnston I, Palace J, Vincent A, Newsom-Davis J. Incidence of serum anti-P/O-type and anti-N-type calcium channel autoantibodies in the Lambert-Eaton myasthenic syndrome. J. Neurol. Sci. 1997;147(1):35–42. doi: 10.1016/s0022-510x(96)05303-8. [DOI] [PubMed] [Google Scholar]
- 117.Stich O, Klages E, Bischler P, et al. SOX1 antibodies in sera from patients with paraneoplastic neurological syndromes. Acta Neurol. Scand. 2011 doi: 10.1111/j.1600-0404.2011.01572.x. [DOI] [PubMed] [Google Scholar]
- 118.Lardone RD, Yuki N, Odaka M, Daniotti JL, Irazoqui FJ, Nores GA. Anti-GM1 IgG antibodies in Guillain-Barre syndrome: fine specificity is associated with disease severity. J. Neurol. Neurosurg. Psychiatry. 2010;81(6):629–633. doi: 10.1136/jnnp.2009.183665. [DOI] [PubMed] [Google Scholar]
- 119.Pittock SJ, Lucchinetti CF, Lennon VA. Anti-neuronal nuclear autoantibody Type 2: paraneoplastic accompaniments. Ann. Neurol. 2003;53(5):580–587. doi: 10.1002/ana.10518. [DOI] [PubMed] [Google Scholar]
- 120.Hoffmann LA, Jarius S, Pellkofer HL, et al. Anti-Ma and anti-Ta associated paraneoplastic neurological syndromes: 22 newly diagnosed patients and review of previous cases. J. Neurol. Neurosurg. Psychiatry. 2008;79(7):767–773. doi: 10.1136/jnnp.2007.118588. [DOI] [PubMed] [Google Scholar]
- 121.Dalmau J, Graus F, Villarejo A, et al. Clinical analysis of anti-Ma2-associated encephalitis. Brain. 2004;127(Pt 8):1831–1844. doi: 10.1093/brain/awh203. [DOI] [PubMed] [Google Scholar]
- 122.Pittock SJ, Lucchinetti CF, Parisi JE, et al. Amphiphysin autoimmunity: paraneoplastic accompaniments. Ann. Neurol. 2005;58(1):96–107. doi: 10.1002/ana.20529. [DOI] [PubMed] [Google Scholar]
- 123.Graus F, Dalmau J, Valldeoriola F, et al. Immunological characterization of a neuronal antibody (anti-Tr) associated with paraneoplastic cerebellar degeneration and Hodgkin’s disease. J. Neuroimmunol. 1997;74(1–2):55–61. doi: 10.1016/s0165-5728(96)00205-6. [DOI] [PubMed] [Google Scholar]
- 124.Peterson K, Rosenblum MK, Kotanides H, Posner JB. Paraneoplastic cerebellar degeneration. I. A clinical analysis of 55 anti-Yo antibody-positive patients. Neurology. 1992;42(10):1931–1937. doi: 10.1212/wnl.42.10.1931. [DOI] [PubMed] [Google Scholar]
- 125.Graus F, Keime-Guibert F, Rene R, et al. Anti-Hu-associated paraneoplastic encephalomyelitis: analysis of 200 patients. Brain. 2001;124(Pt 6):1138–1148. doi: 10.1093/brain/124.6.1138. [DOI] [PubMed] [Google Scholar]
- 126.Adamus G, Guy J, Schmied JL, Arendt A, Hargrave PA. Role of anti-recoverin autoantibodies in cancer-associated retinopathy. Invest. Ophthalmol. Vis. Sci. 1993;34(9):2626–2633. [PubMed] [Google Scholar]
- 127.Shiraga S, Adamus G. Mechanism of CAR syndrome: anti-recoverin antibodies are the inducers of retinal cell apoptotic death via the caspase 9- and caspase 3-dependent pathway. J. Neuroimmunol. 2002;132(1–2):72–82. doi: 10.1016/s0165-5728(02)00314-4. [DOI] [PubMed] [Google Scholar]
- 128.Honnorat J, Antoine JC, Derrington E, Aguera M, Belin MF. Antibodies to a subpopulation of glial cells and a 66 kDa developmental protein in patients with paraneoplastic neurological syndromes. J. Neurol. Neurosurg. Psychiatry. 1996;61(3):270–278. doi: 10.1136/jnnp.61.3.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Eisenbarth GS, Walsh FS, Nirenberg M. Monoclonal antibody to a plasma membrane antigen of neurons. Proc. Natl Acad. Sci. USA. 1979;76(10):4913–4917. doi: 10.1073/pnas.76.10.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Inoko E, Nishiura Y, Tanaka H, et al. Developmental stage-dependent expression of an α2,8-trisialic acid unit on glycoproteins in mouse brain. Glycobiology. 2010;20(7):916–928. doi: 10.1093/glycob/cwq049. [DOI] [PubMed] [Google Scholar]
- 131.Bansal R, Warrington AE, Gard AL, Ranscht B, Pfeiffer SE. Multiple and novel specificities of monoclonal antibodies O1, O4, and R-mAb used in the analysis of oligodendrocyte development. J. Neurosci. Res. 1989;24(4):548–557. doi: 10.1002/jnr.490240413. [DOI] [PubMed] [Google Scholar]