Abstract
Despite the importance of early diagnosis and treatment of HIV, only a small fraction of HIV-exposed infants in low- and middle-income countries are tested for the disease. The gold standard for early infant diagnosis, DNA PCR, requires resources that are unavailable in poor settings, and no point-of-care HIV DNA test is currently available. We have developed a device constructed of layers of paper, glass fiber, and plastic that is capable of performing isothermal, enzymatic amplification of HIV DNA. The device is inexpensive, small, light-weight, and easy to assemble. The device stores lyophilized enzymes, facilitates mixing of reaction components, and supports recombinase polymerase amplification in five steps of operation. Using commercially available lateral flow strips as a detection method, we demonstrate the ability of our device to amplify 10 copies of HIV DNA to detectable levels in 15 minutes. Our results suggest that our device, which is designed to be used after DNA extraction from dried-blood spots, may serve in conjunction with lateral flow strips as part of a point-of-care HIV DNA test to be used in low resource settings.
Introduction
Over 2 million children worldwide are currently infected with HIV, most of whom were infected during pregnancy, birth, or breastfeeding.1 If untreated, the mortality rate of HIV-infected infants may reach 35% by one year of age, and 53% by two years of age.2 Although early infant HIV diagnosis and treatment may reduce infant mortality by 76%,3 only 15% of children born to HIV-positive women in 2009 were screened for HIV within the first two months after birth.1
One reason for the low rate of infant HIV testing is that no accurate, point-of-care test for early infant diagnosis is currently available. Rapid antibody tests, which are widely used in low-resource settings for adult HIV diagnosis, are not appropriate for early infant diagnosis. Maternal anti-HIV antibodies may persist in infant blood for up to 18 months after birth even in HIV-negative infants, causing false positive results in antibody tests.4 The gold standard method for infant HIV diagnosis is DNA PCR, which facilitates the detection of proviral DNA present in peripheral blood mononuclear cells.5 HIV DNA tests are reliable even when HIV viral load in infants is suppressed because of maternal anti-retroviral therapy.5, 6 Unfortunately, HIV DNA PCR is poorly suited for implementation in low-resource settings because PCR requires expensive equipment, electricity, dedicated laboratory space, and trained technicians.
Efforts to improve access to infant HIV diagnosis in developing countries have resulted in dried blood spot (DBS) PCR programs, through which district clinics mail dried blood samples to centralized labs for DNA PCR testing.5, 7 However, PCR remains prohibitively expensive and requires a 3-4 week turnaround time. Many patients never return to the clinic to receive their HIV test results. In some cases, less than half of HIV-positive infants return for follow-up care.8 A point-of-care HIV test based on DNA detection is needed that could provide results rapidly, allowing more patients to learn their HIV status and initiate treatment sooner.
Paper-based microfluidic devices share many of the desired characteristics of a suitable point-of-care HIV DNA test. Paper-based diagnostic tests are rapid, inexpensive, portable, and simple to operate, making them appropriate for low-resource settings.9 In addition, paper devices can perform many of the functions of traditional microfluidic devices but are simpler, do not require pumps, and can easily facilitate mixing.10-12 Most examples of paper-based diagnostic devices have focused on detecting small molecule or protein analytes instead of nucleic acids.13-16 A few exceptions are devices for paper-based sample preparation and detection of nucleic acids.17-20 However, to our knowledge, enzymatic amplification of nucleic acids has been performed only in traditional microfluidic devices,21-23 not in paper-based devices.
We have developed a proof-of-concept device made of plastic and paper that performs nucleic acid amplification of HIV DNA. Our device integrates mixing, reagent storage, and recombinase polymerase amplification (RPA). RPA is an isothermal nucleic acid amplification method that utilizes recombinase enzyme to facilitate the binding of oligonucleotide primers to template DNA. Primers are elongated by strand-displacing DNA polymerase, while single-stranded DNA binding proteins stabilize amplification reaction intermediates.24 We chose RPA because of its likelihood to perform successful amplification in paper: compared to PCR, RPA requires a shorter incubation time, operates at a lower, single incubation temperature, provides much faster amplification, and maintains activity in PCR-inhibiting environments.24 We performed RPA in our device and detected amplified HIV DNA using commercially available lateral flow strips. Our device is designed to be compatible with sample preparation of DBS and detection on lateral flow strips, suggesting its possible use as part of a DNA-based test for HIV diagnosis at the point-of-care.
Experimental
Before developing the amplification device, we first optimized a protocol for performing RPA in solution to amplify HIV DNA. Solution-based amplification was verified both by gel electrophoresis and detection on lateral flow strips. The optimized protocol for solution-based amplification served as a starting place for optimizing matrix-based RPA. Several types of materials were screened for their ability to support RPA in a matrix-based format. Once a suitable matrix was chosen, the optimal conditions for performing RPA were determined. Finally, we constructed a paper and plastic device to perform RPA and used lateral flow strips to demonstrate the successful amplification of HIV DNA.
PCR to generate template for RPA reactions
PCR was performed to generate HIV gag DNA, which served as a template for RPA reactions. The forward and reverse primers used were GAGF1: 5’-TCGGAGAGCGTCGGTATTAA-3’ and GAGR1: 5’-TTATTGTGACGAGGGGTCGC-3’ (Integrated DNA Technologies). The template for PCR was a plasmid containing the HIV gag gene, pHIV-IRES-eYFPΔEnvΔVifΔVpr, a generous gift from the Sutton lab.25 The PCR program consisted of an initial heating step at 94°C for 3 min.; 35 cycles of 94°C for 30 s, 52°C for 30 s, and 72°C for 90 s; and a final elongation step at 72°C for 10 min. PCR products were visualized after agarose gel electrophoresis and ethidium bromide staining. The band containing amplified gag DNA was cut out of the gel using a clean razor blade. The DNA was extracted (Qiagen 28704, QIAquick Gel Extraction Kit) and quantified by measuring absorbance at 260 nm.
RPA reactions in solution
RPA was first performed in solution to screen primers and optimize reaction conditions. RPA was performed using the materials and protocols in the TwistAmp Basic kit (TwistDx, UK). A variety of reaction temperatures, incubation times, and primer sequences were used before the optimal combination was achieved (data not shown). For each optimized reaction, 29.5 μL rehydration buffer, 3.2 μL nuclease-free water, 2.4 μL forward primer [10 μM], and 2.4 μL reverse primer [10 μM] were mixed. The forward and reverse primers used to generate a 135 bp product were RPAF8: 5’-GGACATCAAGCAGCCATGCAAATGTT AAAAGAG-3’ and RPAR1: 5’-TGCTATGTCACTTCCCCTTGGTTCTCTCATCTGGC-3’ (Integrated DNA Technologies). One enzyme pellet included in the kit was added to each solution and vortexed. Ten microliters of template with a total of 0, 10, 102, 103, 104, 105, or 106 copies of HIV gag DNA were added to each reaction. To start the reactions, 2.5 μL of magnesium acetate solution was dispensed into the cap of each tube. The reaction tubes were then recapped, centrifuged briefly, vortexed, and centrifuged again. Samples were incubated in a heat block at 37 °C for 4 min., vortexed, and incubated again for an additional 40 min. Products were purified (Qiagen 28104, QIAquick PCR Cleanup), electrophoresed on a 3% agarose gel (low gelling temperature agarose, Sigma A9414), and visualized with ethidium bromide staining.
Detection of RPA products using lateral flow strips
An RPA-based point-of-care HIV test should include a simple, fast, and user-friendly method for detecting RPA products. As a proof-of-concept detection method, we used commercially available lateral flow strips to detect RPA products (GenLine HybriDetect MGHD1, Milenia Biotec, Germany). The lateral flow strips contain gold nanoparticles conjugated to anti-FAM antibodies and a detection line consisting of anti-biotin antibodies. RPA products that are labeled with a 5’ FAM antigen and a 3’ biotin bind to the gold nanoparticle conjugates and detection line in a sandwich assay, allowing detection. The lateral flow strip also contains a control line consisting of anti-rabbit antibodies, which bind to free gold nanoparticles conjugates to ensure the strip is working properly.
In order to generate labeled RPA products, the TwistAmp nfo kit (TwistDX, UK) must be used in conjunction with a TwistAmp LF probe, an unlabeled forward primer, and a biotin-labeled reverse primer. The TwistAmp LF probe contains a 5’ FAM, an internal abasic site (tetrahydrofuran) that replaces a nucleotide, and a 3’ polymerase extension blocking group. In the reaction, the two primers generate biotin-labeled products. When the LF probe forms a duplex with the biotin-labeled antisense strand, nfo (an endonuclease) recognizes and cuts the abasic site in the probe, which generates a 3’ OH group and allows the blocked end of the probe to unbind. The 3’ OH acts as a target for the polymerase, which extends the probe. The resulting amplicon is labeled with FAM and biotin, allowing detection on the lateral flow strips.26
Labeled RPA products were generated in solution using the TwistAmp nfo kit and LF probe for detection on lateral flow strips. Each reaction contained 29.5 μL rehydration buffer, 3.2 μL nuclease-free water, 2.1 μL forward primer [10 μM], 2.1 μL reverse primer [10 μM], and 0.6 μL LF probe [10 μM]. The forward and reverse primers used were RPAF8: 5’-GGACATCAAGCAGCCATGCAAATGTTAAAAGAG-3’ and RPAR1B: 5’-/biotin/TGCTATGTCACTTCCCCTTGGTTCTCTCATCTGGC-3’ (Integrated DNA Technologies). The probe sequence was PRB1: 5’-/6-FAM/CTGCAGAATGGGATAGATTGCATCCAGTGCA/tetrahydrofuran/GCAGGGCCTATTGCAC/C3-spacer/-3’ (Integrated DNA Technologies). One enzyme pellet included in the kit was added to each solution and vortexed. Ten microliters of template containing a total of 0, 10, 102, 103, 104, 105, or 106 copies of HIV gag DNA were added to each reaction. To start the reactions, 2.5 μL of magnesium acetate solution was dispensed into the cap of each tube. The reaction tubes were then recapped, centrifuged briefly, vortexed, and centrifuged again. Samples were incubated in a heat block at 37 °C for 4 min., vortexed, and incubated again for an additional 30 min. To detect the RPA products, products were diluted 50-fold in running buffer (tris-buffered saline) provided with the Milenia lateral flow strips. Ten microliters of diluted products were dispensed onto the sample pad of each strip. Each strip was placed in a well of a 96-well plate containing 200 μL running buffer. Strips were 3 mm wide and easily fit into the wells, which were 6.7 mm in diameter. Strips rested vertically in the wells for 45 min. before imaging using a flatbed scanner (Epson Perfection V500 Photo).
Screening of materials for RPA device
In order to create a matrix-based device for performing RPA, different materials were screened for compatibility with RPA. Six different materials were chosen to represent a range of thicknesses, matrix compositions, porosity, and fluidic absorbance: glass fiber (GFCP203000, Millipore), cellulose (CFSP223000, Millipore), GF/DVA, MF1, VF2, and Fusion 5 (Whatman). The fluidic absorbance of each material was measured by calculating the difference in weight before and after submerging a 1 cm2 square of the material in water. The fluidic absorbance was used to calculate the area needed to absorb a 50 μL RPA reaction volume. Materials were cut to the appropriate size and placed in 1.5 mL Eppendorf tubes.
RPA reactions were assembled as described above using the TwistAmp Basic kit and 105 copies of HIV gag DNA in 10 μL as template. A 50 μL RPA reaction volume was added to each pad in Eppendorf tubes. Two reactions were incubated in solution: a positive control reaction containing 105 copies of DNA, and a negative control reaction containing no DNA. Reaction tubes were incubated in a heat block at 37 °C for 4 min., vortexed, and incubated again for an additional 40 min. After incubation, 100 μL water was added to all reactions and vortexed. Products were purified from the supernatant (Qiagen, QIAquick PCR Cleanup), electrophoresed on a 3% agarose gel (low gelling temperature agarose, Sigma A9414), and visualized with ethidium bromide staining.
Based on the results of the screening experiment, the incubation time for performing RPA in the best matrix material was optimized. Determining the best incubation time is important for avoiding false positive results, achieving the desired limit of detection, and ensuring that the time needed for performing the assay is reasonable for a point-of-care test. For this experiment, the TwistAmp nfo kit was used as described above. Template was added to the reactions at 0, 10, 100, or 1000 copies of gag DNA in 10 μL of water. A 50 μL RPA reaction volume was added to each pad in Eppendorf tubes and incubated for 10, 20, or 30 min. in a heat block. The reaction tubes were centrifuged at 15,000 × g to elute the reaction products. Products were diluted 50-fold in running buffer, 10 μL of diluted products was added to each Milenia lateral flow strip, and each strip was placed in a well of a 96-well plate containing 200 μL running buffer. Strips were scanned after 3 min.
RPA device fabrication and operation
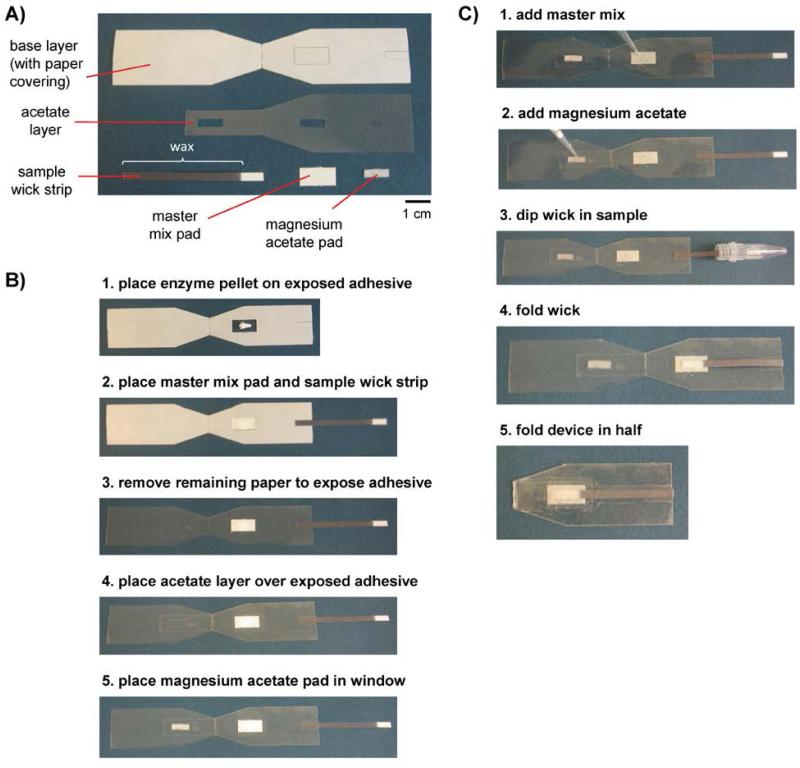
RPA devices were designed to be well-suited for use in low-resource settings by storing reagents, mixing reaction components, and facilitating RPA in a single device. The RPA device was inspired by a microfluidic origami device for sputum sample processing,17 in which folding device components initiates contact and causes reagents to mix together. RPA devices were assembled by stacking components made of acetate, double-sided adhesive, glass fiber matrix, and cellulose. Each device contained five components: a base layer made of acetate and double-sided adhesive, a second acetate layer to aid alignment of other components, a wax-patterned cellulose sample wick strip, a cellulose pad for absorbing master mix, and a glass fiber pad for absorbing magnesium acetate (Fig. 1A). The magnesium acetate is kept separate from the other reagents to reduce primer noise. The sample wick strips consisted of an unpatterned, hydrophilic area that absorbs 10 μL of sample and a long, wax-patterned, hydrophobic arm to facilitate dipping into a microcentrifuge tube to access the sample. The sample wick strip eliminates the need to pipette the sample onto the device and is compatible with a sample volume of 10 μL or more. The sample wick strips were cut from cellulose on which wax had been printed and melted at 150 °C for 3 min. in an oven, a method pioneered by the Whitesides group.27
Fig. 1.
Components, assembly, and operation of a paper-based device that performs recombinase polymerase amplification. (A) Devices were constructed of the five components: a base layer made of acetate and double-sided adhesive covered by a protective layer of paper; a second acetate layer to aid alignment of other components; a sample wick strip patterned with melted wax for absorbing exactly 10 μL of sample; a cellulose pad for holding a master mix solution; and a glass fiber pad for holding magnesium acetate solution. (B) Devices were assembled by peeling off the protective paper layer and assembling each device component in the order shown. The white pellet shown in the first step is composed of lyophilized enzymes and reagents. (C) The user operates the device by pipetting reagents on the appropriate pads, dipping the wick into the sample, and mixing reaction components by folding the device in half.
All components were cut with a 60-watt CO2 laser cutter (Universal Laser Systems Inc). For glass fiber (GFCP203000, Millipore) and cellulose (Whatman No. 1 chromatography paper), the laser was set to power = 3%, speed = 5%. Acetate was cut using power = 3%, speed = 10%, 2 passes (clear acetate, 0.003” thick, K03CL0811, Grafix, Maple Heights, OH). The final component of the device consisted of acetate adhered to one side of double-sided adhesive (double tack archival, KDT912-12, Grafix). The other side of the adhesive sheet is covered by a protective layer of paper, which may be peeled away. This device component was cut on the paper side using power = 10%, speed = 10%, 2 passes for through cuts. Partial cuts, which extended only through the protective paper layer, were cut using power = 5%, speed = 10%, 2 passes.
Devices were assembled according to the process depicted in Fig. 1B. First, the larger rectangle cut into the protective paper of the base layer was peeled away. The enzyme pellet was placed on the exposed adhesive. Second, the master mix pad was placed over the enzyme pellet. The master mix pad completely covered the conical enzyme pellet, which spread out under the pad after applying slight pressure. After placing the master mix pad, the smaller rectangle of protective paper was removed from the base layer, and the wax-patterned end of the sample wick strip was placed on the exposed adhesive. Third, the remaining protective paper was removed from the base layer. Fourth, the acetate layer was placed over the base layer, completely covering the end of the sample wick strip and partially overlapping the master mix pad. Finally, the glass fiber pad was placed in the window cut into the acetate sheet on the side of the device across from the master mix pad.
To evaluate the RPA devices, RPA was performed using the materials found in the TwistAmp nfo kit. RPA reactions were assembled in five steps, shown in Fig. 1C. First, the master mix containing rehydration buffer, water, primers, and probes was dispensed onto the master mix pad. The master mix completely rehydrated the enzyme pellet. Second, 2.5 μL magnesium acetate was dispensed onto the glass fiber pad. Third, the sample wick strip was dipped into the sample to absorb 10 μL of fluid containing varying amounts of template DNA. To evaluate the limit of detection of these devices, samples consisted of 0, 10, 100, or 1000 copies of HIV gag DNA in 10 μL. Fourth, the sample wick strip was folded to overlap the master mix pad. Fifth, the device was folded in half and self-sealed such that the glass fiber pad overlapped the sample wick. Devices were placed on a heat block at 37°C for 15 min and covered by styrofoam box lid for insulation. Devices were then peeled open. The entire sample wick was torn off the device, placed in 90 μL running buffer, and vortexed. Ten microliters of diluted products were added to each Milenia lateral flow detection strip, and each strip was placed in a well of a 96-well plate containing 200 μL running buffer. Strips were scanned after 3 min.
Results
Performance of RPA in solution
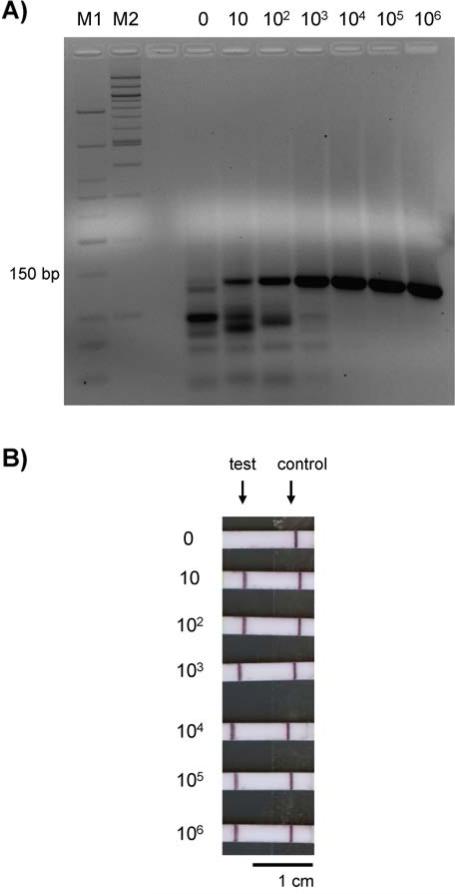
RPA reactions performed in solution yielded detectable products when optimized reaction conditions and primers were used. All reactions to which HIV gag DNA was added produced a clearly visible band of 135 bp after gel electrophoresis (Fig. 2A). The brightness of the bands appeared to be proportional to the amount of template added to the RPA reaction. Although a very faint band of that size may be visible for the no template control, this band is much dimmer than the band for 10 copies of HIV gag DNA, demonstrating a limit of detection of 10 copies.
Fig. 2.
Gel electrophoresis and lateral flow strip detection of RPA reaction products generated in solution. Numbers above the gel lanes and beside the lateral flow strips indicate the total number of HIV DNA copies added as template to each RPA reaction. (A) Gel electrophoresis of 135 bp reaction products. M1 = Low Molecular Weight DNA Ladder, New England BioLabs; M2 = 100 bp DNA Ladder, New England BioLabs. (B) Lateral flow strip results after applying a 1:50 dilution of reaction products. The strip results were positive for all samples except the negative control reaction.
RPA reactions performed in solution using the TwistAmp nfo kit produced amplicons that were detectable on lateral flow strips, demonstrating that the products may be visualized using a point-of-care detection method. The test lines of the lateral flow strips were dark for all RPA reactions to which template DNA was added, indicating a positive result (Fig. 2B). The limit of detection remained at 10 copies, while the negative control reaction appropriately produced no signal at the test line. A negative lateral flow strip result for the negative control suggests that the faint band visible on the gel in the ‘0 copies’ lane may be primer noise instead of template contamination. The structure of the LF probe prevents the amplification of primer-probe dimers, increasing the specificity of amplification and avoiding false positives.26 Therefore, when using an LF probe, RPA is capable of amplifying HIV DNA in solution with adequate specificity to yield products that are clearly visible on lateral flow tests.
Performance of RPA in different materials
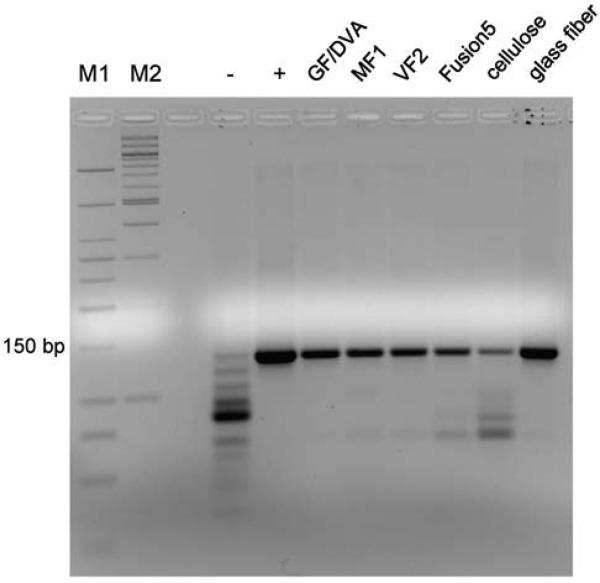
RPA reactions in matrices composed of different materials yielded products that were detectable via gel electrophoresis. A 135 bp band was present in the gel for all matrix materials tested (Fig. 3). However, these bands were all dimmer than the band for the positive control reaction, which was performed in solution. This comparison suggests that the reaction is less efficient in a matrix than in solution, which is expected because the matrix limits diffusion of reaction components. A faint band was present in the gel for the negative control but was much dimmer than any of the bands for which template was added. The brightest band in the negative control lane may consist of primer-dimers or other primer noise. The reaction in the glass fiber pad yielded the most product, perhaps because of its large pore size. The reaction in GF/DVA, which also contains glass fiber, yielded slightly less product than glass fiber alone. The most noise and least product were present for RPA reactions performed in cellulose.
Fig. 3.
Gel elecrophoresis of RPA reaction products generated from 105 copies of HIV DNA in different materials. Gel lanes are labeled with the type of material tested. A 135 bp band was present for all materials tested. The negative control (-) was an RPA reaction in solution to which no HIV DNA was added; the positive control (+) was an RPA reaction in solution containing 105 copies of HIV DNA template.
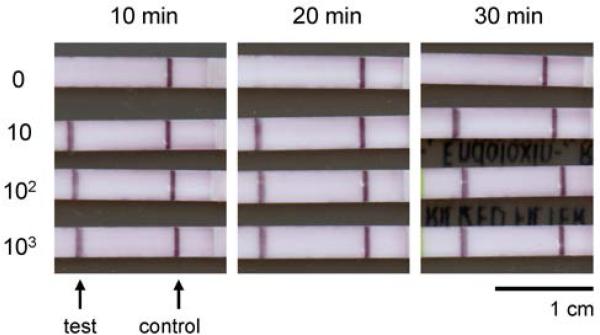
Because glass fiber appeared to be most compatible with RPA, glass fiber was selected as the matrix of choice for developing an amplification device. The incubation time for RPA reactions in this matrix was optimized for future experiments. Products amplified in glass fiber were detectable on lateral flow strips after as little as 10 min. of incubation (Fig. 4). For incubation times of 10, 20, and 30 minutes, the limit of detection remained at 10 copies of HIV gag DNA. Although a 10-minute incubation was sufficient in this experiment, we chose an incubation time of 15 minutes for future experiments to provide maximum sensitivity while keeping assay rapid for use at the point-of-care. All negative control reactions produced a negative lateral flow strip result, showing that specificity is maintained even when RPA is performed in the chosen matrix.
Fig. 4.
Lateral flow strips showing detection of RPA products generated in glass fiber after various incubation times. Numbers beside the lateral flow strips indicate the total number of HIV DNA copies added as template to each RPA reaction. For all incubation times, the limit of detection was 10 copies of HIV gag DNA.
RPA device performance
Using the optimal settings determined in the previous experiments, we constructed fully-assembled RPA devices and demonstrated their ability to successfully support nucleic acid amplification. As shown in Fig. 5, reaction products generated in the RPA devices were easily detectable using lateral flow strips. All reactions to which gag DNA was added produced a positive lateral flow strip result, suggesting that the limit of detection for this amplification assay is 10 copies. The lateral flow strip result for the no target control reaction was negative. A comparison of the lateral flow strip results for reaction products generated in RPA devices, in glass fiber pads alone, and in solution shows that RPA can be performed successfully in any of these formats to detect HIV DNA.
Fig. 5.

Lateral flow strips showing detection of RPA products generated in fully-assembled RPA devices. Numbers beside the lateral flow strips indicate the total number of HIV DNA copies added as template to each RPA reaction. Lateral flow strip results were positive for all reactions to which HIV gag DNA was added, suggesting that the limit of detection is 10 DNA copies.
Discussion
The RPA device presented here is capable of amplifying 10 copies of HIV DNA to detectable levels in 15 minutes while retaining many attributes desired for a point-of-care test. The device is small, light-weight, easy to assemble, and requires only five steps for operation. The only laboratory infrastructure required consists of a micropipette, pipette tips, and a heater. The RPA reagents are stable at room temperature for days and may be transported without refrigeration,28 suggesting that the device could be shipped to remote areas in developing countries for use at the point-of-care. The cost per reaction performed in an RPA device is $4.45, which only 20 cents more than the cost of the RPA reagents alone. The device is also compatible with existing sample preparation and detection methods. Because the sample wick strip is designed to be dipped into a tube containing template DNA, the device should be compatible with standard dried blood spot DNA extraction protocols in which DNA is released from filter paper into solution.7 Alternatively, the sample wick strip could be placed in contact with a filter containing extracted DNA.18 In addition, our results have shown that the amplified products generated in our device may be detected using lateral flow strips. Therefore, our RPA device has the potential to serve as part of a point-of-care HIV DNA test.
Before the RPA device could be used in the field, however, further improvements and testing are required. Currently, the device must be peeled apart to access the reaction products for detection. A more convenient way to remove and apply the products onto a lateral flow strip is needed. Another drawback of the current device is that the RPA device only stores the enzyme pellet; the RPA master mix and magnesium acetate solution must be stored separately and dispensed onto the device with a pipette. Although this will require future evaluation and optimization, storing these buffers in the device may be possible simply by including blister packs in the device or by drying the appropriate buffers into the corresponding pads for future rehydration with water. Including buffer storage in the device would allow all the reagents for RPA to be fully integrated, eliminating the need for tubes and a pipette. The current device also has only been used with an electrically-powered heater. In order to circumvent the need for electricity, the device should be tested for compatibility with a new or existing battery-powered heater.29, 30 Ideally, the RPA device should also be integrated with sample preparation and detection to comprise a sample-to-answer HIV DNA test. Such a test would require evaluation with clinical samples both in the laboratory and in low-resource settings.
Conclusion
We have developed a paper and plastic device that stores enzymes, mixes reaction components, and supports recombinase polymerase amplification of HIV DNA. The device is designed to be compatible with DNA extraction from dried blood spots and detection using lateral flow strips, suggesting its potential to serve as part of a point-of-care HIV DNA test. In addition, the device may be adapted for amplification of other DNA targets for use in other diagnostic systems. This device demonstrates that isothermal, enzymatic amplification of nucleic acids is achievable in a matrix-based format, representing a new application for paper-based microfluidic technologies.
References
- 1.Towards universal access: Scaling up priority HIV/AIDS interventions in the health sector. World Health Organization; 2009. [Google Scholar]
- 2.Newell ML, Coovadia H, Cortina-Borja M, Rollins N, Gaillard P, Dabis F, Ias Lancet. 2004;364:1236–1243. doi: 10.1016/S0140-6736(04)17140-7. [DOI] [PubMed] [Google Scholar]
- 3.Violari A, Cotton MF, Gibb DM, Babiker AG, Steyn J, Madhi SA, Jean-Philippe P, McIntyre JA, Team CS. New England Journal of Medicine. 2008;359:2233–2244. doi: 10.1056/NEJMoa0800971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ciaranello AL, Park JE, Ramirez-Avila L, Freedberg KA, Walensky RP, Leroy V. Bmc Medicine. 2011;9:15. doi: 10.1186/1741-7015-9-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.WHO Recommendations on the Diagnosis of HIV Infection in Infants and Children. World Health Organization; 2010. [PubMed] [Google Scholar]
- 6.Desire N, Dehee A, Schneider V, Jacomet C, Goujon C, Girard PM, Rozenbaum W, Nicolas JC. Journal of Clinical Microbiology. 2001;39:1303–1310. doi: 10.1128/JCM.39.4.1303-1310.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sherman GG, Stevens G, Jones SA, Horsfield P, Stevens WS. Jaids-Journal of Acquired Immune Deficiency Syndromes. 2005;38:615–617. doi: 10.1097/01.qai.0000143604.71857.5d. [DOI] [PubMed] [Google Scholar]
- 8.Braun M, Kabue MM, McCollum ED, Ahmed S, Kim M, Aertker L, Chirwa M, Eliya M, Mofolo I, Hoffman I, Kazembe PN, van der Horst C, Kline MW, Hosseinipour MC. Jaids-Journal of Acquired Immune Deficiency Syndromes. 2011;56:E122–E128. doi: 10.1097/QAI.0b013e31820a7f2f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martinez A, Phillips S, Whitesides G, Carrilho E. Analytical Chemistry. 2010:3–10. doi: 10.1021/ac9013989. [DOI] [PubMed] [Google Scholar]
- 10.Fu E, Lutz B, Kauffman P, Yager P. Lab on a Chip. 2010;10:918–920. doi: 10.1039/b919614e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Osborn J, Lutz B, Fu E, Kauffman P, Stevens D, Yager P. Lab on a Chip. 2010;10:2659–2665. doi: 10.1039/c004821f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fu E, Ramsey S, Kauffman P, Lutz B, Yager P. Microfluidics and Nanofluidics. 2011;10:29–35. doi: 10.1007/s10404-010-0643-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martinez A, Phillips S, Nie Z, Cheng C, Carrilho E, Wiley B, Whitesides G. Lab on a Chip. 2010;10:2499–2504. doi: 10.1039/c0lc00021c. [DOI] [PubMed] [Google Scholar]
- 14.Yu J, Ge L, Huang J, Wang S, Ge S. Lab on a Chip. 2011;11:1286–1291. doi: 10.1039/c0lc00524j. [DOI] [PubMed] [Google Scholar]
- 15.Li X, Tian J, Shen W. Analytical and Bioanalytical Chemistry. 2010;396:495–501. doi: 10.1007/s00216-009-3195-9. [DOI] [PubMed] [Google Scholar]
- 16.Fu E, Kauffman P, Lutz B, Yager P. Sensors and Actuators B-Chemical. 2010;149:325–328. doi: 10.1016/j.snb.2010.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Govindarajan A, Ramachandran S, Vigil G, Yager P, Bohringer K. Lab on a Chip. 2012;12:174–181. doi: 10.1039/c1lc20622b. [DOI] [PubMed] [Google Scholar]
- 18.Jangam S, Yamada D, McFall S, Kelso D. Journal of Clinical Microbiology. 2009;47:2363–2368. doi: 10.1128/JCM.r00092-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He Y, Zhang S, Zhang X, Baloda M, Gurung A, Xu H, Zhang X, Liu G. Biosensors & Bioelectronics. 2011;26:2018–2024. doi: 10.1016/j.bios.2010.08.079. [DOI] [PubMed] [Google Scholar]
- 20.Jordan JA, Ibe CO, Moore MS, Host C, Simon GL. Journal of clinical virology : the official publication of the Pan American Society for Clinical Virology. 2012;54:11–14. doi: 10.1016/j.jcv.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 21.Lee SH, Kim SW, Kang JY, Ahn CH. Lab on a Chip. 2008;8:2121–2127. doi: 10.1039/b811131f. [DOI] [PubMed] [Google Scholar]
- 22.Dimov IK, Garcia-Cordero JL, O'Grady J, Poulsen CR, Viguier C, Kent L, Daly P, Lincoln B, Maher M, O'Kennedy R, Smith TJ, Ricco AJ, Lee LP. Lab on a Chip. 2008;8:2071–2078. doi: 10.1039/b812515e. [DOI] [PubMed] [Google Scholar]
- 23.Asiello P, Baeumner A. Lab on a Chip. 2011:1420–1430. doi: 10.1039/c0lc00666a. [DOI] [PubMed] [Google Scholar]
- 24.Piepenburg O, Williams C, Stemple D, Armes N. Plos Biology. 2006;4:1115–1121. doi: 10.1371/journal.pbio.0040204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Segall H, Yoo E, Sutton R. Molecular Therapy. 2003:118–129. doi: 10.1016/s1525-0016(03)00134-5. [DOI] [PubMed] [Google Scholar]
- 26.TwistAmp™ DNA amplification kits: Combined Instruction Manual. www.twistdx.co.uk.
- 27.Carrilho E, Martinez A, Whitesides G. Analytical Chemistry. 2009;81:7091–7095. doi: 10.1021/ac901071p. [DOI] [PubMed] [Google Scholar]
- 28.Our Technology: Recombinase Polymerase Amplification. www.twistdx.co.uk/our_technology/
- 29.LaBarre P, Hawkins KR, Gerlach J, Wilmoth J, Beddoe A, Singleton J, Boyle D, Weigl B. Plos One. 2011;6:8. doi: 10.1371/journal.pone.0019738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu C, Mauk MG, Hart R, Qiu X, Bau HH. Lab on a Chip. 2011;11:2686–2692. doi: 10.1039/c1lc20345b. [DOI] [PubMed] [Google Scholar]