Abstract
Histophilus somni (Haemophilus somnus) is an important pathogen of cattle that is responsible for respiratory disease, septicemia, and systemic diseases such as thrombotic meningoencephalitis, myocarditis, and abortion. A variety of virulence factors have been identified in H. somni, including compositional and antigenic variation of the lipooligosaccharide (LOS). Phosphorylcholine (ChoP) has been identified as one of the components of H. somni LOS that undergoes antigenic variation. In this study, five genes (lic1ABCDHs and glpQ) with homology to genes responsible for ChoP expression in Haemophilus influenzae LOS were identified in the H. somni genome. An H. somni open reading frame (ORF) with homology to H. influenzae lic1A (lic1AHi) contained a variable number of tandem repeats (VNTR). However, whereas the tetranucleotide repeat 5′-CAAT-3′ is present in lic1AHi, the VNTR in H. somni lic1A (lic1AHs) consisted of 5′-AACC-3′. Due to the propensity of VNTR to vary during replication and cause the ORF to shift in and out of frame with the upstream start codon, the VNTR were deleted from lic1AHs to maintain the gene constitutively on. This construct was cloned into Escherichia coli, and functional enzyme assays confirmed that lic1AHs encoded a choline kinase, and that the VNTR were not required for expression of a functional gene product. Variation in the number of VNTR in lic1AHs correlated with antigenic variation of ChoP expression in H. somni strain 124P. However, antigenic variation of ChoP expression in strain 738 predominately occurred through variable extension/truncation of the LOS outer core. These results indicated that the lic1Hs genes controlled expression of ChoP on the LOS, but that in H. somni there are two potential mechanisms that account for antigenic variation of ChoP.
Keywords: Histophilus somni, Phosphorylcholine, Lipooligosaccharide, Adherence, Antigenic and phase variation
1. Introduction
Histophilus somni (Haemophilus somnus) is a gram-negative coccobacillus that is an important cause of bovine respiratory disease and systemic infections in cattle, including septicemia, thrombotic meningoencephalitis, myocarditis, arthritis, abortion, and others [1]. H. somni possesses a variety of virulence factors, including immunoglobulin binding proteins that are similar to high molecular weight filamentous hemagglutinins [2,3], induction of endothelial cell apoptosis [4], survival in phagocytic cells [5], and production of lipooligosaccharide (LOS). H. somni LOS is an endotoxin, which can undergo phase variation in composition and structure in vitro or in response to a mounting immune response by the host [6,7]. The LOS can also be modified by the incorporation of sialic acid, which is associated with decreased binding by monoclonal antibodies (MAb) to LOS and enhanced resistance to serum killing [8].
The LOS of H. somni undergoes a high rate of random antigenic and compositional phase variation, similar to that of Haemophilus influenzae LOS [9]. However, serum-sensitive isolates from the urogenital tract do not undergo detectable antigenic variation or do so at a substantially lower rate [6]. Antigenic variation in H. somni LOS has been demonstrated in isolates obtained at different time intervals from calves challenged with H. somni. This variation correlates with an immune response to a previous LOS phenotype, indicating that emergence and predominance of new LOS variants are driven by the host’s mounting immune response. However, LOS variation also occurs randomly in vitro at a relatively high rate of about 12% of the population [6].
Choline is a major component of eukaryotic cell membrane phospholipids and is present in the form of phosphatidylcholine. Choline has also been identified in the membranes of many bacterial species in the form of phosphorylcholine (ChoP). ChoP is incorporated into the teichoic acid and lipoteichoic acids of Streptococcus pneumoniae [10], on the LOS of H. influenzae [11], on the LOS and pili of Neisseria species [12,13], on the lipopolysaccharide of Pasteurella multocida [14], and on a 43-kDa protein in Pseudomonas aeruginosa [13]. Among bacterial isolates of different species from the human upper respiratory tract, 15% contained ChoP [15]. Expression of ChoP on H. influenzae LOS undergoes a high rate of reversible antigenic variation. In H. influenzae, ChoP is attached to the primary glycose on one of three heptoses present in the LOS inner core [16], and its expression is associated with bacterial colonization of the upper respiratory tract in an infant rat model [17]. The adherence and invasion of H. influenzae to host cells, including human respiratory cells, is the result of interaction of ChoP with platelet activating factor receptor (PAF-R) [18]. However, in the blood stream, ChoP binds to the acute phase reactant C-reactive protein (CRP), leading to the activation of complement through the classical pathway and killing of the bacteria. Therefore, systemic dissemination of H. influenzae is associated with loss of ChoP expression [17,19]. Thus, on and off expression of ChoP is important for H. influenzae host colonization and dissemination, respectively.
In H. influenzae the lic1ABCDHi locus (lic1ABCDHi) is responsible for expression of ChoP. The gene lic1AHi contains a variable number of tandem repeats (VNTR) of the tetranucleotide unit 5′-CAAT-3′ within its open reading frame (ORF) immediately downstream of potential start codons. Variation in the number of VNTR may occur through slipped strand mispairing (SSM), resulting in shifting of the downstream reading frame in or out of frame with the start codon. When the gene is out of frame translation of a truncated, non-functional protein occurs [20–23]. Therefore, the VNTR in lic1AHi acts as a molecular translational switch responsible for the antigenic variation of ChoP on the LOS [11]. In addition to the lic1Hi locus, H. influenzae glpQ encodes for a glycerophosphoryl diester phosphodiesterase. In the host, and in the absence of free choline, GlpQ allows H. influenzae to obtain ChoP from glycerolphosphorylcholine, which is a degradation product of host cell phospholipids [24].
H. somni also expresses ChoP on its LOS [25]. In pathogenic strain 738, ChoP is expressed on the primary glucose attached to heptose I in the inner core [26]. Antigenic expression of ChoP on strain 738 is also subject to steric interference by expression of the β-galactose-(1–3)-β-GlcNAc (lacto-N-tetraose) outer core [25]. In this study we identified the genes required for expression of ChoP on H. somni LOS, and the molecular mechanisms involved in antigenic variation of ChoP. Our results indicated that a locus with homology to lic1ABCDHi controls expression and antigenic variation of ChoP in H. somni, and that H. somni lic1A (lic1AHs) is a phase variable gene that encodes a choline kinase. We also determined that there are two possible mechanisms of ChoP antigenic variation of H. somni LOS that are strain variable.
2. Results
2.1. Identification of putative ChoP biosynthesis genes
Several attempts were made to amplify a homolog of lic1AHi or lic1DHi from H. somni genomic DNA by PCR using a variety of degenerate and non-degenerate primers under different reaction conditions. The reactions produced either no products or non-specific amplification products. Southern blotting experiments using a digoxigenin-labeled lic1AHi probe with H. somni genomic DNA also did not hybridize to a specific DNA band (data not shown).
A BLAST analysis of the genome sequence of H. somni strain 2336 in comparison to the lic1ABCDHi sequence revealed a locus that contained four ORFs with predicted amino acid homology. The first ORF shared 39% identity over 281 amino acids (AA) with lic1AHi, the second ORF had 35% identity over 301 AAs to lic1BHi, the third ORF shared 50% identity over 230 AAs with lic1CHi, and the forth ORF shared 66% AA identity with lic1DHi. Furthermore, an H. somni ORF that shared 79% identity over 343 AA with the H. influenzae glycerophosphoryl diester phosphodiesterase gene (glpQ) was also identified. H. somni glpQ also shared 80% identity over 363 AA with P. multocida glpQ and 60% identity over 360 AA with Escherichia coli glpQ.
Analysis of lic1AHs predicted the gene to encode a protein containing the sequence HNDLVPENILM, which corresponds to the consensus sequence HXDhXXXNhhh (where h is F, L, I, M, V,W, or Y [a large hydrophobic AA] and X is any AA) [11]. This consensus sequence is reported to contain the catalytic domain for protein kinases and phosphotransferases [11,27], and is found in the sequence of H. influenzae Lic1A [11]. The sequence of lic1AHs contained 25 repeats of the tetranucleotide unit 5′-AACC-3′ three base pairs downstream from the third of three potential start codons. These VNTR would be predicted to cause phase variable expression of ChoP. In contrast, lic1AHi contains the VNTR 5′-CAAT-3′, which begins immediately downstream of a start codon [9]. The first and second potential start codons of lic1AHs are in the same frame while the third start codon is in a different frame. This arrangement was similar to that of the start codons of lic1AHi. When 24 repeats were present in lic1AHs, the third start codon would be in frame with the stop codon at the end of the ORF, and a functional product would be expected to be expressed.
A lic1ABCDHs locus was also identified in the genome sequence of H. somni preputial strain 129Pt, and contained 41 repeats of the VNTR. However, lic1AHs in strain 129Pt was interrupted by an apparent IS1016 insertion sequence that began 61 bp downstream of the VNTR region. This IS1016 element has also been described in bexA of the H. influenzae type b cap locus, requiring a duplication of the locus in order for type b capsule to be expressed [28]. The sequence of the IS1016-like element in strain 129Pt contained 710 bp with 86–95% identity to that of the sequence in H. influenzae.
2.2. Constitutive expression of lic1AHs in E. coli
The vector pSE1 was used for expression of lic1AHs in E. coli BL21DE3pLysS cells (BL21DE3pLysS[pSE1]). Induction of BL21DE3-pLysS[pSE1] with IPTG resulted in expression of lic1AHs, as determined by SDS-PAGE analysis (Fig. 1, lanes 2–4). Maximum levels of expression were achieved 2 h post-induction and remained at the same level for 1 h. To express lic1AHs that was not subject to potential phase variation, the 5′-AACC-3′ repeat region was removed from lic1AHs, as described in Materials and Methods, and was confirmed by PCR amplification (data not shown). Self-ligation of the PCR product resulted in the vector pSE3, which contained lic1AHs lacking the VNTR in addition to three base pairs downstream of the repeat region [lic1AHsΔ(AACC)], thereby leaving the gene in -frame and translated from the start codon immediately upstream of the deleted repeats. The sequence of pSE3 was confirmed by sequencing, and expression of lic1AHsΔ(AACC) by E. coli containing pSE3 was comparable to that E. coli containing pSE1 (data not shown).
Fig. 1.
Electrophoretic profile of E. coli expressing the gene lic1AHs. The plasmid pSE1, which contained lic1AHs was transformed into E. coli. Transformed cells expressed a protein of the approximate molecular size to that of the predicated H. somni choline kinase (Lic1A). Lanes: 1, E. coli containing pSE1 pre-induced with IPTG; 2–4, E. coli containing pSE1 induced with IPTG after 1, 2, and 3 h; 5, Molecular size marker; 6, E. coli control lacking pSE1 pre-induced with IPTG; 7–9, E. coli control lacking pSE1 induced with IPTG after 1, 2, and 3 h.
2.3. Choline kinase assay for Lic1A
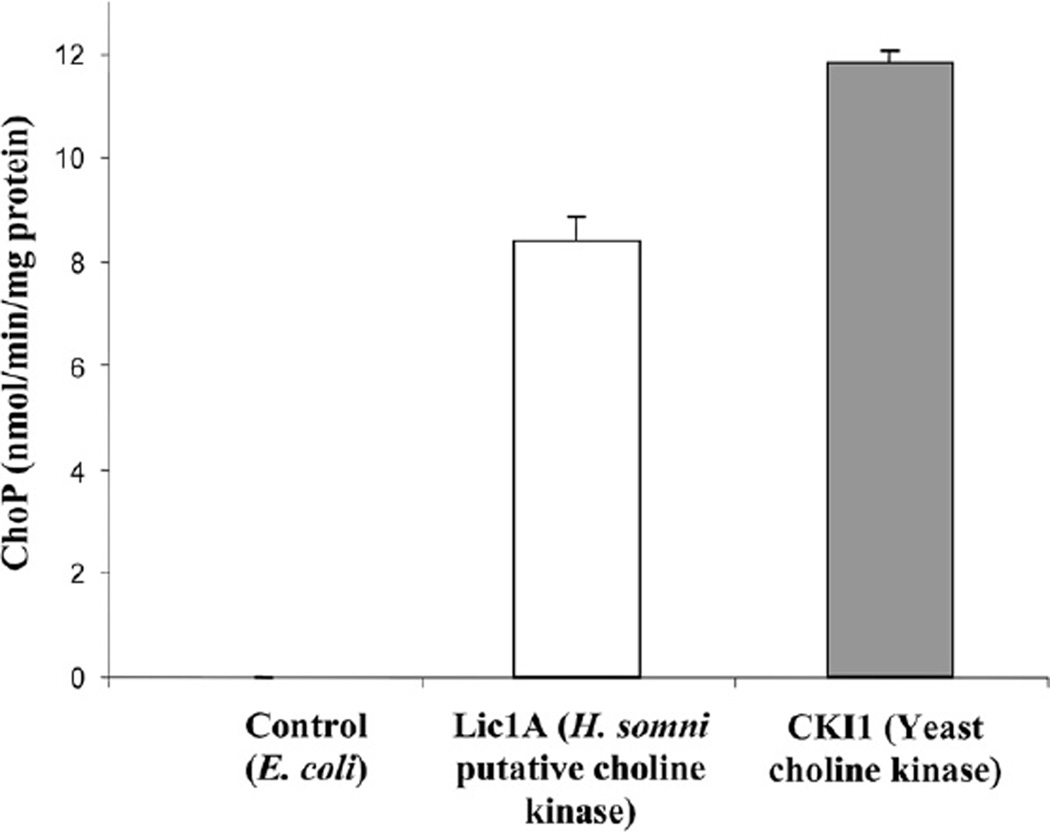
The ability of H. somni Lic1A to phosphorylate choline in the presence of ATP and produce ChoP was determined by comparing the activity of recombinant Lic1AHs to that of yeast choline kinase (CKI) [29]. Choline kinase from yeast strain KS106, a double mutant that does not express ethanolamine kinase and choline kinase, but overexpresses wild type choline kinase, was used [30]. The amount of ChoP produced by Lic1AHs that was expressed in E. coli was 8.39 nmol/min/mg protein, while the control yeast choline kinase produced 11.86 nmol/min/mg protein (Fig. 2), confirming that Lic1AHs was a functional choline kinase.
Fig. 2.
The choline kinase activity of H. somni Lic1A compared to the activity of yeast choline kinase CKI1. Lic1A catalyzed the production of 8.39 nmol ChoP/min/mg protein while CKI1 catalyzed the production of 11.86 mg ChoP/min/mg protein. The results are the average of three experiments.
2.4. LOS composition and phase variation of lic1AHs
ChoP+ and ChoP− clonal derivatives of strains 738 and 124P were selected using MAbs to ChoP and identified as such, as described in Materials and Methods (Table 1). To assess the mechanism of ChoP phase variation in each strain, the number of VNTR in lic1AHs was determined and the LOS composition was analyzed from clonal derivatives of both strains (Table 2). The LOS of the ChoP+ isolate of strain 124P (124P+) contained one glycoform that contained ChoP with 2 hexoses in the outer core. The ChoP− isolate of the same strain (124P−) contained three glycoforms, none of which contained ChoP, but contained 3 hexoses, hexNAc, and sialic acid in the largest glycoform. Thus, there was correlation between a truncated outer core and the presence of ChoP on the LOS. The presence of sialic acid was of particular interest since sialic acid has not previously been found in the LOS of other serum-sensitive preputial isolates of H. somni [8,31,32]. The number of VNTR in lic1AHs of 124P+ was 27. When compared to the sequence of lic1AHs from the genome sequence of strain 2336, this number of VNTR was consistent with the gene being in frame with a stop codon, and expressing a full length and functional product. The number of VNTR in lic1AHs of strain 124P− was 29, which was consistent with the gene translating a truncated, non-functional protein.
Table 1.
H. somni strains used in this study.
| Strain | Source | Reference |
|---|---|---|
| 2336 | Pneumonic lung isolate | [44] |
| 738 | Clonal, calf-passaged isolate of 2336 | [26] |
| 738P | ChoP-positive clonal isolate of 738 | [25,27] |
| 738+ | ChoP-positive clonal isolate of strain 738 | This work |
| 738− | ChoP-negative clonal isolate of strain 738 | This work |
| 7735 | Pneumonic lung isolate | A Potter. Veterinary Infectious Disease Organization. University of Saskatchewan, Canada |
| 7735+ | ChoP-positive clonal isolate of strain 7735 | This work |
| 7735− | ChoP-negative clonal isolate of strain 7735 | This work |
| 93 | Pneumonic lung isolate | A Potter. Veterinary Infectious Disease Organization. University of Saskatchewan, Canada |
| 93+ | ChoP-positive clonal isolate of strain 93 | This work |
| 93− | ChoP-negative clonal isolate of strain 93 | This work |
| 124P | Normal prepuce | [44] |
| 124P+ | ChoP-positive clonal isolate of strain 124P | This work |
| 124P− | ChoP-negative clonal isolate of strain 124P | This work |
| 129Pt | Normal prepuce | [44] |
Table 2.
The number of VNTR in lic1AHs and lob2A of ChoP+ and ChoP− isolates of H. somni strains.
| Straina | Number of VNTR in lic1A (5′-AACC-3′) |
Expression of ChoP |
Number of VNTR in lob2A (5′-GA-3′) |
|---|---|---|---|
| 738Pb | 24 | Yes | 20 |
| 738+ | 24 | Yes | 21 |
| 738− | 24 | No | 21 |
| 7735+ | 43 | Yesc | 21 |
| 7735− | 42 | No | 20 |
| 124+ | 27 | Yes | ND |
| 124− | 29 | Yes | ND |
| 93+ | 24 | Yes | ND |
| 93− | 23 | Yes | ND |
| 2336d | 25 | Yes | 20 |
ND: Not determined.
Strains designated with (+) or (−) are either reactive or non-reactive to anti-ChoP MAb, respectively.
Strain 738P is a ChoP+ derivative obtained from a previous study [30].
The presence of two additional nucleotides downstream of the VNTR place the gene in frame when there are 43 repeats present rather than 42.
Determined from the finished genome sequence of strain 2336.
The LOS of both ChoP+ and ChoP− clonal isolates of strain 738, which were selected based on reactivity with an anti-ChoP MAb, contained ChoP as determined by electrospray mass spectrometry (ES-MS). The ChoP+ isolate (738+) contained three glycoforms, of which two contained ChoP. The ChoP− isolate (738−) contained seven glycoforms, five of which contained ChoP. The LOS of 738+ contained a higher proportion of glycoforms that contained fewer hexose and hexosamine units and was consistent with the LOS being more truncated than that of 738− LOS (Table 3). The number of VNTR in lic1AHs of both 738+ and 738− was 24, indicating that lic1A in both isolates would be in frame with the start codon and express a functional gene product (Table 2). The number of VNTR in lic1AHs of both ChoP+ and ChoP− isolates of strain 738 did not vary, whereas the VNTR number did vary between the ChoP+ and ChoP− isolates of strains 7735, 93, and 124P. However, the number of VNTR in strain 7735 was 42 or 43, almost twice the number in most of the other strains. Furthermore, unlike all other strains examined a polymorphism in the sequence of the coding region downstream of the VNTR in strain 7735 resulted in the presence of an additional nucleotide and a frame-shift in the ORF. That frame-shift resulted in lic1AHs being in frame when 43 VNTR were present, and out of frame in the presence of 42 repeats. In contrast, 43 repeats in the sequence of lic1A from the other strains examined would have resulted in expression of a non-functional product. There were also 41 repeats in lic1AHs of strain 129Pt, but ChoP could not be expressed due to the IS1016 insertion downstream of the VNTR. The number of VNTR in strain 2336, which lacks ChoP, was 25, predicting the gene would be out of frame.
Table 3.
The proposed composition of LOS from ChoP+ and ChoP− isolates of pathogenic strain 738 and commensal strain 124P and the corresponding number of VNTR in lic1AHs of each isolate.
| Clonal isolat | Molecular Mass (Da) | Percent Distribution of glycoforms |
Proposed composition | Number of VNTR | Predicted expression of Lic1A |
|---|---|---|---|---|---|
| 124P+ | 2512 | 100 | ChoP, 2Hex, 2EtnP, 2Hep, 2Kdo, LipA-OH | 27 | Yes |
| 124P− | 3004.0 | 45 | Sial, HexNAc, 3Hex, 2EtnP, 2Hep, 2Kdo, LipA-OH | 29 | No |
| 2712.9 | 32 | HexNAc, 3Hex, 2EtnP, 2Hep, 2Kdo, LipA-OH | |||
| 2509.5 | 23 | 3Hex, 2EtnP, 2Hep, 2Kdo, LipA-OH | |||
| 738+ | 2755 | 38 | ChoP, HexNAc, 3Hex, EtnP, 2Hep, 2Kdo, LipA-OH | 24 | Yes |
| 2590 | 24 | HexNAc, 3Hex, EtnP, 2Hep, 2Kdo, LipA-OH | |||
| 2390 | 38 | ChoP, 2Hex, EtnP, 2Hep, 2Kdo, LipA-OH | |||
| 738− | 2918 | 19 | ChoP, HexNAc, 4Hex, EtnP, 2Hep, 2Kdo, LipA-OH | 24 | Yes |
| 2755 | 12 | ChoP, HexNAc, 3Hex, EtnP, 2Hep, 2Kdo, LipA-OH | |||
| 2714 | 13 | ChoP, 4Hex, EtnP, 2Hep, 2Kdo, LipA-OH | |||
| 2590 | 19 | HexNAc, 3Hex, EtnP, 2Hep, 2Kdo, LipA-OH | |||
| 2552 | 10 | ChoP, 3Hex, EtnP, 2Hep, 2Kdo, LipA-OH | |||
| 2389 | 17 | ChoP, 2Hex, EtnP, 2Hep, 2Kdo, LipA-OH | |||
| 2224 | 10 | 2Hex, EtnP, 2Hep, 2Kdp, LipA-OH |
Kdo: 3-deoxy-d-manno-octulosonic acid. Hep: heptose. Hex: hexose. HexNAc: N-acetylhexosamine. ChoP: phosphorylcholine. EtnP: phosphoethanolamine. LipA-OH: deacylated lipid A.
The number of VNTR in H. somni lob2A, which encodes for an N-acetylglucosamine (GlcNAc) transferase, was examined to determine if there was any correlation between expression of the terminal LOS disaccharide and reactivity with anti-ChoP MAb. The full outer LOS core blocks antigenic reactivity of ChoP with MAb, and lob2A mutants fail to express the terminal lacto-N-tetraose unit [33]. The number of VNTR in lob2A of the strains examined was either 20 or 21. However, the number of repeats in lob2A was independent of, and did not correlate with, ChoP expression (Table 2).
2.5. SDS-PAGE analysis of LOS
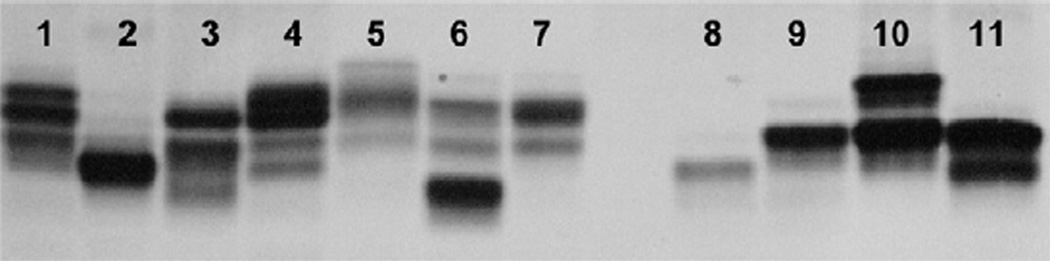
The electrophoretic profile of LOS from ChoP+ and ChoP− isolates of strains 738, 7735, and 124P are shown in Fig. 3. LOS from strains 2336 and 129Pt, neither of which express ChoP [32,34], were included as controls (lanes 10 and 11). The LOS profile from isolate 738− contained high molecular size bands that were similar to those present in parent strain 738 (lanes 4 and 1, respectively). Overall, the LOS of 738+ contained lower molecular size bands compared to the LOS of isolate 738− (lanes 3 and 4). However, the LOS of 738+ expressed bands of higher molecular size compared to LOS from a ChoP+ isolate of strain 738 obtained in a previous study (738P) (lanes 3 and 2, respectively) [25]. The highest molecular size LOS bands of isolate 7735+ were similar to those of the LOS from isolate 7735− (lanes 6 and 7, respectively). However, 7735+ LOS contained a unique lower molecular size band of high intensity. The LOS of isolate 124P+ also contained a single band of a lower molecular size than that of the predominant band present in the LOS of isolate 124P− (lanes 9 and 10, respectively), which was consistent with the results of ES-MS analysis (Table 3).
Fig. 3.
Electrophoretic profiles of LOS from ChoP+ and ChoP− variants of H. somni strains. The ChoP+ variants contain more of the lower molecular size bands than LOS from ChoP− variants and parent strains. Lanes: 1, parent strain 738; 2, 738P (a ChoP+ isolate from a previous study [27]); 3, 738+; 4, 738−; 5, parent strain 7735; 6, 7735+; 7, 7735−; 8, 124P+; 9, 124P−; 10, 2336; 11, 129Pt.
The sequence of lic1AHs from this study is available on GenBank (http//www.nbci.nlm.nih.gov) under the accession number BK001334 and glpQ sequence is available under the accession number BK001335.
3. Discussion
H. influenzae is capable of variable expression of ChoP on its LOS, which plays an important role in the organism’s ability to colonize and invade host tissues [17]. Variable expression of ChoP also occurs at a high rate on H. somni LOS and is reversible [7,25]. In H. influenzae the pathway for incorporation of ChoP into H. influenzae LOS by the lic1Hi locus has been proposed by Weiser et al. [11]. The gene lic1AHi encodes a putative choline kinase, which phosphorylates choline to form ChoP, while lic1BHi encodes a high affinity choline transporter that may be involved in uptake of choline from the environment [35]. The gene lic1CHi encodes a predicted pyrophosphorylase [11,35] that may be involved in activation of ChoP to form nucleoside diphosphocholine. The gene lic1DHi encodes a putative diphosphonucleoside choline transferase that plays a role in transfer of ChoP onto a specific LOS glycose [36]. The amino acid similarity and identical arrangement of the lic1 genes between H. somni and H. influenzae suggest that the choline uptake and utilization pathways in H. somni are similar to those of H. influenzae. However, the nucleotide sequences of these genes did not show high similarities, thereby explaining why PCR and hybridization were not successful in identifying these genes in H. somni. The low nucleotide similarity and differences in the sequence of the VNTR in lic1AHs indicate possible divergent or convergent evolution between the two organisms. However, the identical organization of genes in lic1Hs, and the presence and arrangement of three potential start codons and VNTR in lic1AHs with that of H. influenzae indicates the two species may share a common ancestry. As more genomes of the family Pasteurellaceae become available the evolution of these and other genes may become clearer.
We also identified a gene in H. somni with homology to H. influenzae glpQ, which in H. influenzae encodes an enzyme with glycerophosphoryl diester phosphodiesterase activity. GlpQ enables H. influenzae to obtain choline from glycerolphosphorylcholine, which is a degradation product of mammalian cell phospholipids, allowing the bacteria to obtain choline directly from epithelial cells in the absence of free choline [24]. The presence of a homolog of H. influenzae glpQ in H. somni suggests that the latter pathogen may use a similar mechanism of acquiring choline from the bovine host.
The insertion sequence IS1016 that interrupts lic1AHs in strain 129Pt is the only insertion sequence in lic1Hs and no similar sequences appear to flank the locus. This insertion sequence is also present in the bex capsule gene cluster in H. influenzae and is responsible for duplication of the cap locus in order for the type b capsule to be expressed [28]. The IS1016 in the genome of strain 129Pt may have contributed to the evolution of that strain, but the significance of its presence in lic1AHs is not clear.
The role of lic1AHi in expression of ChoP was determined through sequence homology to eukaryotic choline kinases [11], and generation of a gene deletion mutant that lacked expression of ChoP [36]. Further confirmation of lic1AHi function was achieved through complementing the mutant strain with a copy of the gene that was missing the VNTR, resulting in constitutively restoring ChoP expression. However, the choline kinase activity of lic1AHi has not been biochemically confirmed. In this study a lic1AHs mutant was not generated due to the lack of genetic tools to manipulate H. somni [37]. However, the homology between the H. influenzae and H. somni Lic1A proteins, including amino acid repeats, indicated that lic1AHs likely controls expression of ChoP on H. somni LOS. The absence of ChoP on the LOS of H. somni strain 129Pt [32], which has an interruption in lic1AHs, further supports the role of lic1AHs in expression of ChoP. Finally, choline kinase activity was confirmed for recombinant H. somni Lic1A using a strain with the VNTR removed so that lic1AHs was constitutively on. Furthermore, the translation of an active product from a gene missing the 5′-AACC-3′ VNTR indicated that the repeat region was not required for expression of a functional protein, confirming similar results by High et al., who showed that the 5′-CAAT-3′ VNTR are not necessary for expression of lic2A by H. influenzae [38].
The primary mechanism of antigenic variation of ChoP expression in H. influenzae is lic1AHi phase variation. SSM during DNA replication varies the number of VNTR in lic1AHi resulting in shifting the reading frame downstream of the repeats in or out of frame with the start codon. Therefore, lic1AHi phase varies ON or OFF according to the number of VNTR present [11,23], with concomitant phase variable expression of Lic1A. H. somni lob1 and lob2A contain VNTR in their ORFs and SSM in the repeats of both genes contribute to phase and antigenic variation of H. somni LOS [33,39]. However, phase variation of lic1AHs due to SSM was not entirely responsible for antigenic variation of ChoP expression in strain 738. ChoP+ and ChoP− isolates of strain 738 contained the same number of 5′-AACC-3′ repeats in lic1AHs, consistent with the gene being in the ON phase, which was confirmed by the chemical identification of ChoP on the LOS of both variants. Although the LOS of both variants contained ChoP, the LOS of the ChoP+ variant, as well as another ChoP+ variant from a previous study [25], contained more truncated LOS glycoforms. Furthermore, Western blot analysis of LOS from several H. somni strains containing ChoP showed that only the lowest molecular size glycoforms reacted with MAb to ChoP [25]. Thus, variation in the composition and extension of the oligosaccharide outer core is responsible, at least in part, for the antigenic variation of ChoP expression on strain 738. ChoP is linked to the primary glucose of the LOS outer core in strain 738 [26], and therefore, the addition of glycoses beyond the primary glucose and the change in their linkages may lead to steric interference of ChoP binding to anti-ChoP MAb [25]. Therefore, variation of the LOS outer core may be a mechanism of antigenic variation of ChoP expression in many H. somni strains that are capable of incorporating ChoP into their LOS. When H. influenzae strain RM118 is grown in the presence of sialic acid, the oligosaccharide (Neu5Ac-Gal-GlcNAc-Gal) is added to the primary glucose, which is modified by ChoP [40]. However, whether ChoP is accessible to antibody binding when in this configuration has not been determined (Derek Hood, personal communication).
The selection of isolates with an equal number of VNTR may be due to the random nature of selecting clonal isolates or may reflect a selective preference or stability of the VNTR in lic1AHs of strain 738. Effective DNA repair mechanisms play a role in stability of base pair repeat regions through effective mismatch repair after SSM [41–43]. Therefore, a high fidelity DNA repair mechanism may be responsible for reduced variation of the repeats in strain 738. However, in other strains examined (7735, 93, 124P, and 2336) there was direct correlation between the number of VNTR and expression of ChoP. Nonetheless, all ChoP+ isolates had lower molecular size LOS glycoforms, indicating a shorter oligosaccharide chain. One possibility is that the linkage site for ChoP attachment is blocked by specific glycoses or extension of the outer core. However, this is not the case in strain 738, which is a phase variant of strain 2336 that lacks ChoP [34]. In strain 2336 the glycose extension from the primary glucose is similar to that in 738, but the number of VNTR in strain 2336 lic1AHs was 25, thereby placing the gene out of frame. Therefore, in strain 2336 lack of expression of ChoP is due to phase variation of lic1AHs and not to steric interference. The number of VNTR in strains 124P, 93, and 7735 lic1AHs also correlated with the presence or absence of ChoP in their LOS, further supporting that phase variation of lic1AHs in these strains was due to SSM of the 5′-AACC-3′ repeats, although truncation of their outer core may also be a contributing factor. Of interest was the higher number of VNTR and the polymorphism in the coding sequence of lic1AHs of strain 7735, suggesting that this strain may have undergone some evolutionary divergence compared to the other strains.
In summary, we have identified the genes lic1ABCDHs and glpQ that control expression of ChoP on H. somni LOS. A functional assay of lic1AHs indicated that the gene encoded a choline kinase. Our results also showed that there are two possible mechanisms for antigenic variation of ChoP expression on H. somni LOS: phase variation of lic1AHs expression through variation of the number of VNTR, and phase variable elongation/truncation of the LOS outer core beyond the ChoP-attached glycose. Further investigation is required to understand the interrelationship between LOS composition, phase variation of other LOS biosynthesis genes, and antigenic variation of the ChoP epitope.
4. Materials and methods
4.1. Bacterial strains and growth conditions
The H. somni strains used in this study have been described [44] and are listed in Table 1. H. somni strains were grown on Columbia agar base (Difco culture media, Becton Dickinson and Company, Franklin Lakes, NJ) supplemented with 5% ovine or bovine blood (CBA). CBA plates were incubated 16–24 h at 37°C in a candle extinction jar or in the presence of 5% CO2 [45]. E. coli BL21DE3-pLysS (Invitrogen, Carlsbad, California) was grown on Luria Bertani (LB) agar plates or in LB broth supplemented with 100 µg ml−1 of ampicillin and 34 µg ml−1 of chloramphenicol. Stocks of all bacterial strains were maintained at −80°C in 10% skim milk.
4.2. Gene identification and sequence analysis
To identify putative coding sequences (CDS) in the H. somni genome, the sequences of H. influenzae lic1 and glpQ were compared to the finished genome sequences of H. somni strain 2336 (Laboratory for Genomics and Bioinformatics [Microgen], University of Oklahoma Health Sciences Center at http://www.micro-gen.ouhsc.edu/index.html) and GenBank (NC_010519), and strain 129Pt (Department of Energy Joint Genome Institute (JGI) at http://genome.jgi-psf.org /finished_microbes/haeso/haeso.home.html) and GenBank (NC_008309) using the basic local alignment search tool (BLAST) [46]. Further examination of the sequences was performed on the National Center for Biotechnology Information (NCBI) server at http://www.ncbi.nlm.nih.gov/BLAST.
For analysis and manipulation of DNA sequences, restriction mapping, and designing plasmid constructs, the BioEdit Sequence Alignment Editor version 5.0.9 was used (Tom Hall, North Carolina State, University; http://www.mbio.ncsu.edu/BioEdit/bioedit.html). Lasergene DNA and protein sequence analysis and contig alignment software was used for designing PCR primers (DNASTAR molecular biology software, http://www.dnastar.com). The Artemis DNA sequence viewer and annotation software (Sanger institute, http://www.sanger.ac.uk/Software/Artemis) was used to identify ORFs and annotate sequences from the H. somni genome.
4.3. Polymerase chain reaction (PCR) and DNA sequencing
PCR and sequencing amplification reactions were performed in either a Mastercycler gradient (Eppendorf, Westbury, NY) or a PCRExpress (Hybaid Limited, Thermo Electron Corporation, Waltham, MA) thermocycler. PCR reactions were carried out in a volume of 25–50 µl and included 1–3 units of Taq polymerase (Eppendorf, Westbury, NY), 1.5 mM MgCl2, 2 mM of dNTP, and 20 pM of primers. The primers used in this study and the corresponding PCR annealing temperatures are shown in Table 4. Genomic DNA was purified using the Puregene DNA purification kit (Gentra systems, Minneapolis, MN) according to the manufacturer’s instructions and 10–200 ng was used in PCR reactions. Alternatively single colonies were boiled in distilled water, centrifuged, and the supernatant used as a template for PCR.
Table 4.
Primers used in this study and their corresponding annealing temperatures.
| Primer | Sequence (5′ → 3′)a | Purpose | Annealing temperature |
|---|---|---|---|
| HslicA-F1 | ATCGTTAAGCGGAAAATGACT | Amplification of lic1AHs for sequencing | 50°C |
| HslicA-R1 | CTCCCAAAATCGCTAACAAA | ||
| SE-Hs-lic1A-F-EcoRI | CATTGAATTCTTAGTGTAGTATGTGCGGAG | Amplification of lic1AHs for cloning into pRSET A | 48.8 °C |
| SE-Hs-lic1A-R-HindIII | CTAATCGTTAAGCTTCACTAAATAAACCCAT | ||
| SE-pSE1-Forward-1 | AAATGAACGTTTATTTTCCATAGTA | Amplification of pRSET A (lic1A) without 5′-5′-AACC-3′ repeats | 50.4 °C |
| SE-pSE1-Reverse-1 | AACATGATATTCTTCCTATTTCCAT | ||
| YWC [49] | TATCCGGTTTATCAATGTG | Amplification of lob2A for sequencing | 50 °C |
| YWE [49] | Cy5-GAGCCTGCCATTATTATTCA |
The underlined sequence indicates the restriction endonuclease site designed into the primer.
For analysis of the VNTR repeats in lic1AHs or in lob2A, primers (Table 4) were used to amplify a region that contained the VNTR; one primerwas used for subsequent sequencing. The HslicA-F1 and HslicA-R1 primers were used to amplify lic1AHs while the YWC and YWE primers [33] were used to amplify lob2A as a control. lob2A encodes for an GlcNAc transferase that attaches the GlcNAc of the terminal lacto-N-tetraose unit onto the outer core of the LOS [33]. The amplified products were purified using the QIAquick PCR purification kit (Qiagen, Valencia, CA), and then sequenced. The BigDye Terminator cycle sequencing kit (Applied Biosystems, Foster City, CA) was used for preparing sequencing reactions with the HslicA-F1 primer or the YWC primer (Table 4); extra nucleotides were removed from the PCR product. All sequencing was done at the DNA core sequencing facility at the Virginia Bioinformatics Institute, Virginia Tech.
4.4. Vector construction
The primers SE-Hs-lic1A-F-EcoRI and SE-Hs-lic1A-R-HindIII were used to amplify a 1-kb fragment, which contained lic1AHs from an isolate of H. somni strain 738 that was reactive with MAb to ChoP (738+). The amplified fragment was cloned into the EcoRI and HindIII sites of the inducible expression vector pRSET A (Invitrogen, Carlsbad, California). The resulting vector, designated pSE1, was linearized with HincII, which cut the plasmid immediately upstream of the VNTR (which were later determined to be 5′-AACC-3′) region of lic1AHs. The primers SE-pSE1-Forward-1 and SE-pSE1-Reverse-1 were used to amplify the sequence of the linearized plasmid without including the repeat region. The amplified product was self-ligated to obtain plasmid pSE3, which contained lic1AHs missing the VNTR region (data not shown).
4.5. Biochemical enzyme assay
E. coli BL21DE3pLysS transformed with pSE3 (expressing lic1AHs) was grown to exponential phase at 37°C with shaking. The cells were then induced with 1 mM isopropyl-β-D-thiogalactopyranoside (IPTG) at 30°C for 4 hrs and washed with Tris buffered saline (TBS). The cells were lysed by use of a French Press in buffer containing 50 mM Tris–HCl pH 7.5, 1 mM ethylenediaminetetraacetic acid (EDTA), 0.3 M sucrose, 10 mM b-ME, and protease inhibitors. Unbroken cells were removed by centrifugation (30 min at 15,000 rpm) and the supernatant was used to assay for choline kinase activity. Yeast choline kinase was extracted from yeast strain KS106 (eki1cki1) that over-expressed wild type choline kinase using a multicopy vector. Yeast cells were grown to exponential phase in leucine synthetic media containing 100 mM choline, lysed using a bead beater, and unbroken cells were removed by centrifugation at 1500 × g for 10 minutes. Fifteen µg of cell extract was added to a reaction mixture containing 67 mM glycine–NaOH buffer (pH 9.5), 5 mM [14C] choline (2000 cpm/nmol), 5 mM ATP, 1.3 mM DTT, and 10 mM MgSO4 in a total volume of 30 µl, and the mixture incubated at 30°C for 20 min. Free choline was precipitated using Reinecke salt [47] and ChoP was measured using a Beckman LS 6500 scintillation counter. E. coli BL21DE3pLysS that did not contain any vectors was used as a negative control.
4.6. Colony immunoblotting
Detection of H. somni colonies expressing ChoP was performed by colony immunoblotting as previously described [6]. Blotted colonies were incubated with a 1:10 dilution of MAb 5F5.9 to ChoP [25] or a 1:4000 dilution of MAb TEPC-15 (Sigma–Aldrich, Saint Louis, MO) overnight at 4°C, and then washed with TBS. The specificity of IgG3 MAb 5F5.9 for ChoP was previously confirmed through immunoblotting, inhibition ELISA, and mass spectrometry [25]. The IgA MAb TEPC-15 is also specific for ChoP and has been used to study expression of ChoP on H. influenzae LOS [11,17]. The membranes were incubated with a 1:1000 dilution of horse radish peroxidase (HRP) conjugated to anti-mouse IgG or IgA (Jackson Immunoresearch Laboratories) for detection of MAb 5F5.9 or TEPC-15, respectively. Single ChoP+ or ChoP− colonies were selected from the CBA plates and streaked onto new plates. The colony blotting was repeated to obtain colonies that were either predominantly positive (~95%/plate) or negative (>90%/plate) for ChoP.
4.7. Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE)
E. coli BL21DE3pLysS cells transformed with pSE3 were induced with IPTG and grown in broth cultures. Bacterial samples were obtained from the broth 1, 2, and 3 h post-induction. Samples were washed with PBS, suspended in loading buffer containing β–mercaptoethanol, boiled for 10 minutes, loaded onto NuPAGE 4–12% Bis-Tris pre-cast gels (Invitrogen, Carlsbad, California), and subjected to electrophoresis at 200 volts for 35 minutes.
LOS for SDS-PAGE analysis was extracted using a micro-scale hot phenol/water method, as previously described [48]. For electrophoretic separation of LOS, a discontinuous 14% polyacrylamide gel was used [49]. After fixation and periodate oxidation, gels were stained with ammoniacal silver for visualization of LOS bands [50].
4.8. Electrospray mass spectrometry (ES-MS) analysis
LOS was O-deacylated by mild hydrazinolysis and treatment with 4 M KOH, as previously described [26]. After washing twice with cold acetone, O-deacylated LOS was redissolved in water and lyophilized. Deacylated samples were dissolved in an aqueous solvent containing 50% acetonitrile and 0.1% formic acid and analyzed on a VG Quattro triple quadrupole mass spectrometer (Fisons Instruments). The mass spectrometer was scanned from m/z 150 to 2,500 with a scan time of 10 s; the electrospray tip voltage was 2.5 kV [26]. Percentage distribution of glycoforms was determined by comparing the intensities of the doubly and triply charged ions corresponding to each glycoform in the mass spectra.
Acknowledgements
We thank Drs. Elena S. Lysenko and Jeffrey N. Weiser from the Department of Microbiology, School of Medicine, University of Pennsylvania, for their valuable advice and technical information. This work was funded by USDA-NRI grants 2001-52100-11314 and 2003-35204-13637 (to T.J.I) and by United States Public Health Service Grant GM-50679 from the National Institutes of Health (to G.M.C.).
References
- 1.Inzana TJ, Corbeil LB. Haemophilus. In: Gyles CLT, Prescott JF, Songer JG, Thoen CO, editors. Pathogenesis of bacterial infections of animals. 3rd ed. Oxford, UK: Blackwell Publishing; 2004. pp. 243–257. [Google Scholar]
- 2.Corbeil LB, Bastida-Corcuera FD, Beveridge TJ. Haemophilus somnus immunoglobulin binding proteins and surface fibrils. Infect Immun. 1997;65:4250–4277. doi: 10.1128/iai.65.10.4250-4257.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yarnall M, Widders PR, Corbeil LB. Isolation and characterization of Fc receptors from Haemophilus somnus. Scand J Immunol. 1988;28:129–137. doi: 10.1111/j.1365-3083.1988.tb02424.x. [DOI] [PubMed] [Google Scholar]
- 4.Sylte MJ, Corbeil LB, Inzana TJ, Czuprynski CJ. Haemophilus somnus induces apoptosis in bovine endothelial cells in vitro. Infect Immun. 2001;69:1650–1660. doi: 10.1128/IAI.69.3.1650-1660.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gomis SM, Godson DL, Wobeser GA, Potter AA. Effect of Haemophilus somnus on nitric oxide production and chemiluminescence response of bovine blood monocytes and alveolar macrophages. Microb Pathog. 1997;23:327–333. doi: 10.1006/mpat.1997.0162. [DOI] [PubMed] [Google Scholar]
- 6.Inzana TJ, Gogolewski RP, Corbeil LB. Phenotypic phase variation in Haemophilus somnus lipooligosaccharide during bovine pneumonia and after in vitro passage. Infect Immun. 1992;60:2943–2951. doi: 10.1128/iai.60.7.2943-2951.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Inzana TJ, Hensley J, McQuiston J, Lesse AJ, Campagnari AA, Boyle SM, et al. Phase variation and conservation of lipooligosaccharide epitopes in Haemophilus somnus. Infect Immun. 1997;65:4675–4681. doi: 10.1128/iai.65.11.4675-4681.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inzana TJ, Glindemann G, Cox AD, Wakarchuk W, Howard MD. Incorporation of N-acetylneuraminic acid into Haemophilus somnus lipooligosaccharide (LOS): enhancement of resistance to serum and reduction of LOS antibody binding. Infect Immun. 2002;70:4870–4879. doi: 10.1128/IAI.70.9.4870-4879.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weiser JN, Love JM, Moxon ER. The molecular mechanism of phase variation of Haemophilus influenzae lipopolysaccharide. Cell. 1989;59:657–666. doi: 10.1016/0092-8674(89)90011-1. [DOI] [PubMed] [Google Scholar]
- 10.Fischer W, Behr T, Hartmann R, Peter-Katalinic J, Egge H. Teichoic acid and lipoteichoic acid of Streptococcus pneumoniae possess identical chain structures. A reinvestigation of teichoid acid (C polysaccharide) Eur J Biochem/FEBS. 1993;215:851–857. doi: 10.1111/j.1432-1033.1993.tb18102.x. [DOI] [PubMed] [Google Scholar]
- 11.Weiser JN, Shchepetov M, Chong ST. Decoration of lipopolysaccharide with phosphorylcholine: a phase-variable characteristic of Haemophilus influenzae. Infect Immun. 1997;65:943–950. doi: 10.1128/iai.65.3.943-950.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Serino L, Virji M. Phosphorylcholine decoration of lipopolysaccharide differentiates commensal Neisseriae from pathogenic strains: Identification of licA-type genes in commensal Neisseriae. Mol Microbiol. 2000;35:1550–1559. doi: 10.1046/j.1365-2958.2000.01825.x. [DOI] [PubMed] [Google Scholar]
- 13.Weiser JN, Goldberg JB, Pan N, Wilson L, Virji M. The phosphorylcholine epitope undergoes phase variation on a 43-kilodalton protein in Pseudomonas aeruginosa and on pili of Neisseria meningitidis and Neisseria gonorrhoeae. Infect Immun. 1998;66:4263–4267. doi: 10.1128/iai.66.9.4263-4267.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harper M, Cox A, St Michael F, Parnas H, Wilkie I, Blackall PJ, et al. Decoration of Pasteurella multocida lipopolysaccharide with phosphocholine is important for virulence. J Bacteriol. 2007;189:7384–7391. doi: 10.1128/JB.00948-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gillespie SH, Ainscough S, Dickens A, Lewin J. Phosphorylcholine-containing antigens in bacteria from the mouth and respiratory tract. J Med Microbiol. 1996;44:35–40. doi: 10.1099/00222615-44-1-35. [DOI] [PubMed] [Google Scholar]
- 16.Schweda EK, Brisson JR, Alvelius G, Martin A, Weiser JN, Hood DW, et al. Characterization of the phosphocholine-substituted oligosaccharide in lipopolysaccharides of type b Haemophilus influenzae. Eur J Biochem/FEBS. 2000;267:3902–3913. doi: 10.1046/j.1432-1327.2000.01426.x. [DOI] [PubMed] [Google Scholar]
- 17.Weiser JN, Pan N, McGowan KL, Musher D, Martin A, Richards J. Phosphorylcholine on the lipopolysaccharide of Haemophilus influenzae contributes to persistence in the respiratory tract and sensitivity to serum killing mediated by C-reactive protein. J Exp Med. 1998;187:631–640. doi: 10.1084/jem.187.4.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Swords WE, Buscher BA, Ver SK, II, Preston A, Nichols WA, Weiser JN, et al. Non-typeable Haemophilus influenzae adhere to and invade human bronchial epithelial cells via an interaction of lipooligosaccharide with the PAF receptor. Mol Microbiol. 2000;37:13–27. doi: 10.1046/j.1365-2958.2000.01952.x. [DOI] [PubMed] [Google Scholar]
- 19.Tong HH, Blue LE, James MA, Chen YP, DeMaria TF. Evaluation of phase variation of nontypeable Haemophilus influenzae lipooligosaccharide during nasopharyngeal colonization and development of otitis media in the chinchilla model. Infect Immun. 2000;68:4593–4597. doi: 10.1128/iai.68.8.4593-4597.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Henderson IR, Owen P, Nataro JP. Molecular switches - the ON and OFF of bacterial phase variation. Mol Microbiol. 1999;33:919–932. doi: 10.1046/j.1365-2958.1999.01555.x. [DOI] [PubMed] [Google Scholar]
- 21.van der Woude MW, Baumler AJ. Phase and antigenic variation in bacteria. Clin Microbiol Rev. 2004;17:581–611. doi: 10.1128/CMR.17.3.581-611.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levinson G, Gutman GA. Slipped-strand mispairing: a major mechanism for DNA sequence evolution. Mol Biol Evol. 1987;19:1198–1202. doi: 10.1093/oxfordjournals.molbev.a040442. [DOI] [PubMed] [Google Scholar]
- 23.Moxon ER, Wills C. DNA microsatellites: agents of evolution? Sci Am. 1999;280(1):94–99. doi: 10.1038/scientificamerican0199-94. [DOI] [PubMed] [Google Scholar]
- 24.Fan X, Goldfine H, Lysenko E, Weiser JN. The transfer of choline from the host to the bacterial cell surface requires glpQ in Haemophilus influenzae. Mol Microbiol. 2001;41:1029–1036. doi: 10.1046/j.1365-2958.2001.02571.x. [DOI] [PubMed] [Google Scholar]
- 25.Howard MD, Cox AD, Weiser JN, Schurig GG, Inzana TJ. Antigenic diversity of Haemophilus somnus lipooligosaccharide: phase-variable accessibility of the phosphorylcholine epitope. J Clin Microbiol. 2000;38:4412–4419. doi: 10.1128/jcm.38.12.4412-4419.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cox AD, Howard MD, Brisson J-R, Van Der Zwan M, Thibault P, Perry MB, et al. Structural analysis of the phase-variable lipooligosaccharide from Haemophilus somnus strain 738. Eur J Biochem. 1998;253:507–516. doi: 10.1046/j.1432-1327.1998.2530507.x. [DOI] [PubMed] [Google Scholar]
- 27.Brenner S. Phosphotransferase sequence homology. Nature. 1987;329:21. doi: 10.1038/329021a0. [DOI] [PubMed] [Google Scholar]
- 28.Kroll JS, Loynds BM, Moxon ER. The Haemophilus influenzae capsulation gene cluster: a compound transposon. Mol Microbiol. 1991;5:1549–1560. doi: 10.1111/j.1365-2958.1991.tb00802.x. [DOI] [PubMed] [Google Scholar]
- 29.Kim KH, Voelker DR, Flocco MT, Carman GM. Expression, purification, and characterization of choline kinase, product of the CKI gene from Saccharomyces cerevisiae. J Biol Chem. 1998;273:6844–6852. doi: 10.1074/jbc.273.12.6844. [DOI] [PubMed] [Google Scholar]
- 30.Kim K, Kim KH, Storey MK, Voelker DR, Carman GM. Isolation and characterization of the Saccharomyces cerevisiae EKI1 gene encoding ethanolamine kinase. J Biol Chem. 1999;274:14857–14866. doi: 10.1074/jbc.274.21.14857. [DOI] [PubMed] [Google Scholar]
- 31.Cox AD, Howard MD, Inzana TJ. Structural analysis of the lipooligosaccharide from the commensal Haemophilus somnus strain 1P. Carbohydr Res. 2003;338:1223–1228. doi: 10.1016/s0008-6215(03)00113-7. [DOI] [PubMed] [Google Scholar]
- 32.St Michael F, Howard MD, Li J, Duncan AJ, Inzana TJ, Cox AD. Structural analysis of the lipooligosaccharide from the commensal Haemophilus somnus genome strain 129Pt. Carbohydr Res. 2004;339:529–535. doi: 10.1016/j.carres.2003.10.032. [DOI] [PubMed] [Google Scholar]
- 33.Wu Y, McQuiston JH, Cox A, Pack TD, Inzana TJ. Molecular cloning and mutagenesis of a DNA locus involved in lipooligosaccharide biosynthesis in Haemophilus somnus. Infect Immun. 2000;68:310–319. doi: 10.1128/iai.68.1.310-319.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.St Michael F, Li J, Howard MD, Duncan AJ, Inzana TJ, Cox AD. Structural analysis of the oligosaccharide of Histophilus somni (Haemophilus somnus) strain 2336 and identification of several lipooligosaccharide biosynthesis gene homologues. Carbohydr Res. 2005;340:665–672. doi: 10.1016/j.carres.2004.12.029. [DOI] [PubMed] [Google Scholar]
- 35.Fan X, Pericone CD, Lysenko E, Goldfine H, Weiser JN. Multiple mechanisms for choline transport and utilization in Haemophilus influenzae. Mol Microbiol. 2003;50:537–548. doi: 10.1046/j.1365-2958.2003.03703.x. [DOI] [PubMed] [Google Scholar]
- 36.Lysenko E, Richards JC, Cox AD, Stewart A, Martin A, Kapoor M, et al. The position of phosphorylcholine on the lipopolysaccharide of Haemophilus influenzae affects binding and sensitivity to C-reactive protein-mediated killing. Mol Microbiol. 2000;35:234–245. doi: 10.1046/j.1365-2958.2000.01707.x. [DOI] [PubMed] [Google Scholar]
- 37.Sandal I, Seleem MN, Elswaifi SF, Sriranganathan N, Inzana TJ. Construction of a high-efficiency shuttle vector for Histophilus somni. J Microbiol Methods. 2008;74:106–109. doi: 10.1016/j.mimet.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 38.High NJ, Jennings MP, Moxon ER. Tandem repeats of the tetramer 5′-CAAT-3′ present in lic2A are required for phase variation but not lipopolysaccharide biosynthesis in Haemophilus influenzae. Mol Microbiol. 1996;20:165–174. doi: 10.1111/j.1365-2958.1996.tb02498.x. [DOI] [PubMed] [Google Scholar]
- 39.McQuiston JH, McQuiston JR, Cox AD, Wu Y, Boyle SM, Inzana TJ. Characterization of a DNA region containing 5′-(CAAT)n-3′ DNA sequences involved in lipooligosaccharide biosynthesis in Haemophilus somnus. Microb Pathog. 2000;28:301–312. doi: 10.1006/mpat.1999.0351. [DOI] [PubMed] [Google Scholar]
- 40.Hood DW, Randle G, Cox AD, Makepeace K, Li J, Schweda EK, et al. Biosynthesis of cryptic lipopolysaccharide glycoforms in Haemophilus influenzae involves a mechanism similar to that required for O-antigen synthesis. J Bacteriol. 2004;186:7429–7439. doi: 10.1128/JB.186.21.7429-7439.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bayliss CD, van dVT, Moxon ER. Mutations in polI but not mutSLH destabilize Haemophilus influenzae tetranucleotide repeats. EMBO J. 2002;21:1465–1476. doi: 10.1093/emboj/21.6.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Strand M, Prolla TA, Liskay RM, Petes TD. Destabilization of tracts of simple repetitive DNA in yeast by mutations affecting DNA mismatch repair. Nature. 1993;365:274–276. doi: 10.1038/365274a0. [DOI] [PubMed] [Google Scholar]
- 43.van Belkum A, Scherer S, van Alphen L, Verbrugh H. Short-sequence DNA repeats in prokaryotic genomes. Microbiol Mol Biol Rev. 1998;62:275–293. doi: 10.1128/mmbr.62.2.275-293.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Corbeil LB, Blau K, Prieur DJ, Ward ACS. Serum susceptibility of Haemophilus somnus from bovine clinical cases and carriers. J Clin Microbiol. 1985;22:192–198. doi: 10.1128/jcm.22.2.192-198.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Inzana TJ, Corbeil LB. Development of a defined medium for Haemophilus somnus isolated from cattle. Am J Vet Res. 1987;48:366–369. [PubMed] [Google Scholar]
- 46.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 47.Porter TJ, Kent C. Choline/ethanolamine kinase from rat liver. Methods Enzymol. 1992;209:134–146. doi: 10.1016/0076-6879(92)09017-w. [DOI] [PubMed] [Google Scholar]
- 48.Inzana TJ. Electrophoretic heterogeneity and inter strain variation of the lipopolysaccharide of Haemophilus influenzae. J Infect Dis. 1983;148:492–499. doi: 10.1093/infdis/148.3.492. [DOI] [PubMed] [Google Scholar]
- 49.Inzana TJ, Apicella MA. Use of a bilayer stacking gel to improve resolution of lipopolysaccharides and lipooligosaccharides in polyacrylamide gels. Electrophoresis. 1999;20:462–465. doi: 10.1002/(SICI)1522-2683(19990301)20:3<462::AID-ELPS462>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 50.Tsai CM, Frasch CE. A sensitive silver stain for detecting lipopolysaccharides in poly acrylamide gels. Anal Biochem. 1982;119:115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]