Abstract
An extensive literature shows that astrocytes exhibit metabotropic glutamate receptor 5 (mGluR5)–dependent increases in cytosolic calcium ions (Ca2+) in response to glutamatergic transmission and, in turn, modulate neuronal activity by their Ca2+-dependent release of gliotransmitters. These findings, based on studies of young rodents, have led to the concept of the tripartite synapse, in which astrocytes actively participate in neurotransmission. Using genomic analysis, immunoelectron microscopy, and two-photon microscopy of astrocytic Ca2+ signaling in vivo, we found that astrocytic expression of mGluR5 is developmentally regulated and is undetectable after postnatal week 3. In contrast, mGluR3, whose activation inhibits adenylate cyclase but not calcium signaling, was expressed in astrocytes at all developmental stages. Neuroglial signaling in the adult brain may therefore occur in a manner fundamentally distinct from that exhibited during development.
The concept that electrically nonresponsive astrocytes can sense glutamatergic transmissions dates to 1990, when it was first described that cultured astrocytes exhibit increases in cytosolic Ca2+ in response to glutamate (1). This observation, in combination with the finding that astrocytic Ca2+ increases are linked to release of gliotransmitters, forms the basis for the concept of the tripartite synapse (2–4). Ample evidence has supported the observation that excitatory transmission evokes Ca2+ signaling in astrocytes by activation of metabotropic glutamate receptors (mGluRs). For example, stimulation of glutamatergic neuronal afferents in hippocampal slices triggers Ca2+ waves in astrocytes inhibited by mGluR5 antagonists (2, 5). However, almost all studies to date have employed either slices prepared from young rodents or cultured astrocytes in their analysis of neuron-glia signaling. Transcriptome analyses suggest that expression of mGluR5 is developmentally regulated and that astrocytic mGluR5 peaks at postnatal day 7 (fig. S1) (6, 7). To assess whether astrocytes express Gq-linked mGluR beyond this young age, we harvested adult human and murine astrocytes using fluorescently activated cell sorting followed by microarray analysis (FACS-array). Adult cortical or hippocampal tissue was dissociated and fluorescently tagged with an antibody directed against the astrocyte-specific glutamate transporter EAAT2 (GLT1) (8, 9). Two distinct populations of cells, GLT1+ (astrocytes) and GLT1− (all other cell types), were isolated, and the relative enrichment of genes known to be selectively expressed by astrocytes was evaluated in the two postsorted populations by quantitative real-time fluorescence polymerase chain reaction (qPCR) (fig. S2, A and B). Aldehyde dehydrogenase 1 family member L1 (ALDH1L1), the water channel aquaporin 4 (AQP4), the excitatory amino acid transporter 2 (GLT1, SLC1A2), and glial fibrillary acidic protein (GFAP) exhibited a relative enrichment of 20- to 1000-fold in GLT1+ cells (fig. S2, C and D). Conversely, the GLT1+ pool was depleted of genes expressed by other cell types (fig. S2, C and D). The pattern of expression of these genes was confirmed in both human and mouse astrocytes (fig. S2, C and E) (8). Moreover, immunolabeling and qPCR analysis confirmed that ALDH1L1 and AQP4 are enriched in the GLT1+ pool of cells isolated from 1-, 2-, 3-, and 12-week-old mice (fig. S3, A and B).
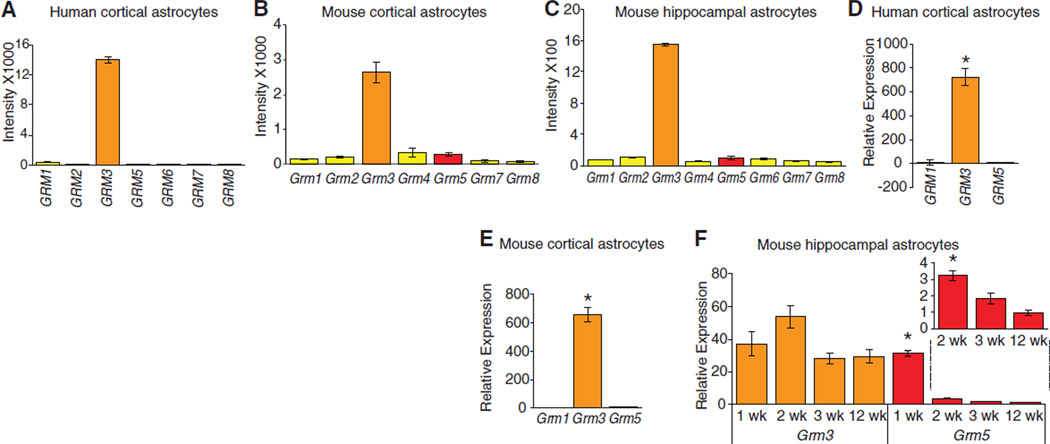
We next asked which mGlu receptors are expressed by cortical human astrocytes. The analysis suggested that mGluR3 is the major mGlu receptor expressed by adult cortical human astrocytes, whereas the expression of other mGluRs was low or absent (Fig. 1A). Similar data were collected from murine cortical and hippocampal astrocytes (Fig. 1, B and C). qPCR confirmed that only mGluR3 is enriched in adult human and mouse astrocytes (Fig. 1, D to F). In contrast, mGluR5 is only significantly expressed in astrocytes harvested from 1-week-old mice, with mGluR5 levels falling sharply by the end of week 2 (Fig. 1F) and displaying a continued decline through adulthood (Fig. 1F, inset). To validate isolation of astrocytes in young pups based on their Glt1+ expression, we also isolated Aldh1l1-eGFP+ cells in 1-week-old pups. Aldh1l1 is the most homogeneously expressed astrocyte marker (10). qPCR analysis showed that both ALDH1L1+ and GLT1+ cells’ populations in 1-week-old pups exhibited comparable levels of mGluR3 and mGluR5 mRNA expression (fig. S3C).
Fig. 1.
Expression profile of mGluRs in human and mouse astrocytes. (A to C) Microarray analysis of the expression of mGluR in (A) adult human cortical astrocytes, (B) adult mouse cortical astrocytes, and (C) adult mouse hippocampal astrocytes. Grm3 is the major mGluR expressed by human cortical astrocytes and mouse cortical and hippocampal astrocytes. (D) qPCR analysis of GLT1+ populations confirmed that GRM3 is highly enriched in human cortical astrocytes. (E) qPCR analysis of GLT1+ cell populations showed that Grm3 also is highly enriched in mouse cortical astrocytes. (F) Expression of Grm3 and Grm5 in mouse hippocampal astrocytes isolated from 1-, 2-, 3-, and 12-week-old mice. Error bars, mean ± SEM; n = 3 biological samples. *P < 0.05, one-way ANOVA, Bonferroni multiple comparisons test.
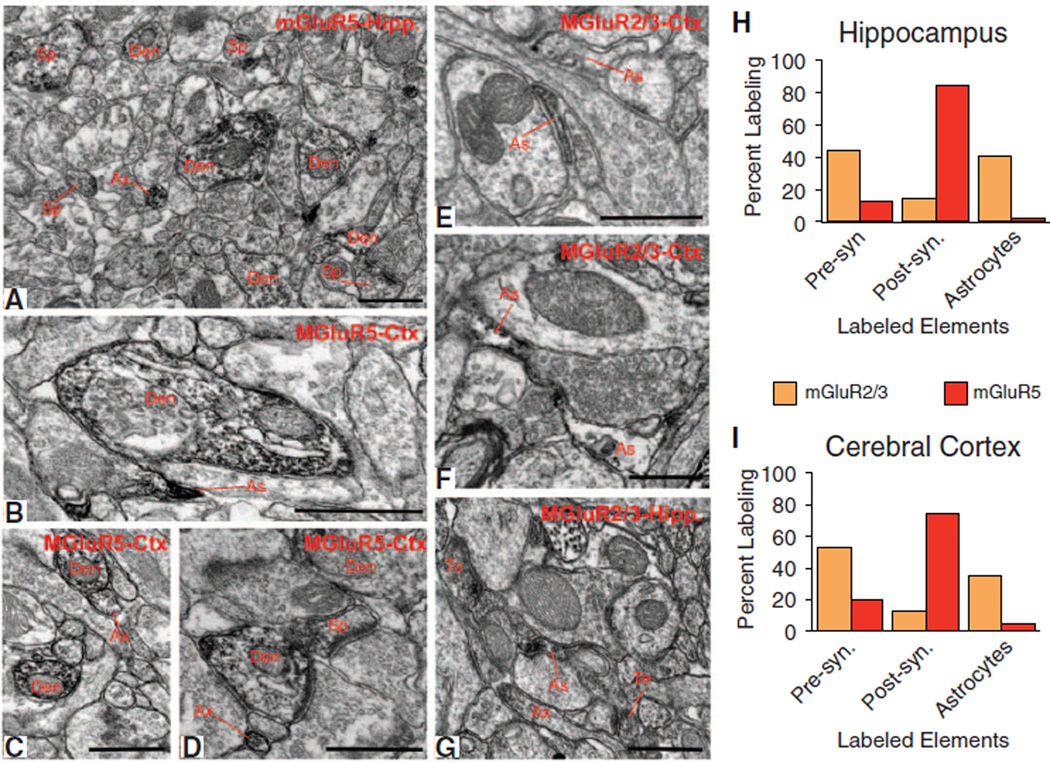
To further validate our observations of the expression of mGluR5 and mGluR3 in developing astrocytes, we immunostained cortical and hippocampal tissue of adult mice with mGluR5 or mGluR2/3 antibodies and examined the relative distribution of labeling across different neuronal and glial elements identified by their respective ultrastructural features (11). In both the cerebral cortex and hippocampus, the overall pattern of labeling for the two receptor subtypes was very similar (i.e., 70 to 80% immunoreactivity for mGluR5 was associated with postsynaptic elements such as spines and dendritic shafts), whereas less than 5% labeled structures were accounted for by glial processes (Fig. 2, A to D, H, and I). In contrast, labeling of group II mGluRs was rarely encountered in postsynaptic structures but was frequently seen in astrocytic processes (35 to 45% labeling) and preterminal axonal segments (Fig. 2, E to I) (12, 13).
Fig. 2.
Electron micrographic analysis of mGluR2/3 and mGluR5 in the adult mouse cortex and hippocampus. (A to D) Electron micrographs showing examples of mGluR5-immunoreactive elements, and (E to G) mGluR2/3-immunoreactive elements in the mouse hippocampus (Hipp) and cerebral cortex (Ctx). Ax, unmyelinated axons; Den, dendrites; As, astrocytes; Te, axon terminals. Scale bars, 0.5 µm. (H and I) Histograms showing the relative percentage of mGluR5-or mGluR2/3-immunoreactive elements categorized as presynaptic (terminals and unmyelinated axons) or postsynaptic (dendrites and spines) neuronal elements or glia. For each region and antibody, data were collected in three mice. A total of 2215 µm2 of mGluR5 or mGluR2/3 immunostained tissue was examined in both cortex and hippocampus.
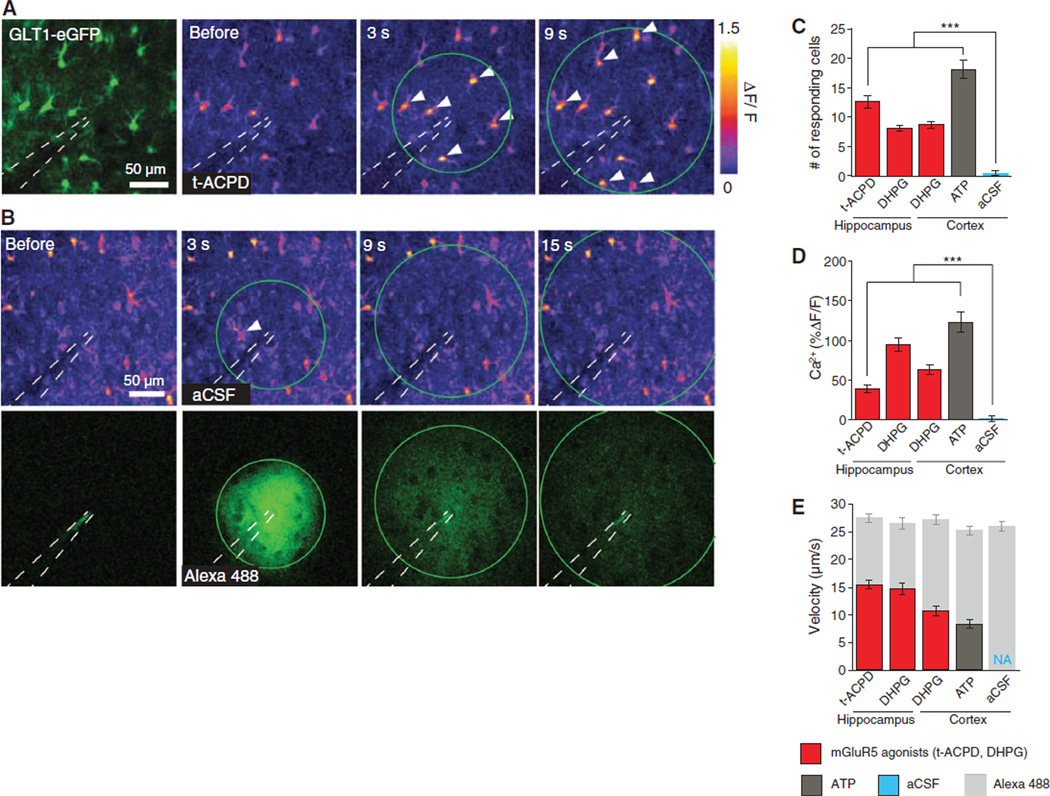
To compare the efficacy by which group I mGluR1/5 Gq-coupled receptors mobilize intracellular Ca2+ stores in young and adult mice, we used two-photon imaging to quantify astrocytic Ca2+ signaling in response to two mGluR5 agonists: 3,5-dihydroxyphenylglycine (DHPG) and (±)-1-aminocyclopentane-trans-1,3-dicarboxylic acid (t-ACPD). Acute hippocampal and cortical slices were prepared from mice pups on postnatal (P) days P12 to P15 and loaded with rhod-2 acetoxymethyl ester (AM) (Fig. 3A). The agonists were microinjected with a pipette filled with artificial cerebrospinal fluid (aCSF) containing Alexa 488 (100 µM) (Fig. 3B). Pressure injection of vehicle (aCSF) was adjusted to trigger Ca2+ increases in 0 to 2 cells per field (Fig. 3, B and C). DHPG (200 µM) or t-ACPD (500 µM) triggered robust Ca2+ waves in both hippocampal and cortical astrocytes (Fig. 3, C to E, and fig. S4). Adenosine triphosphate (ATP) (500 µm) also evoked robust increases in astrocytic Ca2+ (Fig. 3, C to E).
Fig. 3.
mGluR5 agonists trigger astrocytic Ca2+ signaling in slices prepared from mice pups. (A) The left panel shows enhanced green fluorescent protein (eGFP) driven by the GLT1 promoter; the three right panels depict relative increase in rhod-2 fluorescence signal (ΔF/F) in response to microinjection of t-ACPD in a hippocampal slice prepared from a 15-day-old mouse pup. The injection pipette is outlined. Green circle represents Alexa 488 wavefront (not shown). White arrows indicate astrocytes displaying an increase in Ca2+. (B) aCSF injection in a hippocampal slice. (Top) Changes in rhod-2 fluorescence emission (ΔF/F); (bottom) Alexa 488 diffusion. (C to E) Histograms comparing (C) number of responding astrocytes, (D) Ca2+ amplitude, and (E) Ca2+ wave velocity and Alexa 488 diffusion velocity (internal control for consistency of agonist injection). ***P < 0.001, one-way ANOVA, Bonferroni test; n = 8 to 9 trials.
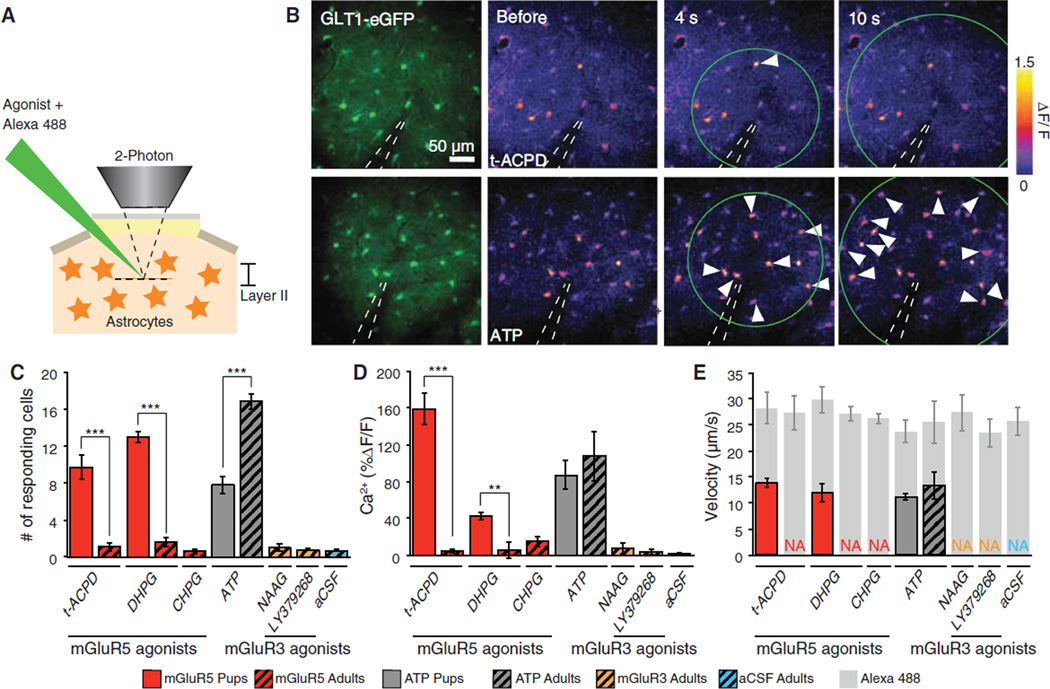
We next asked whether mGluR1/5 agonists also induced Ca2+ signaling in vivo (Fig. 4, A and B). The agonists were microinjected into the cortex of ketamine/xylazine-anesthetized pups (P12 to P15) or adult mice. Cortical astrocytes in mice pups displayed potent increases in Ca2+ in response to mGluR5 agonists (Fig. 4C), which were directly comparable to observations in acute cortical slices (Fig. 4, D and E). When the same experiments were repeated in adult anesthetized mice, astrocytes failed to respond to the mGluR5 agonists, DHPG or t-ACPD, as well as (RS)-2-chloro-5-hydroxyphenylglycine (CHPG, 500 µM). The number of astrocytes responding to either of these mGluR5 agonists was not statistically different from control aCSF injections (Fig. 4, C to E, and figs. S5 and S6). Analysis with electroencephalography showed that t-ACPD or DHPG did not significantly alter local neuronal activity (fig. S7). Because anesthesia can suppress astrocytic Ca2+ signaling (14, 15), t-ACPD injections were repeated in awake, nonanesthetized mice. Microinjection of t-ACPD in awake mice triggered an increase in Ca2+ in an average of 0.67 ± 0.33 cells (n = 7 trials), which is not significantly different from either vehicle control or t-ACPD injection in anesthetized mice [analysis of variance (ANOVA), Bonferroni test]. Application of the Gi-coupled mGluR3 receptor agonists, N-acetylaspartylglutamate (NAAG, 1 mM) or LY379268 (100 µM) failed, as expected, to elicit astrocytic Ca2+ signaling in the mouse brain (Fig. 4, C and D). The lack of agonist-induced response did not reflect an in-ability of adult astrocytes to mobilize cytosolic Ca2+ because ATP potently induced Ca2+ increases (Fig. 4C).
Fig. 4.
Multiple mGluR5 agonists fail to trigger astrocytic Ca2+ signaling in adult mice. (A) Experimental setup used for microinjection of agonist in cerebral cortex layer II in anesthetized mice loaded with rhod-2 AM. (B) (Top left) EGFP driven by the astrocyte-specific GLT1 promoter in an adult mouse. (Top right) Time lapse of changes in rhod-2 fluorescence signal (ΔF/F) in response to microinjection of t-ACPD (500 µM). One astrocyte (white arrow) exhibited an increase in rhod-2 signal. (Bottom) Rhod-2 signal changes (ΔF/F) in response to ATP (500 µM) in an adult mouse. White arrows indicate astrocytes that exhibited an increase in Ca2+. (C to E) Histograms comparing (C) number of astrocytes displaying an increase in Ca2+ in response to agonist injections in pups and adult mice, (D) Ca2+ amplitude, and (E) Ca2+ wave velocity and Alexa 488 diffusion velocity (internal control for consistency of agonist injection). **P < 0.01, ***P < 0.001, one-way ANOVA, Bonferroni test; n = 4 to 19 trials.
The tripartite synapse forms a central element in work implicating astrocytes in long-term potentiation and complex neurocognitive functions such as sleep (2, 5, 16, 17). Our analysis has broad implications because it shows that Gq-coupled group I mGluRs are not expressed by adult murine cortical and hippocampal astrocytes or by adult cortical human astrocytes. Astrocytic Ca2+ signaling evoked by synaptic release of glutamate may thus be confined to the young rodent pups, which most commonly are employed in studies of neuron-glia signaling (3). The observations reported here do not call into question that astrocytes can be indirectly activated by neural activity. A number of transmitters, including endocannabinoids, purines, norepinephrine, and acetylcholine, as well as changes in extracellular Ca2+, can trigger astrocytic Ca2+ signaling (14, 18–21). Yet, activation of these pathways is typically limited to episodes of intense glutamatergic transmission or to the global release of neuromodulators that occur in the setting of arousal or awakening.
Our analysis shows that adult astrocytes express mGluR3, which is a Gi/Go receptor negatively coupled to adenylate cyclase. Can activation of mGluR3 trigger gliotransmitter release? This seems unlikely, because mGluR3 agonist failed to trigger Ca2+ increases in astrocytes (Fig. 4, C and D), and regulated exocytosis generally is enhanced by cyclic adenosine monophosphate (cAMP)–dependent protein kinase (PKA). The slow time scale of Gi/Go-coupled processes likely excludes their involvement in rapid synaptic events. Thus, the existing literature plus the lack of mGluR5-induced Ca2+ increases suggest that mGluR3 receptors are not involved in gliotransmitter release.
In particular, our observations indicate that the Gi/Go-coupled suppression of cAMP signaling is the principal intracellular pathway by which astrocytes monitor local glutamatergic transmission in the adult CNS and further suggest that glutamatergic signaling per se is insufficient to trigger astrocytic Ca2+ signaling.
Supplementary Material
Acknowledgments
This work was supported by the National Institute of Neurological Disorders and Stroke, NIH (NS075177 and NS078304), the NIH National Center for Research Resources base grant of the Yerkes National Primate Research Center (RR00165), and the National Center for Research Resources P51RR000165 the Office of Research Infrastructure Programs/OD P51OD011132 to the Yerkes National Primate Research Center, and an American Heart Association Predoctoral Fellowship (12PRE12030048). We thank A. Cornwell, P. Kammermeier, T. Takano, and B. Kress for discussions; J. Rothstein for transgenic mice; and B. Barres and N. Heintz for generously sharing their transcriptome data.
Footnotes
Supplementary Materials
www.sciencemag.org/cgi/content/full/339/6116/197/DC1
Materials and Methods
Figs. S1 to S7
References (22–25)
References and Notes
- 1.Cornell-Bell AH, Finkbeiner SM, Cooper MS, Smith SJ. Science. 1990;247:470. doi: 10.1126/science.1967852. [DOI] [PubMed] [Google Scholar]
- 2.Araque A, Parpura V, Sanzgiri RP, Haydon PG. Trends Neurosci. 1999;22:208. doi: 10.1016/s0166-2236(98)01349-6. [DOI] [PubMed] [Google Scholar]
- 3.Nedergaard M, Verkhratsky A. Glia. 2012;60:1013. doi: 10.1002/glia.22288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rusakov DA, Zheng K, Henneberger C. Neuroscientist. 2011;17:513. doi: 10.1177/1073858410387304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Panatier A, et al. Cell. 2011;146:785. doi: 10.1016/j.cell.2011.07.022. [DOI] [PubMed] [Google Scholar]
- 6.Cahoy JD, et al. J. Neurosci. 2008;28:264. doi: 10.1523/JNEUROSCI.4178-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doyle JP, et al. Cell. 2008;135:749. doi: 10.1016/j.cell.2008.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lovatt D, et al. J. Neurosci. 2007;27:12255. doi: 10.1523/JNEUROSCI.3404-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lovatt D, Nedergaard M. In: Neuroglia. ed. 3. Kettenmann H, Ransom BR, editors. New York: Oxford Univ. Press; 2012. pp. 347–357. [Google Scholar]
- 10.Zhang Y, Barres BA. Curr. Opin. Neurobiol. 2010;20:588. doi: 10.1016/j.conb.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 11.Peters A, Palay SL, Webster HD. The Fine Structure of the Nervous System: Neurons and Their Supporting Cells. vol. 18. New York: Oxford Univ. Press; 1991. [Google Scholar]
- 12.Petralia RS, Wang YX, Niedzielski AS, Wenthold RJ. Neuroscience. 1996;71:949. doi: 10.1016/0306-4522(95)00533-1. [DOI] [PubMed] [Google Scholar]
- 13.Shigemoto R, et al. J. Neurosci. 1997;17:7503. doi: 10.1523/JNEUROSCI.17-19-07503.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schummers J, Yu H, Sur M. Science. 2008;320:1638. doi: 10.1126/science.1156120. [DOI] [PubMed] [Google Scholar]
- 15.Thrane AS, et al. Proc. Natl. Acad. Sci. U.S.A. 2012;109:18974. doi: 10.1073/pnas.1209448109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Halassa MM, et al. Neuron. 2009;61:213. doi: 10.1016/j.neuron.2008.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pascual O, et al. Science. 2005;310:113. [Google Scholar]
- 18.Torres A, et al. Sci. Signal. 2012;5:ra8. doi: 10.1126/scisignal.2002160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Navarrete M, Araque A. Neuron. 2008;57:883. doi: 10.1016/j.neuron.2008.01.029. [DOI] [PubMed] [Google Scholar]
- 20.Bekar LK, He W, Nedergaard M. Cereb. Cortex. 2008;18:2789. doi: 10.1093/cercor/bhn040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takata N, et al. J. Neurosci. 2011;31:18155. doi: 10.1523/JNEUROSCI.5289-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.