Abstract
We report on the use of shock waves delivered by a shock-tube to permeabilize cancer cells and potentiate the cytotoxicity of the type-1 ribosome-inactivating protein, saporin. We studied human colorectal cancer HT29 and ovarian cancer OVCAR-5 cells and used two different cytotoxicity assays, colony formation and loss of mitochondrial activity. A single shock wave and saporin (10−9 M) produced significant toxicity not seen with either shock wave or drug alone. Increasing the number of shock waves up to five further increased cytotoxicity. Higher toxicity was seen with the clonogenic assay compared to MTT assay. Shock waves may have applications in promoting cytoplasmic delivery of toxins into cancer cells after intratumoral injection.
Keywords: Shock tube, Saporin, Cancer, Ribosome-inactivating protein, Cytoplasmic delivery
1. Introduction
Type-1 ribosome-inactivating proteins (RIP) such as saporin have the potential to be highly cytotoxic agents against cancer. However, in contrast to type-2 RIP such as ricin they lack the lectin necessary to bind to cell-surface lectin receptors and cause cellular internalization [1]. They are therefore not very toxic to cancer cells unless action is taken to allow the molecules to penetrate into cells; in addition a means of selectively delivering the RIPs to tumor cells and sparing normal cells is desirable. Covalent conjugation of saporin to monoclonal antibodies that recognize tumor antigens produces immunotoxins that possess both cancer cell selectivity and are internalized [2, 3], and these molecules have recently entered phase I clinical trials for leukemia and multiple myeloma [4].
However another strategy that may be used to both give both tumor selectivity and toxin internalization is the local deposition of energy into the tumor in such a manner that transient pores are opened in the plasma membrane of the tumor cells allowing the RIP molecules to gain access to the cytoplasm [5]. This strategy has the additional advantage of avoiding the lysosomal compartments into which immunotoxins may be delivered and where the active RIP may be degraded before it reaches its target ribosomes [1]. This transient membrane permeabilization has been accomplished by electroporation [6] or by shock waves generated by an extracorporeal lithotripter [5].
We have shown that a single shock wave generated by a shock tube has the correct parameters to deliver fluorescent dextran molecules into the cytoplasm of cells without toxicity [7, 8]. In this report we show that from one to five shock waves from a shock tube potentiates the cytotoxicity of saporin towards human cancer cells as determined by two different assays for cell viability, chosen to provide measures of loss of viability in both the short term (24 h) and the long term (2 weeks).
2. Materials and methods
2.1. Materials
Saporin (Sigma St. Louis MO) was dissolved in phosphate-buffered saline (PBS) without Mg2+ and Ca2+ to form a stock solution of 10−5 M. The concentration in complete media containing the cell pellet was varied from 10−12 M to 10−6 M. All cell samples were washed with PBS without Mg2+ and Ca2+ (5 ml at 233 × g).
2.2. Cell culture
NIH: OVCAR-5 (OVCAR-5) human epithelial ovarian cancer cells were obtained from Dr. T. Hamilton (Fox Chase Cancer Institute, Philadelphia, PA) and HT-29 human colorectal adenocarcinoma cells came from ATCC. OVCAR-5 cells were grown in RPMI-1640 medium while HT-29 cells were cultured in McCoy’s 5A both supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin in 250-ml T-flasks at 37 °C under an atmosphere of 5% CO2. Total viable cell counts were obtained with a hemocytometer using trypan blue dye exclusion before and after the shock wave experiments. For all experiments cells were grown to 80–90% confluence with > 98% viability, and were harvested by use of trypsin–EDTA. For the shock tube experiments, flattop snap-cap microcentrifuge tubes were used. The centrifuge tube was covered with a triple layer of Parafilm (Parafilm ‘M’, American National Can Chicago, IL). The tubes were filled with a mixture of saporin solution and 2:0 × 106 cells in serum-containing medium. A cell pellet was gently formed by centrifugation (5 min at 233 × g).
2.3. Shock waves
Shock waves were generated with a double diaphragm shock tube that was obtained from Pharma Wave (Boston, MA) and is described in detail elsewhere [7, 8]. The shock wave was delivered into the tube containing the cell pellet through the covering of parafilm. The obtained pressure at the bottom of the microcentrifuge tube was 11.6 ± 1.6 MPa (n = 3) and the pulse duration (positive pressure) was 32.1 ± 7.1 µs (n = 3, mean ± SD) as measured with a PVDF needle hydrophone (model 80-0.5-4.0, Imotec Messtechnik, Warendorh, Germany) and a digital oscilloscope (9360, 600 MHz, 1 MΩ (15 pF), LeCroy Co., New York, NY).
2.4. Cell viability
After the experiment the cells were resuspended and washed with PBS without Mg2+ and Ca2+. OVCAR-5 cells were plated in 24-well tissue culture plates in complete medium and incubated for 24 h. Cell viability was determined by 3-[4,5-dimethylthiazol- 2-yl]-2,5-diphenyltetrazolium bromide (MTT) assay as previously described [9] and survival fractions were expressed relative to cells treated with neither saporin nor shock waves but pelleted resuspended and washed. The viability of HT-29 cells was also measured by clonogenic assay. Resuspended washed cells were plated in 35-mm culture dishes incubated for 10–14 days with medium changes every other day. The number of colonies larger than 50 cells were counted using an inverted microscope [10] and survival fractions were calculated from controls as described above.
2.5. Statistical analysis
All measurements are given as mean ± standard error (SE) of 3–10 separate experiments. Each experiment contained 2–4 replicate samples. Differences between means were assessed by one-way factorial analysis of variance. A value of P 0:05 was considered to be statistically significant. Fractional product analysis as recommended by Greco was employed [11] to analyze the data with number of shock waves and HT29 cells. The raw data consisted of mean survival fractions (s.f.) from saporin alone and from shock waves alone and from combination of saporin and shock waves. Cytotoxic fractions (c.f.) were calculated as 1 2 s.f. Bliss synergism was then tested by calculating the fractional product parameter according to the fractional product analysis method of Webb [12]. The fractional product value is defined as c.f.[combination saporin and shock wave]/{c.f. [saporin] + c.f. [shock wave] – (c.f. [saporin] × c.f. [shock wave])}. Values > 1 indicate Bliss synergism, values ≈ 1 indicate additivity and values > 1 indicate Bliss antagonism.
3. Results
We studied two human cancer cell lines that are typical of highly malignant poorly differentiated tumors that are resistant to chemotherapeutic agents. We also used two different methods of measuring cytotoxicity; the MTT assay that is widely used to measure mitochondrial dehydrogenase activity in the short term after the cytotoxic insult and a clonogenic assay that is more applicable to anti-tumor therapy and gives a long-term measure of loss of the ability of the cells to proliferate. Although saporin is expected to have low toxicity to cancer cells in the absence of membrane permeabilization, the toxicity is not expected to be zero. Therefore, differences in cell survival attributed to the potentiation by shock wavemediated cell membrane permeabilization may be best measured by comparing differences in the concentration of saporin necessary to kill cells with and without shock waves.
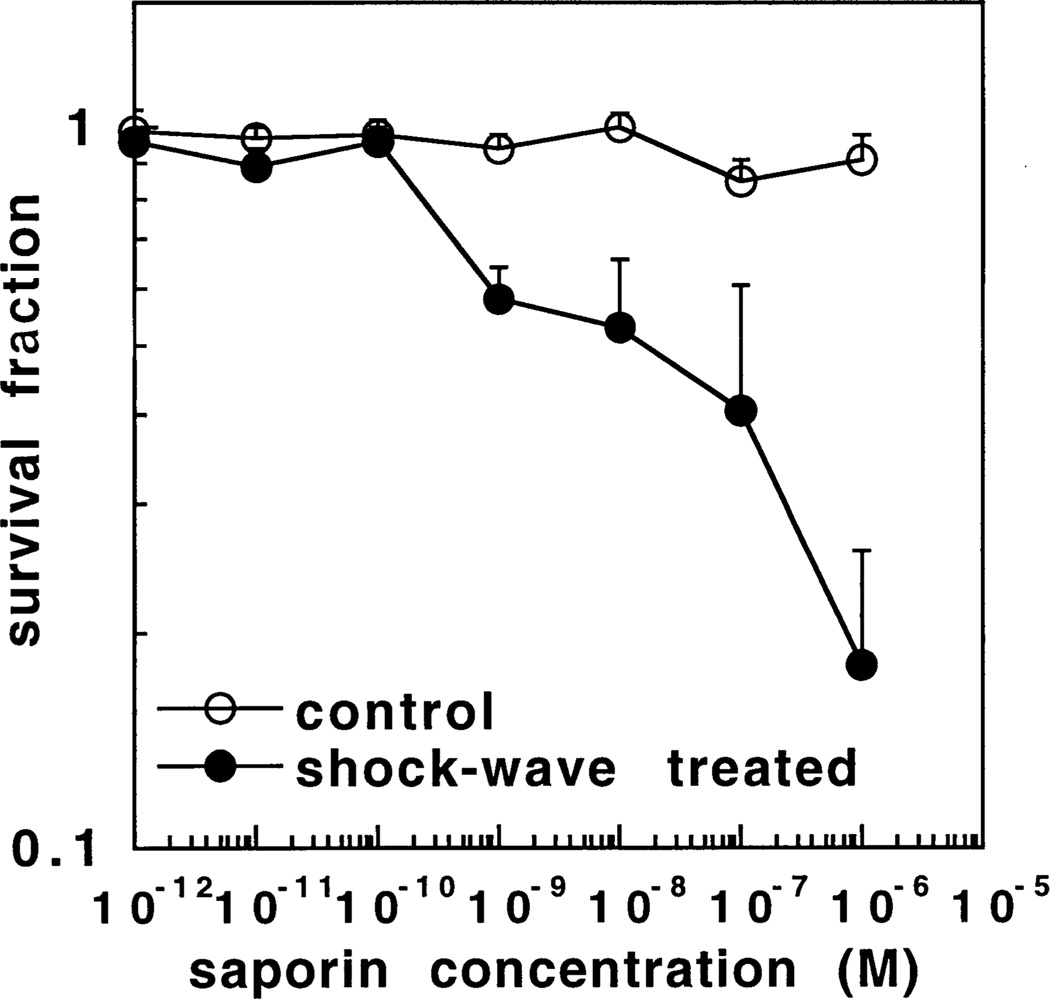
Fig. 1 shows the survival fraction of OVCAR-5 ovarian cancer cells in the presence of increasing concentrations of saporin both with and without a single shock wave measured by the MTT assay. A single shock wave alone produced no toxicity (survival fraction = 0:98 ± 0:3) in the absence of saporin. There was no significant toxicity to cells in the absence of a shock wave with increasing saporin concentrations up to 10−6 M (the highest concentration tested). In the presence of a shock wave however cytotoxicity began to be evident at 10−9 M saporin (P < 0:005 vs. no shock wave) and increased steadily up to 10−6 M where the survival fraction was 0.18 ± 0.08 (P < 0:0005 vs. no shock wave).
Fig. 1.
Survival fractions determined by MTT assay of OVCAR-5 cells treated with increasing concentrations of saporin either with or without a single shock wave and compared to cells treated by pelleting and resuspending alone. Values are means ± SE of at least six independent experiments.
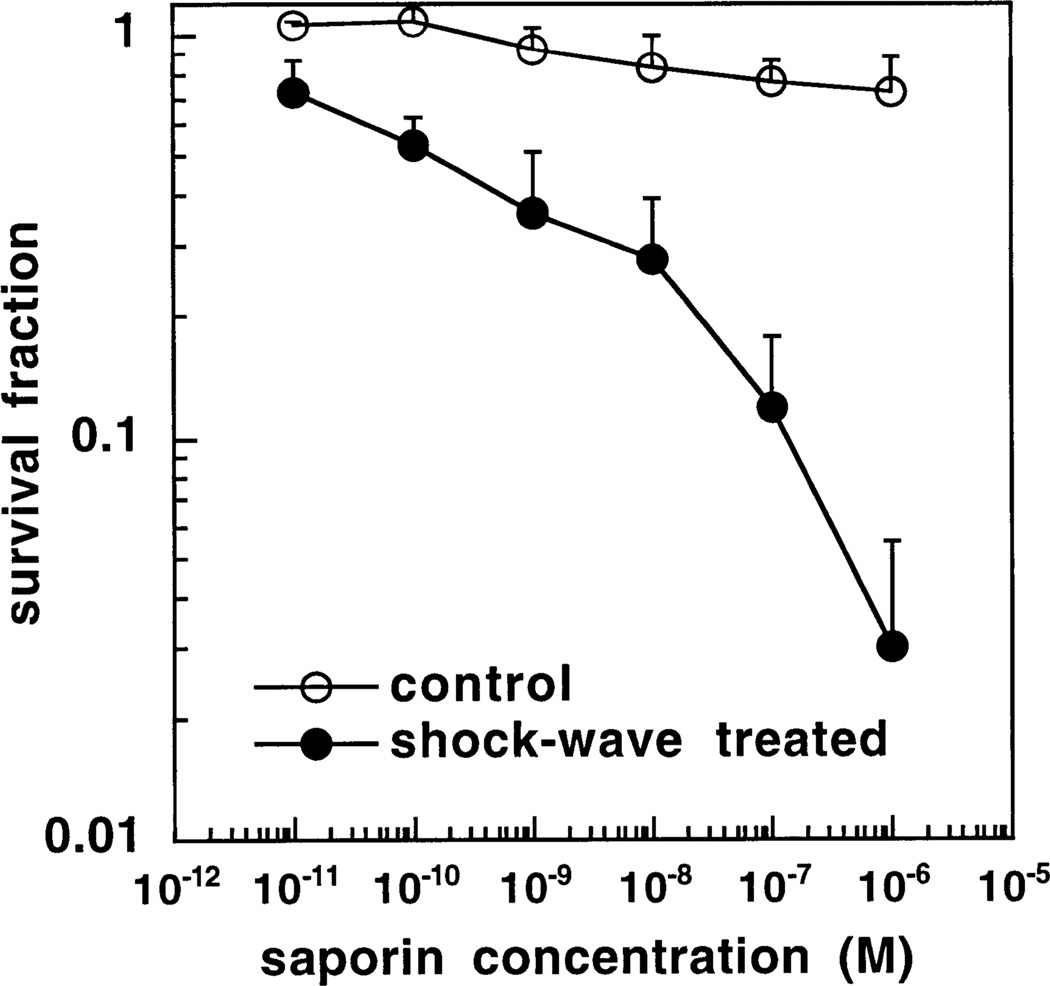
Fig. 2 shows the results for HT-29 colorectal cancer cells measured by colony-formation assay. In this case there was a small but significant degree of toxicity with a single shock wave in the absence of saporin (survival fraction = 0:73 ± 0:13 P < 0:05 vs. control). When cells were incubated with saporin without shock waves significant toxicity became evident at a saporin concentration of 10−7 M and 10−6 M (survival fractions of 0.77 ± 0.1 and 0.72 ± 0.12 both P < 0:05 vs. control). When cells were incubated with saporin in the presence of a shock wave significant additional toxicity compared to both shock wave alone and saporin without shock wave became evident at 10−9 M (P < 0:05 vs. both shock wave alone or saporin alone) and increased further to a survival fraction of 0.03 ± 0.025 (P < 0:001) at 10−6 M saporin.
Fig. 2.
Survival fractions determined by clonogenic assay of HT29 cells treated with increasing concentrations of saporin either with or without a single shock wave and compared to cells treated by pelleting and resuspending alone. Values are means ± SE of six independent experiments.
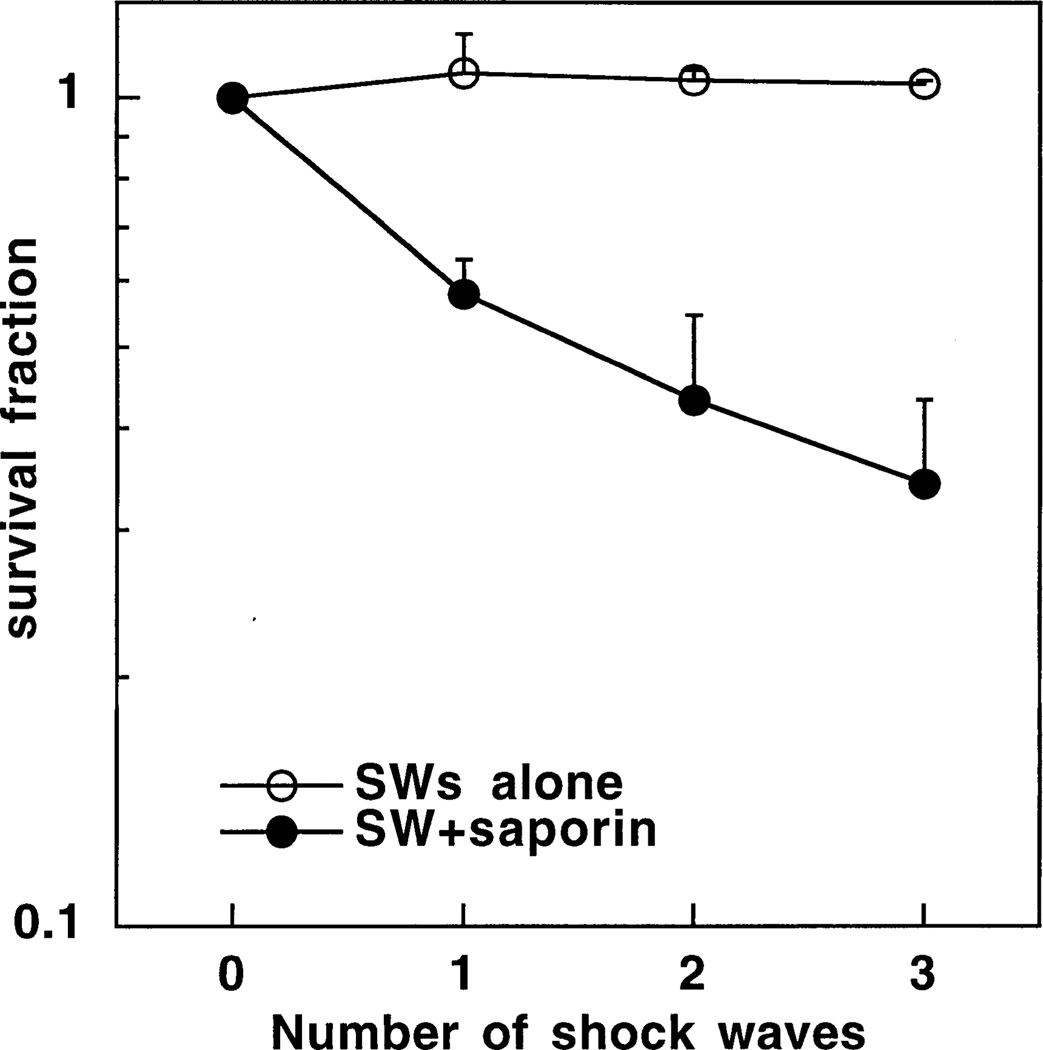
We next asked whether increasing the number of shock waves delivered to the cells would further potentiate the cytotoxicity of saporin compared to a single shock wave. Fig. 3 shows the results obtained when OVCAR-5 cells were incubated either with or without 10−7 M saporin and exposed to either one two or three shock waves and the survival was measured using the MTT assay. Using this cell line and assay there was no observed toxicity with even three shock waves alone. Two shock waves plus saporin gave more cytotoxicity than one shock wave plus saporin, and the difference between three shock waves plus saporin and one shock wave plus saporin was significant (P < 0:05).
Fig. 3.
Survival fractions determined by MTT assay of OVCAR-5 cells treated with one two or three shock waves either with or without 10−7 M saporin and compared to cells treated by pelleting and resuspending alone in the absence of saporin or shock wave. Values are means ± SE of six independent experiments.
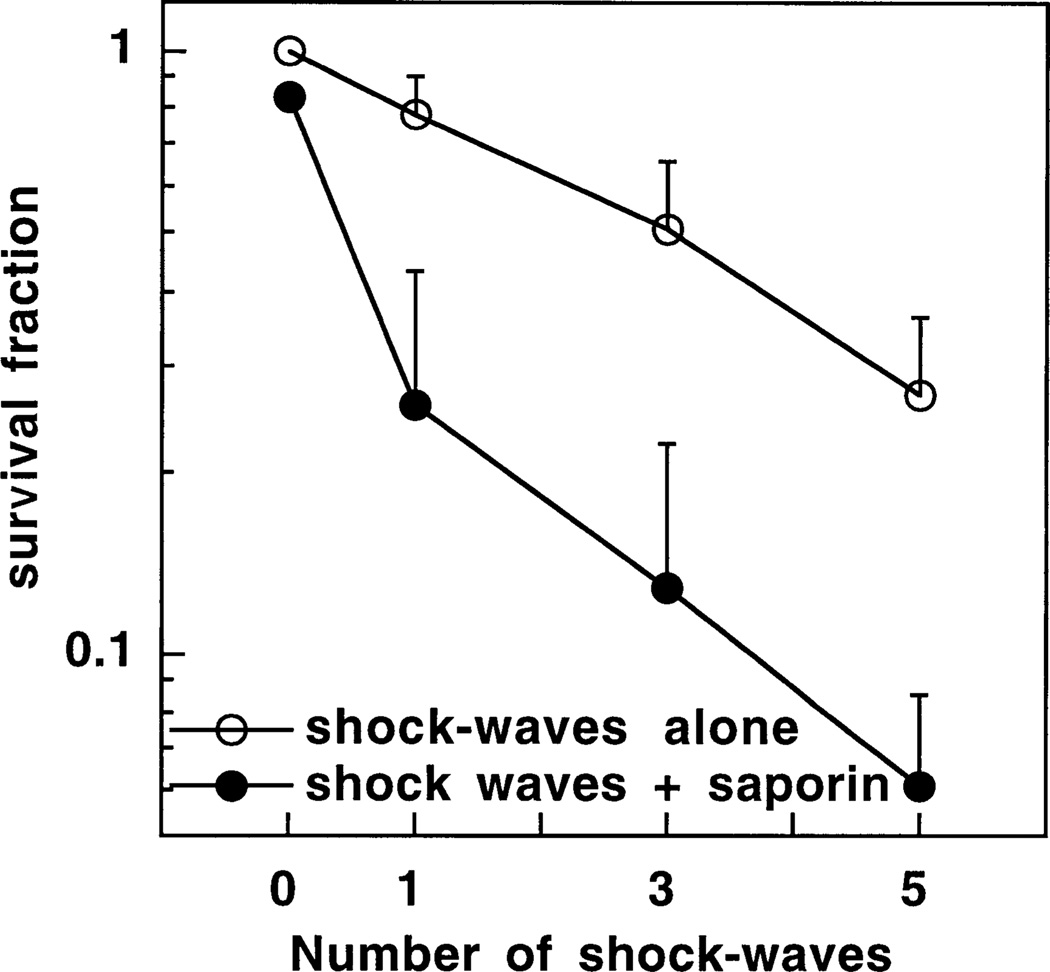
When this experiment was repeated with HT29 cells and the colony formation assay, the cytotoxicity seen previously with one shock wave in the absence of saporin increased further after three and five shock waves were delivered (Fig. 4). Since under these experimental conditions both saporin alone and shock waves alone have significant toxicity, the appropriate analysis method for the toxicity of the combination of shock waves and saporin is to test for antagonistic, additive or synergistic effects. The values of the fractional product parameter for the combination of saporin and one three or five shock waves were 1.86, 1 43, 1.31, all significantly greater than one (P < 0:05), indicating Bliss synergism.
Fig. 4.
Survival fractions determined by clonogenic assay of HT29 cells treated with one teo or three shock waves either with or without 10−7 M saporin and compared to cells treated by pelleting and resuspending alone in the absence of saporin or shock wave. Values are means ± SE of six independent experiments.
We then asked whether the sensitivity of HT29 cells to shock waves alone (without saporin) observed with the colony-forming assay, was due to increased sensitivity of HT29 cells to shock waves compared to OVCAR-5 cells, or to the ability of the clonogenic assay to detect losses of viability not seen with the MTT assay. When one, three, and five shock waves were delivered to HT29 cells in the absence of saporin and the mitochondrial activity measured with the MTT assay, we obtained survival fractions of 0.98 ± 0.1, 0.96 ± 0.07, and 0.95 ± 0.09, respectively. These numbers were compared to the survival fractions of 0.78 ± 0.13, 0.5 ± 0.15, and 0.27 ± 0.09 measured by clonogenic assay after one, three, and five shock waves, respectively, and were significantly different for three shock waves (P < 0:05) and for five shock waves (P = 0:0005).
4. Discussion
The local deposition of energy into a tumor has been used to potentiate the effect of cytotoxic molecules by increasing the molecular uptake by tumor cells. This is thought to occur by the creation of temporary pores in the cytoplasmic membrane, thus allowing exogenous molecules to diffuse through and accumulate in the cytoplasm. This methodology may be especially useful for macromolecules such as RIPs that would otherwise be routed via endosomal and lysosomal compartments where they may be degraded by intralysosomal proteases. We have previously shown that shock waves created by ablation of a target material by a short laser pulse may be used to deliver fluorescent macromolecules into cells in vitro [13–15]. We went on to demonstrate that shock waves generated by a shock tube could also accomplish this permeabilization with no toxicity to the cells as determined by the MTT assay. Molecules as large as 2 MDa fluoresceinlabeled dextran were delivered into the cytoplasm [8], and it was proposed that the shock tube-generated shock waves had the correct parameters (pressure, rise-time, and duration) to give a threshold of impulse (defined as the integral of pressure with duration) for molecular delivery into cells [7]. Cellular deformation and membrane effects due to varying shear stresses produced by the impulse of the shock wave on the relative motion of cells and fluid phase was proposed to be responsible for the permeabilization observed.
The present study has demonstrated that shock-tube generated shock waves could effectively potentiate the cytotoxicity of the type 1 RIP saporin in two different cell lines as determined by two viability assays. In contrast with our previous studies [7, 8] and our present results with OVCAR-5 cells, when we measured the cell viability of HT29 cells after shock waves alone using a clonogenic assay, we found a significant loss of viability using shock waves alone and saporin alone, not seen with the MTT assay. Although many workers have shown that the MTT assay correlates well with clonogenic assays for measuring loss of viability in vitro after several toxic treatments [16–18], others have found discrepancies (reviewed in Ref. [19]). There can be a substantial increase in cytotoxicity when survival is measured by long-term clonogenic assays compared to short term mitochondrial activity assays, particularly for cells with mutant or absent p53 [19], such as is the case with HT29 cells [20].
Delius and Adams have used shock waves generated by an extracorporeal lithotripter to potentiate the cytotoxicity of type 1 RIPs both in vitro and in tumors growing in mice [5]. Gelonin and saporin were used with L1210 and HeLa cancer cells in vitro, and the application of 250 shock waves demonstrated significant potentiation of toxicity. SSK2 fibrosarcoma tumors grown in C3H mice were treated with up to three batches of 500 shock waves each after i.p. administration of gelonin or saporin (25 and 2.5 mg/kg, respectively). Shock wave application delayed the tumor growth and long-term remissions lasting > 180 days were induced in 40% of the animals. Mashiba et al. [21] studied the use of electroporation in combination with saporin to inhibit the proliferation of PC9 and ASPC-1 human cancer cells. A combination of 1 µg/ml saporin and eight electroporation pulses (80–90 V for 10 ms) completely inhibited proliferation. They also demonstrated the effectiveness of the procedure in nude mice bearing these tumors after intratumoral injection of 1 mg of saporin, and produced several complete regressions. Another novel strategy using local energy delivery to allow type 1 RIPs to treat tumors has been reported termed photochemical internalization [22]. In this method the RIP such as gelonin is combined with a photosensitizer and these molecules are taken up into lysosomes together, and upon illumination, allows the gelonin to escape from the lysosomal compartment and reach the ribosomes. This procedure was demonstrated in vitro in several cell lines [22], and in vivo where the photosensitizer aluminum phthalocyanine disulfonate was injected i.p. into nude mice bearing the WiDr human colon cancer, followed by an intratumoral injection of 50 µg gelonin and illumination of the tumor with 135 J/cm2 of red light [23]. Significant tumor responses (including cures) were obtained with the combination of RIP, photosensitizer and light.
We have demonstrated the use of the combination of shock tube-generated shock waves and saporin to potentiate the cytotoxic activity of the RIP in vitro. Although we have not yet tested this approach in tumors growing in mice, it should be entirely feasible. The shock tube could be positioned on top of a subcutaneous tumor that has previously been locally injected with saporin and an appropriate number of shock waves launched into the tissue. The ability of a small number of shock waves (1–5) generated either by a shock-tube [7, 8], or by laser ablation of a target [13, 14] to accomplish the same degree of in vitro permeabilization that requires 250 lithotripter-generated shock waves [5, 24], suggests that the efficiency of permeabilization depends critically on the shockwave parameters (pressure, rise-time, and duration), and that the shock-wave parameters needed to fracture kidney stones may not be optimal for cellular permeabilization. If a repetitive shock wave generator (diaphragmless shock tube capable of delivering shock waves with a repetition rate similar to extracorporeal lithotripters [25]), or a system of repetitive laser ablation of a target were developed it may used in combination with a type 1 RIP as a practical therapeutic modality.
Acknowledgements
T.K. was supported in part by the Cell Science Research Foundation Japan. M.R.H. was supported by the Department of Defense Medical Free Electron Laser Program (contract N 00014-94-1-0927) and the National Institutes of Health (grant R01 CA/ AI838801). This work was partly supported by a contract from Mile Creek Capital, LLC, Boston, MA.
References
- 1.Barbieri L, Battelli MG, Stirpe F. Ribosome-inactivating proteins from plants. Biochim. Biophys. Acta. 1993;1154:237–282. doi: 10.1016/0304-4157(93)90002-6. [DOI] [PubMed] [Google Scholar]
- 2.Flavell DJ. Saporin immunotoxins. Curr. Top. Microbiol. Immunol. 1998;234:57–61. doi: 10.1007/978-3-642-72153-3_4. [DOI] [PubMed] [Google Scholar]
- 3.Flavell DJ, Boehm DA, Noss A, Warnes SL, Flavell SU. Therapy of human T-cell acute lymphoblastic leukaemia with a combination of anti-CD7 and anti-CD38-SAPORIN immunotoxins is significantly better than therapy with each individual immunotoxin. Br. J. Cancer. 2001;84:571–578. doi: 10.1054/bjoc.2000.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Foon KA. Monoclonal antibody therapies for lymphomas. Cancer J. 2000;6:273–278. [PubMed] [Google Scholar]
- 5.Delius M, Adams G. Shock wave permeabilization with ribosome inactivating proteins: a new approach to tumor therapy. Cancer Res. 1999;59:5227–5232. [PubMed] [Google Scholar]
- 6.Mashiba H, Ozaki Y, Matsunaga K. Augmented inhibition of tumor cell proliferation in combined use of electroporation with a plant toxin, saporin. Ann. N. Y. Acad. Sci. 1999;886:233–235. doi: 10.1111/j.1749-6632.1999.tb09424.x. [DOI] [PubMed] [Google Scholar]
- 7.Kodama T, Hamblin MR, Doukas AG. Cytoplasmic molecular delivery with shock waves: importance of impulse. Biophys. J. 2000;79:1821–1832. doi: 10.1016/S0006-3495(00)76432-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kodama T, Doukas AG, Hamblin MR. Shock wave-mediated molecular delivery into cells. Biochim. Biophys. Acta. 2002;1542:186–194. doi: 10.1016/s0167-4889(01)00177-x. [DOI] [PubMed] [Google Scholar]
- 9.Hamblin MR, Miller JL, Ortel B. Scavenger-receptor targeted photodynamic therapy. Photochem. Photobiol. 2000;72:533–540. doi: 10.1562/0031-8655(2000)072<0533:srtpt>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 10.Del Governatore M, Hamblin MR, Piccinini EE, Ugolini G, Hasan T. Targeted photo-destruction of human colon cancer cells using charged 17.1A chlorin e6 immuno-conjugates. Br. J. Cancer. 2000;82:56–64. doi: 10.1054/bjoc.1999.0877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greco WR, Bravo G, Parsons JC. The search for synergy: a critical review from a response surface perspective. Pharmacol. Rev. 1995;47:331–385. [PubMed] [Google Scholar]
- 12.Webb JL. Effect of more than one inhibitor, antagonism, summation, and synergism. Enzyme and Metabolic Inhibitors. 1963:488–512. [Google Scholar]
- 13.McAuliffe DJ, Lee S, Flotte TJ, Doukas AG. Stress-wave-assisted transport through the plasma membrane in vitro. Lasers Surg. Med. 1997;20:216–222. doi: 10.1002/(sici)1096-9101(1997)20:2<216::aid-lsm14>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 14.Mulholland SE, Lee S, McAuliffe DJ, Doukas AG. Cell loading with laser-generated stress waves: the role of the stress gradient. Pharm. Res. 1999;16:514–518. doi: 10.1023/a:1018814911497. [DOI] [PubMed] [Google Scholar]
- 15.Lee S, McAuliffe DJ, Zhang H, Xu Z, Taitelbaum J, Flotte TJ, Doukas AG. Stress-wave-induced membrane permeation of red blood cells is facilitated by aquaporins. Ultrasound Med. Biol. 1997;23:1089–1094. doi: 10.1016/s0301-5629(97)00083-5. [DOI] [PubMed] [Google Scholar]
- 16.Wasserman TH, Twentyman P. Use of a colorimetric microtiter (MTT) assay in determining the radio-sensitivity of cells from murine solid tumors. Int. J. Radiat. Oncol. Biol. Phys. 1988;15:699–702. doi: 10.1016/0360-3016(88)90314-8. [DOI] [PubMed] [Google Scholar]
- 17.McHale AP, McHale L. Use of a tetrazolium based colorimetric assay in assessing photoradiation therapy in vitro. Cancer Lett. 1988;41:315–321. doi: 10.1016/0304-3835(88)90293-5. [DOI] [PubMed] [Google Scholar]
- 18.Carmichael J, Mitchell JB, DeGraff WG, Gamson J, Gazdar AF, Johnson BE, Glatstein E, Minna JD. Chemosensitivity testing of human lung cancer cell lines using the MTT assay. Br. J. Cancer. 1988;57:540–547. doi: 10.1038/bjc.1988.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brown JM, Wouters BG. Apoptosis, p53, and tumor cell sensitivity to anticancer agents. Cancer Res. 1999;59:1391–1399. [PubMed] [Google Scholar]
- 20.Nagasawa H, Li CY, Maki CG, Imrich AC, Little JB. Relationship between radiation-induced G1 phase arrest and p53 function in human tumor cells. Cancer Res. 1995;55:1842–1846. [PubMed] [Google Scholar]
- 21.Mashiba H, Ozaki Y, Ikuno S, Matsunaga K. Augmentation of antiproliferative and antitumor effect on human cancer cells in combined use of electroporation with a plant toxin, saporin. Cancer Biother. Radiopharm. 2001;16:495–499. doi: 10.1089/10849780152752092. [DOI] [PubMed] [Google Scholar]
- 22.Berg K, Selbo PK, Prasmickaite L, Tjelle TE, Sandvig K, Moan J, Gaudernack G, Fodstad O, Kjolsrud S, Anholt H, Rodal GH, Rodal SK, Hogset A. Photochemical internalization: a novel technology for delivery of macromolecules into cytosol. Cancer Res. 1999;59:1180–1183. [PubMed] [Google Scholar]
- 23.Selbo PK, Sivam G, Fodstad O, Sandvig K, Berg K. In vivo documentation of photochemical internalization, a novel approach to site specific cancer therapy. Int. J. Cancer. 2001;92:761–766. doi: 10.1002/1097-0215(20010601)92:5<761::aid-ijc1238>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 24.Gambihler S, Delius M, Ellwart JW. Permeabilization of the plasma membrane of L1210 mouse leukemia cells using lithotripter shock waves. J. Membr. Biol. 1994;141:267–275. doi: 10.1007/BF00235136. [DOI] [PubMed] [Google Scholar]
- 25.Hosokawa T, Yamamoto M, Teshima K. Destruction of mouse lymphoma-cells with high-pressure pulses generated by a diaphragmless shock-tube. Shock Waves. 1995;5:19–23. [Google Scholar]