Abstract
Background
Breast cancer (BC) patients often experience cancer-related fatigue (CRF) before, during, and after their chemotherapy. Circadian rhythms are 24-hour cycles of behavior and physiology that are generated by internal pacemakers and entrained by zeitgebers (e.g., light). A few studies have suggested a relationship between fatigue and circadian rhythms in some clinical populations.
Methods
One hundred and forty-eight women diagnosed with stage I-III breast cancer and scheduled to receive at least four cycles of adjuvant or neoadjuvant chemotherapy, and 61 controls (cancer-free healthy women) participated in this study. Data were collected before (Baseline) and after four cycles of chemotherapy (Cycle-4). Fatigue was assessed with the Short Form of Multidimensional Fatigue Symptom Inventory (MFSI-SF); circadian activity rhythm (CAR) was recorded with wrist actigraphy (six parameters included: amplitude, acrophase, mesor, up-mesor, down-mesor and F-statistic). A mixed model analysis was used to examine changes in fatigue and CAR parameters compared to controls, and to examine the longitudinal relationship between fatigue and CAR parameters in BC patients.
Results
More severe CRF (total and subscale scores) and disrupted CAR (amplitude, mesor and F-statistic) were observed in BC patients compared to controls at both Baseline and Cycle-4 (all p's<0.05); BC patients also experienced more fatigue and decreased amplitude and mesor, as well as delayed up-mesor time at Cycle-4 compared to Baseline (all p's<0.05). The increased total MFSI-SF scores were significantly associated with decreased amplitude, mesor and F-statistic (all p's<0.006).
Conclusion
CRF exists and CAR is disrupted even before the start of chemotherapy. The significant relationship between CRF and CAR indicate possible underlying connections. Re-entraining the disturbed CAR using effective interventions such as bright light therapy might also improve CRF.
Keywords: breast cancer, fatigue, circadian activity rhythm, chemotherapy
Introduction
Studies show that breast cancer (BC) patients often experience fatigue before, during, and after their chemotherapy.[1-6] Cancer-related fatigue (CRF) interferes with patients’ daily life and impacts their quality of life,[7-10] and is often an important cause of discontinuation of their treatment.[11] CRF is also associated with significant disability and increased utilization of health care resources.[12] The causes of CRF are not clear but are believed to be multifactorial, as CRF is highly correlated to physiological factors (e.g., pain, anemia, neuroendocrine changes, altered energy metabolism, inflammation), psychological factors (e.g., stress, depression, anxiety), socio-cultural factors (e.g., education, cognitive and behavioral response), and chronobiological factors (e.g., sleep and circadian rhythms).[13-15, 12, 6] Ancoli-Israel et al. hypothesized that in BC patients, CRF might be associated with decreased exposure to bright light and desynchronized activity-related circadian rhythms.[13] Our data collected in BC patients indeed found a significant correlation with more fatigue associated with less bright light exposure.[16]
Circadian rhythms are 24-hour cycles of behavior and physiology that are generated by one or more internal pacemakers and exist in all mammals. In humans, the suprachiasmatic nucleus (SCN) in the hypothalamus serves as the central neural pacemaker. Humans possess a circadian rhythm of approximately 24 hours. Examples of circadian rhythms include alternations of hormone secretion, body temperature, and sleep-wake cycles. The 24-hour circadian rhythms are entrained by a number of environmental stimuli (zeitgebers), such as the timing of activity, exercise, social interaction, and the sleep-wake schedule, etc., but the predominant entraining agent is light, including daylight and artificial light.[17, 18]
A few studies have suggested a relationship between fatigue and circadian rhythms in different types of clinical populations, while some other studies failed to identify a relationship. Attarian et al.[19] found a significant correlation between fatigue and disrupted sleep cycles in multiple sclerosis patients. Shibui et al. published a case report on periodic fatigue due to desynchronization in a patient with non-24-hour sleep-wake syndrome.[20] In cancer patients, CRF has been associated with a few circadian activity rhythm (CAR) parameters, such as mesor (a circadian rhythm-adjusted mean of the daily activity), acrophase (timing of the peak of the activity rhythm), amplitude (peak of the activity), and disruptions of the 24-hour autocorrelation coefficient during chemotherapy and radiotherapy.[21-23] Bower et al.[24] found that in BC survivors those with persistent fatigue showed a significantly flattered cortisol slope than those without. In two studies [25, 26] focusing on the correlation between CAR and quality of life in patients with metastatic colorectal cancer, the desynchronized rest-activity rhythms prior to chemotherapy were associated with more fatigue, poor quality of life and shorter survival; however, the relationships were only examined cross-sectionally. On the other hand, two other studies found no association between fatigue and the 24-hour autocorrelation coefficient in either BC survivors or other cancer patients.[27, 28]
Most of these reports explored the relationship between fatigue and circadian rhythms during therapy or in cancer survivors and none were compared to non-cancer controls in longitudinal studies. Therefore, in this study, utilizing a group of innovative actigraphy-based CAR parameters,[29] we examined both CRF and CAR before and after four cycles of chemotherapy in a group of women with BC, and compared their results with those of cancer-free women. We hypothesized that women with BC would experience more fatigue and disrupted CAR than controls, and that there would be a relationship between this CRF and CAR.
METHODS
Participants
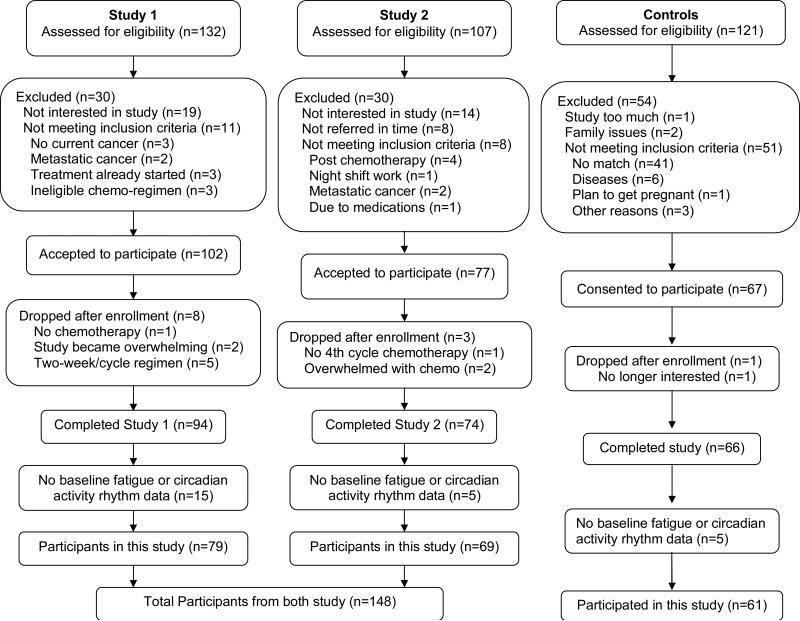
One hundred and forty-eight women with newly diagnosed stage I-III BC receiving at least four cycles of adjuvant or neoadjuvant chemotherapy participated in this study. These BC patients came from two studies which had identical protocols and data collection began before the start of chemotherapy and spanned at least 4 cycles of treatment: the first study focused on fatigue, sleep and circadian rhythms (Study 1; N=79), and the second focused on chemotherapy related cognitive impairments (Study 2; N=69). Sixty-one cancer-free women who were age- and socio-economic status matched to BC patients in Study 2 also participated in this study as healthy controls (see Figure 1 for the Screening and Enrollment processes).
Figure 1.
Screening and Enrollment Flowchart
Pregnant women, those undergoing bone marrow transplants, and those with metastatic breast cancer, with confounding underlying medical illnesses, with significant pre-existing anemia or with other physical or psychological impairments were excluded from both studies. The University of California Committee on Protection of Human Subjects and the UCSD Moores Cancer Center's Protocol Review and Monitoring Committee approved both studies, and an informed consent was obtained from each woman at the beginning of her participation.
Measures
Fatigue
Fatigue was assessed using the Short Form of Multidimensional Fatigue Symptom Inventory (MFSI-SF).[30] The MFSI-SF is a 30-item questionnaire, which is comprised of five subscales: General Fatigue, Physical Fatigue, Emotional Fatigue, Mental Fatigue, and Vigor. Each subscale includes 6 items and each item is rated on a 5-point scale indicating how true the statement was during the last week (0=not at all, 4=extremely). The sum of the General, Physical, Emotional, and Mental subscale scores minus the Vigor subscale score generates a total score. The range of possible scores for each subscale is 0 to 24, and the range for total score is –24 to 96, with higher scores indicating severer fatigue, except for the Vigor subscale, where larger scores indicate less fatigue. The MFSI-SF has been validated and is a reliable tool for assessing the full spectrum of cancer-related fatigue symptoms in both clinical and research applications.[31]
Circadian activity rhythm (CAR)
CAR was analyzed by fitting each subject's activity data to a 5-parameter extended cosine model.[29] The activity data were measured with two types of actigraphs: the Actillume-II (Ambulatory Monitoring, Inc., Ardsley, New York) for Study 1 and Actiwatch-Light (Philips Respironics Mini Mitter, Bend, OR) for Study 2. The Actillume-II contains a microprocessor, 32K RAM memory, and associated circuitry and uses a piezoelectric linear accelerometer (sensitivity <.003 g-force) with a sampling rate of 20Hz to measure and record wrist movement. The Actiwatch-Light is similar in size to a watch (37×29×9 mm; weight 17 grams). It uses a piezoelectric linear accelerometer (sensitivity <.01 g-force) with a sampling rate of 32Hz to measure and record wrist movement. A 1-minute epoch setting was used for both actigraphy devices. The epoch-by-epoch activity data were downloaded to a desktop computer for the further CAR analysis. To establish equivalency between the two devices, a validation study in eight volunteers was conducted with both devices worn concurrently on the same wrist for 72-hours. The Actillume-II-derived SUMACT (summary activity) count and the Actiwatch-Light-derived activity count data were highly correlated (both r >0.85), and therefore deemed equivalent for the purpose of this study.
This 5-parameter extended cosine model is an anti-logistic transformation of the standard cosine curve, and it allows for estimation of parameters describing the shape of the 24-hour rest/activity rhythm.[29] The following six CAR parameters were generated from the model in this study: the amplitude (peak or maximum of the curve, with a lower amplitude suggesting a less rhythmic rhythm); the acrophase (time of day of the peak activity, with a later time suggesting more phase-delay); the mesor (half-way between minimum and maximum of the curve, a circadian rhythm-adjusted mean of the daily activity); the time of day that the subject switched from low activity to high activity, (i.e., the time of day that activity increased from below the mesor to above the mesor, called “up-mesor”, a later time suggesting that the subject became active later in the day); the time of day where the subject switched from high activity to low activity (i.e., the time of day that activity decreased from above the mesor to below the mesor, called “down-mesor”, a later time suggesting that the subject settled down later in the evening); and the F-statistic (the “goodness of fit” of the fitted function with a larger value indicating more robust rhythms). In brief, data sets that were most rhythmic or had the strongest rhythms yielded the largest F-statistic and the smallest standard error.
Procedure
Participants were referred by oncologists in the San Diego area, and were mainly from the UCSD Moores Cancer Center. After consent forms were signed, medical records were abstracted for medical history and current medication use. Data reported in this study were collected after diagnosis but before the start of chemotherapy (Baseline), and at the end of the fourth cycle of treatment (Cycle-4).
At both data collection time points, women completed a set of questionnaires, including MFSI-SF, wore an actigraphy for three consecutive 24-hour periods (i.e., 72 hours), and completed a sleep log used for editing actigraphy data. For each woman, actigraphy was recorded on the same days of the week at each time point. The day chosen was based on the day of the chemotherapy administration. While the ideal recording time for an actigraph is generally one week, due to potential subject burden, the minimum of three days suggested by the AASM practice parameters for actigraphy [32] was used in both studies.
Cancer-free controls were age and education matched to the cancer patients in Study 2. Potential cancer-free controls were friends referred by the cancer patient or were recruited independently by study personnel. Once consented, controls were given the same questionnaires and wore actigraphs at the same two time points as the patients.
Data analysis
Descriptive statistics (mean, standard deviation and standard errors) were calculated for all outcomes at both time points. T-test and Chi-square test were used to examine the differences between the two samples of BC patients in terms of demographic and disease/treatment characteristics. Analysis of covariance (ANOVA) was performed between the patients and controls groups in fatigue and CAR measurements while controlling for BMI and Study (1 or 2).
A mixed model analysis [33] was used to examine changes in fatigue and CAR parameters from Baseline to Cycle-4 in BC patients comparing with controls, and to examine the longitudinal relationship between fatigue and CAR parameters for BC patients only. This modeling approach accounts for correlations in repeated measures within a subject, and also allows for partially missing data (i.e., subjects with missing data at some, but not all time-points can be included in the model). A random intercept was included in each mixed model to account for subject-specific effects. In the mixed models examining the changes of fatigue or CAR over time, the chemotherapy week (time), case-control status (group), and the time*group interaction were modeled as fixed effects. Since a few CAR parameters (amplitude, mesor and F-statistic) were significantly different between the two studies, the Study (1 or 2) variable was also adjusted in these mixed models.
In those mixed models examining the longitudinal relationship between fatigue (response) and CAR parameters (predictor), total MFSI-SF scores was the dependent variable, and CAR parameters (amplitude, acrophase, mesor, up-mesor, down-mesor, or F-statistic) was the independent variable. In addition to a random intercept, the coefficient of the CAR variable was included as a random effect, thereby allowing for subject-specific slope terms for CAR parameter in the model. These mixed models were adjusted for BMI, chemotherapy week (time) and Study (1 or 2). Adjusted regression coefficients (ß-value) with standard errors and associated p-values are presented.
All analyses were performed using version 9.2 of SAS (SAS Institute Inc. 2008). Given that there were 7 outcomes of interest (total MFSI-SF score and six CAR variables; the analyses of the five subscale of MFSI-SF were secondary), a Bonferroni correction would yield p<0.0071 as statistically significant. However, we present all p-values in Table 2 and note which tests meet a Bonferroni adjustment.
Table 2.
Mixed model results for BC patients with fatigue as the dependent variable and circadian activity rhythm parameters as the independent variable
| Fatigue | Circadian activity rhythms | Mixed model results |
||
|---|---|---|---|---|
| Adj. ß-value | Standard Error | p-value | ||
| Total MFSI-SF score | Amplitude | -11.834 | 3.033 | 0.0002 |
| Acrophase | 0.476 | 0.887 | 0.6 | |
| Mesor | -14.257 | 5.244 | 0.006 | |
| Up-mesor | 0.307 | 0.738 | 0.7 | |
| Down-mesor | 0.308 | 0.696 | 0.7 | |
| F-statistic | -0.00652 | 0.00225 | 0.005 | |
Note: a adjusted for BMI, time and Study. MFSI-SF=Multidimensional Fatigue Symptom Inventory-Short Form, higher scores indicate more fatigue. The p-values in bold meet the Bonferroni adjustment of p<0.0071. Amplitude was the maximum activity of the day, acrophase was the time the of the peak activity of the day, mesor was the mean activity, up-mesor or down-mesor was the time when the activity rose from below the average to above the average, or from above the average to below the average, and F-statistic measured the overall rhythmicity of circadian activity, a smaller F-statistic indicates less robust or disrupted circadian activity rhythm.
RESULTS
Study 1 data were collected between 2000 and 2005, and as was recommended at that time, women in Study 1 all received 3-week chemotherapy cycles. Study 2 data were collected between 2005 and 2010, at which point the recommended treatment regimen started to change to a 2-week cycle; therefore 37 (63%) women in Study 2 received a 2-week cycle regimen and 22 (37%) received a 3-week cycle regimen of chemotherapy. Since there were no significant differences between those with 2-week and those with 3-week regimens in all measurements (demographics, disease/treatment, questionnaires and CAR parameters) (all p's>0.05), the different length of treatment cycles was not considered as a confounder in this study.
There were no significant differences between the two samples of BC patients in terms of age, race, body mass index (BMI), education level, marital status, annual household income, baseline menopausal status, cancer stage, surgery type, chemotherapy regimen and fatigue (all p's>0.05), the two samples were therefore merged together in this study. There were also no significant differences between the merged BC patients group and control group in age, race, education, marital, income, baseline menopausal status; however, BC patients’ BMI was significantly higher than that in controls (p=0.04), BMI was then adjusted when comparing the two groups. Detailed demographic and disease characteristics of all women are listed in Table 1.
Table 1.
Demographic, disease and treatment characteristics of the participants (n=148)
| Variable | Study 1 (n=79) | Study 2 (n=69) | Controls (n=61) |
|---|---|---|---|
| Age (years) | |||
| Mean (SD) | 50.7 (9.8) | 51.3 (9.0) | 52 (9.4) |
| Range | 34 – 79 | 31 – 76 | 29 –81 |
| BMI (kg/m2) | |||
| Mean (SD) | 28.8 (6.9) | 27.5 (7.4) | 26.0 (7.0) |
| Range | 17.4 – 51.8 | 19.3 – 61.9 | 19.1 – 56.7 |
| Race [n (%)] | |||
| Caucasian | 60 (76.0) | 61 (88.4) | 52 (85.3) |
| Non-Caucasian | 19 (24.0) | 8 (11.6) | 9 (14.7) |
| Education [n (%)] | |||
| Some college and below | 40 (50.6) | 32 (46.4) | 22 (36.1) |
| Completed college and above | 39 (46.4) | 37 (53.6) | 39 (63.9) |
| Marital status [n (%)] | |||
| Never married | 8 (10.1) | 3 (4.4) | 7 (11.5) |
| Divorced/separated/widowed | 15 (19.0) | 19 (27.5) | 12 (19.7) |
| Married | 56 (70.9) | 47 (68.1) | 42 (68.8) |
| Household annual income [n (%)] | |||
| ≤ $30,000 | 11 (13.9) | 11 (15.9) | 2 (3.3) |
| > $30,000 | 58 (73.4) | 49 (71.0) | 50 (82.0) |
| Refused to answer | 10 (12.7) | 9 (13.1) | 9 (14.7) |
| Baseline menopausal status [n (%)] | |||
| Pre-menopause | 32 (42.7) | 28 (40.6) | 21 (35.0) |
| Peri-menopause | 7 (9.3) | 9 (13.0) | 8 (13.3) |
| Post-menopause | 21 (28.0) | 27 (39.1) | 22 (36.7) |
| Hysterectomy | 4 (20.0) | 5 (7.3) | 9 (15.0) |
| Not available | 4 | 0 | 1 |
| Cancer stage [n (%)] | |||
| Stage I | 22 (30.6) | 15 (25.9) | - |
| Stage II | 36 (50.0) | 24 (41.4) | |
| Stage III | 14 (19.4) | 19 (32.8) | |
| Not available | 7 | 11 | |
| Surgery type | |||
| Lumpectomy | 27 (37.5) | 24 (41.4) | - |
| Mastectomy | 32 (44.4) | 28 (48.3) | |
| Double mastectomy | 4 (5.6) | 3 (5.2) | |
| No surgery before Chemotherapy | 9 (12.5) | 3 (5.2) | |
| Not available | 7 | 11 | |
| Chemotherapy regimen [n (%)] | |||
| AC based | 62 (86.1) | 47 (78.3) | - |
| Others | 10 (13.9) | 13 (21.7) | |
| Not available | 7 | 9 | |
Note: AC = Doxorubicin + Cyclophosphamide.
Fatigue
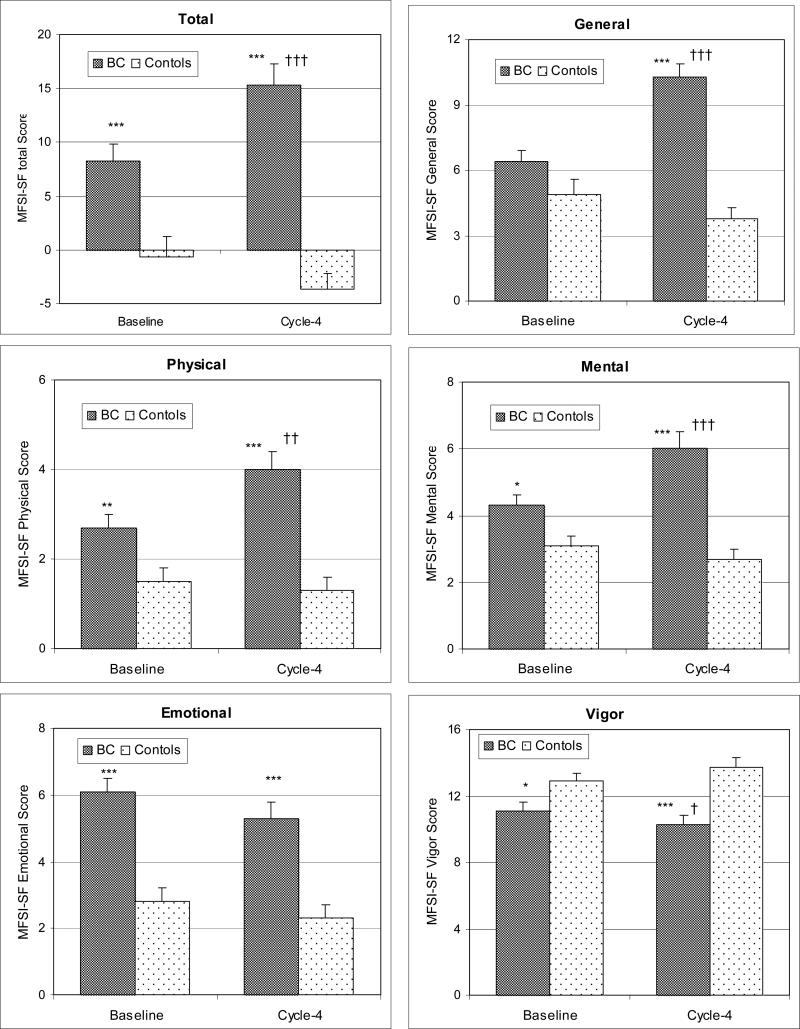
Patients experienced more fatigue than controls at both time points as well as more fatigue at Cycle-4 than at Baseline. As shown in Figure 2, for BC patients, total MFSI-SF scores increased significantly from Baseline to Cycle-4 (p<0.0001), as well as the scores of General (p<0.0001), Physical (p=0.0004) and Mental (p<0.0001) subscales; meanwhile, the Vigor subscale score decreased significantly from Baseline to Cycle-4 (p=0.03), but there were no significant changes for the Emotional subscale scores (p=0.2). For controls, there were no significant changes from Baseline to Cycle-4 in any scores of MFSI-SF (all p's>0.1).
Figure 2.
Comparisons of total and subscale (General, Physical, Mental, Emotional and Vigor) scores of the Multidimensional Fatigue Symptom Inventory-Short Form (MFSI-SF) between BC patients and controls over time (mean+SE). Higher scores indicate more fatigue, except Vigor subscale, where a higher score indicates less fatigue. Baseline=before the start of chemotherapy, Cycle-4=at the end of cycle 4 of chemotherapy. Compared to controls at the same time point: * p<0.05, ** p<0.01, *** p<0.0001; compared to Baseline for the same group: † p<0.05, †† p<0.01, ††† p<0.0001.
Compared to controls, at Baseline, BC patients reported significantly more total (p=0.001), Emotional (p<0.0001) and Mental (p=0.006) fatigue, and less Vigor (p=0.05), but there were no significant differences in the General (p=0.1) and Physical (p=0.07) subscales scores. At Cycle-4, BC patients reported significantly higher total and General, Physical, Emotional and Mental subscale scores and less Vigor subscale scores of MFSI-SF (all p's<0.006).
When comparing the changes over time, there was a significant time*group interaction in MFSI-SF total (p=0.0002), General (p<0.0001), Physical (p=0.02), Mental (p=0.0002 and Vigor (p=0.04) scores, indicating more increases of fatigue in BC patient than that in controls.
Circadian activity rhythm (CAR)
Patients experienced more disrupted rhythms compared to controls at both time points and had more disruption at Cycle-4 than at Baseline. The changes of the six CAR parameters for BC patients vs. controls are shown in Figure 3. For BC patients, from Baseline to Cycle-4, the amplitude (p=0.005), mesor (p=0.005) and F-statistic (p=0.03) decreased significantly, and the up-mesor time delayed significantly (p=0.04); for controls, there were no significant changes between the two time points in any of the six parameters (all p's>0.3).
Figure 3.
Comparisons of circadian activity rhythm measures (amplitude, acrophase, mesor, up-mesor, down-mesor and F-statistic) between BC patients and controls over time (mean+SE). Smaller values of amplitude and F-statistic indicate disrupted circadian activity rhythm; later times of acrophase, up-mesor and down-mesor indicate delayed circadian activity rhythms. Baseline=before the start of chemotherapy, Cycle-4=at the end of cycle 4 of chemotherapy. Compared to controls at the same time point: * p<0.05, ** p<0.01, *** p<0.0001; compared to Baseline for the same group: † p<0.05, †† p<0.01, ††† p<0.0001.
Compared to controls, BC patients showed significantly lower amplitude and smaller f-statistic at Baseline (both p=0.01), and lower amplitude (p=0.0004), lower mesor (p=0.02), and smaller f-statistic (p<0.0001) at Cycle-4 , suggesting disrupted CAR. There were no significant differences between the two groups in acrophase, up-mesor and down-mesor.
When comparing the changes over time, there was a significant time*group interaction in the amplitude (p=0.05) and mesor (p=0.03), indicating more disruptions in CAR in BC patients that that in controls.
Associations between fatigue and CAR in BC patients
For BC patients only, using total MFSI-SF score as the dependent variable and CAR parameters as the independent variable, after adjusting for time and Study, mixed model analyses revealed that the changes of total MFSI-SF scores were significantly associated with the changes of amplitude, mesor, and F-statistic (all p's<0.01). Specifically, women with lower amplitude, smaller mesor and smaller F-statistics experienced more fatigue, suggesting that more fatigue was associated with more disrupted CAR.
DISCUSSION
This study confirmed that BC patients not only reported significant more CRF than cancer-free controls both before the start of and after four cycles of chemotherapy, they also reported significantly more fatigue after than prior to treatment. BC patients’ CAR showed the same pattern as fatigue, with disrupted CAR present prior to the start of chemotherapy and becoming worse after treatment. Furthermore, increased fatigue was significantly associated with disrupted CAR.
Compared to cancer-free controls, BC patients reported significant worse scores in total and all five subscale scores of the MFSI-SF both before and after chemotherapy, except for the General and Physical scores at baseline, where the difference between the two groups was not significant. These results indicate that before chemotherapy, BC patients were already mentally and emotionally fatigued and experienced lack of energy. Since the General subscale includes six questions asking about the general feeling of fatigue, such as “I feel tired”, “I feel fatigued” and “I am pooped”, which do not refer to any cancer-specified field of fatigue, this subscale cannot discriminate BC patients from controls before the start of chemotherapy. As the other scales distinguished the two groups, asking questions about general fatigue in cancer patients may not be enough to identify CRF in this population. After four cycles of chemotherapy, BC patients experienced more CRF in all five domains of fatigue compared to controls, as well as experiencing more fatigue than at baseline suggesting that chemotherapy is a significant contributor to fatigue in cancer patients.
When CAR was examined, BC patients demonstrated significantly lower amplitude and smaller F-statistic than controls at both time points. BC patients’ amplitude also significantly decreased after four cycles of chemotherapy. In addition, BC patients’ up-mesor time was significantly delayed after chemotherapy. Lower amplitude generally indicates less movement during the day and/or more movement during the night. Delayed up-mesor time plus stable down-mesor time suggest BC patients’ active time during the day decreased after chemotherapy. Together with the decreased F-statistic, these data indicate that BC patients experienced disrupted CAR before the start for chemotherapy, and this disrupted CAR became more disrupted after four cycles’ of chemotherapy. These results confirm our previously reported findings which were based on data from Study 1.[34]
Mixed model analysis revealed that BC patients experienced more increases of CRF (total and 4 of 5 subscale scores of MFSI-SF) and more disruptions of CAR (amplitude, mesor) from baseline to after four cycles of chemotherapy than controls, and the increased CRF was significantly associated with disrupted CAR, i.e., the increased fatigue was significantly associated with decreases in amplitude, mesor and F-statistic. This relationship suggests that more fatigue was predicted by more disrupted CAR in women with BC undergoing chemotherapy, or vice versa. Although a few studies have identified a relationship between fatigue and CAR before [21, 25, 26] or during/after cancer treatments,[22-24] the current study is the first to report findings of increased CRF and disturbed CAR in women with BC compared to cancer-free women, and a significant relationship between the CRF and CAR both before and after chemotherapy.
Another strength of this study is that a group of innovative CAR parameters were used. This group of CAR parameters were derived from an extended cosine model,[29] the curve generated by this model has a more rectangular-like wave shape than the regular cosine curve, and thus has a better fit to the pattern of daily activity rhythm recorded by actigraphy, i.e., nearly flattened and steady activity levels during both the day and night. These CAR parameters have been utilized in other studies of BC which showed that CAR gets progressively more desynchronized during chemotherapy [34] and that bright light treatment improved CAR.[35, 36]
The current study, along with previous findings, suggests that there is a significant relationship between CRF and circadian rhythms, especially CAR. CRF and circadian rhythms may share some common potential mechanisms, or a cause-and-effect relationship may exist. Sleep problems are common among cancer patients [37] and these sleep problems are associated and may also share some underlining mechanisms with fatigue.[38] Our current and previously published data confirm that in addition to disturbed sleep both before and during chemotherapy in BC patients,[1, 39, 40] CAR is disrupted before the start of chemotherapy and becomes more disrupted during treatment.[34] The disruptions in circadian rhythms can affect sleep quality and thus disrupt a variety of physiological mechanisms pertaining to fatigue.[13] It is possible therefore that the circadian rhythms disruption plays a role in the psychological experience of fatigue.[41] However, on the other hand, both CRF and CAR could be consequences of cancer treatment. Chemotherapy has been reported be a major cause of CRF, and chemotherapy induced side effects (nausea, dizziness, dyspnea, etc.), or changes of daily routine of activity, might also contributed to alternations of CAR. Therefore, as concluded in a recent review by Payne,[42] few studies have focused on the relationship between fatigue and circadian rhythms, and the exact interacting mechanisms, including possible mediators and neurotransmitter mechanisms still remain unknown, further studies are needed to answer these questions.
Light therapy has been shown to be effective for the treatment of circadian-rhythms-related disorders such as delayed and advanced sleep phase syndromes, jet lag syndrome, shift work syndrome, even fatigue symptoms, by synchronizing circadian rhythms.[43-45] Preliminary data from our laboratory also showed that bright light prevented fatigue and CAR from deteriorating in women with BC undergoing chemotherapy.[36, 46] Since light is the predominant entraining agent for circadian rhythms, these findings support the possible cause-and-effect relationship between circadian rhythms and fatigue, although studies with larger sample sizes and among other clinical populations are needed to confirm the effect of light therapy on fatigue.
This study had some limitations. The data were collected only in women with stage I to stage III BC, so conclusions cannot be extended to patients with other stages of BC or with other types of cancer. Patients and controls were all volunteers and were not randomly selected; thus there might have been some sample selection bias which could limit the generalizability of the study.
Only Study 2 had one year post-chemotherapy follow-up data (not reported here), so the long term relationship between fatigue and CAR after completing the treatment could not be determined by this study. Circadian rhythms were only measured using actigraphy; other measurements of circadian rhythms, such as salivary melatonin and core body temperature, also need to be studied regarding the relationships between fatigue and circadian rhythms in cancer patients.
In summary, BC patients experienced more fatigue and disrupted CAR than cancer-free controls both before and after four cycles of chemotherapy; this fatigue and disruption of CAR became even worse after four cycles compared to pre-chemotherapy in BC patients. In addition, the increase of fatigue was significantly associated with disruption in CAR. Fatigue may be caused by disturbed circadian rhythms, or vice versa. Studies to examine the effect of light therapy on fatigue among cancer patients through the possible mechanism of re-entraining circadian rhythms are warranted.
Acknowledgments
Supported by: NCI CA112035, UL1RR031980 (CTRI), the UCSD Stein Institute for Research on Aging and the Department of Veterans Affairs Center of Excellence for Stress and Mental Health (CESAMH).
References
- 1.Ancoli-Israel S, Liu L, Marler M, Parker BA, Jones V, Robins Sadler G, et al. Fatigue, sleep and circadian rhythms prior to chemotherapy for breast cancer. Support. Care Cancer. 2006;14(3):201–209. doi: 10.1007/s00520-005-0861-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berger AM, Farr LA, Kuhn BR, Fischer P, Agrawal S. Values of Sleep/Wake, Activity/Rest, Circadian Rhythms, and Fatigue Prior to Adjuvant Breast Cancer Chemotherapy. J Pain Symptom Manage. 2007;33:398–409. doi: 10.1016/j.jpainsymman.2006.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Byar KL, Berger AM, Bakken SL, Cetak MA. Impact of adjuvant breast cancer chemotherapy on fatigue, other symptoms, and quality of life. Oncol.Nurs Forum. 2006;33(1):E18–26. doi: 10.1188/06.ONF.E18-E26. [DOI] [PubMed] [Google Scholar]
- 4.Nieboer P, Buijs C, Rodenhuis S, Seynaeve C, Beex LV, van der WE, et al. Fatigue and relating factors in high-risk breast cancer patients treated with adjuvant standard or high-dose chemotherapy: a longitudinal study. J Clin Oncol. 2005;23(33):8296–8304. doi: 10.1200/JCO.2005.10.167. [DOI] [PubMed] [Google Scholar]
- 5.Berger AM, Gerber LH, Mayer DK. Cancer-related fatigue: implications for breast cancer survivors. Cancer. 2012;118(8 Suppl):2261–2269. doi: 10.1002/cncr.27475. [DOI] [PubMed] [Google Scholar]
- 6.Campos MP, Hassan BJ, Riechelmann R, Del GA. Cancer-related fatigue: a practical review. Ann.Oncol. 2011;22(6):1273–1279. doi: 10.1093/annonc/mdq458. [DOI] [PubMed] [Google Scholar]
- 7.Visser MRM, Smets EMA. Fatigue, depression and quality of life in cancer patients: how are they related? Support.Care Cancer. 1998;6:101–108. doi: 10.1007/s005200050142. [DOI] [PubMed] [Google Scholar]
- 8.Tchen N, Juffs HG, Downie FP, Yi Q-L, Hu H, Chemerynsky I, et al. Cognitive function, fatigue, and menopausal symptoms in women receiving adjuvant chemotherapy for breast cancer. Journal of Clinical Oncology. 2003;21(22):4175–4183. doi: 10.1200/JCO.2003.01.119. [DOI] [PubMed] [Google Scholar]
- 9.Lindley C, Vasa S, Sawyer WT, Winer EP. Quality of life and preferences for treatment following systemic adjuvant therapy for early-stage breast cancer. J Clin Oncol. 1998;16(4):1380–1387. doi: 10.1200/JCO.1998.16.4.1380. [DOI] [PubMed] [Google Scholar]
- 10.Bower JE, Ganz PA, Desmond KA, Rowland JH, Meyerowitz BE, Belin TR. Fatigue in breast cancer survivors: occurrence, correlates, and impact on quality of life. J.Clin.Oncol. 2000;18(4):743–753. doi: 10.1200/JCO.2000.18.4.743. [DOI] [PubMed] [Google Scholar]
- 11.Winningham ML, Nail LM, Burke MB, Brophy L, Cimprich B, Jones LS, et al. Fatigue and the cancer experience; the state of the knowledge. Oncology Nursing Forum. 1994;21(1):23–36. [PubMed] [Google Scholar]
- 12.Goldstein D, Bennett BK, Webber K, Boyle F, de Souza PL, Wilcken NR, et al. Cancer-related fatigue in women with breast cancer: outcomes of a 5-year prospective cohort study. J Clin Oncol. 2012;30(15):1805–1812. doi: 10.1200/JCO.2011.34.6148. [DOI] [PubMed] [Google Scholar]
- 13.Ancoli-Israel S, Moore P, Jones V. The relationship between fatigue and sleep in cancer patients: A review. Eur J Cancer Care (Engl) 2001;10(4):245–255. doi: 10.1046/j.1365-2354.2001.00263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morrow GR, Andrews PLR, Hickok JT, Roscoe JA, Matteson S. Fatigue associated with cancer and its treatment. Support.Care Cancer. 2002;10:389–398. doi: 10.1007/s005200100293. [DOI] [PubMed] [Google Scholar]
- 15.Ryan JL, Carroll JK, Ryan EP, Mustian KM, Fiscella K, Morrow GR. Mechanisms of cancer-related fatigue. Oncologist. 2007;12(Suppl 1):22–34. doi: 10.1634/theoncologist.12-S1-22. [DOI] [PubMed] [Google Scholar]
- 16.Liu L, Marler M, Parker BA, Jones V, Johnson S, Cohen-Zion M, et al. The relationship between fatigue and light exposure during chemotherapy. Support.Care Cancer. 2005;13(12):1010–1017. doi: 10.1007/s00520-005-0824-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Czeisler CA, Buxton OM. The human circadian timing system and sleep-week regulation. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. Elsevier Saunders; St. Louis: 2011. pp. 402–419. [Google Scholar]
- 18.Küller R. The influence of light on circarhythms in humans. J Physiol Anthropol Appl Human Sci. 2002;21(2):87–91. doi: 10.2114/jpa.21.87. [DOI] [PubMed] [Google Scholar]
- 19.Attarian HP, Brown KM, Duntley SP, Carter JD, Cross AH. The relationship of sleep disturbances and fatigue in multiple sclerosis. Arch Neurol. 2004;61(4):525–528. doi: 10.1001/archneur.61.4.525. [DOI] [PubMed] [Google Scholar]
- 20.Shibui K, Uchiyama M, Iwama H, Ozaki S, Takahashi K, Okawa M. Periodic fatigue symptoms due to desynchronization in a patient with non-24-h sleep-wake syndrome. Psychiatry and Clinical Neurosciences. 1998;52(5):477–477. doi: 10.1046/j.1440-1819.1998.00424.x. [DOI] [PubMed] [Google Scholar]
- 21.Miaskowski C, Lee K, Dunn L, Dodd M, Aouizerat BE, West C, et al. Sleep-wake circadian activity rhythm parameters and fatigue in oncology patients before the initiation of radiation therapy. Cancer Nurs. 2011;34(4):255–268. doi: 10.1097/NCC.0b013e3181f65d9b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roscoe JA, Morrow GR, Hickok JT, Bushunow P, Matteson S, Rakita D, et al. Temporal interrelationships among fatigue, circadian rhythm and depression in breast cancer patients undergoing chemotherapy treatment. Supportive Care in Cancer. 2002;10(4):329–336. doi: 10.1007/s00520-001-0317-0. [DOI] [PubMed] [Google Scholar]
- 23.Berger AM, Wielgus K, Hertzog M, Fischer P, Farr L. Patterns of circadian activity rhythms and their relationships with fatigue and anxiety/depression in women treated with breast cancer adjuvant chemotherapy. Support.Care Cancer. 2009 doi: 10.1007/s00520-009-0636-0. [DOI] [PubMed] [Google Scholar]
- 24.Bower JE, Ganz PA, Dickerson SS, Peterson L, Aziz N, Fahey JL. Diurnal cortisol rhythm and fatigue in breast cancer survivors. Psychoneuroendocrinology. 2005;30(1):92–100. doi: 10.1016/j.psyneuen.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 25.Mormont MC, Waterhouse J, Bleuzen P, Giacchetti S, Jami A, Bogdan A, et al. Marked 24-h rest/activity rhythms are associated with better quality of life, better response and longer survival in patients with metastatic colorectal cancer and good performance status. Clinical Cancer Research. 2000;6:3038–3045. [PubMed] [Google Scholar]
- 26.Innominato PF, Focan C, Gorlia T, Moreau T, Garufi C, Waterhouse J, et al. Circadian rhythm in rest and activity: a biological correlate of quality of life and a predictor of survival in patients with metastatic colorectal cancer. Cancer Research. 2009;69(11):4700–4707. doi: 10.1158/0008-5472.CAN-08-4747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alexander S, Minton O, Andrews P, Stone P. A comparison of the characteristics of disease-free breast cancer survivors with or without cancer-related fatigue syndrome. Eur.J.Cancer. 2009;45(3):384–392. doi: 10.1016/j.ejca.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fernandes R, Stone P, Andrews P, Morgan R, Sharma S. Comparison between fatigue, sleep disturbance, and circadian rhythm in cancer inpatients and healthy volunteers: evaluation of diagnostic criteria for cancer-related fatigue. J.Pain Symptom.Manage. 2006;32(3):245–254. doi: 10.1016/j.jpainsymman.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 29.Marler MR, Martin JL, Gehrman PR, Ancoli-Israel S. The Sigmoidally-transformed Cosine Curve: A Mathematical Model for Circadian Rhythms with Symmetric Non-sinusoidal Shapes. Statistics in Medicine. 2006;25(22):3893–3904. doi: 10.1002/sim.2466. [DOI] [PubMed] [Google Scholar]
- 30.Stein KD, Martin SC, Hann DM, Jacobsen PB. A multidimensional measure of fatigue for use with cancer patients. Cancer Pract. 1998;6(3):143–152. doi: 10.1046/j.1523-5394.1998.006003143.x. [DOI] [PubMed] [Google Scholar]
- 31.Stein KD, Jacobsen PB, Blanchard CM, Thors C. Further validation of the multidimensional fatigue symptom inventory-short form. J Pain Symptom Manage. 2004;27(1):14–23. doi: 10.1016/j.jpainsymman.2003.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ancoli-Israel S, Cole R, Alessi CA, Chambers M, Moorcroft WH, Pollak C. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26(3):342–392. doi: 10.1093/sleep/26.3.342. [DOI] [PubMed] [Google Scholar]
- 33.Diggle PJ, Liang KY, Zeger SL. Analysis of Longitudinal Data. 1994:1–254. [Google Scholar]
- 34.Savard J, Liu L, Natajaran L, Rissling M, Neikrug AB, He F, et al. Breast cancer patients have progressively impaired sleep-wake activity rhythms during chemotherapy. Sleep. 2009;32(9):1155–1160. doi: 10.1093/sleep/32.9.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ancoli-Israel S, Martin JL, Kripke DF, Marler M, Klauber MR. Effect of light treatment on sleep and circadian rhythms in demented nursing home patients. Journal of the American Geriatrics Society. 2002;50(2):282–289. doi: 10.1046/j.1532-5415.2002.50060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Neikrug AB, Rissling M, Trofimenko V, Liu L, Natajaran L, Lawton S, et al. Bright light therapy protects women from circadian rhythm desynchronization during chemotherapy for breast cancer. Behavioral Sleep Medicine. 2012;10(3):202–216. doi: 10.1080/15402002.2011.634940. [DOI] [PubMed] [Google Scholar]
- 37.Lee K, Cho M, Miaskowski C, Dodd M. Impaired sleep and rhythms in persons with cancer. Sleep Medicine Reviews. 2004;8(3):199–212. doi: 10.1016/j.smrv.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 38.Liu L, Mills PJ, Rissling MB, Fiorentino L, Natarajan L, Dimsdale JE, et al. Fatigue and sleep quality are associated with changes in inflammatory markers in breast cancer patients undergoing chemotherapy. Brain Behav Immun. 2012;26(5):706–713. doi: 10.1016/j.bbi.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu L, Fiorentino L, Natarajan L, Parker BA, Mills PJ, Sadler GR, et al. Pre-treatment symptom cluster in breast cancer patients is associated with worse sleep, fatigue and depression during chemotherapy. Psycho-oncology. 2009;18(2):187–194. doi: 10.1002/pon.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu L, Rissling M, Natajaran L, Fiorentino L, Mills PJ, Dimsdale JE, et al. The Longitudinal Relationship between Fatigue and Sleep in Breast Cancer Patients Undergoing Chemotherapy. Sleep. 2012;35(2):237–245. doi: 10.5665/sleep.1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morrow G, Tian L, Roscoe J, Griggs JG, Hickok JT, Smith B, Kramer Z, Kim Y. The relationship between circadian rhythm and fatigue in breast cancer patients. Annals of Behav Med. 2000;22:S188. [Google Scholar]
- 42.Payne JK. Altered circadian rhythms and cancer-related fatigue outcomes. Integr.Cancer Ther. 2011;10(3):221–233. doi: 10.1177/1534735410392581. [DOI] [PubMed] [Google Scholar]
- 43.Terman M, Lewy AJ, Dijk DJ, Boulos Z, Eastman CI, Campbell SS. Light Treatment for Sleep Disorders: Consensus report. IV. Sleep phase and duration disturbances. Journal of Biological Rhythms. 1995;10(2):135–147. doi: 10.1177/074873049501000206. [DOI] [PubMed] [Google Scholar]
- 44.Eastman CI, Boulos Z, Terman M, Campbell SS, Dijk DJ, Lewy AJ. Light Treatment for Sleep Disorders: Consensus report. VI. Shift work. Journal of Biological Rhythms. 1995;10(2):157–164. doi: 10.1177/074873049501000208. [DOI] [PubMed] [Google Scholar]
- 45.Boulos Z, Campbell SS, Lewy AJ, Terman M, Dijk DJ, Eastman CI. Light Treatment for Sleep Disorders: Consensus report. VII. Jet lag. Journal of Biological Rhythms. 1995;10(2):167–176. doi: 10.1177/074873049501000209. [DOI] [PubMed] [Google Scholar]
- 46.Ancoli-Israel S, Rissling M, Neikrug AB, Trofimenko V, Natajaran L, Parker BA, et al. Light treatment prevents fatigue in women undergoing chemotherapy for breast cancer. Support.Care Cancer. 2011;20:1211–1219. doi: 10.1007/s00520-011-1203-z. [DOI] [PMC free article] [PubMed] [Google Scholar]